Abstract
Background
There is growing evidence to suggest that delusions associated with schizophrenia arise from altered structural brain connectivity. The present study investigated whether structural changes in three major fasciculi that interconnect the limbic system – the cingulum bundle, uncinate fasciculus and fornix – are associated with delusions in chronic schizophrenia patients.
Methods
Free-water corrected Diffusion Tensor Imaging was used to investigate the association between delusions and both microstructural changes within these three fasciculi and extracellular changes in the surrounding free-water. Clinical data and diffusion MRI scans were obtained from 28 healthy controls and 86 schizophrenia patients, of whom 34 had present state delusions, 35 had a lifetime history but currently remitted delusions, and 17 had never experienced delusions.
Results
While present state and remitted delusions were found to be associated with reduced free-water corrected fractional anisotropy (FAT) and increased free-water corrected radial diffusivity (RDT) in the cingulum bundle bilaterally, extracellular free-water (FW) in the left cingulum bundle was found to be specifically associated with present state delusions in chronic schizophrenia. No changes were observed in the remaining tracts.
Conclusions
These findings suggest that state and trait delusions in chronic schizophrenia are associated with microstructural processes, such as myelin abnormalities (as indicated by decreased FAT and increased RDT) in the cingulum bundle and that state delusions are additionally associated with extracellular processes such as neuroinflammation or atrophy (as indicated by increased FW) in the left cingulum bundle.
Keywords: Free-water imaging, Schizophrenia, Delusions, Limbic system, Diffusion Tensor Imaging
Highlights
-
•
Free-water imaging was used to differentiate microstructural and extracellular processes.
-
•
Patients with delusions showed increased RDT and FW in the cingulum bundle.
-
•
Myelin abnormalities and neuroinflammation may be involved in the manifestation of delusions.
1. Introduction
Delusions are described as “fixed beliefs that are not amenable to change in light of conflicting evidence” (American Psychiatric Association, 2013) and are one of the most distinctive and common symptoms of schizophrenia. It has long been suggested that delusions represent a misinterpretation or misperception of sensory experiences resulting from abnormal neural connectivity. Frith et al. (2000) suggested that certain delusions might result from an abnormal connectivity between the frontal lobe and the parietal cortex, leading to a misattribution of internally generated events to external sources. It has been proposed that this abnormal fronto-parietal communication might be the result of structural changes in frontally extending white matter tracts that connect these distant cortical regions (Whitford et al., 2014a).
Other studies have focused on connectivity within the limbic system. The limbic system is a complex network of interconnected gray matter structures including the amygdala, hippocampus, hypothalamus, thalamus, basal ganglia, and cingulate gyrus (Mega et al., 1997). While the limbic system plays a role in behavior, motivation and olfaction, its principal functions are in emotional regulation and memory (Salcman, 1978). The limbic system is interconnected by three major white matter fasciculi, namely the cingulum bundle, uncinate fasciculus, and fornix. The cingulum bundle connects the anterior cingulate cortex with the nucleus accumbens, the amygdala and the medial dorsal thalamus (Nestor et al., 2007), and is involved in memory, emotion and attention (Catani and Thiebaut de Schotten, 2008). The uncinate fasciculus is a ventral limbic connection that originates in the temporal lobe and projects into orbital, medial and ventral regions of the frontal cortex (Catani et al., 2002, Price et al., 2008). The uncinate fasciculus has been reported to be involved in memory (Cohen, 2011, Schott et al., 2011), emotion, and inhibition (Price et al., 2008). The fornix connects the hippocampus with the mammillary bodies, thalamus and nucleus accumbens (Davies et al., 2001, Takei et al., 2008), and is thought to be primarily involved in memory functions (Catani and Thiebaut de Schotten, 2008, Takei et al., 2008).
There is growing evidence to suggest that abnormalities in the structural and functional connectivity of the limbic system may be a causal factor in the development of delusions in individuals with schizophrenia. With regards to functional connectivity, Javanbakht (2006) suggested that delusions emerge from a fronto-limbic imbalance resulting from an increase in limbic dopamine, which has been observed during psychotic episodes of schizophrenia but not during remission. According to this account, a hyperactive limbic system attributes enhanced emotional importance to internal and external events (Javanbakht, 2006). This in turn leads to a breakdown of the prefrontal cortex's ability to differentiate internally from externally generated events and thereby gives rise to the development of delusions. Support for the involvement of the limbic system in the development of delusions comes from an fMRI study which reported that disrupted functional connectivity in fronto-limbic structures were associated with acute psychotic states (Schott et al., 2015). Additionally, a positron emission tomography (PET) study reported an association between metabolic changes in brain circuits of the limbic system and the development of psychosis in schizophrenia (Tamminga et al., 1992).
With regards to structural connectivity, findings from several Diffusion Tensor Imaging (DTI) studies have noted correlations between severity of white matter abnormalities in the constituent fasciculi of the limbic system and severity of delusions in patients with schizophrenia (Bracht et al., 2014, Chan et al., 2010, Fitzsimmons et al., 2014, Whitford et al., 2014a, Whitford et al., 2014b). Given the established association between structural and functional connectivity within the limbic system (Cohen et al., 2008), it is feasible that abnormalities in structural connectivity could underpin the observed abnormalities in functional connectivity, which could, in turn, lead to the development of delusions.
Reduced fractional anisotropy (FA; Mori and Zhang, 2006) and increased radial diffusivity (RD; Song et al., 2005) have repeatedly been reported in patients with schizophrenia (Prasad et al., 2015, Seal et al., 2008). Abnormal axial diffusivity (AD), on the other hand, has not been linked to schizophrenia as reliably as FA and RD changes (Seal et al., 2008). Two of the more common interpretations of these white matter findings in schizophrenia are changes to the myelin sheath surrounding the axons (Kubicki et al., 2005, Muller and Schwarz, 2006, Uranova et al., 2007) and neuroinflammation (Laan et al., 2009, Pasternak et al., 2012, van Berckel et al., 2008). Distinguishing between these two pathologies is of utmost importance to our understanding of the neurobiological basis of schizophrenia and for the development of more effective treatments (Pasternak et al., 2009).
At this time, it is not possible to differentiate myelin changes from neuroinflammation with common DTI metrics. Both pathologies lead to a decrease in FA (Pasternak et al., 2009), and while an increase in RD is typically associated with myelin alterations (Song et al., 2005), RD measures can also be contaminated by inflammation (Lodygensky et al., 2010, Wang et al., 2014). Pasternak et al. (2009) developed a novel technique, termed free-water (FW) imaging, to address this problem by differentiating between diffusion properties of brain tissue, such as white matter fiber bundles, and surrounding free-water such as cerebrospinal fluid. Changes to the myelin sheath impact the diffusion of water molecules in close proximity to the axon (Song et al., 2005), whereas neuroinflammation increases the fractional volume of water molecules diffusing freely in the extracellular space (Syková and Nicholson, 2008) where microglia modulate immune defense (Schwartz et al., 2006). Thus, neuroinflammation is associated with excessive extracellular free-water, which can be partialled out to yield improved DTI indices such as free-water corrected fractional anisotropy (FAT), free-water corrected radial diffusivity (RDT) and free-water corrected axial diffusivity (ADT), all of which provide a more precise estimation of tissue changes (Pasternak et al., 2012).
