Abstract
Introduction:
Congenital heart defects (CHDs) are an important cause of mortality and morbidity in children representing a major global health burden. It is thus important to determine their prevalence and spectrum and identify risk factors associated with the development of heart defects.
Materials and Methods:
A case-control study was carried out in the Department of Pediatrics and Center of Cardiology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India, from February 2014 to August 2015. All patients referred with complaints or clinical examination suggestive of CHDs were further evaluated with echocardiography. On Echocardiography, patients having CHDs were included as cases and those having a normal echocardiographic study were included as controls. Healthy controls were also included. 400 cases and 400 controls were thus identified; preterms having patent ductus arteriosus and patent foramen ovale and those with acquired heart defects were excluded. Risk factors among cases and controls were further studied.
Results:
Acyanotic heart defects were 290 (72.50%) of the total heart defects, whereas the contribution of cyanotic heart defects was 110 (27.50%). Out of all CHDs, ventricular septal defect was the most common lesion with contribution of 152 (38%) cases, whereas among the cyanotic heart defects, Tetralogy of Fallot was the most common lesion (18% of total cases). Out of the total 400 cases, 261 were males (65.25%). On univariate analysis, paternal age (odds ratio, OR, 2.01), bad obstetric history (OR, 2.65), antenatal febrile illness (OR, 4.12), and advanced maternal age (OR, 3.28) were found to increase the risk of CHD whereas intake of multivitamin (OR, 3.02) was found to be protective. The risk factors were further analyzed with multivariate logistic regression analysis and all the above factors were found to be significantly associated.
Conclusion:
We noted that the profile of CHD in our population was similar to the published literature although many were missed during infancy and detected later in life. Several antenatal factors were found to be associated with the incidence of congenital heart disease emphasizing the need to prioritize antenatal care and counseling to pregnant mothers along with good maternal nutrition and folic acid supplementation.
Keywords: Age, congenital heart disease, profile, risk factors
INTRODUCTION
Congenital heart defect (CHD) is the most common defect among all birth defects representing a major global health problem. Twenty-eight percent of all major congenital anomalies consist of heart defects.[1] The worldwide prevalence of CHD is estimated to be eight to ten per 1000 live births[2] but the prevalence greatly varies between regions. Prevalence of CHD in India is reported to be between 2.5 to 5/1000 live births but recent studies by Bhat et al.[3] and Smitha et al.[4] have suggested the prevalence to be between 8.5 and 13.6. These are primarily seen in neonates, infants, and children; although in our country, it is not uncommon to see adults with uncorrected CHD. The reported prevalence of CHD in our country seems to have dramatically increased in recent decades, but this is most likely because of better diagnostic procedures, especially echocardiography. In India, as large numbers of births are conducted at home by unskilled health workers, we do not have many studies related to profile and risk factors of various CHDs.
In most of the studies, it has been found that ventricular septal defect (VSD) is the most common lesion followed by patent ductus arteriosus (PDA). The other common lesions are atrial septal defect (ASD), pulmonary stenosis (PS), and coarctation of aorta. A community-based study by Bhat et al.[3] in Uttarkhand reported VSD (30.4%) as the most common congenital heart disease followed by ASD (17.63%), PDA (9.62%), and PS (6.41%). Commonly identified risk factors for CHD includes multivitamins and folic acid-deficient diet during pregnancy, maternal diabetes, febrile illnesses, consanguinity, systemic lupus erythematosus (SLE) in the mother, bad obstetric history (including previous history of abortions and stillbirths), advanced paternal/maternal age, and maternal drug exposure.
NEED OF STUDY
CHD is a group of diseases that is fairly common though largely unmanaged in our country; it accounts for good proportion of neonatal and infant mortality in the country. The burden of CHD in India is likely to be the largest among all nations in the world simply because of the fact that there are more children born in India than anywhere else.[5] The majority of children with CHDs in our region escape detection, reasons being lack of awareness, poor socioeconomic status, and poor availability of Echocardiography. Uttar Pradesh is India’s most populous state: It would be the world’s fifth most populous nation, next to China, India, the United States, and Indonesia, with infant mortality rate (IMR) of 53 (India-42) (Census 2011). As the infant mortality from readily preventable causes declines, contribution of CHD to IMR would likely to increase.[6] This study was undertaken to study the profile of patients with CHD in western Uttar Pradesh and also the risk factors associated with it so that preventive measures can be taken to prevent CHD during pregnancy.
