Abstract
The basal ganglia, a network of subcortical structures, play a critical role in movements, sleep and mental behavior. Basal ganglia disorders such as Parkinson’s disease and Huntington’s disease affect sleep. Deep brain stimulation (DBS) to treat motor symptoms in Parkinson’s disease can ameliorate sleep disturbances. Our series of previous studies lead the hypothesis that dopamine, acting on D2 receptors on the striatopallidal terminals, enhances activity in the external globus pallidus (GPe) and promotes sleep. Here, we tested if DBS in the GPe promotes sleep in rats. We found that unilateral DBS (180 Hz at 100 μA) in the GPe in rats significantly increased both non-rapid eye movement and rapid eye movement sleep compared to sham DBS stimulation. The EEG power spectrum of sleep induced by DBS was similar to that of the baseline sleep, and sleep latency was not affected by DBS. The GPe is potentially a better site for DBS to treat both insomnia and motor disorders caused by basal ganglia dysfunction.
Keywords: deep brain stimulation, globus pallidus externa, Sleep, insomnia
Introduction
One of the most common basal ganglia disorders is Parkinson’s disease, which is characterized by muscle rigidity, tremor and bradykinesia. These motor symptoms mostly result from the loss of substantia nigra pars compacta (SNc) dopamine signaling to the basal ganglia. Parkinson’s disease also has prominent non-motor symptoms, including sleep abnormalities like insomnia, sleep fragmentation, and daytime sleepiness. The neural substrate for these sleep symptoms in Parkinson’s disease has recently explored in animal studies. MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) lesions of the SNc in primates reduce total sleep amounts and flatten circadian rhythm (Belaid et al., 2014). SNc dopamine signaling in the basal ganglia promotes sleep in rodents (Qiu et al., 2010, Qiu et al., 2014). We postulate that dopamine from the SNc acts on D2 receptors on striatum inputs to the globus pallidus externa (GPe), disinhibiting the GPe and promoting sleep. The loss of this SNc dopaminergic input in Parkinson’s disease may disrupt sleep via these basal ganglia connections.
Deep brain stimulation (DBS) effectively treats parkinsonian motor symptoms, and DBS can also improve sleep dysfunction such as insomnia (Amara et al., 2012). The most common sites targeted for DBS in Parkinson’s disease are the globus pallidus interna (GPi) and subthalamic nucleus (STN). How DBS in these sites improves sleep problems is unclear, but an intriguing possibility is DBS in the STN and GPi may improve sleep and motor disorders through the GPe. Both STN and GPi project to the GPe, and electrophysiological studies have showed an increased activity in STN target neurons in the GPe during STN DBS (Hashimoto et al., 2003, Kita et al., 2005, Miocinovic et al., 2006, Hahn et al., 2008). Lesions of the GPe in monkeys exacerbates parkinsonian symptoms (Zhang et al., 2006), while DBS in the GPe works as effectively as DBS in the GPi for motor symptoms (Hahn et al., 2008, Vitek et al., 2012).
The GPe is a major basal ganglia hub that has the most extensive efferent connections of basal ganglia structures, including a unique direct projection to the frontal cortex (Chen et al., 2015, Saunders et al., 2015). The interconnectivity of major DBS target sites and the GPe raises the possibility that direct GPe stimulation with DBS may also be an effective treatment for sleep dysfunction in Parkinson’s disease. We hypothesized that DBS directly in the GPe may promote sleep. We examined the effects of DBS in the GPe on sleep in rodents and found that it increased both NREM and REM sleep.
Experimental procedures
Animals
Experiments were performed on male Sprague-Dawley rats (300-325 g, Harlan). Animals were individually housed with ad libitum access of food and water, and under light-controlled conditions (12 h light/12 h dark cycle, with light on at 07:00 h; 100 lux) in isolated ventilated chambers maintained at 20-22 °C. All protocols were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and these experiments were carried out in accordance with guidelines laid down by the U.S. National Institutes of Health regarding the care and use of animals for experimental procedures. Every effort was made to minimize the number of animals used and any pain and discomfort experienced by the subjects.
