ABSTRACT
Cytokine-induced Killer (CIK) cells are a heterogeneous population of ex vivo expanded T lymphocytes capable of MHC-unrestricted antitumor activity, which share phenotypic and functional features with both NK and T cells. Preclinical data and initial clinical studies demonstrated their high tolerability in vivo, supporting CIK cells as a promising cell population for adoptive cell immunotherapy. In this study, we report for the first time that CIK cells display a donor-dependent expression of CD16, which can be engaged by trastuzumab or cetuximab to exert a potent antibody-dependent cell-mediated cytotoxicity (ADCC) against ovarian and breast cancer cell lines, leading to an increased lytic activity in vitro, and an enhanced therapeutic efficacy in vivo. Thus, an efficient tumor antigen-specific retargeting can be achieved by a combination therapy with clinical-grade monoclonal antibodies already widely used in cancer therapy, and CIK cell populations that are easily expandable in very large numbers, inexpensive, safe and do not require genetic manipulations. Overall, these data provide a new therapeutic strategy for the treatment of Her2 and EGFR expressing tumors by adoptive cell therapy, which could find wide implementation and application, and could also be expanded to the use of additional therapeutic antibodies.
KEYWORDS: Antibody-dependent cell-mediated cytotoxicity (ADCC), cytokine induced killer (CIK) cells, immunotherapy, monoclonal antibodies
Abbreviations
- ACT
Adoptive cell transfer
- ADCC
Antibody-Dependent Cell-mediated Cytotoxicity
- CAR
Chimeric antigen receptor
- CIK
Cytokine Induced Killer cells
- E/T ratio
Effector/Target ratio
- EGFR
Human epidermal growth factor 1
- Her2
Human epidermal growth factor 2
- i.p.
intraperitoneal
- IFNγ
Interferon-γ
- IL-2
Interleukin-2
- mAbs
Monoclonal Antibodies
- MHC
Major Histocompatibility Complex
- NKG2D
Natural-Killer group 2 member D
- NSG
NOD/SCID common γ chain knockout
- PBMCs
Pheripheral Blood Mononuclear Cells
Introduction
Recent years have witnessed a renewed interest for immunotherapy in cancer, mainly due to the successes of checkpoint inhibitors1,2 and adoptive cell therapy (ACT) with chimeric antigen receptor (CAR)-redirected T cells.3 These latter, in particular in hematological malignancies, outperformed previous strategies with tumor-infiltrating lymphocytes (TIL)4 or NK cells.5 However, CAR-modified T cells involve genetic manipulation of donor lymphocytes, require very specialized facilities for generation and are extremely expensive, all features that strongly limit the clinical diffusion of this approach. Overall, three critical issues need to be addressed by all ACT strategies in order to be effective in clinical applications: first, sufficient numbers of immune effector cells must be obtained; second, they must reach the tumor site and recognize the targets and third, kill the tumor cells.6 These obstacles could be overcome by using Cytokine-Induced Killer (CIK) cells, a population of effector cells obtained from peripheral blood mononuclear cells (PBMCs), which show unique and interesting features supporting their role as a promising tool for ACT approaches. CIK cells are a heterogeneous population of lymphocytes, which can be obtained from PBMCs by the timed addition of interferon-γ (IFNγ), monoclonal antibodies (mAb) directed against CD3 (OKT3) and interleukin-2 (IL-2).7 During the first 24 h, the addition of IFNγ induces the expression of IL-2 receptors, and increases cytotoxicity. After 2–3 weeks of expansion, the bulk population is composed mainly by CD3+CD56+ CIK cells and CD3+CD56− T cells, and only a small fraction of CD3−CD56+ NK cells.8 The CD3+CD56+ subset can exert a MHC-independent antitumor activity against both hematological and solid malignancies, but not against normal tissues or haematopoietic precursors.9-11 In vitro-generated CD3+CD56+ CIK cells are mostly CD8+, and express the αβ and γδ TCRs in proportions similar to those found in the peripheral blood T cells. The molecule that plays the most important role in tumor recognition is likely NKG2D, a member of the c-type lectin-activating receptor family that is normally expressed on all NK cells. Interestingly, the ligands for NKG2D appear to have an expression pattern relatively restricted to malignant tissues.12,13 NKG2D has a significant role in the triggering of IL-2-activated NK cells inducing calcium flux, cytokine release and cytotoxicity.14 Its expression is upregulated in CIK cells by the presence of IFNγ, high-dose IL-2 and TCR-crosslinking with OKT3 mAb. Studies with anti-NKG2D blocking antibodies, small interfering RNA or redirected cytolysis, indicated that most CIK cell cytotoxicity is exerted through NKG2D rather than TCR engagement.15,16 Several clinical trials have proved the therapeutic efficacy together with low toxicity of CIK cell infusion in patients affected by different malignancies,17 thus supporting CIK cells as a very promising cell population for adoptive immunotherapy. Nevertheless, retargeting and improvement of CIK cell activity still remain unmet needs. Here, we report the remarkable observation that CIK cells present a donor-dependent expression of CD16, which can be engaged by clinically relevant antibodies, such as trastuzumab and cetuximab. This leads to the triggering of a potent antibody-dependent cell-mediated cytotoxicity (ADCC) that has been never reported before, and has strong relevance both in vitro and in vivo. Thus, the combination of clinical-grade antibodies with a genetically unmodified effector cell population provides new opportunities for redirected ACT.
Results
CIK cells express CD16 in a donor-dependent manner
CIK cells were obtained by in vitro stimulation of healthy donor PBMCs with IFNγ, anti-CD3 antibody and IL-2.7,9 At day 28 of expansion, the resulting effector cell population comprised about 40% of cells co-expressing CD3 and CD56, while NK cells not only did not expand under these culture conditions, but progressively declined (Fig. 1A). Multi-color cytometry analysis of the CD3+CD56+ subpopulation was carried out throughout the culture period to evaluate the expression of different markers (Fig. 1B). Results essentially confirmed the already reported phenotype.11,15,18,19 In particular, the CD8+ component of CD3+CD56+ CIK cells readily expanded reaching 82.7 ± 4.2% of positivity at day 28, whereas the CD4+ subset progressively decreased to 8.9 ± 1.0%. A similar trend was observed for the TCR-expressing subpopulations: TCRα/β+ CIK cells increased during culture from 63.3 ± 19.5% to 83.7 ± 7.9%; in contrast, TCRγ/δ+ CD3+CD56+ cells decreased from 29.2 ± 17.7% to 11.9 ± 4.1%. At the end of culture almost all cells expressed NKG2D (96.1 ± 2.0%), while both CD28 (22.8 ± 12.9%) and CD11 (94.2 ± 5.0%) did not underwent substantial modifications.