MATERIAL AND METHODS
This is an analytical case-control study done in the Department of Pediatrics and Center of Cardiology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India, from February 2014 to September 2015, with the aim to determine the profile of different CHDs in children and newborns and the risk factors associated with CHD. All children suspected of congenital heart disease presenting to pediatric OPD/IPD/Nursery, on the basis of history and clinical examination, were included. A suspected case was defined as any child with SpO2 (Saturation) < 93% in room air or visible cyanosis, history of frequent chest infections/unexplained failure to thrive/feeding difficulty/effort intolerance, unexplained congestive heart failure, murmur, abnormal electrocardiography (ECG), abnormal heart sounds, abnormal blood pressure, differential peripheral pulses, and abnormal chest x-ray. Parents of children who had not given consent of participating in the study and cases of acquired heart disease (eg, RHD) were excluded. All children were then screened through ECG and chest x-ray and the diagnosis was confirmed by echocardiography. Patients with any structural heart disease on echocardiography were grouped as cases, whereas those found with a structurally normal heart were grouped as controls. Healthy controls (with no suspicion of a heart defect on clinical examination) were recruited from immunization clinic and a questionnaire was filled taking a detailed antenatal and maternal history for risk factors without subjecting them to echocardiography. Cases and controls were compared for various risk factors for the development of heart diseases. The risk factors analyzed were age of the parents, history of consanguinity, any febrile illness during pregnancy, multivitamin or any drug intake, bad obstetrics history, maternal diabetes, and any systemic illness in the mother such as SLE. Spectrum of various CHDs was then analyzed along with their gender distribution, age of presentation, and clinical presentation. Data collected were analyzed through SPSS version 17 (Stata Corp, College Station, Texas, USA). Pearson χ2 test was used to compare the risk factor among cases and controls. Odds ratio (OR) for risk factor was calculated. All cases were followed up after surgical/medical management in the pediatric cardiology clinic.
RESULTS
A total of 1154 patients were screened by Echocardiography of whom 754 presented with normal Echocardiography study whereas 400 were detected to have CHDs [Table 1]. Acyanotic heart defects were 290 (72.5%) of the total heart defects, whereas the contribution of cyanotic heart defects was 110 (27.5%). Out of all CHDs, VSD was the most common lesion with contribution of 152 (38%) cases, whereas among the cyanotic heart defects, Tetralogy of Fallot (TOF) was the most common lesion (18% of total cases). Out of the total 400 cases, 261 were males (65.25%). VSD was the most common lesion in both the sexes. In 14 CHDs, there was male preponderance. In five CHDs, there was female preponderance, whereas with tricuspid atresia, there were equal number of cases among males and females. Among the controls, 239 were recruited from immunization clinic and the remaining had been referred to the pediatric cardiology clinic but were found to have normal hearts on echocardiogram.
Table 1.
Spectrum of congenital heart defects according to gender and age of presentation
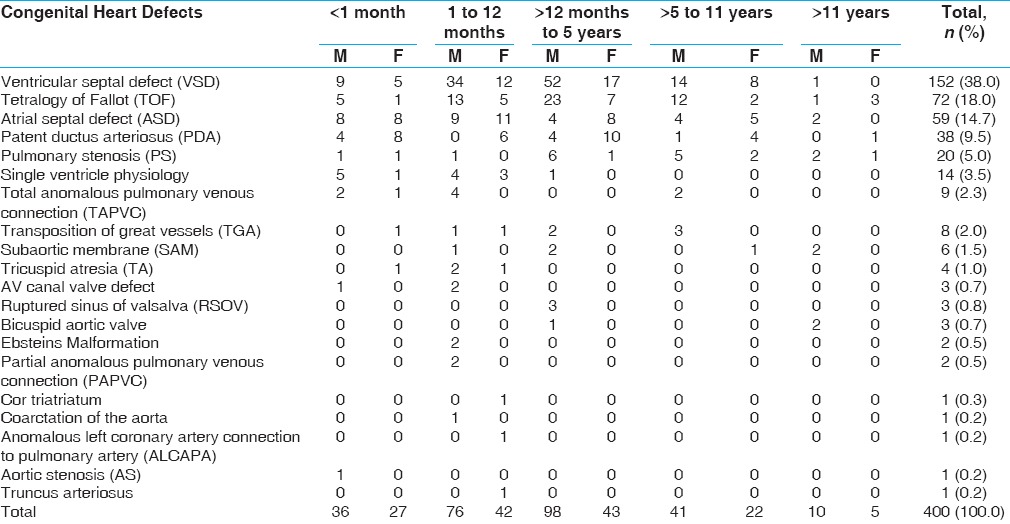
Among acyanotic defects, the distribution of various defects was VSD (52.4%) followed by ASD (20.3%), PDA (13%), PS (6.9%), subaortic membrane (2%), AV canal defect (1%), ruptured sinus of valsalva (1%), bicuspid aortic valve (1%), PAPVC (0.7%), cor triatriatum (0.33%), coarctation of aorta (0.33%), ALCAPA (0.33%), and aortic stenosis (0.33%). Age of presentation for most of the children was between 1 and 5 years.
