ABSTRACT
MDSC undergo metabolic reprogramming in the tumor resulting in an increased fatty acid β oxidation that supports their immunosuppressive functions. Fatty acid oxidation inhibitors, used to treat coronary disease, significantly delayed tumor growth and had a significantly increased antitumor effect when combined with adoptive cell therapy or low dose chemotherapy.
Keywords: Cancer, Immunometabolism, MDSC
Myeloid cells normally play important roles in the initiation of protective immune responses and in the healing of damaged tissues. However, under the influence of tumor-derived factors (and certain chronic infections), they are hijacked to become myeloid-derived suppressor cells (MDSC) that inhibit antitumor T cell functions and protect tumors from the effects of chemotherapy and radiation therapy.1,2 Inhibiting MDSC increases the efficacy of immunotherapy and/or radiation and chemotherapy. However, blocking MDSC has been difficult because they can respond to multiple tumor-derived factors, and they have at least three distinct mechanisms to block T cell function, namely the depletion of the amino-acids L-arginine (through arginase I) and L-cysteine, the production of nitric oxide (NO; through NOS2) and peroxynitrite (ONOO−; through the combined effect of NO + H2O2).3,4 The plasticity of MDSC and the redundancy of their immunosuppressive mechanisms have made it difficult to inhibit them using targeted approaches such as arginase or nitric oxide inhibitors and tyrosine kinase inhibitors. Therefore, anti-MDSC therapies are mostly limited to high dose cyclophosphamide or gemcitabine, which block MDSC as a result of their myelosuppressive effects. Unfortunately, this results in a rapid increase in MDSC as the bone marrow recovers from the myelosuppressive effects of chemotherapy, reestablishing a highly immunosuppressive microenvironment.
During several decades, researchers have studied the energy metabolism of tumor cells with the goal of developing novel treatments of cancer.5,6 More recently, several groups have studied the metabolic pathways used by immune cells at rest and after activation. The results have shown that different subpopulations have distinct metabolic requirements depending on their state of activation.7,8 For example, resting CD8+ T cells mostly use fatty acid oxidation (FAO) to maintain their “house keeping” functions, but during their activation and effector stages they become highly glycolytic, finally returning to fatty acid oxidation when they develop into long-term memory cells. In contrast, CD4+ CD25+ FoxP3+ regulatory T cells use FAO to support their immunosuppressive functions. Similarly, myeloid cells, as represented by macrophages and granulocytes, also have unique metabolic characteristics. While M1 macrophages and granulocytes preferentially use glycolysis, M2 macrophages primarily use FAO. In cancer, Gabrilovich's group showed that the synthesis and oxidation of lipids in CD11c+ dendritic cells infiltrating tumors blocked the assembly of antigen onto Class II MHC and impaired antigen presentation, thus preventing the stimulation and activation of T cells.9 This effect could be blocked if dendritic cells were treated with an inhibitor of fatty acid synthesis, TOFA.
As reported in our recent publication,10 we studied the metabolic changes and characteristics of CD11b+ GR1+ MDSC (both Ly6G+ granulocytic and Ly6C+ monocytic MDSC). Normal granulocytes primarily use glycolysis to sustain their functions. Our results showed that tumor-associated MDSC, both granulocytic and monocytic, undergo metabolic reprogramming by significantly increasing fatty acid β oxidation. This metabolic reprogramming was characterized by an increased expression of the lipid uptake receptors CD36 and Msr1, an increase in the FAO enzymes carnitine palmitoyltransferase 1 (CPT1) and 3-hydroxyacyl-CoA dehydrogenase (HADHA), an increase in mitochondrial mass, and an increase in oxygen consumption rate. These events were paralleled with an activation of all three immunosuppressive mechanisms, namely an increased production of arginase I, an increased expression of NOS2 (together with NO production), and an increased production of ONOO−, which resulted in an enhanced ability to block T cell proliferation and IFNγ production (Fig. 1).
Figure 1.