Figure 1.
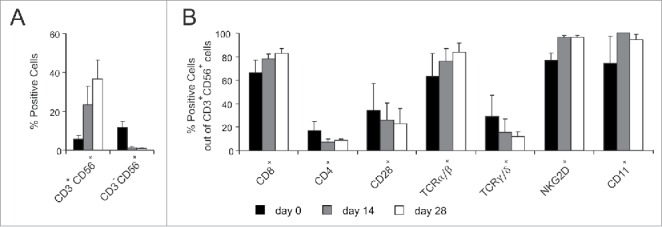
Phenotypic characterization of CIK cells. CIK cells were generated in vitro and analyzed for their phenotype by flow cytometry throughout the culture period. In the figure, histograms refer to three distinct time points, namely day 0 (black), day 14 (gray) and day 28 (white). (A) Percentage of CD3+CD56+ CIK cells and CD3−CD56+ NK cells in the bulk cultures at the reported time points. (B) Expression of different cell surface markers on CD3+CD56+ cells at the reported time points. Results show the mean expression ± SD of 5 to 15 independent experiments performed on PBMCs and related cultures from distinct donors.
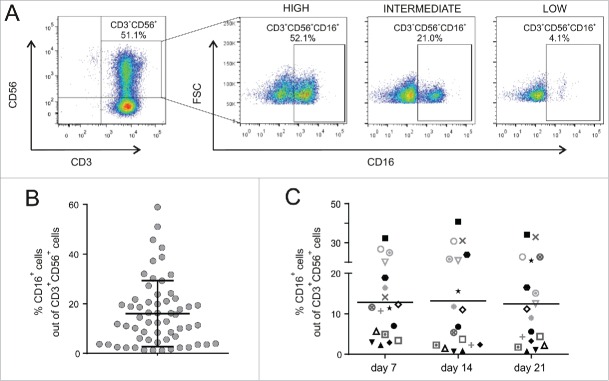
Interestingly, there is not a full agreement about the expression of CD16, since some groups reported that CIK cells do not express this receptor,9,11,20 while others detected its expression at some extent, but did not further investigated such issue.16,21,22 In this study, CD16-expressing cells were clearly detected within the CD3+CD56+ CIK population, as they formed a defined and distinct subset in the dot plot graphs (Fig. 2A). Notably, cytometry analysis was carried out with the 3G8 antibody clone, which had been already used by others,11,16 and showed that CD16 expression in CIK cells was donor-dependent and characterized by a marked variability among donors, who were arbitrarily classified as low (<5 %), intermediate (>5 % and <25%), and high (>25 %), according to the percentage of CD16 expression within their CD3+CD56+ populations. Overall, the expression in 60 different healthy donors was in the range of 2.3–54.2%, mean 16.0 ± 13.3% (Fig. 2B); to the best of our knowledge, this is the first report with such a high number of donors tested. Interestingly, the fraction of CIK cells expressing the CD16 receptor remained stable during the entire period of culture (Fig. 2C).
Figure 2.
CD16 expression on CIK cells. CIK cells were analyzed for CD3, CD56 and CD16 co-expression by flow cytometry. (A) Bulk cultures were first gated for CD3+ and CD56+ co-expression to identify the CIK subset (left panel), followed by analysis of CD16 expression (right panels). CIK cells were arbitrarily stratified as having a high (>25%), intermediate (<25% and >5%) or low (<5%) expression of CD16. (B) CD16 expression in CIK cells from different donors (n = 60) between the second and third week in vitro. Bar indicates mean value ± SD. (C) Comparison of the CD3+CD56+CD16+ component in distinct donors (n = 18, one symbol each) at different time points of culture (p > 0.5).
CIK cells can be retargeted to exert ADCC by antigen-specific mAbs engaging CD16
The observation of CD16 expression on CIK cells led us to investigate whether this receptor could mediate ADCC, and thus if the antitumor activity could be improved by combination with mAb, as it occurs in NK cells. We first evaluated Her2 and EGFR expression on target cell lines using trastuzumab and cetuximab as primary antibodies (Fig. 3A). SKOV-3 cells showed a relevant expression of both receptors, while IGROV-1, MDA-MB-231 and MDA-MB-468 cell lines expressed only EGFR. Thus, these cell lines were left untreated or pre-incubated for 30 min with 10 µg of trastuzumab or cetuximab, and used as targets in a standard cytotoxicity assay. Consistently with the receptor expression on target cells, a significant antigen-specific increase of cytotoxicity was observed when mAbs were present in the assay (Fig. 3B). Indeed, both trastuzumab and cetuximab significantly enhanced CIK cell cytotoxicity against SKOV-3 cells, which express both receptors, and increased basal specific lysis (9.1 ± 5.2%) to 35.4 ± 13.8% and to 16.1 ± 3.9%, respectively (Fig. 3B). On the other hand, only cetuximab increased CIK cell lytic activity toward IGROV-1, MDA-MB-231 and MDA-MB-468, as EGFR only is expressed on these cells; in this case, basal specific lysis (35.3 ± 14.2%, 19.7 ± 12.1% and 13.0 ± 12.3%, respectively) increased to 59.0 ± 7.7%, 45.2 ± 14.4% and 41.3 ± 21.3%, respectively (Fig. 3 B). Importantly, trastuzumab did not modify the killing against Her2 non-expressing target cell lines (Fig. 3B), thus suggesting that the increased cytotoxic activity is indeed mediated by the engagement of CD16 and subsequent ADCC.
Figure 3.