Cyanotic heart defects constituted 27.5% of total CHDs. Spectrum of various cyanotic lesions were as follows: TOF 65.5%, single ventricle 12.72%, TAPVC 8.2%, TGA 7.3%, tricuspid atresia 3.63%, Ebstein malformation 1.8%, and truncus arteriosus 0.9%. TOF was the second most common lesion among all CHDs and most common lesion among cyanotic heart defects. Out of total 72 cases of TOF, 60 (83.3%) cases had classical TOF whereas 10 (13.9%) cases were of TOF with pulmonary atresia and 2 (2.8%) of TOF with absent pulmonary valve.
In-house deliveries
Total numbers of newborns (live births) at our hospital were 5911 during the study period, out of which 238 were screened and 52 were found to have CHD. Preterms with PDA (spontaneously or after stopping medications) or patent foramen ovale were excluded. Prevalence of CHD was found to be 8.79/1000 live births during the study period which is higher than study by Khalil et al.[17] Acyanotic heart defects contributed to 35 (67.30%) cases whereas cyanotic heart defects contributed 17 (32.70%) cases.
Risk factors for congenital heart defect
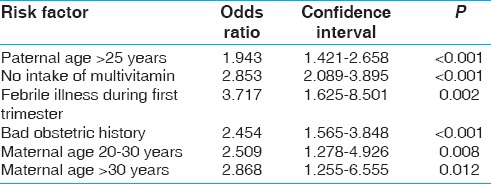
Cases and controls were compared for various risk factors for the development of heart diseases. On univariate analysis, paternal age, bad obstetric history, antenatal febrile illness, and advanced maternal age were found to increase the risk of CHD whereas intake of multivitamin decreased the risk, as shown in Table 2. History of consanguinity among parents, drug intake and maternal diabetes were not found to be significantly associated with heart defect. All the risk factors found to be significant on univariate analysis were further subjected to multivariate logistic regression analysis [Table 3]; it was found that advanced age of the parents, febrile illness in the mother during pregnancy, history of previous abortions/stillbirths, and absence of multivitamins and folic acid in the diet were significantly associated with the development of heart defect in the offspring.
Table 2.
Risk factors of congenital heart defects on univariate analysis
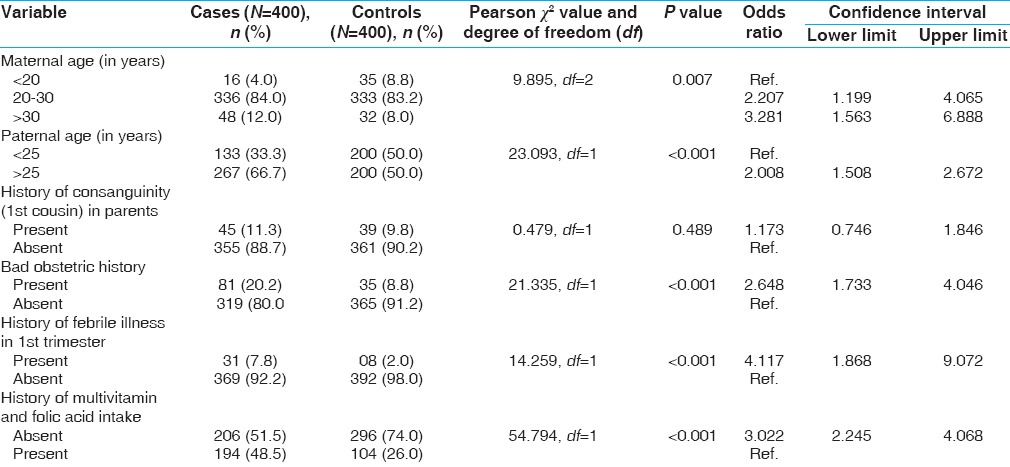
Table 3.
