Abstract
Background:
Hirsutism is one of the most important diseases that lead women to refer to dermatology clinic. Hyperprolactinemia is one of the causes of hirsutism. The aim of this study was to determine prolactin (PRL) levels in hirsute women.
Materials and Methods:
In this cross-sectional study, hirsute patients were evaluated. For all of the patients, 2 or 3 days after mense starting, hormone level tests were performed, and 200 patients that had not polycystic ovary syndrome enrolled to the study. A questionnaire of history and physical examination were performed. Data have been analyzed with SPSS version 21.
Results:
Hyperprolactinemia were seen in 25 patients (12.5%). There was no significant relation between marital statuses, galactorrhoea, positive family history, and infertility with hyperprolactinemia. But significant relation was seen between irregular mense and hyperprolactinemia.
Conclusion:
Although hyperprolactinemia is the rare cause of hirsutism, the prevalence of hyperprolactinemia was high in our study. Thus, PRL level in hirsute patients should be evaluate.
Keywords: Hirsutism, hyperprolactinemia, polycystic ovary syndrome
INTRODUCTION
Hirsutism is an abnormal growth of terminal hairs in areas dependent on androgen in women and is referred as an increase of unwanted hairs more than what is acceptable in a race or society.[1] Hirsutism is a cultural attitude and what is considered hirsutism in one culture may be considered typical in another. Therefore, the frequency of hirsutism is different in different societies.[2] For example, a woman who is regarded hirsute in a culture may be completely normal in another culture.[3] In the conducted studies, the frequency of hirsutism is approximately 13% in Iran.[4] The difference in Iran from the foreign studies is probably due to racial difference and difference of age groups of the studies. Hirsutism is a symptom of hyperandrogenism and different causes such as polycystic ovary syndrome (PCOS), congenital adrenal hyperplasia, androgen-secreting ovarian and adrenal tumors, Cushing syndrome, pregnancy, hyperprolactinemia, gonads disorders, drug intake, and idiopathic causes can induce it.[5,6] Its prevalent symptoms include obesity, acne, menstrual disorders, and alopecia which can cause affliction of the person with PCOS.[7]
About half of the hirsute people are obese, and there is a significant relationship between obesity and hirsutism in different studies.[8,9]
Hyperprolactinemia is observed in about 5–10% of the patients with amenorrhoea which is almost accompanied by galactorrhoea and one of its important complications is infertility. Of the causes of hyperprolactinemia are drug intake and thyroid gland disorders.[10,11]
Considering frequency of hirsutism and failure to study prolactin (PRL) level in patients, the performance of this study about hirsutism causes can help confront with the hirsute patients to find the main cause and determine a suitable solution for patients.
MATERIALS AND METHODS
In this cross-sectional study, all patients with hirsutism referring to the Dermatology Clinic of Babol city, Northern Iran, were included in the study. After giving necessary explanations about this study and receiving a letter of consent, a questionnaire including a history of the disease and physical examination was completed. History of disease included age, marital status, term of affliction with hirsutism, menstrual disorder (<21 days and more than 35 days), history of galactorrhoea, history of androgenic alopecia, history of hirsutism, history of thyroid disease, PCOS, and treatment for infertility (bromocriptine, clomiphene, and metformin).
Morning venous blood samples were obtained after a 12 h overnight fasting from the subjects at days 2 to 3 of a menstrual cycle, and immediately centrifuged, and the serum was frozen and stored until all the samples from all the cases had been collected. Follicle stimulating hormone (FSH), luteinizing hormone (LH), PRL, dehydroepiandrosterone sulfate (DHEA-S), 17-OH progesterone, testosterone, sex hormone-binding globulin (SHBG), thyroid-stimulating hormone (TSH), T3, and T4 tests were performed for all patients.[12]
In case, there was suspicion about PCOS syndrome, pelvic ultrasonography was requested for the patients. According to the definition, the cases with more than ten cysts with dimensions of 2–8 mm in ovary were called PCOS. In the case of confirming the diagnosis, these patients were excluded from the study. Data obtained from tests were also recorded in the related questionnaire.