The aim of the present study was to investigate the diffusion properties of white matter fiber tracts of the limbic system in relation to delusions in patients with schizophrenia. For this purpose, FAT, RDT, ADT and FW of the cingulum bundle, uncinate fasciculus and fornix were compared between schizophrenia patients with present state delusions, schizophrenia patients with remitted delusions, schizophrenia patients without a lifetime history of delusions, and healthy controls.
2. Materials and methods
2.1. Participants
The data for this study were obtained from the Australian Schizophrenia Research Bank (ASRB). Details on the original data collection process are provided elsewhere (Loughland et al., 2010). Data were analyzed for 115 participants, consisting of 28 healthy controls (HC) and 87 individuals who had been diagnosed with schizophrenia according to the DSM-IV (American Psychiatric Association, 1994). Diagnostic and clinical information were acquired by conducting the Diagnostic Interview for Psychosis (DIP; Castle et al., 2006). Exclusion criteria included an inability to converse fluently in English, intellectual disability (IQ < 70), movement disorders, a present diagnosis of substance dependence, electroconvulsive therapy within the past six months, brain injury and/or organic brain disorders. One statistical outlier, defined as a value greater than three standard deviations from the sample mean, was identified for FW in the SZD + PS group and was therefore excluded from further analyses. A subset of the participant sample has been reported on previously in the context of two studies exploring associations between auditory verbal hallucinations and FA in the arcuate fasciculus (McCarthy-Jones et al., 2015), and in the inferior occipito-frontal fasciculus (Oestreich et al., 2015).
Of the 86 schizophrenia patients, 69 had a lifetime history of delusions, which was operationalized as a total score > 0 on the lifetime ratings of the DIP items #58 (other primary delusions), #59 (delusions of passivity), #60 (persecutory delusions), #61 (delusions of influence), #62 (primary delusional perception), #63 (grandiose delusions) and #64 (bizarre delusions). Of these 69 patients, 34 (SZD + PS) had present state delusions (i.e. delusions present during the past month), while 35 (SZD + LT) had a lifetime history but currently remitted delusions (i.e., no delusions within the past month). The remaining 17 schizophrenia patients (SZD −) reported no lifetime history or present state delusions.
Data on the duration of antipsychotic drug use were available for 81 out of the 86 patients with schizophrenia (see Table 1). Lifetime history of alcohol or substance abuse was assessed with DIP items #74 (lifetime diagnosis of alcohol abuse/dependence) and #78 (lifetime diagnosis of drug abuse/dependence), respectively. Hallucinations were assessed with DIP items #49–53. Thought disorder was assessed with the DIP items #54–57. Depression was measured by the DIP items #20 (dysphoria), #21 (loss of pleasure) and #22 (suicide). Negative symptoms were assessed using the DIP items #90 (restricted affect), #91 (blunted affect) and #97 (negative formal thought disorder).
Table 1.
Demographic and clinical data for the participant sample.
| Variables | SZD + PS (n = 34) | SZD + LT (n = 35) | SZD − (n = 17) | HC (n = 28) | Group difference |
|---|---|---|---|---|---|
| Demographics | |||||
| Age: years (M, SD) | 40.24 (8.98) | 37.94 (9.77) | 41.12 (11.97) | 37.86 (9.66) | F(3,110) = 0.70, p = 0.56 |
| Gender (% male) | 85% | 77% | 65% | 86% | χ2(3) = 3.80, p = 0.28 |
| Scanner location (Melbourne/Sydney/Brisbane/Perth/Newcastle) | (7/10/13/2/2) | (8/8/14/1/4) | (1/7/5/1/3) | (8/8/7/4/1) | χ2(12) = 11.37, p = 0.50 |
| Drugs | |||||
| Antipsychotics: months (M, SD) | 49.56 (37.85)a | 45.53 (35.14)b | 55.08 (36.59)c | – | F(2,76) = 0.34, p = 0.72 |
| Substance abuse (%) | 13%a | 15%b | 6%d | – | χ2(2) = 0.67, p = 0.71 |
| Alcohol abuse (%) | 39%a | 33%b | 20%d | – | χ2(2) = 1.61, p = 0.45 |
| Psychopathology | |||||
| Illness duration: years (M, SD) | 15.97 (8.46) | 13.43 (7.84) | 16.47 (10.18) | – | F(2,83) = 1.05, p = 0.35 |
| Hallucinations: current (M, SD) | 1.5 (2.11) | 0.74 (2.02) | 1.82 (2.63) | – | F(2,83) = 1.75, p = 0.18 |
| Hallucinations: remitted (M, SD) | 2.20 (2.29) | 2.86 (3.32) | 3.00 (2.74) | – | F(2,83) = 0.54, p = 0.59 |
| Thought disorder (M, SD) | 0.88 (1.23) | 1.03 (1.64) | 0.24 (0.56) | – | F(2,83) = 2.13, p = 0.13 |
| Depression (M, SD) | 3.85 (3.40) | 4.34 (3.69) | 3.47 (3.68) | – | F(2,83) = 0.37, p = 0.69 |
| Negative symptoms (M, SD) | 1.21 (1.34) | 1.49 (1.48) | 1.71 (1.72) | – | F(2,83) = 0.71, p = 0.50 |
Note. SZD + PS = schizophrenia group with present state delusions; SZD + LT = schizophrenia group with lifetime history of delusions; SZD − = schizophrenia group without a lifetime history or present state delusions; HC = healthy controls; M = mean; SD = standard deviation.
n = 31 due to missing data.
n = 34 due to missing data.
n = 15 due to missing data.
n = 13 due to missing data.
The 28 healthy control participants were screened for a family history of mental disorders and did not have a history of movement or neurological disorders, organic brain disorders, psychotic disorders, dementias, epilepsy, seizures and/or brain injury.
In order to minimize the possibility that the findings of this study were driven by demographic or clinical variables other than delusions, all groups were matched on demographic variables and the three schizophrenia groups were matched on all other levels of psychopathology (see Table 1). Specifically, all groups were matched on the demographic variables age, gender and scanner location. The three schizophrenia groups were additionally matched on the clinical symptoms hallucinations (current), hallucinations (remitted), thought disorder, depression and negative symptoms, as well as antipsychotics, substance abuse, alcohol abuse and illness duration. Given that the SZD- group is a rare and unique sample, we matched all of the other groups to this group.