Risk factors for congenital heart disease by multivariate logistic regression analysis
DISCUSSION
In our study, out of total 400 cases, acyanotic CHDs constituted 290 cases, which was 72.5% of total CHDs [Tables 4 and 5], similar to other investigators.[7,8,9,10]
Table 4.
Comparison of profile of congenital heart defects with other studies (outside India)
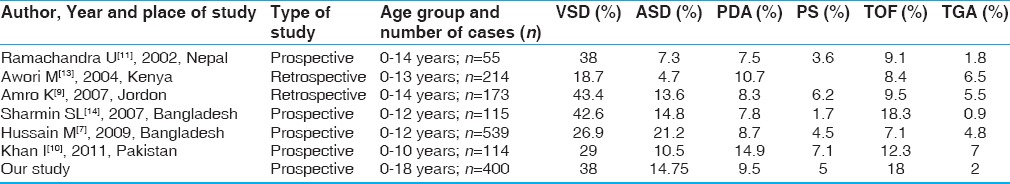
Table 5.
Comparison of profile of congenital heart defects with other Indian studies
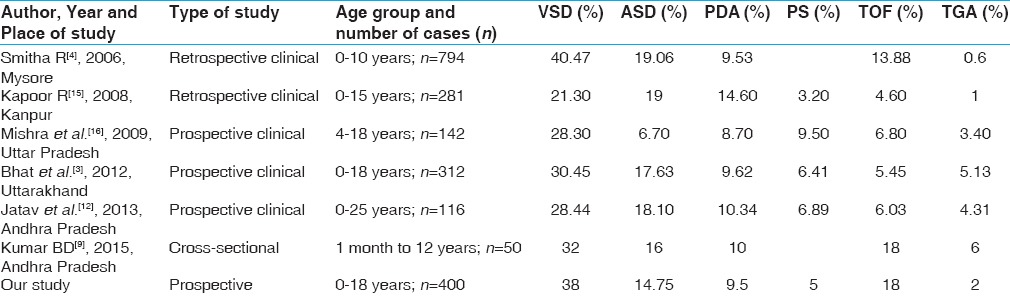
As most of the studies had shown male preponderance, similarly our study also found M: F ratio of 1.9 that was similar to that of Hussain et al.[7] and Kumar et al.[9] who had found the ratio as 2.08:1 and 1.78:1, respectively. In contrast, Amro[8] and Khan et al.[10] did not find much of gender disparity.
Forty-five percent cases of CHD presented during infancy; this was lower than reported in other published studies.[7,10] In the preschool age group (>1 to 5 years), presentation of CHDs was higher (35%) than the published data.[7,11,12] School-going children (between >5 and 11 years) constituted 15.75% whereas less number of adolescents (>11 years) presented to our clinic (3.75%) as compared with the studies by Ramachandra et al.[11] and Jatav et al.[12] [Table 5].
The most common indication for cardiac evaluation was detection of a murmur and visible cyanosis noticed by the parents. The age of presentation was also quite variable both for cyanotic and acyanotic heart diseases. Two cases of TOF presented with brain abscess and another with infective endocarditis with a large vegetation on the mitral valve.
RISK FACTORS
Maternal age
In our study, we found that in maternal age groups between 20 and 30 years and more than 30 years, there was more risk of having CHDs in their offspring in comparison to the group less than 20 years. As compared with maternal age group less than 20 years, in maternal age group between 20 and 30 years, odds of having CHDs was 2.509 (95% confidence interval, CI, 1.278-4.926) whereas in group > 30 years, odds of having CHDs was 2.868 (95% CI, 1.255-6.555). The study done by Miller et al.[18] found that advanced maternal age >35 years was associated with increased prevalence of CHDs. Advanced maternal age is associated with several genetic abnormalities association with Downs syndrome that has a high incidence of CHD is also well documented.
Paternal age
We found significant association of CHDs with paternal age (>25 years). Offspring having paternal age >25 years had increased risk of CHDs in comparison to those with paternal age <25 years (OR, 1.943; 95% CI, 1.421-2.658). This is consistent with the study done by Olshan et al.[19] and Lian et al.[20] who also found a similar association. In contrast, a Chinese study[21] found no relationship between advancing paternal age and CHDs. This is an interesting observation as lot of attention is given to maternal age although advanced paternal age may also be a predisposing factor for the development of heart defects.