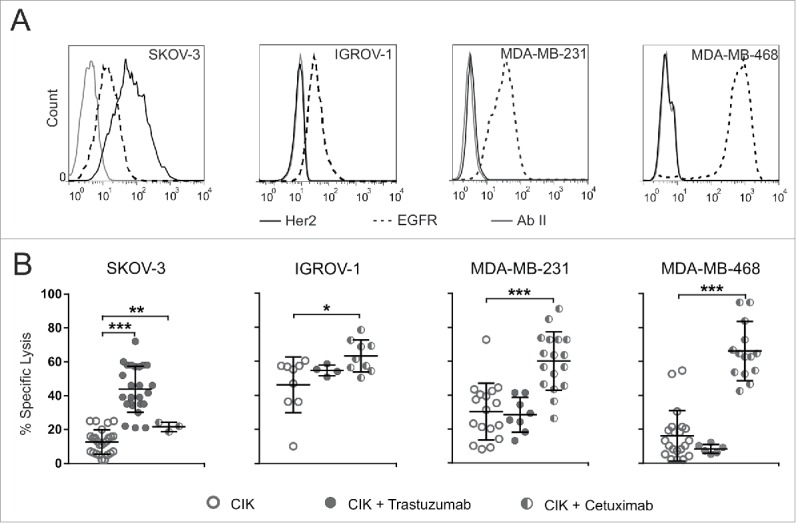
CIK cells engage CD16 and exert ADCC. (A) Target cells were analyzed for Her2 (black line) and EGFR (dotted line) expression by flow cytometry analysis. Fluorochrome-conjugated secondary antibody alone (gray line) served as negative control. Results are representative of five experiments. (B) CIK cells were challenged against SKOV-3, IGROV-1, MDA-MB-231 and MDA-MB-468 tumor cell lines. Lytic activity was measured using bulk CIK cells (day 21 of culture) in the absence (open circles) or presence of trastuzumab (gray circles) or cetuximab (half-filled circles). The symbols refer to the specific lysis of individual CIK cell cultures from different donors at an E/T ratio of 100:1, and bars indicate mean values ± SD. Data were analyzed by Student's t-test (***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05; not statistically significant (p > 0.05) if not indicated).
To formally prove such hypothesis, CIK cells were pretreated with an anti-CD16 blocking antibody to prevent the specific interaction between CD16 and the Fc region of therapeutic mAbs. CD16 masking completely abrogated the additional CIK cell lytic activity ascribable to the addition of trastuzumab or cetuximab, leading the cytotoxicity to return to the basal levels observed with untreated CIK cells (Fig. 4). In particular, the cytotoxicity against SKOV-3, which was enhanced by the addition of trastuzumab, reduced from 35.4 ±13.8% to 19.8 ± 11.2% with the addition of anti-CD16 antibody, this latter value being not significantly different from basal specific lysis (Fig. 4A). Similarly, CD16 masking led to a specific lysis reduction to 28.3 ± 15.3%, 24.5 ± 5.8% and 28.3 ± 7.1% for cetuximab-treated IGROV-1, MDA-MB-231 and MDA-MB-468, respectively, as the interaction between the receptor and mAb is prevented (Fig. 4B–D). As a control, CIK cells were pretreated with an irrelevant mouse IgG1 isotype antibody, in the presence or absence of mAbs, to rule out potential interferences in the tests (Fig. 4). In all cases, the addition of such control antibody nor reduced the increased activity mediated by trastuzumab or cetuximab, nor interfered with basal CIK cell functions.
Figure 4.
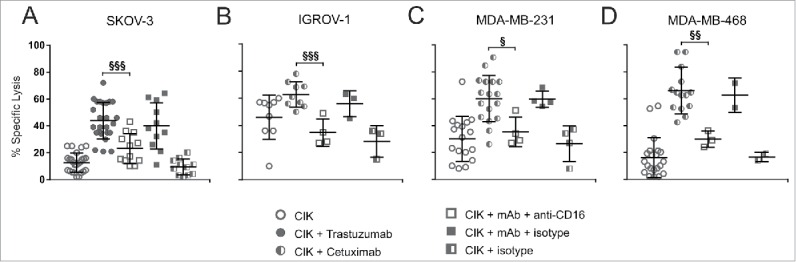
CD16 engagement is critically required for CIK cell-mediated ADCC. CIK cells were challenged against SKOV-3 (A), IGROV-1 (B), MDA-MB-231 (C) and MDA-MB-468 (D) tumor cell lines. Lytic activity was measured using bulk CIK cells (day 21 of culture) in the absence (open circles) or presence of trastuzumab (gray circles) or cetuximab (half-filled circles), adding an anti-CD16 blocking antibody (open squares) or an isotype antibody (gray squares) to the corresponding specific mAb (trastuzumab in A, and cetuximab in B, C and D). An isotype antibody served as negative control (half-filled square). The symbols refer to the specific lysis of individual CIK cell cultures from different donors at an E/T ratio of 100:1, and bars indicate mean values ± SD. Data were analyzed by Student's t-test (§§§, p ≤ 0.001; §§, p ≤ 0.01; §, p ≤ 0.05; not statistically significant (p > 0.05) if not indicated).
As different levels of CD16 expression were detected on CIK cells obtained from different donors, we wanted to unveil whether cytotoxic activity was dependent on the amount of CD16 present on cells. To this aim, we performed cytotoxic assays by using CIK cells presenting different extent of CD16 expression. An antibody-mediated increase of the cytotoxic activity could be detected in all CIK cultures tested, irrespective of the levels of CD16 expression. Fig. 5 compares the cytotoxicity of CIK cultures stratified for CD16 expression in the presence of the mAbs, and no statistically significant differences were found against both ovarian and breast cancer cell lines.
Figure 5.