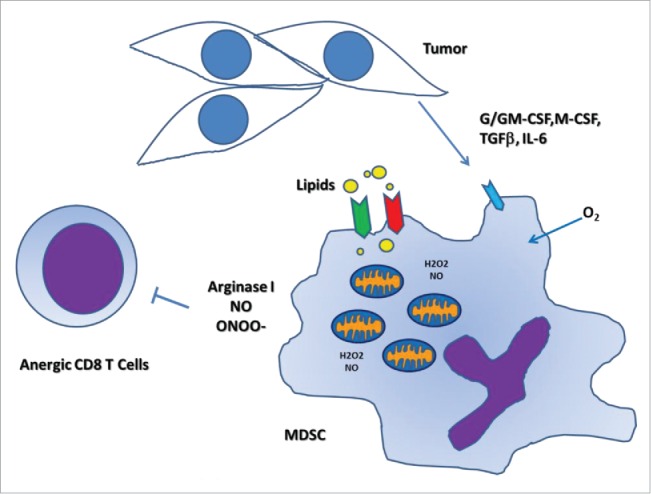
Tumor-associated MDSC increase fatty acid uptake and activate FAO. Tumor-infiltrating MDSC show a heightened expression of lipid uptake receptors, such as CD36 and Msr1, thereby increasing fatty acid uptake. MDSC display an increased mitochondrial biogenesis and fatty acid oxidation. These events coincide with elevated immunosuppressive pathways, which block antitumor T cell responses.
We then tested the effect of FAO inhibition in vitro and in vivo using several transplantable tumor models. FAO inhibitors developed to treat severe coronary disease (unstable angina) decreased the incorporation of fatty acids, decreased ATP production and blocked the development of all immunosuppressive functions of MDSC without causing apoptosis. Mice treated with FAO inhibitors had a significant decrease in tumor growth, which was dependent on CD4+ and CD8+ T cells. Furthermore, FAO inhibition enhanced the antitumor effect of single-dose chemotherapy and adoptive cellular therapy. The latter was accompanied by an increase in the number of CD8+ T cells infiltrating the tumor and an increased production of IFNγ. Therefore, targeting FAO may represent a new approach to globally inhibiting the function of tumor-associated MDSC without causing myelosuppression. It also appears to significantly enhance the effect of various forms of cancer therapy. These results support the need to continue understanding the metabolic reprogramming of immune cells in cancer and the possibility of using FAO inhibitors to potentiate the effect of immunotherapies, such as checkpoint inhibitors and adoptive cellular therapy, as well as standard cancer treatments, such as chemotherapy and radiation therapy. In addition, given its ability to modulate chronic inflammatory cells such as MDSC, it may be possible to use FAO inhibition as a way of treating other immune-mediated diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded in part by R01AI112402, R01CA082689, R01CA107974, P20GM103501, and P30GM114732 (to A.C. Ochoa) and partially supported from LA CaTS Center (U54GM104940; to A.A. Al-Khami and A.C. Ochoa) and the Al Copeland Foundation funds.
References
- 1.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA et al.. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011; 1:54-67; PMID:22039576; http://dx.doi.org 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macatangay BJ, Landay AL, Rinaldo CR. MDSC: a new player in HIV immunopathogenesis. AIDS 2012; 26:1567-9; PMID:22810370; http://dx.doi.org/ 10.1097/QAD.0b013e328355e682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev 2008; 222:180-91; PMID:18364002; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 2008; 13:472-82; PMID:18538731; http://dx.doi.org/ 10.1016/j.ccr.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Warburg O. On respiratory impairment in cancer cells. Science 1956; 124:269-70; PMID:13351639 [PubMed] [Google Scholar]
- 7.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38:633-43; PMID:23601682; http://dx.doi.org/ 10.1016/j.immuni.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science 2013; 342:1242454; PMID:24115444; http://dx.doi.org/ 10.1126/science.1242454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B et al.. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 2010; 16:880-6; PMID:20622859; http://dx.doi.org/ 10.1038/nm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W et al.. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res 2015; 3:1236-47; PMID:26025381; http://dx.doi.org/ 10.1158/2326-6066.CIR-15-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]