Intake of multivitamin containing folic acid
We found that intake of multivitamin and folic acid during the first trimester had protective role against CHDs. Mothers who had taken multivitamin and folic acid during first trimester had reduced risk of CHDs (OR, 2.853; 95% CI, 2.089-3.895). Both Hungarian randomized trial[22] and population-based study conducted in Atlanta[23] showed similar association in which risk of having CHDs in offspring was reduced by 60% and 25% in mothers who took multivitamin and folic acid, respectively, during the first trimester of pregnancy. This is important as supplementation of folic acid could be helpful in preventing both neural tube defects and CHD.
Febrile illness during first trimester
We found significant association of CHDs with febrile illness during the first trimester. Those mothers who had history of febrile illness in first trimester had increased odds of having CHD in their offspring (OR, 3.717; 95% CI, 1.625-8.501), which was similar to the study done by Shi et al.[24]
Bad obstetric history
Our study found the odds of having CHD to be 2.454 (95% CI, 1.565-3.848) with an underlying bad obstetric history whereas a study by Hasan et al.[25] found no such association. This association raises the possibility that previous abortions or stillbirths might have been suffering with significant CHD that has led to early fetal demise; therefore, routine fetal screening is strongly indicated especially in high-risk pregnancies.
Pregestational diabetes
Number of mothers with diagnosed pregestational diabetes was low in our study (n = 6); the reason might be poor antenatal detection and followup of majority of mothers leading to undiagnosed diabetes. It is pregestational diabetes that is strongly associated with the development of heart defects. We had either no or single antenatal checkup for the majority of mothers, and screening for diabetes remains a neglected area.
Similarly, we could not find any association of drug intake although majority of mothers had received at least one drug during pregnancy. There was no documentary evidence in most of the cases and majority of them were native medications, the composition of which was not known.
CONCLUSIONS
The profile of various CHDs in our study was largely similar to preexisting literature. Although many of the CHDs were detected during infancy, a large number were missed and presented late because of lack of awareness and delayed referral. We found a significant association between incidence of CHD and advanced parental age, bad obstetric history, febrile illness during pregnancy, and a folic acid-deficient diet. There is need to prioritize antenatal care and counseling to pregnant mothers that includes multivitamin and folic acid supplementation, screening for diabetes, and, if possible, provision of detailed fetal cardiac evaluation in mothers with bad obstetric history or those having febrile illness during first trimester. Injudicious use of drugs during antenatal period is to be avoided and proper documentation of any drug intake during pregnancy has to be done to assess possible teratogenic effect.
Limitation of the study
Data are not the true representative of profile of CHD in community as these have been collected from a specialized clinic. Prevalence cannot be evaluated from our study as we have analyzed only patients referred to our clinic. Most of the mothers did not have rigorous antenatal care. Risk factors were assessed using a questionnaire rather than documented evidence and could be subject to recall bias.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dolk H, Loane M, Garne E for the European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–9. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 2.Saxena A. Congenital heart disease in India: A status report. Indian J Paediatr. 2005;72:595–8. doi: 10.1007/BF02724185. [DOI] [PubMed] [Google Scholar]
- 3.Bhat NK, Dhar M, Kumar R, Patel A, Rawat A, Kalra BP. Prevalence and pattern of congenital heart disease in Uttakhand, India. Indian J Pediatr. 2013;80:281–5. doi: 10.1007/s12098-012-0738-4. [DOI] [PubMed] [Google Scholar]