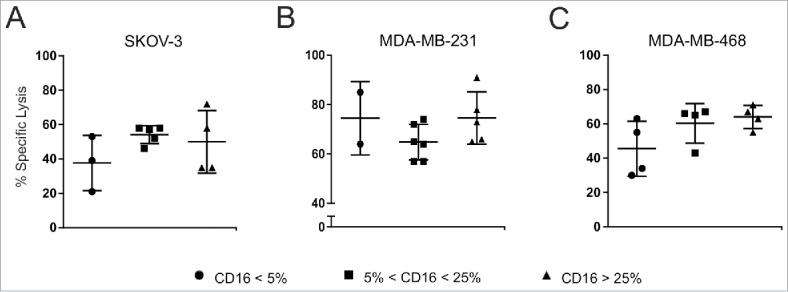
Correlation between CD16 expression and ADCC activity. CIK cells samples were arbitrarily stratified as low (<5%, circles), intermediate (>5 % and <25%, squares) and high (>25 %, triangles), according to their CD16 expressing levels. Their lytic activity against SKOV-3 (A), MDA-MB-231 (B) and MDA-MB-468 (C) tumor cell lines was measured in a standard cytotoxicity assay, in the presence of the corresponding specific mAb (trastuzumab in A, and cetuximab in B and C). The results are reported as the percentage of specific lysis at an E/T ratio of 100:1. Each symbol represents a different donor, and bars indicate mean values ± SD. Data were analyzed by Student's t-test. No statistical significant differences were found between different groups.
ADCC is accountable to CD3+CD56+CD16+ CIK cell fraction
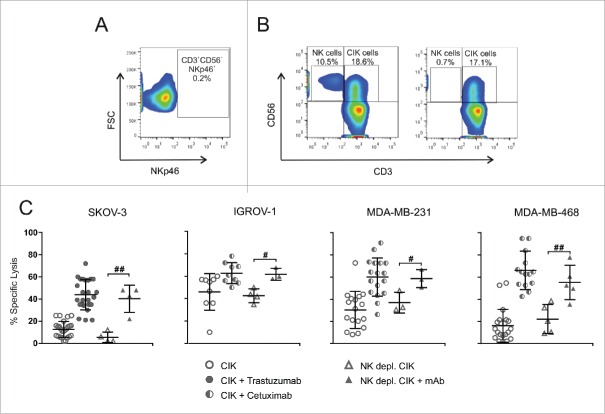
It is well known that CD16 is expressed by CD3−CD56+ NK cells, which also have the ability to kill tumor cells by ADCC.23 Moreover, most of the studies on CIK cells, including clinical trials of adoptive immunotherapy, used bulk-expanded CIK cultures where NK cells were still present as contaminants. NK cells were found in PBMCs at the beginning of ex vivo CIK cell expansion phase, while their percentage progressively decreased during culture (Fig. 1A). Therefore, to verify that the ADCC activity observed was not due to the residual NK subpopulation but rather to CD3+CD56+CD16+ CIK cells, NK cells were removed from cultures by immunomagnetic depletion with a mAb against NKp46,24,25 an antigen exclusively expressed by NK cells, but not CIK cells (Fig. 6A–B).11,21 Thus, bulk and NK-depleted CIK cells were challenged against target cell lines in a standard 51Cr-release assay, in the presence or absence of the corresponding specific therapeutic mAb (Fig. 6C). Against all target cell lines tested, the cytotoxicity of NK-depleted and bulk CIK cells fully overlapped, both alone and in combination with the mAbs. Indeed, the addition of mAbs to NK-depleted populations was still able to produce a significant increase of cytotoxicity.
Figure 6.
ADCC is accountable to CD3+CD56+CD16+ CIK cells. (A) Flow cytometry analysis was performed in triple fluorescence to determine the absence of NKp46 expression on CD3+CD56+ cells. (B) NKp46+ NK cells were removed by magnetic beads depletion. The panels show one representative experiment out of 5 performed, where flow cytometry analysis was carried out before (left) and after (right) NK depletion. (C) CIK cells were challenged against SKOV-3, IGROV-1, MDA-MB-231 and MDA-MB-468 tumor cell lines. Lytic activity was measured using bulk CIK cells (circles) or NK-depleted CIK cells (triangles), in the absence (open symbols) or presence (gray symbols) of the corresponding mAb (trastuzumab for SKOV-3, and cetuximab for IGROV-1, MDA-MB-231 and MDA-MB-468). The symbols refer to the specific lysis of individual CIK cell cultures from different donors at an E/T ratio of 100:1, and bars indicate mean values ± SD. Data were analyzed by Student's t-test (##, p ≤ 0.01; #, p ≤ 0.05).
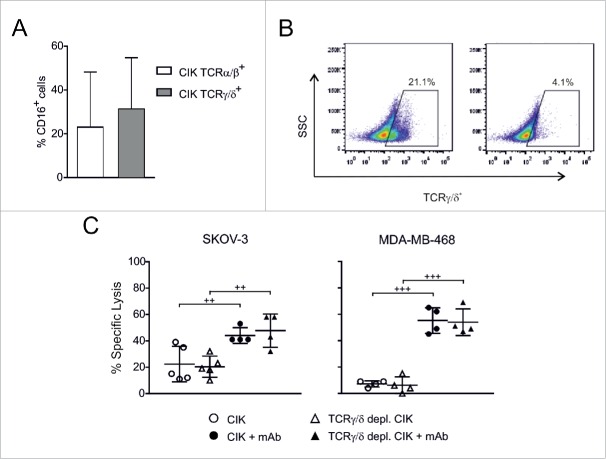
On the other hand, CD3+CD56+ CIK cells comprise two subsets characterized by the expression of the TCRα/β and TCRγ/δ receptors, respectively (Fig. 1B). Since the TCRγ/δ+ cells have been reported to express CD16 and exert ADCC,26,27 the relative expression of CD16 on the two CIK cell subpopulations was assessed by flow cytometry. As reported in Fig. 7A, a mean of 23.0 ± 25.2% and 31.3 ± 23.5% of TCRα/β+ and TCRγ/δ+ CIK cells, respectively, expressed also CD16. Thereafter, to gain insights into the contribution of TCRγ/δ+ subset of CIK cells to ADCC, these cells were removed from bulk CIK cell cultures by immunomagnetic depletion (Fig. 7B), and TCRγ/δ-depleted CIK cells were tested for lytic activity against Her2+ or EGFR+ tumor cell lines, in the presence or absence of the specific therapeutic mAb. Basal and mAb-enhanced cytotoxicity were totally comparable prior and after cell depletion (Fig. 7C), thus indicating that the triggered ADCC activity could not be ascribed only to the TCRγ/δ+ CIK cell subset.
Figure 7.