2.2. Data acquisition and preprocessing
Diffusion weighted images were acquired from five identical 1.5 T Siemens Avanto scanners (Siemens, Erlangen, Germany) from different locations within Australia. The imaging parameters across all five scanners were identical: TR = 8400 ms, TE = 88 ms, FOV = 25 cm, 104 × 104 matrix and 2.4 mm slice thickness. One volume was acquired with diffusion weighting b = 0 s/mm2, and 64 volumes with non-collinear gradients and diffusion-weighting b = 1000 s/mm2 were acquired in the axial plane.
2.3. Preprocessing
Intra-scan misalignments due to head movements and eddy currents were removed for each individual participant through affine registration of the diffusion weighted images to the baseline image (FSL, Functional MRI of the Brain [FMRIB] Software Library [FSL]). After masking and manually editing a label map to remove non-brain tissue using 3D Slicer software (version 4.3.1; www.slicer.org), diffusion tensor maps were estimated from the eddy current and motion corrected diffusion-weighted images based on least-squares estimation.
2.4. Diffusion tensor tractography
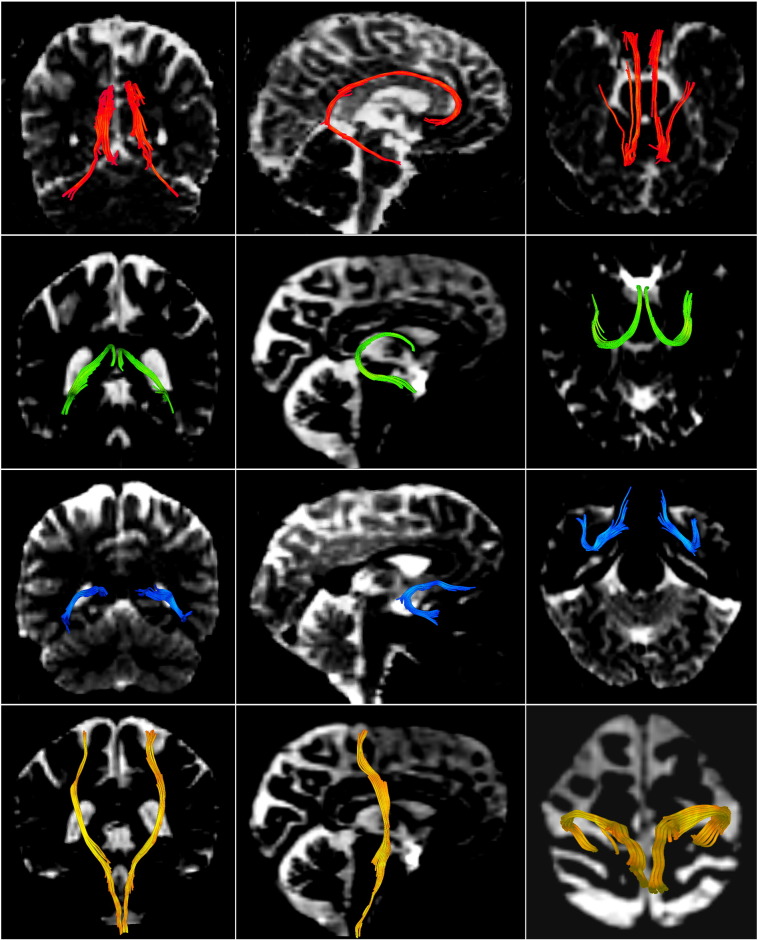
The cingulum bundle, fornix and uncinate fasciculus were extracted with deterministic (streamline) tractography using 3D Slicer software (see Fig. 1). The motor fibers of the internal capsule were also extracted as a control region. Tracts were manually seeded by placing a fiducial (2.50 mm) in the fasciculus-of-interest (identified on a color-by-orientation map) by a single rater who was blind to participant diagnosis. Fiber tracking was repeated by an independent rater on five subjects, randomly selected from the SZD − group, to assess inter-rater reliabilities. Tracts were generated using an eigenvector-tracking algorithm from the seedpoints defined by the fiducial. A step size of 1.00 mm was applied and tractography was terminated upon reaching the stopping criterion (FA < 0.25). Minimum path length was set to 20.00 mm, maximum path length to 800.00 mm and integrated step length to 0.50 mm. The spatial positions of fiducials were manually adjusted until the distinctive fibers of the tracts of interest became apparent, based on the atlas by Catani and Thiebaut de Schotten (2008). A binary label map was generated for each fiber bundle by labeling all voxels through which any fibers passed.
Fig. 1.
The cingulum bundle (red), the fornix (green), the uncinate fasciculus (blue) and the motor fibers of the internal capsule (yellow), extracted with deterministic tractography. Coronal (left column), sagittal (middle column) and axial (right column) views. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Free-water maps and free-water corrected DTI maps were generated directly from the diffusion weighted images by fitting the following model in each voxel (Pasternak et al., 2009): Aq(D, f) = f exp(− bqTDq) + (1 − f)exp(− bdwater). Whereby Aq is the attenuated signal, normalized by the b0 for the diffusion gradient q, and b represents the b-value. The first term represents the tissue compartment, where f is the fractional volume of the tissue compartment (Pasternak et al., 2009). This tissue compartment accounts for water molecules in close proximity to tissue membranes and is modeled with the diffusion tensor D, which can then be converted to scalar measures that are corrected for free-water, such as free-water corrected fractional anisotropy (FAT), free-water corrected radial diffusivity (RDT), and free-water corrected axial diffusivity (ADT; Pasternak et al., 2009). The second term, which represents the isotropic free-water compartment, is modeled by the diffusion coefficient, dwater set to the diffusivity of water in body temperature (3 × 10− 3 mm2/s; Pasternak et al., 2009). This free-water compartment accounts for water molecules that diffuse freely and isotropically, which, with current diffusion times, may only be found in the extracellular space. It is defined by a single parameter, namely the fractional volume of free-water (FW) quantified as 1 − f (Pasternak et al., 2009).
After extraction of the tracts, FAT, RDT, ADT and FW were calculated for each of the four fiber bundles of interest by averaging across all voxels in each participant's label map.
2.5. Statistical analysis
Statistical analyses were performed using SPSS (version 22, www.spss.com). A mixed analysis of covariance (ANCOVA) – with group (4 levels: HC; SZD −; SZD + LT; SZD + PS) as the between-subjects factor and tract (4 levels: cingulum bundle, fornix, uncinate fasciculus, internal capsule) and hemisphere (2 levels: left, right) as within-subjects factors – was performed for each of the four diffusion metrics (FAT, RDT, ADT, FW). To control for the different scanner locations, four dummy variables were created for the five scanner locations and added as covariates into all analyses. In the case of a group ∗ tract interaction, two-tailed follow-up contrasts were used to examine the underlying simple effects. The effect size measure partial eta squared (ƞp2) was reported for the ANCOVA analyses and the effect size measure Cohen's d (d) was reported for the follow-up contrasts. Bonferroni correction was used to correct for multiple comparisons, whereby a comparison of 4 groups across 4 tracts involved 24 contrasts. Corrections to alpha with k = 24 comparisons were therefore applied by multiplying the uncorrected p-values of the follow-up contrasts by 24. Statistical significance was defined as p < 0.05, and a trend towards significance was defined as 0.05 < p < 0.1. Intra-class correlation coefficients (ICC) were calculated to assess the inter-rater reliability between two raters across five subjects from the SZD − group for all tracts (cingulum bundle, fornix, uncinate fasciculus, internal capsule), across all measures (FAT, RDT, ADT, FW) and both hemispheres (left, right).