Surgery
Under anesthesia (ketamine, 100mg/kg; xylazine, 10mg/kg), rats were implanted with electrodes for recording electroencephalogram (EEG) and electromyographic (EMG) as described previously (Lu et al., 2000, Qiu et al., 2010). In brief, four EEG screw electrodes were implanted into the skull (two in the frontal and two in the parietal bones on each side) and two flexible EMG wire electrodes were placed into the nuchal muscles. And a twisted bipolar stimulation electrode (part# MS303/1-B/SPC, Plastics One, Roanoke, VA, USA) was targeted to the GPe (coordinate: AP = −0.92 mm, ML = +2.9 mm, DV = 6 mm) unilaterally, as per the atlas of Paxinos and Watson (Paxinos and Watson, 2009). The free ends of the EEG/EMG electrodes leads were soldered into a head socket, together with the stimulation electrode, were then affixed to the skull with dental cement. Rats were allowed 10 days for recovery after the surgery.
EEG recording and stimulation
Each rat was transferred from the holding room to a recording chamber and habituated to the flexible EEG/EMG and stimulation electrode connection cables and conditions for two days. Following this habituation period, the EEG/EMG cables were connected to the amplifier (CED, Micro1401) and the stimulation electrode cable was connected to the stimulator (GRASS Technologies, Model S88) through a commutator (Plastics One). The stimulation signals were performed through a photoelectric stimulus isolation unit at a constant current output mode. The stimulation conditions were: frequency 180 Hz, duration 100 μs. stimulus intensity 100 μA. Each animal received a 180 Hz at 1.0 μA stimulation as baseline control and a 180 Hz at 100 μA of DBS from 19:00 to 22:00 in two consecutive days.
The EEG and EMG signals were amplified, digitized at a sampling rate of 256 Hz and recorded using Spike 2 (CED, Cambridge, UK). When complete, EEG/EMG recording data were transformed to EDF data format, filtered (EEG: 0.1-40 Hz; EMG: 10-100 Hz), and automatically scored with SleepSign (Kissei Comtec, Nagano, Japan) by 10-sec epochs as wake, non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep, according to previously established criteria (Lu et al., 2000, Lu et al., 2001). After automatic scoring, sleep-wake stages were examined and corrected manually. The amount spent in wake, NREM sleep and REM sleep, sleep stage transitions, bouts and mean duration of each stage were determined from the scored data. In addition, EEG power spectra for NREM and REM sleep epochs were analyzed offline using FAST Fourier Transformation (512 point, Hanning window, 0.5-24.5 Hz with 0.5 Hz resolution using SleepSign).
Histology
Rats after two hour DBS were deeply anesthetized with chloral hydrate (500 mg/kg) and perfused with saline followed by 500 ml 10% formalin through the heart. The brains were removed, post-fixed for 4 hr in 10% formalin, and then equilibrated in 20% sucrose in PBS overnight. The brains were sectioned in the coronal plane on a freezing microtome into four series at 40 μm. One series of sections were processed for Nissl staining to verify the location of the tip of the stimulate electrode in the GPe. One series of sections were processed for c-Fos immunocytochemistry to observe whether GPe DBS would affect the activity of the neurons in the nucleus which are related with sleep and wake, such as ventrolateral preoptic area (VLPO) and tuberomammillary nucleus (TMN). Immunohistochemistry was performed in accordance with the free floating method described previously (Qiu et al., 2010). Sections were incubated with 0.3% H2O2 for 15 min to quench the endogenous peroxidase activity. After washing in 0.1M PBS (Ph 7.4), the sections were incubated with a rabbit polyclonal primary antibody against c-Fos (Ab5, Oncogene Research Products) at a 1:10000 dilution in PBS containing 0.25% Triton X-100 for 24 hr at room temperature. On the second day, the sections were washed in PBS and incubated in biotinylated donkey anti-rabbit secondary antiserum (Jackson ImmunoResearch Laboratories, PA, USA; 1:1000 dilution) for 1 hr followed by a 1:1000 dilution of avidin-biotin-peroxidase (Vector Laboratories, CA, USA) for 1 hr at room temperature. The peroxidase reaction was visualized with 0.05% 3,3-diaminobenzidine tetrahydrochloride (Sigma, MO, USA) in PBS and 0.01% H2O2 and strengthened with 0.002% Ni, 0.001% CoCl2. After terminating the reaction by PBS-azide, sections were mounted, dehydrated and cover slipped. As controls, adjacent sections were incubated without the primary antibody to confirm that non-specific staining had occurred.
Statistical analysis
The quantitative data were presented as the mean ± standard error of mean (SEM). Time course of the hourly amounts of each stage, histograms of sleep/wake amounts, sleep/wake stage transition number, number and duration of sleep/wake bouts were analyzed by pared t-test, with each animal serving as its own control. In all case, difference were considered statistically significant at the 95% level (p < 0.05).