CIK cell-mediated ADCC is independent of TCRγ/δ+ subpopulation. (A) Flow cytometry analysis was performed in quadruple fluorescence to determine the expression of CD16 in TCRα/β (n = 8) and TCRγ/δ (n = 14) subpopulations of CD3+CD56+ CIK cells from different donors. (B) TCRγ/δ+ cells were removed by magnetic beads depletion. The panels show one representative experiment out of 5 performed, in which flow cytometry analysis was carried out before (left) and after (right) depletion. (C) CIK cells were tested for cytotoxicity against SKOV-3 and MDA-MB-468 tumor cell lines. Lytic activity was measured using bulk CIK cells (circles) or TCRγ/δ-depleted CIK cells (triangles), in the absence (open symbols) or presence (black symbols) of the corresponding mAb (trastuzumab for SKOV-3, and cetuximab for MDA-MB-468). The symbols refer to the specific lysis of individual CIK cell cultures from different donors at an E/T ratio of 50:1, and bars indicate mean values ± SD. Data were analyzed by Student's t-test (+++, p ≤ 0.001; ++, p ≤ 0.01).
Overall, these results demonstrate that the enhanced cytotoxicity induced by mAbs is associated with the CD3+CD56+CD16+ subpopulation in CIK cell cultures and is irrespective of the TCR type expressed, thus confirming that CIK cells can exert ADCC through the engagement of CD16.
mAb retargeting enhances CIK cell activity in vivo
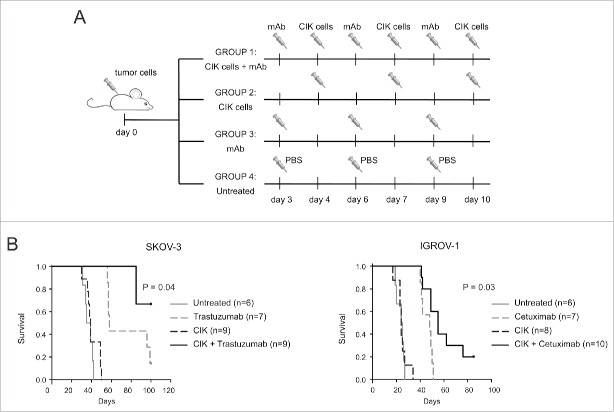
CIK cell-mediated ADCC was assessed for therapeutic implications in mouse models of human ovarian carcinoma xenografted in NSG mice. On day 0, female mice were injected intraperitoneally with 1 × 106 SKOV-3 or IGROV-1 tumor cells. Then, animals were randomly assigned to experimental groups, and treated i.p. with CIK cells in combination or not with the mAbs, as reported in (Fig. 8A). As showed in Kaplan–Meier survival curves (Fig. 8B), co-administration of CIK cells and mAbs significantly increased the survival of both SKOV-3 and IGROV-1 tumor-bearing mice, as compared to animals receiving CIK cells or mAb alone.
Figure 8.
Combining mAb and CIK cells improves the in vivo antitumor activity. (A) On day 0, mice received intraperitoneally 1 × 106 SKOV-3 or IGROV-1 cells. Depending on the experimental group, treatments consisted of a combination of 1 mg trastuzumab or 1.5 mg cetuximab on day 3, 6, 9 and 1 × 107 CIK cells on day 4, 7, 10 (n = 9 and n = 10, respectively), or mAbs only (n = 7) or CIK cells only (n = 9 and n = 8, respectively) at the same doses and on the same days of the combined treatment. The untreated group (n = 6) received three injections of PBS. (B) Kaplan–Meier survival curves of NSG mice bearing intraperitoneal SKOV-3 or IGROV-1 tumors. Combination therapy (black lines) significantly increased survival compared to any other treatment (mAbs only, gray-dashed lines; CIK only, black-dashed lines; untreated, gray lines), both in SKOV3 (P = 0.04) and IGROV-1-injected mice (p = 0.03).
Discussion
In this study, CIK cells were obtained from healthy donor PBMCs according to standard protocols.7-9 Phenotype of the CD3+CD56+ subpopulation was evaluated by cytometry analysis, and results essentially confirmed what already described by other groups.11,15,18,19 In particular, CD3+CD56+ CIK cells are mostly CD8+, and express a TCR, which has been shown to be functional and responsive to specific MHC-dependent stimuli but not directly involved in tumor cell killing.18 On the other hand, CIK cells are characterized by high levels of NKG2D that is considered the main mediator of their antitumor activity, as it binds MICA/B and members of the ULBP family, leading to target cell recognition and lysis.15 Moreover, we detected high levels of CD11, which is a requisite for target cell binding and lysis.18
However, our attention focused on CD16 expression, because we repeatedly observed results that were discordant from those reported in the literature. Indeed, flow cytometry highlighted a remarkable and donor-dependent expression of CD16 on CD3+CD56+ cells, which remained stable during the entire period of culture, notwithstanding the enormous expansion of the CD3+CD56+ population.
Based on these phenotypical observations, we were prompted to investigate the potential functional relevance of CD16 in improving the CIK cell intrinsic cytotoxic activity to exert ADCC when combined with antitumor therapeutic mAbs. Indeed, it has been widely demonstrated that ADCC plays an essential role in mediating the antitumor effects of routinely used therapeutic mAbs, such as trastuzumab and cetuximab,28,29 which are FDA and EMA-approved for the treatment of Her2+ and EGFR+ tumors, such as breast, gastric, colorectal, and head and neck cancers.30
The combination of mAbs with CIK cells produced a significant antigen-specific increase of cytotoxicity that was consistent with Her2 and EGFR expression on target cells. The direct involvement of CD16 was demonstrated by using a blocking monoclonal antibody against the CD16, which was able to bring about the cytotoxicity to return to the basal levels, thus indicating that the CD16 receptor is functional and directly involved in the ADCC.
An important observation is that the ability of mAbs to increase the CIK cell cytotoxic activity is irrespective of the levels of CD16 expression. This finding suggests that even a small fraction of CD16+ CIK cells can exert a relevant ADCC activity, likely due to the recycling potentialities of the effectors, and leads to a significant enhancement of the whole population cytotoxicity. Translated into a potential clinical application, this aspect could represent a critical issue, since patients generating CIK cell cultures with even a low expression of CD16 might strongly benefit from CIK and mAbs combination therapy.