3. Results
The four groups did not significantly differ from each other on the demographic variables age, gender or scanner location (see Table 1). Furthermore, aside from delusions, the three schizophrenia groups did not significantly differ from each other on any other measures of psychopathology, including negative symptoms, thought disorder, depression, hallucinations, lifetime history of hallucinations, medication, or alcohol and antipsychotic drug use. High intra-class correlation coefficients were observed across all tracts and measures, with ICCs(3,2) ranging from 85.6% to 99.9% (see Table 2).
Table 2.
Intra-class correlation coefficients.
| Tract |
||||
|---|---|---|---|---|
| Cingulum bundle | Fornix | Uncinate fasciculus | Internal capsule | |
| FAT left | 0.984 | 0.999 | 0.952 | 0.973 |
| FAT right | 0.965 | 0.998 | 0.935 | 0.977 |
| RDT left | 0.970 | 0.980 | 0.946 | 0.919 |
| RDT right | 0.987 | 0.973 | 0.939 | 0.992 |
| ADT left | 0.934 | 0.995 | 0.915 | 0.905 |
| ADT right | 0.946 | 0.955 | 0.924 | 0.967 |
| FW left | 0.998 | 0.991 | 0.982 | 0.984 |
| FW right | 0.998 | 0.979 | 0.856 | 0.965 |
Note. FAT = free-water corrected fractional anisotropy; RDT = free-water corrected radial diffusivity; ADT = free-water corrected axial diffusivity; FW = free-water.
3.1. Free-water corrected fractional anisotropy (FAT)
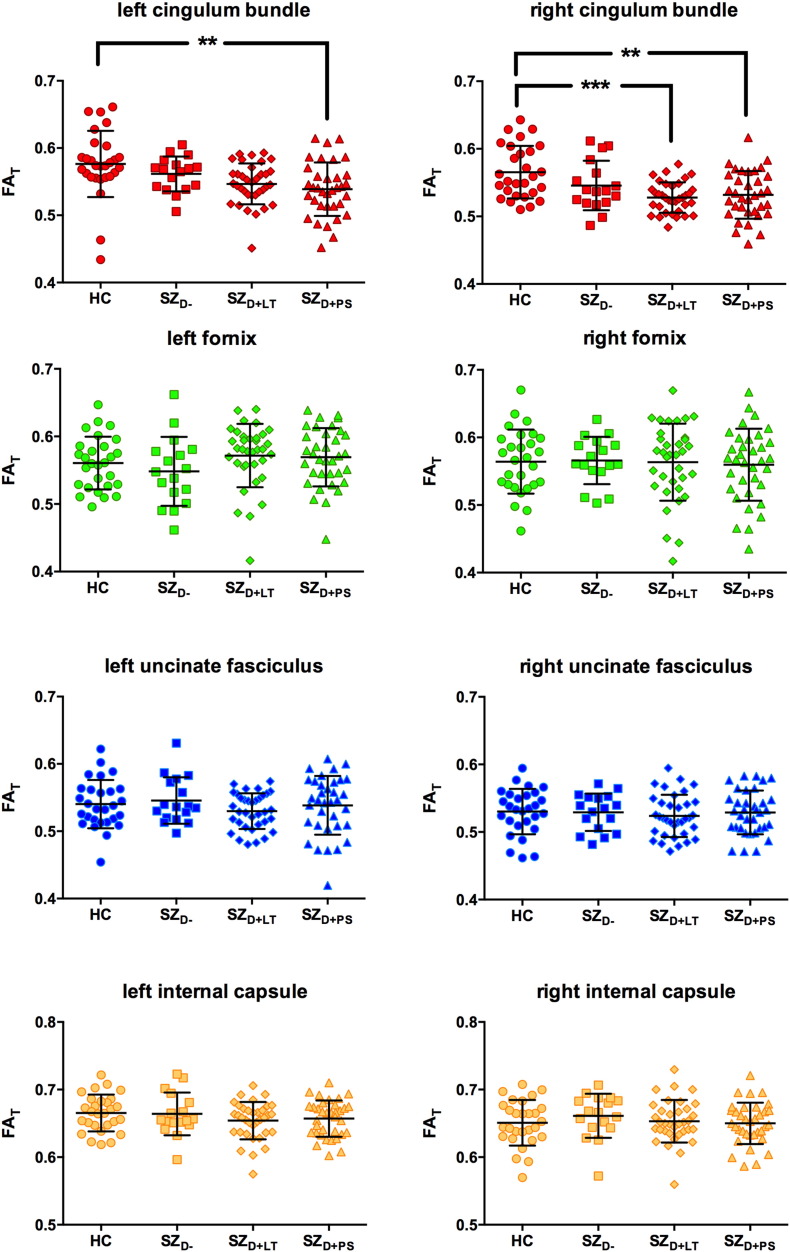
Scatterplots illustrating the between-group differences in FAT are provided in Fig. 2. A mixed ANCOVA identified a significant main effect for tract [F(3,318) = 182.579, p < 0.001, ƞp2 = 0.633] and a significant tract ∗ group interaction [F(9,318) = 3.871, p < 0.001, ƞp2 = 0.099]. A trend towards a main effect for group was also observed [F(3,106) = 2.185, p = 0.094, ƞp2 = 0.058]. No main effect was observed for hemisphere [F(1,106) = 0.621, p = 0.432, ƞp2 = 0.006] and no hemisphere ∗ group interaction [F(3,106) = 0.223, p = 0.880, ƞp2 = 0.006] was present. The SZD + PS group had significantly reduced FAT in the cingulum bundle bilaterally compared to the HC group (see Table 3). Additionally, the SZD + LT group had significantly reduced FAT in the right cingulum bundle compared to the HC group (see Table 3).
Fig. 2.
Free-water corrected fractional anisotropy (FAT) of the left and right cingulum bundle (red), fornix (green), uncinate fasciculus (blue) and the motor fibers of the internal capsule (yellow).