Results
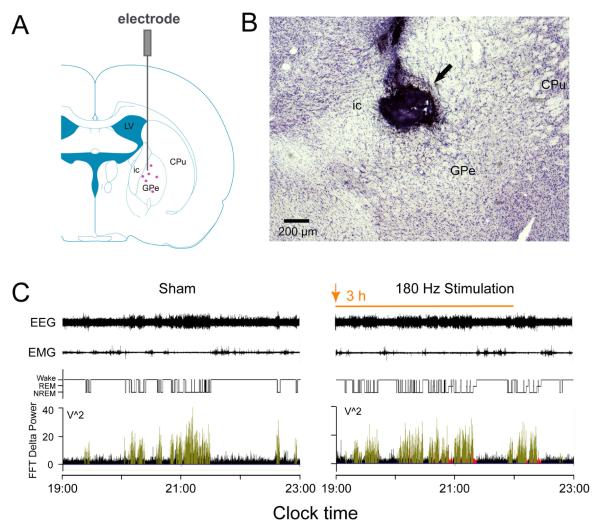
Rats with DBS electrode in the GPe received either a real (180 Hz at 100 μA) or sham (100 Hz, 1.0 μA) stimulation from 19:00-22:00 (ZT12-15) while recording EEG/EMG/video from 19:00-23:00. Compared to sleep during sham stimulation, DBS in the GPe (n=6) significantly increased both NREM sleep and REM sleep amounts (Figure 1C, Figure 2A and B); however, DBS did not change the FFT delta power (Figure 1C, lower panel).
Figure 1. DBS in GPe promotes sleep.
A: Coronal section outline shows the placement site of the electrode in the rats. The purple dots in the GPe illustrate the stimulated points. B: CV staining was used to verify the location of the electrode, the arrow indicate the electrode tip above the GPe. C: Typical examples of EEG/EMG recordings and corresponding hypnograms and delta power of a rat at sham condition or DBS in GPe during 19:00 -22:00 (The orange arrow and bar represent the beginning and duration of DBS, respectively). Compared to baseline, DBS in GPe induced promotes both NREM and REM sleep without changes in EEG delta power (black columns: Wake; green columns: NREM sleep; Red columns: REM sleep) and sleep latency (the time from the beginning of DBS to the appearance of the first NREM sleep episode lasting for at least 20 sec.). Although DBS increases sleep significantly, wake is still present. CPu, caudate putamen; GPe, globus pallidus externa; ic, internal capsule; LV, lateral ventricle
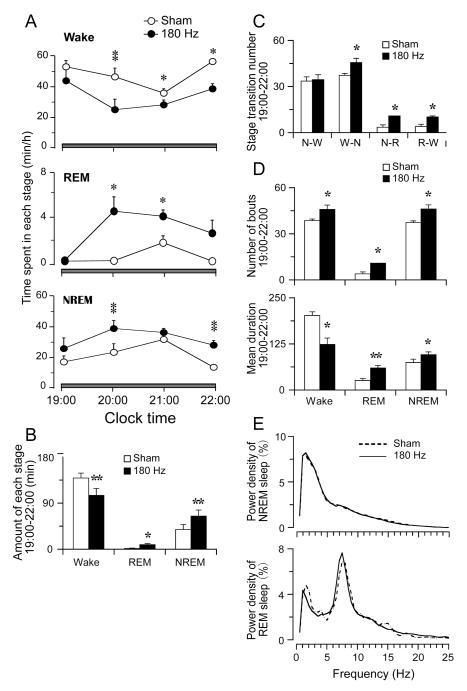
Figure 2. Detailed sleep-wake profile by DBS in GPe.
Hourly amounts (A), total amounts (B) and state transitions, number, and mean duration (C and D) of wake, REM and NREM sleep of baseline and DBS in GPe during 19:00 -22:00, respectively. DBS in GPe increases both NREM sleep and REM sleep and induces more transitions from wake to NREM sleep, NREM sleep to REM sleep and REM sleep to wakefulness and increases average duration of NREM and REM sleep. Wake bout duration is reduced. (E) EEG power densities of NREM and REM sleep of control and DBS group are similar. *P < 0.05, **P < 0.01.
Compared with sham stimulation, 3 hrs of DBS in the GPe markedly increased the amount of NREM and REM sleep, and the sleep promotion effect lasted at least for one hour post-DBS (Figure 2A and B). DBS in GPe significantly increased the total amounts of NREM and REM sleep by 67.3% (65.2 min ± 11.7 vs. 38.9 min ± 9.4 control, p < 0.01) and 400.0% (9.5 min ± 1.9 vs. 1.9 ± 0.7 control, p < 0.05), and decreased the total amount of wakefulness by 24.3% (105.3 min ± 13.6 vs. 139.1 min ± 9.7 control, p < 0.01).