Another relevant aspect is that most of the CIK cell studies reported in the literature, including clinical trials of adoptive immunotherapy, used bulk-expanded CIK cultures where NK cells were still present as contaminants. To exclude that the ADCC activity observed was due to the presence of a residual NK subpopulation, NK cells were removed from cultures. Performing a depletion for NKp46, instead of a positive selection using CD5, which is not expressed by NK cells,31 allowed us to obtain “untouched” CIK cells, thus preventing unwanted nonspecific effector cell stimulation in the subsequent cytotoxicity assays. Results excluded that the observed ADCC is mediated by a residual NK cell subpopulation, but is rather truly exerted by CIK cells.
Besides NK cells, CD16 has been shown to mediate ADCC also in γδ T cells.26,27 Flow cytometry analysis of CIK cells highlighted the expression of both TCRα/β and TCRγ/δ receptors on CD3+CD56+ cells, and the relative expression of CD16 in either subpopulations was characterized. Interestingly, the ADCC induced by the addition of trastuzumab and cetuximab was not impaired by depletion of the TCRγ/δ+ component, indicating that cytotoxicity is due to the engagement of CD16 on both TCRα/β+ and TCRγ/δ+ CIK cell subsets, and thus that it is independent of the TCR type expressed. Therefore, analysis of lytic activity confirmed that the ADCC observed is accountable to the CD3+CD56+CD16+ subpopulation, demonstrating that CIK cells can induce ADCC by the engagement of CD16.
This result was further supported by in vivo experiments using human ovarian carcinoma xenografts in NSG mice, in which the co-administration of CIK cells and mAbs significantly increased the survival of treated mice. The inefficacy of the treatment with CIK cells as a single agent is consistent with our in vitro results, and mostly with reports from other groups where SKOV-3 cells have been described to be resistant to CIK cell-mediated killing.22,32,33 Nevertheless, when treated with the combination of CIK cells and mAbs, even these resistant cells become responsive to therapy. These results are even more remarkable considering that, in this setting, CIK cell monotherapy was not effective in improving the outcome of treated mice; conversely, when combined with trastuzumab or cetuximab CIK cells appeared even more efficient than the antibodies used alone.
Overall, we advance that the combination of adoptive CIK cell therapy with clinical-grade mAbs can be proposed as a very promising strategy to improve the therapeutic approaches against different types of tumors. Indeed, several clinical studies demonstrated the feasibility and high tolerability of CIK cell infusions; 17 moreover, trastuzumab and cetuximab are well established and widely used in clinical practice and, therefore, no particular safety and clinical concerns should be raised for this combination approach. This novel strategy could address one of the critical issues of current adoptive immunotherapy approaches, i.e. the rapid obtainment of sufficient numbers of targeted effector cells to be administered, because CIK cells are easy to produce and do not require genetic manipulations for expansion; moreover, the relevant enhancement of CIK antitumor potential mediated by mAb combination could allow to reduce the total cell dose and the number of infusions required for treatment, with positive implications for patients with limited ex vivo expansion rates of CIK cells.6 The finding of no correlation between the expression level of CD16 on CIK cells and the cytotoxicity outcome when combined with mAbs, further broadens the number of patients that could potentially benefit from this therapy.
In conclusion, we demonstrated for the first time that CIK cells express CD16 in a donor-dependent manner, and that the concurrent administration of therapeutic mAbs, such as trastuzumab or cetuximab, leads to a significant improvement of their antitumor activity, triggering a potent ADCC activity.
Taken together, the results produced in this study describe a novel function of CIK cells, which confirm their outstanding potential as a promising tool for ACT approaches. These data lead us to envisage new perspectives for adoptive immunotherapy where antigen-specific retargeting of T cells can be achieved by the simple combination of non-antigen-specific effector cells and tumor-specific mAbs, already widely employed in clinical practice.
Materials and methods
Generation and characterization of CIK cells
CIK cells were obtained from healthy donors as previously reported by other groups.7–9 Briefly, PBMCs were isolated from buffy coats by means of Ficoll-Paque PLUS (GE Healthcare) density gradient centrifugation. The monocytes were depleted by adhesion to tissue culture flask and the non-adherent cells were resuspended and cultured at a density of 1 to 2 × 106 cells/mL in RPMI 1640 (Euroclone), 10% heat-inactivated FBS (Gibco), 1% Ultraglutamine, 1% Hepes buffer, 1% penicillin/streptomycin (Lonza) at 37°C, 5% CO2. At day 0, medium was supplemented with rhIFNγ (PeproTech) at 1000 U/mL and after 24 h of incubation, anti-CD3 mAb (OKT-3, Ortho Biotech Inc.) at 50 ng/mL and rhIL-2 (Proleukin, Novartis) at 300 IU/mL were added to the culture medium. Every 2–3 d medium was replenished by adding fresh rhIL-2 at 450 IU/mL. CIK cells phenotype was analyzed using multi-color flow cytometry. Briefly, cells were harvested between day 7 and 28 and stained for 30 min at 4°C, using the following antibodies: CD3-BV510 (clone UCHT1), CD8-BV421 (clone RPA-T8), CD4-APC-H7 (clone RPA-T4), CD28-PE-Cy™7 (clone CD28.2), TCRα/β-FITC (clone T10B9.1A-31), TCRγ/δ-BV421 (clone B1), CD11a-FITC (clone G43-25B) from BD Bioscience; CD56-PE (clone HCD56), NKG2D-APC (clone 1D11), CD16a-FITC (clone 3G8), from BioLegend. For CD16 staining, cells were pre-incubated with 2.5 µg/106 cells of Human BD Fc Block (BD PharMingen) to avoid non-specific Fc Receptor-mediated antibody binding. Flow cytometry analysis was performed either on FACScalibur or LSRII, using CellQuest software (BD Bioscience). Data analyses were performed using FlowJo software (Treestar).
Depletion of NK and TCRγ/δ+ CIK cells
Where reported, NK or TCRγ/δ+ CIK cells were removed from bulk cultures by immunomagnetic depletion at day 14 to 21 of culture. For NK cells depletion, CIK cells were stained with APC-conjugated anti-NKp46 antibody (clone 9EJ, Miltenyi Biotec), and then washed and mixed with anti-APC microbeads (Miltenyi Biotec). For TCRγ/δ+ CIK cells depletion, the Anti-TCR γ/δ+ MicroBead Kit (Miltenyi Biotec) was used according to the manufacturer's instructions. MS MACS Columns and MACS Separator from Miltenyi Biotec were used according to the manufacturer's instructions. The unlabeled cell fraction, containing NKp46− or TCRγ/δ− CIK cells, were collected and stained with fluorochrome-conjugated antibodies to verify purity of fractions.