A chemiluminescence immunoassay system was used for the determination of sex hormones (DiaSorin, Germany). Testosterone of more than 1.2 ng/ml, DHEA-S of more than 340 μg/ml, PRL of more than 27 ng/ml, LH of more than 27 IU/L, and FSH of more than 15 IU/L, 17-hydroxy progesterone of more than 300 ng/ml, SHBG of more than 20 nmol/L, TSH of more than 5.1 mIU/ml, T3 of more than 3.1 nmol/L, and T4 of more than 160 nmol/L were regarded abnormal.
Data were collected and coded. After being recorded in the designed tables, it was entered in version 21 of SPSS version 21 for Windows (IBM Corp, Armonk, NY, USA) and then statistically analyzed. The obtained information was described by presenting tables of frequency and the related figures. To describe qualitative characteristics, frequency and percent were used and for quantitative characteristics, mean and range of variations were used. To compare qualitative variables, Chi-square test was used and to determine the relationship between quantitative variables, a t-test was used in case of normal distribution. P < 0.05 was regarded significant.
RESULTS
During the study period, 200 patients were included. The mean age of the studied people was 26.17 ± 6.05 years. The minimum age of patients was 16 years, and the maximum age was 45 years. The majority of patients (37%) were in age group of 21–25 years. The mean duration of hirsutism was 4.96 ± 3.75 years in the studied people. Eighty-five patients (42.5%) were single, 115 (57.5%) were married, and 23 married patients (20%) underwent pharmacotherapy due to infertility. Half of the patients (100 patients) had regular menses, whereas the remaining 100 patients had irregular menses. Sixty percent found to have a first-degree family history of hirsutism. In studied patients, 18 patients (9%) had galactorrhoea, 45 patients (22.5%) had androgenic alopecia, and 29 patients (14.5%) had the history of ovary cyst.
Out of the 200 patients in the study, 25 patients (12.5%) had hyperprolactinemia. The mean level of PRL in the studied people was 16.69 ± 7.24 ng/ml.
Table 1 shows the comparison of the patients’ complaint with hyperprolactinemia.
Table 1.
Comparing the studied variables with hyperprolactinemia in the studied patients
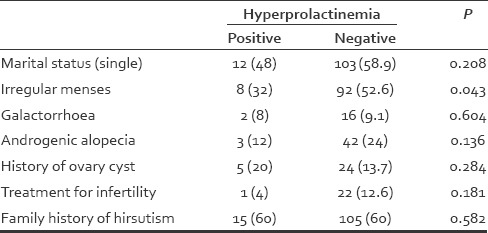
The mean age of the people with hyperprolactinemia was 26.16 ± 5.51 years, and the mean age of the people without hyperprolactinemia was 26.17 ± 6.13 years. The mean age between the people with hyperprolactinemia and the people without hyperprolactinemia did not show a significant difference (P = 0.914). Twelve patients with hyperprolactinemia (48%) were single. Hyperprolactinemia was found in two patients with galactorrhoea (8%), three patients with androgenic alopecia (12%), five patients with androgenic alopecia (12%), five patients with the history of ovary cyst (20%), one patient receiving drug for infertility (among 115 married women), and 15 patients with positive family history of hirsutism. There was no significant difference between these cases and hyperprolactinemia, but there was a significant difference between regular menses and hyperprolactinemia [Table 1].
In general, hormone tests were normal in 106 patients (53%).
Measurement of DHEA-S level showed that 18 patients (9%) had a serum level more than 340 μg/ml, and there was hyperprolactinemia in two patients.
Twenty-two patients (11%) had hypothyroidism, and all of them underwent treatment. Thyroid serum tests were normal in all treated cases, but three cases of these patients had hyperprolactinemia.
After measuring LH and FSH and calculating the ratio of LH to FSH, LH/FSH ratio was more than 3 in four patients who did not have hyperprolactinemia, and ovary lesions were not found in these four patients after performing ultrasonography.