Transitions from wake to NREM, from NREM to REM, and from REM to wake were significantly increased (Figure 2C). We further analyzed the NREM and REM sleep, and wake bout number and duration, and we found that DBS in GPe significantly increased the bout number of NREM and REM sleep and mean duration of NREM and REM sleep. The number of wake bouts was also increased, although the mean duration of wake was significantly decreased (Figure 2D). EEG power spectrum of NREM and REM sleep by DBS was similar to that of the baseline NREM and REM sleep (Figure 2E). Sleep latency, or time to the first sleep bout, was not affected by the DBS (p > 0.05).
We obtained four cases with DBS electrodes misplaced in the outside of the GPe such as in the caudoputamen (CPu) or in the internal capsule. We found DBS in those areas did not cause changes in sleep.
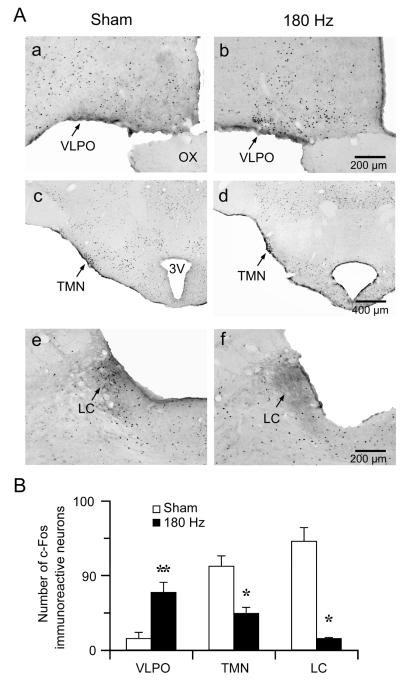
To see if DBS in the GPe activates endogenous sleep systems, we examined c-Fos expression after two hours of DBS (19:00-21:00). Animals (n=4) after DBS showed high c-Fos expression in the VLPO, a sleep-active region, and low c-Fos expression in arousal systems such as the locus coeruleus (LC) and TMN. In contrast, control animals (n=3) perfused at 21:00 showed high c-Fos in arousal systems such as the LC and TMN but not in the sleep-active VLPO (Figure 3). Because there were still substantial wake episodes during DBS, as the time of stimulation is normally a period of activity for rats, c-Fos expression was not completely eliminated in the TMN during DBS.
Figure 3. DBS in GPe activates endogenous sleep system.
A: Representative photomicrographs of c-Fos immunostaining in the VLPO, TMN and LC of control (a, c and e) and DBS (b, d and f) rats. Compared to control, DBS in GPe induces c-Fos in the VLPO but not in the LC or TMN. 3V: third ventricle; OX: optic chiasm; VLPO: ventrolateral preoptic nucleus.
B: The number of c-Fos-immunoreactive neurons in VLPO, TMN and LC after control or DBS in GPe. Values are means ± SEM. *P < 0.05, **P < 0.01.
Discussion
This is the first study reporting that DBS in the GPe promotes NREM and REM sleep. DBS increasing both NREM and REM sleep with normal sleep EEG power spectrum and normal sleep latency, suggesting that DBS in the GPe promotes natural sleep, mostly by sleep consolidation. Interestingly, the sleep effects may extend beyond the DBS termination. Our prior research has established the neural circuitry in the basal ganglia regulating sleep. Specifically, we have identified the GPe as the key structure promoting sleep. Inputs that influence GPe neuronal activity regulate sleep. For example, dopamine from the SNc acting on D2 receptors in the striatum enhances GPe activity and promotes sleep (Qiu et al., 2014). We propose that pallidocortical projections regulate sleep (Chen et al., 2015).
How DBS affects targeted neuronal populations is not clear. On the one hand, high frequency DBS may disrupt normal neuronal firing patterns, acting like inhibition. This inhibitory property is consistent with lack of Fos expression in the GPe after DBS. On the other hand, DBS may stimulate some local neurons or entrain them to the stimulation frequency. Sleep promotion (increase in duration and bout number) by DBS in the GPe mimics sleep effects by optogenetic stimulation of the GPe (Qiu et al., 2014) , suggesting that DBS in the GPe has stimulatory property. Based on animal studies, we hypothesize that stimulatory property by DBS in the GPe is critical for correcting sleep disorder in PD. One possibility is that DBS inhibits the GPe neurons but stimulates GPe efferents. A computational model predicts that DBS stimulates GPi and GPe efferent axons (Johnson and McIntyre, 2008).