- 4.Smitha R, Karat SC, Narayanappa D, Krishnamurthy B, Prasanth SN, Ramachandra B, et al. Prevalence of congenital heart diseases in Mysore. Indian J Hum Genet. 2006;12:11–6. [Google Scholar]
- 5.Kumar RK. Universal heart coverage for children with heart disease in India. Ann Pediatr Cardiol. 2015;8:177–83. doi: 10.4103/0974-2069.164674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar RK, Shrivastava S. Pediatric heart care in India. Heart. 2008;94:984–90. doi: 10.1136/hrt.2007.139360. [DOI] [PubMed] [Google Scholar]
- 7.Hussain M, Tahura S, Sayeed MA, Rahman MM, Rahman MM, Kar SK. Past and present pattern of congenital heart disease at DSH: A situation analysis. Bangladesh J Child Health. 2010;34:51–5. [Google Scholar]
- 8.Amro K. Pattern of congenital heart disease in Jordan. Eur J Gen Med. 2009;6:1615. [Google Scholar]
- 9.Kumar BD, Reddy KR, Elizabeth B. Study of incidence of congenital heart diseases in children of age group 1 month to 12 yrs. J Evol Med Dental Sci. 2015;4:1151–9. [Google Scholar]
- 10.Khan I, Muhammad A, Muhammad T. Pattern of congenital heart disease. Gomal J of Med Sci. 2011;9:174–7. [Google Scholar]
- 11.Ramachandran U, Alurkar V, Thaplia A. Pattern of cardiac diseases in children in Pokhara, Nepal. Kathmandu Univ Med J. 2006;4:222–7. [PubMed] [Google Scholar]
- 12.Jatav RK, Kumbhare MB, Srinivas M, Rao DR, Kumar PG, Reddy PR, et al. Prevalence and pattern of congenital heart diseases in Karimnagar, Andhra Pradesh, India: diagnosed clinically and by trans-thoracic-two-dimensional echocardiography. Int J Res Med Sci. 2014;2:186–92. [Google Scholar]
- 13.Awori M, Ogendo S. The spectrum of paediatric congenital heart disease at the Kenyatta National Hospital: Implications for surgical care. Ann Afr Surg. 2013;10:9–11. [Google Scholar]
- 14.Sharmin LS, Haque MA, Bari MI, Ali MA. Pattern and clinical profile of congenital heart disease in a teaching hospital. TAJ. 2008;21:58–62. [Google Scholar]
- 15.Kapoor R, Gupta S. Prevalence of congenital heart disease, Kanpur, India. Indian Pediatr. 2008;45:309–11. [PubMed] [Google Scholar]
- 16.Mishra M, Mittal M, VermaAM, Rai R, Chandra G, Singh DP, et al. Prevalence and pattern of congenital heart disease in school children of Eastern Uttar Pradesh. Indian Heart J. 2009;61:58–60. [PubMed] [Google Scholar]
- 17.Khalil A, Aggarwal R, Thirupuram S, Arora R. Incidence of congenital heart disease among hospital live births in India. Indian Pediatr. 1994;31:519–27. [PubMed] [Google Scholar]
- 18.Miller A, Riehle-Colarusso T, Siffel C, Frías JL, Correa A. Maternal age and prevalence of isolated congenital heart defects in an urban area of the United States. Am J Med Genet A. 2011;155A:2137–45. doi: 10.1002/ajmg.a.34130. [DOI] [PubMed] [Google Scholar]
- 19.Olshan AF, Schnitzer PG, Baird PA. Paternal age and the risk of congenital heart defects. Teratology. 1994;50:80–4. doi: 10.1002/tera.1420500111. [DOI] [PubMed] [Google Scholar]
- 20.Lian ZH, Zack MM, Erickson JD. Paternal age and the occurrence of birth defects. Am J Hum Genet. 1986;39:648–60. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhan SY, Lian ZH, Zheng DZ, Gao L. Effect of fathers' age and birth order on occurrence of congenital heart disease. J Epidemiol Community Health. 1991;45:299–301. doi: 10.1136/jech.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botto LD, Mulinare J, Erickson JD. Occurrence of congenital heart defects in relation to maternal multivitamin use. Am J Epidemiol. 2000;151:878–84. doi: 10.1093/oxfordjournals.aje.a010291. [DOI] [PubMed] [Google Scholar]
- 23.Scanlon KS, Ferencz C, Loffredo CA, Wilson PD, Correa-Villasenõr A, Khoury MJ, Willett WC the Baltimore-Washington Infant Study Group. Preconceptional and folate intake and malformations of the cardiac outflow tract. Epidemiology. 1998;9:95–8. [PubMed] [Google Scholar]
- 24.Shi QY, Zhang JB, Mi YQ, Song Y, Ma J, Zhang YL. Congenital heart defects and maternal fever: Systematic review and meta-analysis. J Perinatol. 2014;34:677–82. doi: 10.1038/jp.2014.76. [DOI] [PubMed] [Google Scholar]
- 25.Hasan I, Haleem AA, Bhutta ZA. Profile and risk factor for congenital heart disease. JPMA. 1997;47:78–81. [PubMed] [Google Scholar]