HC = healthy control subjects (n = 28), SZD − = schizophrenia patients without a lifetime history or present state delusions (n = 17), SZD + LT = schizophrenia patients with a lifetime history but currently remitted delusions (n = 35), SZD + PS = schizophrenia patients with present state delusions (n = 34). Error bars represent standard deviations.**p ≤ 0.01, ***p ≤ 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Significant follow-up contrasts in the cingulum bundle.
| FAT |
RDT |
FW |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| df | hemisphere | t | p | d | t | p | d | t | p | d | |
| HC vs SZD + PS | 60 | left | 3.794 | 0.006 | 0.875 | 4.647 | < 0.001 | 1.061 | 4.359 | < 0.001 | 1.184 |
| right | 4.031 | 0.003 | 0.726 | 4.340 | < 0.001 | 1.024 | |||||
| HC vs SZD + LT | 61 | left | 3.333 | 0.03 | 0.850 | ||||||
| right | 4.339 | < 0.001 | 1.181 | 4.440 | < 0.001 | 1.165 | |||||
Note. All p-values adjusted with the Bonferroni correction, based on 24 comparisons. FAT = free-water corrected fractional anisotropy, RDT = free-water corrected radial diffusivity, FW = free-water, df = degrees of freedom, t = t-statistic, p = p-values, d = Cohen’s d HC = healthy controls, SZD + PS = schizophrenia patients with present state delusions. SZD + LT = schizophrenia patients with lifetime history of delusions.
3.2. Free-water corrected radial diffusivity (RDT)
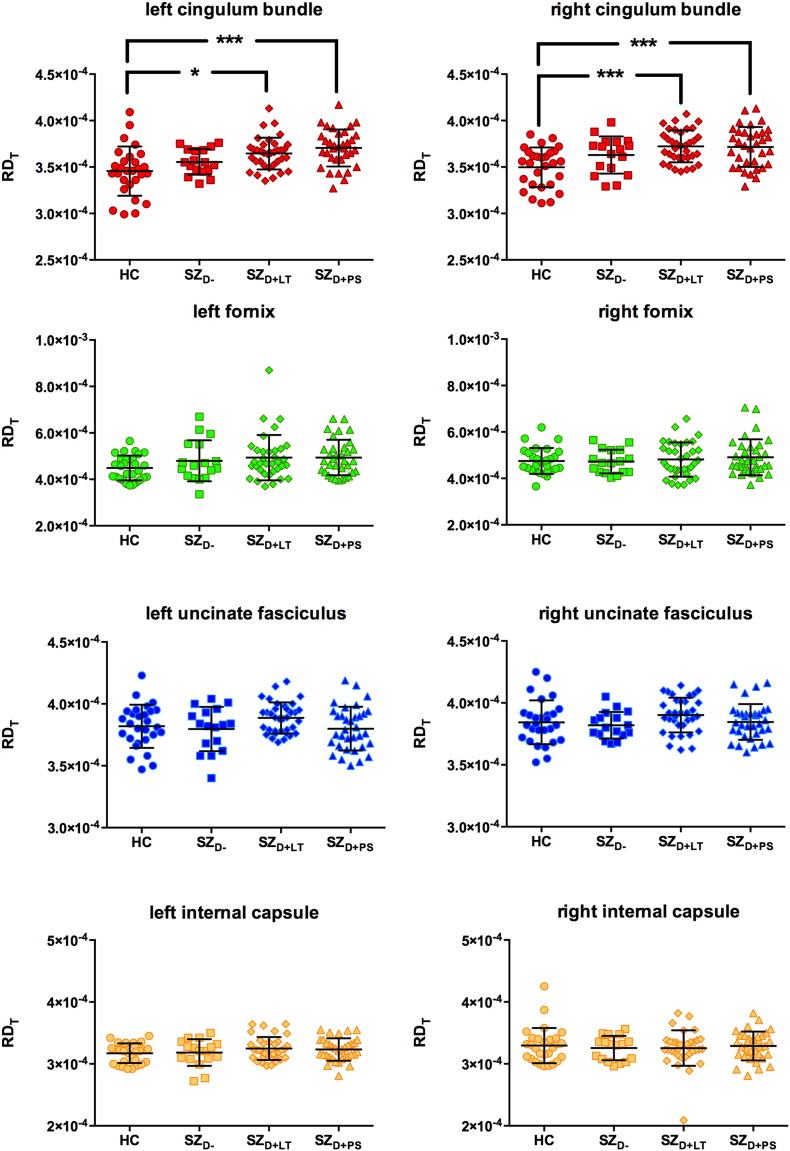
Scatterplots illustrating the between-group differences in RDT are provided in Fig. 3. A mixed ANCOVA identified a significant main effect for tract [F(3,318) = 172.808, p < 0.001, ƞp2 = 0.620] and a significant main effect for group [F(3,106) = 3.638, p = 0.015, ƞp2 = 0.093]. A trend towards a tract ∗ group interaction [F(9,318) = 1.901, p = 0.095, ƞp2 = 0.051] was observed. There was no main effect for hemisphere [F(1,106) = 0.900, p = 0.345, ƞp2 = 0.008] and no hemisphere ∗ group interaction [F(3,106) = 1.193, p = 0.316, ƞp2 = 0.033]. The SZD + PS group had increased RDT compared to the HC group in the cingulum bundle bilaterally (see Table 3). The SZD + LT group had significantly increased RDT in the cingulum bundle bilaterally compared to the HC group (see Table 3).
Fig. 3.
Free-water corrected radial diffusivity (RDT) of the left and right cingulum bundle (red), fornix (green), uncinate fasciculus (blue) and the motor fibers of the internal capsule (yellow).
HC = healthy control subjects (n = 28), SZD − = schizophrenia patients without a lifetime history or present state delusions (n = 17), SZD + LT = schizophrenia patients with a lifetime history but currently remitted delusions (n = 35), SZD + PS = schizophrenia patients with present state delusions (n = 34). Error bars represent standard deviations. *p ≤ 0.05,***p ≤ 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Free-water corrected axial diffusivity (ADT)
A mixed ANCOVA identified a significant main effect for tract [F(3,318) = 175.362, p < 0.001, ƞp2 = 0.623]. A trend towards a tract ∗ group interaction [F(9,318) = 1.882, p = 0.077, ƞp2 = 0.051] was also observed. There was no main effect for hemisphere [F(1,106) = 0.856, p = 0.357, ƞp2 = 0.008], no main effect for group [F(3,106) = 0.156, p = 0.925, ƞp2 = 0.004] and no hemisphere ∗ group interaction [F(3,106) = 1.035, p = 0.380, ƞp2 = 0.028]. No significant between-group differences were observed for ADT.