Testosterone level was abnormal in 62 patients (31%) among whom 19 patients had hyperprolactinemia in 11 patients with high testosterone, DHEA-S was high but they did not have hyperprolactinemia. None of the studied people had abnormal SHBG and 17-hydroxy progesterone level.
DISCUSSION
Hirsutism makes different problems for women due to the creation of hairs in some parts of the body which are visible. For this reason, many patients who refer to treat hirsutism are young women so that mean age of the studied people was 26.18 ± 6.05 in this study, and most patients were in age group of 21–25 years. The mean term of affliction with hirsutism in the studied people was 4.96 ± 3.75 years.
Similar to our study, the patients with hirsutism are young women in similar studies. In a study by Farnaghi et al.,[13] which investigated 110 patients with hyperprolactinemia in the Razi Hospital of Tehran, mean age of the patients was 29.7 ± 3.2 years with an age range of 16–38 years. The term of affliction with hirsutism was 8 months to 7 years with the mean age of 4.2 ± 1.2 years. In the study by Tirgar-Tabari et al.,[14] most patients (70.1%) were in age group of 20–23 years. In the study by Rahimnejad et al.[15] in Southern Iran, which 81 patients with hirsutism were studied, mean age was 22.5 ± 0.5 years (between 13 and 41 years). About 90.1% of the patients were below the age of 30 years.
Considering that one of the symptoms of endogenous glands diseases particularly ovary diseases is irregular menses, irregular menses was also investigated in this study. In our study, half of the patients (50%) had irregular menses. In other studies, irregular menses was 38% in study by Farnaghi et al.,[13] 38% in study by Tirgar-Tabari,[14] 23.4% in study by Yazdanfar et al.,[16] 19.8% in study by Ghaderi et al.,[17] and 52% in study by Rahimnejad et al.[15] Difference in the reported percent can be attributed to other diseases and racial and hereditary causes.
In our study, 60% of the patients had a positive family record in the first degree relatives in terms of affliction with hirsutism. In different studies, different percent have been reported in terms of positive family record among people with hirsutism. A positive family record of hirsutism was found in 29% of patients in study by Farnaghi et al.,[13] 28% of patients in study by Tirgar-Tabari,[14] 72.8% in study by Rahimnejad et al.,[15] 40.7% in study by Yazdanfar et al.,[16] and 67.5% in study by Ghaderi et al.[17] Considering high positive family record in our study, it can be said that genetic has played an effective role in hirsutism. It should be considered that there is a strong family relation in some endogenous glands such as PCOS or adrenal congenital hyperplasia one of which is hirsutism.[18]
In this study, 9% of the patients had galactorrhoea, 22.5% had androgenic alopecia, and 14.5% had ovary cyst record. Moreover, 23 out of 115 married patients (20%) underwent pharmacotherapy due to infertility. In a study by Tirgar-Tabari et al.,[14] 34% of the patients had androgenic alopecia. Low rate of androgenic alopecia in our study can be due to the exclusion of the patients with PCO because androgenic alopecia is one of the manifestations of PCOS syndrome.[19]
In our study, 25 patients (12.5%) had hyperprolactinemia. Hyperprolactinemia was found in two patients (8%) with galactorrhoea, three patients (12%) with androgenic alopecia, five patients (20%) with a history of ovary cyst, one patient receiving the drug for infertility (115 married women) and 15 patients with a positive family record of hirsutism. There was a significant difference between menstrual disorder and hyperprolactinemia. Comparison of mean age between the people with hyperprolactinemia and the people without hyperprolactinemia and comparison of the mean term of affliction with hirsutism between the people with hyperprolactinemia and the people without hyperprolactinemia did not show a significant difference.
There are many differences in PRL level in our study and other similar studies. Hyperprolactinemia was 12.5% in our study, 22.8% in the study by Ahmad et al.,[20] 14.9% in the study by Heidarieh et al.,[21] 0.3% in the study by Azziz et al.,[22] 0% in the study by Carmina et al.,[23] and 10.1% in the study by Rahimnejad et al.[15] Hereditary and racial issues can be the causes of these differences. The important point of our study is that the patients with PCOS, which may be accompanied by hyperprolactinemia,[24,25] were excluded from the study unlike other studies so that high PRL level is not be a confounding factor due to affliction with PCO.