Cell lines and cultures
Ovarian (SKOV-3 and IGROV-1) and breast (MDA-MB-231 and MDA-MB-468) cancer cells lines were used as target cells. Ovarian cancer cell lines were cultured in RPMI 1640 (Euroclone) supplemented with 1% Sodium pyruvate (Lonza), while breast tumor cell lines were kept in DMEM (Euroclone). Both media were supplemented with 10% heat-inactivated FBS (Gibco), 1% Ultraglutamine, 1% Hepes buffer, 1% penicillin/streptomycin (all from Lonza). All cell lines were authenticated by STR sequences analysis.
Assessment of HER2 and EGFR expression
The expression of Her2 and EGFR was assessed on cell lines by flow cytometry using as primary antibodies the humanized anti-Her2 monoclonal IgG1 antibody trastuzumab (Roche) and the chimeric mouse-human anti-EGFR monoclonal IgG1 antibody cetuximab (MerckSerono), respectively, and a PE-conjugated anti-IgG1 antibody (Miltenyi Biotec) as secondary antibody.
Cytotoxicity assays
Cytotoxic activity of CIK cells was assessed using a standard 4-h 51Cr-release assay. A total of 106 target cells were labeled with 100 µCi 51Cr for 1 h at 37°C, washed twice with culture medium. Depending on the experiment, target cells were incubated for 30 min with 10 µg of trastuzumab or cetuximab. For blocking experiments, effector cells were incubated for 30 min with 10 µg anti-CD16 blocking antibody. Target and effector cells were then plated at the indicated E/T ratio in a 96-well U-bottom plate in a total volume of 200 µL. After 4 h incubation at 37°C, 30 µL supernatant was transferred on a scintillation plate (Perkin Elmer), and measured using a Top Count gamma counter (Perkin Elmer). Each test was performed in triplicate. The results are expressed as the percentage of lysis, which is calculated as follows: % Specific Lysis = (experimental release–spontaneous release)/(maximal release–spontaneous release) × 100. Spontaneous and maximal release were obtained by incubating target cells in medium alone or in PBS 2% SDS, respectively.
In vivo studies
On day 0, 6–8 week-old female NOD/SCID common γ chain knockout (NSG, Charles River) mice were injected intraperitoneally with 1 × 106 SKOV-3 or IGROV-1 cells, and randomly assigned to four experimental groups. Combination treatments consisted of 1 mg trastuzumab or 1.5 mg cetuximab on days 3, 6 and 9, followed by 1 × 107 CIK cells on day 4, 7 and 10 after tumor injection; control mice received mAbs or CIK cells only, at the same doses and with the same schedule as in the combined treatment, or PBS.
Statistics
Results were analyzed for statistical significance by using paired or unpaired Student's t-test, as appropriate (***p < 0.001, **p < 0.01, *p < 0.05). Mice survival was compared using log-rank survival statistics. Histograms represent mean values ± standard deviation. In scatter-plot graphs, symbols indicate different samples or assays, and horizontal bars represent means ± standard deviation. Statistical analysis was performed using GraphPad Prism 4.0 software.
Study approval
Anonymized human buffy coats were obtained from the Blood Bank of Padova Hospital, and donors provided their written informed consents to participate in this study. Procedures involving animals and their care were in conformity with institutional guidelines that comply with national and international laws and policies (D.L. 26/2014 and subsequent implementing circulars), and the experimental protocol (Authorization n. 1143/2015-PR) was approved by the Italian Ministry of Health.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was partly supported by grants from the Italian Association for Cancer Research (AIRC, IG-17035 and Special Program Molecular Clinical Oncology 5 per mille ID 10016), and Progetto di Ricerca di Ateneo 2015, University of Padova, to AR.
References
- 1.Aranda F, Buqué A, Bloy N, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Spisek R et al.. Trial Watch: Adoptive cell transfer for oncological indications. Oncoimmunology [Internet] 2015; 4:e1046673; PMID:26451319; http://dx.doi.org/ 10.1080/2162402X.2015.1046673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Drake CG, Pardoll DM. Perspective immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell [Internet] 2015; 27:450-61; PMID:25858804; http://doi.org/ 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: A race to the finish line. Sci Transl Med [Internet] 2015; 7:280ps7-280ps7; PMID:25810311; http://dx.doi.org/ 10.1126/scitranslmed.aaa3643 [DOI] [PubMed] [Google Scholar]
- 4.Feldman SA, Assadipour Y, Kriley I, Goff SL, Rosenberg SA. Adoptive cell therapy-Tumor-Infiltrating Lymphocytes, T-Cell receptors, and chimeric antigen receptors. Semin Oncol [Internet] 2015; 42:626-39; PMID:26320066; http://dx.doi.org/ 10.1053/j.seminoncol.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim O, Jung MY, Hwang YK, Shin E-C. Present and future of allogeneic natural killer cell therapy. Front Immunol [Internet] 2015; 6:286; PMID:26089823; http://dx.doi.org/ 10.3389/fimmu.2015.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer 2011; 2:363-8; PMID:21716717; http://dx.doi.org/ 10.7150/jca.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med 1991; 174:139-49; PMID:1711560; http://dx.doi.org/ 10.1084/jem.174.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Introna M, Franceschetti M, Ciocca A, Borleri G, Conti E, Golay J, Rambaldi A. Rapid and massive expansion of cord blood-derived cytokine-induced killer cells: an innovative proposal for the treatment of leukemia relapse after cord blood transplantation. Bone Marrow Transplant 2006; 38:621-7; PMID:16980990; http://dx.doi.org/ 10.1038/sj.bmt.1705503 [DOI] [PubMed] [Google Scholar]
- 9.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol 1994; 153:1687-96; PMID:7519209 [PubMed] [Google Scholar]