DBS in the STN and GPi is effective at improving motor symptoms and insomnia of Parkinson’s disease. The GPe is a key basal ganglia hub and also directly interacts with the cortex, and we hypothesize that DBS in the GPi and STN may act through the GPe for the regulation of sleep and motor behavior (Qiu et al., 2010, Qiu et al., 2014, Chen et al., 2015). GPe lesions can produce or exacerbate both motor and non-motor symptoms of Parkinson’s disease, including sleep dysfunction, while lesions to other basal ganglia output pathways like the SNr or thalamus have minimal effects on sleep in rats (Zhang et al., 2006, Qiu et al., 2010, Fuller et al., 2011). DBS in the thalamus does not improve PD motor symptoms with the exception of tremor (Fasano et al., 2012). Given that the GPe has extensive projections, including direct projections to cortex, this structure may be an ideal DBS site for treating both motor and non-motor behaviors in neurological disorders.
Highlights.
● DBS in the GPe promotes natural sleep in rats.
● DBS in GPe activates endogenous sleep system.
● GPe is an intriguing candidate site for DBS to treat both insomnia and motor dysfunction.
Acknowledgments
This work was supported by Metronic Inc (01026067), the National Institutes of Health (NS061841 and NS062727), National Natural Science Foundation of China (31171049) and by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Abbreviations
- CPu
caudate putamen
- DBS
deep brain stimulation
- EEG
electroencephalogram
- EMG
electromyographic
- GPe
globus pallidus externa
- GPi
globus pallidus interna
- ic
internal capsule
- LC
locus coeruleus
- NREM
non-rapid eye movement
- REM
rapid eye movement
- SNc
substantia nigra pars compact
- STN
subthalamic nucleus
- VLPO
ventrolateral preoptic area
- TMN
tuberomammillary nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amara AW, Standaert DG, Guthrie S, Cutter G, Watts RL, Walker HC. Unilateral subthalamic nucleus deep brain stimulation improves sleep quality in Parkinson's disease. Parkinsonism & related disorders. 2012;18:63–68. doi: 10.1016/j.parkreldis.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaid H, Adrien J, Laffrat E, Tande D, Karachi C, Grabli D, Arnulf I, Clark SD, Drouot X, Hirsch EC, Francois C. Sleep disorders in Parkinsonian macaques: effects of L-dopa treatment and pedunculopontine nucleus lesion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:9124–9133. doi: 10.1523/JNEUROSCI.0181-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Ferrari L, Sacchet MD, Foland-Ross LC, Qiu MH, Gotlib IH, Fuller PM, Arrigoni E, Lu J. Identification of a direct GABAergic pallidocortical pathway in rodents. The European journal of neuroscience. 2015;41:748–759. doi: 10.1111/ejn.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson's disease with deep brain stimulation. The Lancet Neurology. 2012;11:429–442. doi: 10.1016/S1474-4422(12)70049-2. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. The Journal of comparative neurology. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn PJ, Russo GS, Hashimoto T, Miocinovic S, Xu W, McIntyre CC, Vitek JL. Pallidal burst activity during therapeutic deep brain stimulation. Experimental neurology. 2008;211:243–251. doi: 10.1016/j.expneurol.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, McIntyre CC. Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. Journal of neurophysiology. 2008;100:2549–2563. doi: 10.1152/jn.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8611–8619. doi: 10.1523/JNEUROSCI.1719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. Journal of neurophysiology. 2006;96:1569–1580. doi: 10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. In stereotaxic coordinates. Elsevier Inc.; 2009. The rat brain. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. The European journal of neuroscience. 2010;31:499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MH, Yao QL, Vetrivelan R, Chen MC, Lu J. Nigrostriatal Dopamine Acting on Globus Pallidus Regulates Sleep. Cerebral cortex. 2014 doi: 10.1093/cercor/bhu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliott HL, Xie T, Gerfen CR, Sabatini BL. A direct GABAergic output from the basal ganglia to frontal cortex. Nature. 2015 doi: 10.1038/nature14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL, Zhang J, Hashimoto T, Russo GS, Baker KB. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Experimental neurology. 2012;233:581–586. doi: 10.1016/j.expneurol.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Russo GS, Mewes K, Rye DB, Vitek JL. Lesions in monkey globus pallidus externus exacerbate parkinsonian symptoms. Experimental neurology. 2006;199:446–453. doi: 10.1016/j.expneurol.2006.01.006. [DOI] [PubMed] [Google Scholar]