3.4. Free-water (FW)
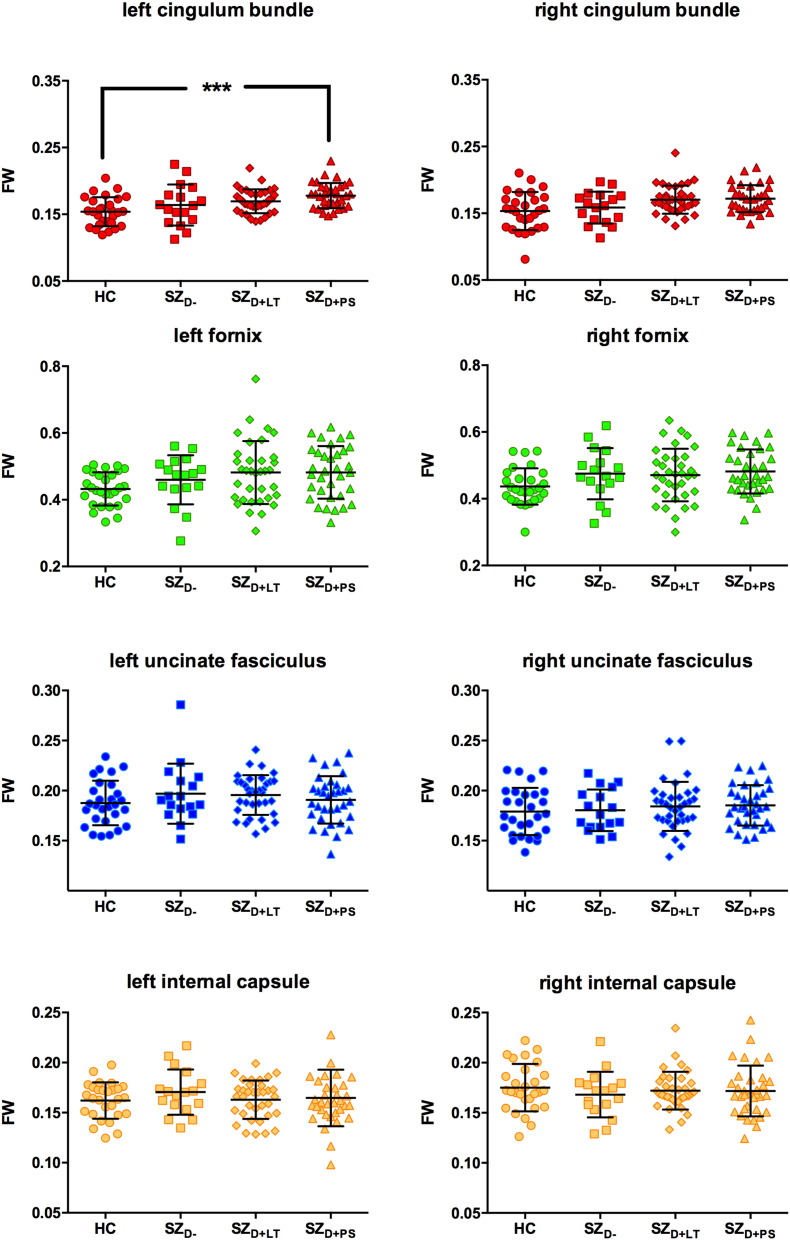
Scatterplots illustrating the between-group differences in FW are provided in Fig. 4. A mixed ANCOVA identified a significant main effect for tract [F(3,318) = 610.133, p < 0.001, ƞp2 = 0.852] and a significant main effect for group [F(3,106) = 3.858, p = 0.012, ƞp2 = 0.098]. A trend towards a tract ∗ group interaction was observed [F(9,318) = 2.061, p = 0.091, ƞp2 = 0.055]. No significant main effect for hemisphere [F(1,106) = 0.936, p = 0.335, ƞp2 = 0.009] and no significant hemisphere ∗ group interaction [F(3,106) = 0.544, p = 0.653, ƞp2 = 0.015] were observed. The SZD + PS group had significantly increased FW compared to the HC group in the left cingulum bundle (see Table 3).
Fig. 4.
Free-water (FW) of the left and right cingulum bundle (red), fornix (green), uncinate fasciculus (blue) and the motor fibers of the internal capsule (yellow).
HC = healthy control subjects (n = 28), SZD − = schizophrenia patients without a lifetime history or present state delusions (n = 17), SZD + LT = schizophrenia patients with a lifetime history but currently remitted delusions (n = 35), SZD + PS = schizophrenia patients with present state delusions (n = 34). Error bars represent standard deviations. ***p ≤ 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
No significant between-group differences were observed for any of the diffusion metrics for the fornix, uncinate fasciculus or internal capsule.
4. Discussion
The present study used diffusion tensor tractography in combination with free-water correction to investigate the diffusion properties of the white matter fasciculi of the limbic system – namely, the cingulum bundle, fornix, and uncinate fasciculus – in relation to the presence and history of delusions in patients with chronic schizophrenia. The findings from this study suggest that state and trait delusions in chronic schizophrenia are associated with reduced FAT, and can be further explained by increased RDT. Additionally, increased FW in the left cingulum bundle seems to be specifically associated with state delusions. Taken together, these results provide preliminary evidence that microstructural processes, such as myelin abnormalities (as indicated by increased RDT) of the cingulum bundle may be related to delusions in chronic schizophrenia. Furthermore, extracellular processes, such as neuroinflammation or atrophy (as indicated by increased FW) of the left cingulum bundle may be involved during active delusional states in chronic schizophrenia.
The findings of reduced FAT and increased RDT are in line with previous studies, which observed a correlation between positive symptoms in schizophrenia patients and structural white matter changes in the cingulum bundle (Fitzsimmons et al., 2014, Fujiwara et al., 2007, Tang et al., 2010, Whitford et al., 2014a). However, in the present study, the diffusion measures in the fornix and the uncinate fasciculus were preserved in all patient groups. This result is inconsistent with many (Abdul-Rahman et al., 2011, Kawashima et al., 2009, Kitis et al., 2012, Kuroki et al., 2006, McIntosh et al., 2008, Price et al., 2008, Takei et al., 2008), though not all (Highley et al., 2002, Jones et al., 2006, Kubicki et al., 2002), previous studies, which have identified decreased FA and increased RD in schizophrenia patients. One potential explanation as to why previous studies reported significant white matter changes in the uncinate fasciculus and fornix, contrary to the present study, is that these studies employed uncorrected FA and RD measures, whereas the present study employed free-water corrected FAT and RDT, thus eliminating biases of partial volume effects. This is likely particularly true for the fornix, as changes in the ventricle size have been shown to affect diffusion measures in the fornix in the absence of free-water correction (Metzler-Baddeley et al., 2012).
The structural changes in the cingulum bundle observed in the present study are in line with findings from functional studies that report fronto-limbic imbalances during delusional states (Javanbakht, 2006). The cingulum bundle connects several areas of the limbic system with the prefrontal cortex and is therefore a likely communication route of these brain regions (Nestor et al., 2007). It is possible that recurrent delusions, which are thought to be induced by an increase in limbic dopamine, ultimately lead to a change in the structural integrity of the connections to and from the limbic system. Alternatively, it is also possible that preexisting structural changes are responsible for abnormal functional connectivity between the limbic system and other brain areas such as the prefrontal cortex, which might represent a vulnerability for functional disconnectivity and as such could lead to the development of delusions. It remains to be seen whether an increase in limbic dopamine, which has been associated with active delusional states (Javanbakht 2006), is related to the increase in extracellular FW, such as we observed in the left cingulum bundle of schizophrenia patients with active delusional states in the present study. However, it must be acknowledged that the proposed account is, at the present time, highly speculative and would benefit from empirical validation in future studies.