- 10.Sangiolo D, Martinuzzi E, Todorovic M, Vitaggio K, Vallario A, Jordaney N, Carnevale-Schianca F, Capaldi A, Geuna M, Casorzo L et al.. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: Implications for their infusion across major HLA barriers. Int Immunol 2008; 20:841-8; PMID:18469328; http://dx.doi.org/ 10.1093/intimm/dxn042 [DOI] [PubMed] [Google Scholar]
- 11.Franceschetti M, Pievani A, Borleri G, Vago L, Fleischhauer K, Golay J, Introna M. Cytokine-induced killer cells are terminallydifferentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol 2009; 37:616-28; PMID:19375652; http://dx.doi.org/ 10.1016/j.exphem.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 12.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 2002; 17:19-29; PMID:12150888; http://dx.doi.org/ 10.1016/S1074-7613(02)00333-3 [DOI] [PubMed] [Google Scholar]
- 13.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol 2000; 1:119-26; PMID:11248803; http://dx.doi.org/ 10.1038/77793 [DOI] [PubMed] [Google Scholar]
- 14.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999; 285:727-9; PMID:10426993; http://dx.doi.org/ 10.1126/science.285.5428.727 [DOI] [PubMed] [Google Scholar]
- 15.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 2004; 103:3065-72; PMID:15070686; http://dx.doi.org/ 10.1182/blood-2003-06-2125 [DOI] [PubMed] [Google Scholar]
- 16.Karimi M, Cao TM, Baker JA, Verneris MR, Soares L, Negrin RS. Silencing Human NKG2D, DAP10, and DAP12 Reduces Cytotoxicity of Activated CD8+ T Cells and NK Cells. J Immunol [Internet] 2005; 175:7819-28; PMID:16339517; http://dx.doi.org/ 10.4049/jimmunol.175.12.7819 [DOI] [PubMed] [Google Scholar]
- 17.Schmeel LC, Schmeel FC, Coch C, Schmidt-Wolf IGH. Cytokine-induced killer (CIK) cells in cancer immunotherapy: report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol [Internet] 2014; 141:839-49; PMID:25381063; http://dx.doi.org/ 10.1007/s00432-014-1864-3 [DOI] [PubMed] [Google Scholar]
- 18.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3 +CD56 + CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood 2011; 118:3301-10; PMID:21821703; http://dx.doi.org/ 10.1182/blood-2011-02-336321 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol [Internet] 1993; 21:1673-9; PMID:7694868 [PubMed] [Google Scholar]
- 20.Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol 1997; 74:51-6; PMID:9063373; http://dx.doi.org/ 10.1007/s002770050257 [DOI] [PubMed] [Google Scholar]
- 21.Bonanno G, Iudicone P, Mariotti A, Procoli A, Pandolfi A, Fioravanti D, Corallo M, Perillo A, Scambia G, Pierelli L et al.. Thymoglobulin, interferon-γ and interleukin-2 efficiently expand cytokine-induced killer (CIK) cells in clinical-grade cultures. J Transl Med [Internet] 2010; 8:129; PMID:21138560; http://dx.doi.org/ 10.1186/1479-5876-8-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science 2006; 311:1780-4; PMID:16556847; http://dx.doi.org/ 10.1126/science.1121411 [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G. Biology of natural killer cells. Adv Immunol [Internet] 1989; 47:187-376; PMID:2683611; http://dx.doi.org/ 10.1016/S0065-2776(08)60664-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bottino C, Biassoni R, Millo R, Moretta L, Moretta A. The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Hum Immunol 2000; 61:1-6; PMID:10658972; http://dx.doi.org/ 10.1016/S0198-8859(99)00162-7 [DOI] [PubMed] [Google Scholar]
- 25.Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A. P46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med 1997; 186:1129-36; PMID:9314561; http://dx.doi.org/ 10.1084/jem.186.7.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braakman E, van de Winkel J, van Krimpen B, Jansze M, Bolhuis R. CD16 on human gamma delta T lymphocytes: expression, function, and specificity for mouse IgG isotypes. Cell Immunol 1992; 143:97-107; PMID:1377991; http://dx.doi.org/ 10.1016/0008-8749(92)90008-D [DOI] [PubMed] [Google Scholar]
- 27.Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, Fai-So H, Moriyasu F, Nieda M, Nicol AJ. Vγ9Vδ2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs-Rituximab and trastuzumab. Int J Cancer [Internet] 2008; 122:2526-34; PMID:18307255; http://dx.doi.org/ 10.1002/ijc.23365 [DOI] [PubMed] [Google Scholar]
- 28.Sliwkowski M, Lofgren J, Lewis G, Hotaling T, Fendly B, Fox J. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol 1999; 26:60-70; PMID:10482195 [PubMed] [Google Scholar]
- 29.Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, Touge H, Makino H, Takata M, Miyata M et al.. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res [Internet] 2007; 13:1552-61; PMID:17332301; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1726 [DOI] [PubMed] [Google Scholar]
- 30.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer [Internet] 2012; 12:278-87; PMID:22437872; http://dx.doi.org/ 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 31.Ishiyama T, Watanabe K, Fukuchi K, Yajima K, Koike M, Tomoyasu S, Tsuruoka N. The presence of CD5low+ NK cells in normal controls and patients with pulmonary tuberculosis. Immunol Lett 1993; 37:139-44; PMID:7505001; http://dx.doi.org/ 10.1016/0165-2478(93)90023-U [DOI] [PubMed] [Google Scholar]
- 32.Scheffold C, Kornacker M, Scheffold YC, Contag CH, Negrin RS. Visualization of effective tumor targeting by CD8 + Natural Killer T cells redirected with bispecific antibody F(ab′)2 HER2xCD3. Cancer Res 2002; 62:5785-91; PMID:12384539 [PubMed] [Google Scholar]
- 33.Yoon SH, Lee JM, Woo SJ, Park MJ, Park JS, Kim HS, Park MY, Sohn HJ, Kim TG. Transfer of Her-2/neu specificity into cytokine-induced killer (CIK) cells with RNA encoding chimeric immune receptor (CIR). J Clin Immunol 2009; 29:806-14; PMID:19517218; http://dx.doi.org/ 10.1007/s10875-009-9308-6 [DOI] [PubMed] [Google Scholar]