PRL hormone is produced by pituitary gland. Prolactinoma is the most prevalent pituitary gland tumor which secrets high amount of PRL and can cause hirsutism, infertility, galactorrhoea, acne, and headache.[26,27] Pituitary gland tumors should be considered in the people with high PRL. Although increase in the concentration of blood PRL can be due to pregnancy, primary hypothyroidism, intake of some drugs (chlorpromazine, perphenazine, haloperidol, metoclopramide, alpha methyldopa, resertin, opioids, amitriptyline, fluoxetine, verapamil, estrogen, and antiandrogen) and PCOS.[27]
CONCLUSION
In our study, despite the exclusion of patients with PCO, the prevalence of hyperprolactinemia was high. Therefore, high serum PRL level should be considered in the referring people with hirsutism. Considering the importance of hyperprolactinemia as one of the causes of infertility and unawareness of patients with hirsutism, the hirsute patients particularly those with a positive family record of hirsutism are recommended to inform the physician and necessary studies should be conducted in this regard.
Financial support and sponsorship
The present work was supported by a grant from the Babol University of Medical Sciences. No additional external funding received for this study.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lumachi F, Basso SM. Medical treatment of hirsutism in women. Curr Med Chem. 2010;17:2530–8. doi: 10.2174/092986710791556005. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R. The evaluation and management of hirsutism. Obstet Gynecol. 2003;101(5 Pt 1):995–1007. doi: 10.1016/s0029-7844(02)02725-4. [DOI] [PubMed] [Google Scholar]
- 3.Somani N, Turvy D. Hirsutism: An evidence-based treatment update. Am J Clin Dermatol. 2014;15:247–66. doi: 10.1007/s40257-014-0078-4. [DOI] [PubMed] [Google Scholar]
- 4.Tehrani FR, Rashidi H, Azizi F. The prevalence of idiopathic hirsutism and polycystic ovary syndrome in the Tehran lipid and glucose study. Reprod Biol Endocrinol. 2011;9:144. doi: 10.1186/1477-7827-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setji TL, Brown AJ. Polycystic ovary syndrome: Update on diagnosis and treatment. Am J Med. 2014;127:912–9. doi: 10.1016/j.amjmed.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Unluhizarci K, Kaltsas G, Kelestimur F. Non polycystic ovary syndrome-related endocrine disorders associated with hirsutism. Eur J Clin Invest. 2012;42:86–94. doi: 10.1111/j.1365-2362.2011.02550.x. [DOI] [PubMed] [Google Scholar]
- 7.Brewer M, Pawelczak M, Kessler M, Shah B. A review of polycystic ovarian syndrome in adolescents. Minerva Pediatr. 2010;62:459–73. [PubMed] [Google Scholar]
- 8.Mustaqeem M, Sadullah S, Waqar W, Farooq MZ, Khan A, Fraz TR. Obesity with irregular menstrual cycle in young girls. Mymensingh Med J. 2015;24:161–7. [PubMed] [Google Scholar]
- 9.Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: Skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901–16. doi: 10.1016/j.jaad.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Onal ED, Saglam F, Sacikara M, Ersoy R, Cakir B. Thyroid autoimmunity in patients with hyperprolactinemia: An observational study. Arq Bras Endocrinol Metabol. 2014;58:48–52. doi: 10.1590/0004-2730000002846. [DOI] [PubMed] [Google Scholar]
- 11.Wang AT, Mullan RJ, Lane MA, Hazem A, Prasad C, Gathaiya NW, et al. Treatment of hyperprolactinemia: A systematic review and meta-analysis. Syst Rev. 2012;1:33. doi: 10.1186/2046-4053-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maleki A, Rashidi N, Aghaei Meybodi H, Montazeri M, Montazeri M, Falsafi F, et al. Metabolic syndrome and inflammatory biomarkers in adults: A population-based survey in western region of Iran. Int Cardiovasc Res J. 2014;8:156–60. [PMC free article] [PubMed] [Google Scholar]
- 13.Farnaghi F, Seyrafi H, Zarrinpour N. Descriptive study of 110 patients with hirsutism in Tehran Razi Hospital during the years 2000-2001. Iran J Dermatol. 2002;6:21–5. [Google Scholar]
- 14.Tirgar-Tabari S, Haji Ahmadi M, Gholi Nejad N, Talebzadeh Noori Z. Frequency of hirsutism among females students in Babol University of Medical Sciences, 1999. J Babol Univ Med Sci. 2002;4:20–4. [Google Scholar]
- 15.Rahimnejad M, Saleh A, Jandaghi A, Amirabadi Y, Shayan Z. Evaluation of serum level of testosterone, dehydropiandrosterone sulphate (DHEA-S) prolactine, luteinizing hormone (LH) follicle stimulating hormone (FSH) in women with hirsutism. J Pars Med Univ. 2007;5:7–14. [Google Scholar]
- 16.Yazdanfar A, Beyhaghi Z, Manouchehrian N. Frequency of hirsutism in female students of Hamadan university of medical sciences. J Hamadan Univ Med Sci. 1997;4:26–32. [Google Scholar]
- 17.Ghaderi R, Sharifzadeh G, Faramarzi R, Faramarzi R. Relationship between hirsutism and sex hormones. J Birjand Univ Med Sci. 2004;11:11–7. [Google Scholar]
- 18.Bazarganipour F, Taghavi SA, Montazeri A, Ahmadi F, Chaman R, Khosravi A. The impact of polycystic ovary syndrome on the health-related quality of life: A systematic review and meta-analysis. Iran J Reprod Med. 2015;13:61–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Agapova SE, Cameo T, Sopher AB, Oberfield SE. Diagnosis and challenges of polycystic ovary syndrome in adolescence. Semin Reprod Med. 2014;32:194–201. doi: 10.1055/s-0034-1371091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad QM, Shah IH, Sameem F, Kamili QU, Sultan J. Hirsutism in Kashmir: An etiological study. Indian J Dermatol. 2009;54:80–2. doi: 10.4103/0019-5154.48997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidarieh M, Niroumanesh S, Hajizadeh A. Evaluation the grade of hirsutism in hyperprolactinemic hirsute women. Daneshvar. 2004;49:13–8. [Google Scholar]
- 22.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: Experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–62. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 23.Carmina E, Rosato F, Jannì A, Rizzo M, Longo RA. Extensive clinical experience: Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab. 2006;91:2–6. doi: 10.1210/jc.2005-1457. [DOI] [PubMed] [Google Scholar]
- 24.Robin G, Catteau-Jonard S, Young J, Dewailly D. Physiopathological link between polycystic ovary syndrome and hyperprolactinemia: Myth or reality? Gynecol Obstet Fertil. 2011;39:141–5. doi: 10.1016/j.gyobfe.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Su HW, Chen CM, Chou SY, Liang SJ, Hsu CS, Hsu MI. Polycystic ovary syndrome or hyperprolactinaemia: A study of mild hyperprolactinaemia. Gynecol Endocrinol. 2011;27:55–62. doi: 10.3109/09513590.2010.487606. [DOI] [PubMed] [Google Scholar]
- 26.Glintborg D, Altinok M, Mumm H, Buch K, Ravn P, Andersen M. Prolactin is associated with metabolic risk and cortisol in 1007 women with polycystic ovary syndrome. Hum Reprod. 2014;29:1773–9. doi: 10.1093/humrep/deu133. [DOI] [PubMed] [Google Scholar]
- 27.Housman E, Reynolds RV. Polycystic ovary syndrome: A review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847. doi: 10.1016/j.jaad.2014.05.007. [DOI] [PubMed] [Google Scholar]