The association between delusions and RDT (but not ADT) could be indicative of altered myelination in this tract. Although increased extracellular volume can also be caused by a range of pathologies such as atrophy, a breakdown of the cellular membrane, low dendritic quantity and low cell density (Pasternak et al., 2012, Selemon et al., 2002), previous research suggests that a plausible reason for the increased extracellular volume in schizophrenia is neuroinflammation (Pasternak et al., 2015). Neuroinflammation results from a cellular response to injury of the central nervous system by the activation of microglia and astrocytes (Pasternak et al., 2012, Streit, 2006). The activation of microglia is responsible for multiple metabolic and biochemical changes, which lead to profuse osmosis of water from blood and therefore increased extracellular volume (Schwartz et al., 2006, Syková and Nicholson, 2008). This increase in extracellular volume is reflected in increased free-water values, whereas the fiber structure itself, and therefore the free-water corrected values, remain unaffected (Pasternak et al., 2012). In a recent PET study, microglial activity was found to be enhanced in patients with schizophrenia as well as in individuals at ultra-high risk for psychosis (Bloomfield et al., 2016), which supports the involvement of neuroinflammatory processes in the illness progression of schizophrenia. Moreover, and in line with the present study, neuroinflammation has been reported to be related to symptom severity and subclinical psychotic symptoms (Bloomfield et al., 2016). In order to have greater confidence in these tentative conclusions, better-powered studies are required, as well as studies that correlate free-water imaging findings with additional measures of neuroinflammation, such as PET or blood-markers of neuroinflammation.
The present study has a number of limitations. First, while data on duration of antipsychotic drugs are reported, dosage data were not available. Second, the data used in this study were acquired from five scanners. While all scanners were the same model and build, it is possible that disparities between the scanners had an impact on DTI measures. However, it should be noted that the same imaging sequences were used across the five sites, there were no significant group differences in terms of scanner site, and scanner location was included as a covariate in all analyses. Third, there are disadvantages associated with deterministic tractography, including the fact that it does not provide an index of confidence as to how likely a voxel is to occur within a given fasciculus, and the fact that one-tensor tractographic techniques (such as we employed in the current study) can have difficulty resolving crossing fibers (Jones, 2008).
In conclusion, the present study found reduced FAT and increased RDT to be associated with both present-state delusions and a lifetime history of delusions in patients with chronic schizophrenia. Additionally, increased FW in the left cingulum bundle was found to be associated with active delusional states in chronic schizophrenia. These results are consistent with a combination of altered myelination and neuroinflammation or atrophy in this tract. If confirmed by future studies, this would have important clinical implications, as it suggests that potential treatments for delusions in schizophrenia could aim to both increase myelination and reduce neuroinflammation and atrophy in the cingulum bundle, which would likely require polypharmacy.
Acknowledgements
This study was supported by the Schizophrenia Research Institute using data from the Australian Schizophrenia Research Bank, funded by NHMRC Enabling Grant (No. 386500) held by V Carr, U Schall, R Scott, A Jablensky, B Mowry, P Michie, S Catts, F Henskens and C Pantelis (Chief Investigators), and the Pratt Foundation, Ramsay Health Care, the Sylvia and Charles Viertel Charitable Foundation, as well the Schizophrenia Research Institute, using an infrastructure grant from the NSW Ministry of Health. Thomas Whitford is supported by a Discovery Project from the Australian Research Council (DP140104394) and a Career Development Fellowship from the National Health and Medical Research Council of Australia (APP1090507). Simon McCarthy-Jones is supported by an Australian Research Council Discovery Early Career Research Award (DE140101077). Ofer Pasternak is supported by the National Institutes of Health grants R01MH108574, R01MH085953, R01MH074794, 2P41EB015902, 1R01AG042512, R01MH102377, and a NARSAD young investigator award. Martha Shenton is supported by a VA merit award. Marek Kubicki is supported by a National Institutes of Health grant R01 MH102377. This work is part of Lena Oestreich's doctorate thesis (PhD).
References
- Abdul-Rahman M.F., Qiu A., Sim K. Regionally specific white matter disruptions of fornix and cingulum in schizophrenia. PLoS One. 2011;6(4):e18652. doi: 10.1371/journal.pone.0018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fourth ed. The Association; Washington (DC): 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, D.C.: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- van Berckel B.N., Bossong M.G., Boellaard R., Kloet R., Schuitemaker A., Caspers E., Luurtsema G., Windhorst A.D., Cahn W., Lammertsma A.A., Kahn R.S. Microglia activation in recent-onset schizophrenia: a quantitative (R) [11C]PK11195 positron emission tomography study. Biol. Psychiatry. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Bloomfield P.S., Selvaraj S., Veronese M., Rizzo G., Bertoldo A., Owen D.R., Bloomfield M.A., Bonoldi I., Kalk N., Turkheimer F., McGuire P., de Paola V., Howes O.D. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C]PBR28 PET brain imaging study. Am. J. Psychiatry. 2016;173(1):44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Horn H., Strik W., Federspiel A., Razavi N., Stegmayer K., Wiest R., Dierks T., Müller T.J., Walther S. White matter pathway organization of the reward system is related to positive and negative symptoms in schizophrenia. Schizophr. Res. 2014;153(1–3):136–142. doi: 10.1016/j.schres.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Castle D.J., Jablensky A., McGrath J.J., Carr V., Morgan V., Waterreus A., Valuri G., Stain H., McGuffin P., Farmer A. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol. Med. 2006;36(1):69–80. doi: 10.1017/S0033291705005969. [DOI] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Chan W.Y., Yang G.L., Chia M.Y., Lau I.Y., Sitoh Y.Y., Nowinski W.L., Sim K. White matter abnormalities in first-episode schizophrenia: a combined structural MRI and DTI study. Schizophr. Res. 2010;119(1–3):52–60. doi: 10.1016/j.schres.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Cohen M.X. Hippocampal-prefrontal connectivity predicts midfrontal oscillations and long-term memory performance. Curr. Biol. 2011;1(22):1900–1905. doi: 10.1016/j.cub.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Cohen M.X., Elger C.E., Weber B. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. NeuroImage. 2008;39(3):1396–1407. doi: 10.1016/j.neuroimage.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Davies D.C., Wardell A.M., Woolsey R., James A.C. Enlargement of the fornix in early-onset schizophrenia: a quantitative MRI study. Neurosci. Lett. 2001;301(3):163–166. doi: 10.1016/s0304-3940(01)01637-8. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons J., Schneiderman J.S., Whitford T.J., Swisher T., Niznikiewicz M.A., Pelavin P.E., Terry D.P., Mesholam-Gately R.I., Seidman L.J., Goldstein J.M., Kubicki M. Cingulum bundle diffusivity and delusions of reference in first episode and chronic schizophrenia. Psychiatry Res. 2014;224(2):124–132. doi: 10.1016/j.pscychresns.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D., Blakemore S., Wolpert D.M. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res. Rev. 2000;31(2–3):357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Namiki C., Hirao K., Miyata J., Shimizu M., Fukuyama H., Sawamoto N., Hayashi T., Murai T. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr. Res. 2007;95(1–3):215–222. doi: 10.1016/j.schres.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Highley J.R., Walker M.A., Esiri M.M., Crow T.J., Harrison P.J. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb. Cortex. 2002;12(11):1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Javanbakht A. Sensory gating deficits, pattern completion, and disturbed fronto-limbic balance, a model for description of hallucinations and delusions in schizophrenia. Med. Hypotheses. 2006;67(5):1173–1184. doi: 10.1016/j.mehy.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Jones D.K. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44(8):936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Catani M., Pierpaoli C., Reeves S.J., Shergill S.S., O'Sullivan M., Golesworthy P., McGuire P., Horsfield M.A., Simmons A., Williams S.C., Howard R.J. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum. Brain Mapp. 2006;27(3):230–238. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T., Nakamura M., Bouix S., Kubicki M., Salisbury D.F., Westin C.F., McCarley R.W., Shenton M.E. Uncinate fasciculus abnormalities in recent onset schizophrenia and affective psychosis: a diffusion tensor imaging study. Schizophr. Res. 2009;110(1–3):119–126. doi: 10.1016/j.schres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitis O., Ozalay O., Zengin E.B., Haznedaroglu D., Eker M.C., Yalvac D., Oguz K., Coburn K., Gonul A.S. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatry Clin. Neurosci. 2012;66(1):34–43. doi: 10.1111/j.1440-1819.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- Kubicki M., Westin C.F., Maier S.E., Frumin M., Nestor P.G., Salisbury D.F., Kikinis R., Jolesz F.A., McCarley R.W., Shenton M.E. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am. J. Psychiatry. 2002;159(5):813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M., Park H., Westin C.F., Nestor P.G., Mulkern R.V., Maier S.E., Niznikiewicz M., Connor E.E., Levitt J.J., Frumin M., Kikinis R., Jolesz F.A., McCarley R.W., Shenton M.E. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. NeuroImage. 2005;26(4):1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki N., Kubicki M., Nestor P.G., Salisbury D.F., Park H.J., Levitt J.J., Woolston S., Frumin M., Niznikiewicz M., Westin C.F., Maier S.E., McCarley R.W., Shenton M.E. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol. Psychiatry. 2006;60(1):22–31. doi: 10.1016/j.biopsych.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan W., Smeets H., de Wit N.J., Kahn R.S., Grobbee D.E., Burger H. Glucocorticosteroids associated with a decreased risk of psychosis. J. Clin. Psychopharmacol. 2009;29(3):288–290. doi: 10.1097/JCP.0b013e3181a44575. [DOI] [PubMed] [Google Scholar]
- Lodygensky G.A., West T., Stump M., Holtzman D.M., Inder T.E., Neil J.J. In vivo MRI analysis of an inflammatory injury in the developing brain. Brain Behav. Immun. 2010;24(5):759–767. doi: 10.1016/j.bbi.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughland C., Draganic D., McCabe K., Richards J., Nasir A., Allen J., Catts S., Jablensky A., Henskens F., Michie P., Mowry B., Pantelis C., Schall U., Scott R., Tooney P., Carr V. Australian schizophrenia research bank: a database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophrenia. Aust. N Z J. Psychiatry. 2010;44(11):1029–1035. doi: 10.3109/00048674.2010.501758. [DOI] [PubMed] [Google Scholar]
- McCarthy-Jones S., Oestreich L.K.L., Australian Schizophrenia Research Bank, Whitford T.J. Reduced integrity of the left arcuate fasciculus is specifically associated with hallucinations in the auditory verbal modality in schizophrenia. Schizophr. Res. 2015;162(1–3):1–6. doi: 10.1016/j.schres.2014.12.041. [DOI] [PubMed] [Google Scholar]
- McIntosh A.M., Muñoz Maniega S., Lymer G.K., McKirdy J., Hall J., Sussmann J.E., Bastin M.E., Clayden J.D., Johnstone E.C., Lawrie S.M. White matter tractography in bipolar disorder and schizophrenia. Biol. Psychiatry. 2008;64(12):1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Mega M.S., Cummings J.L., Salloway S., Malloy P. The limbic system: an anatomic, phylogenetic, and clinical perspective. J. Neuropsychiatry Clin. Neurosci. 1997;9(3):315–330. doi: 10.1176/jnp.9.3.315. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C., O'Sullivan M.J., Bells S., Pasternak O., Jones D.K. How and how not to correct for CSF-contamination in diffusion MRI. NeuroImage. 2012;59(2):1394–1403. doi: 10.1016/j.neuroimage.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Mori S., Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Muller N., Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurochem. Res. 2006;10(2):131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- Nestor P.G., Kubicki M., Spencer K.M., Niznikiewicz M., McCarley R.W., Shenton M.E. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr. Res. 2007;90(1–3):308–315. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich L.K.L., McCarthy-Jones S., Bank A.S.R., Whitford T.J. Decreased integrity of the fronto-temporal fibers of the left inferior occipito-frontal fasciculus associated with auditory verbal hallucinations in schizophrenia. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9421-5. [DOI] [PubMed] [Google Scholar]
- Pasternak O., Sochen N., Gur Y., Intrator N., Assaf Y. Free water elimination and mapping from diffusion MRI. Magn. Reson. Med. 2009;62(3):717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- Pasternak O., Westin C.F., Bouix S., Seidman L.J., Goldstein J.M., Woo T.U., Petryshen T.L., Mesholam-Gately R.I., McCarley R.W., Kikinis R., Shenton M.E., Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J. Neurosci. 2012;32(48):17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O., Westin C.F., Dahlben B., Bouix S., Kubicki M. The extent of diffusion MRI markers of neuroinflammation and white matter deterioration in chronic schizophrenia. Schizophr. Res. 2015;161(1):113–118. doi: 10.1016/j.schres.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K.M., Upton C.H., Schirda C.S., Nimgaonkar V.L., Keshavan M.S. White matter diffusivity and microarchitecture among schizophrenia subjects and first-degree relatives. Schizophr. Res. 2015;161(1):70–75. doi: 10.1016/j.schres.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G., Cercignani M., Parker G.J., Altmann D.R., Barnes T.R., Barker G.J., Joyce E.M., Ron M.A. White matter tracts in first-episode psychosis: a DTI tractography study of the uncinate fasciculus. NeuroImage. 2008;39(3):949–955. doi: 10.1016/j.neuroimage.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcman M. A hypothesis concerning the structural relationship between memory and emotion. Med. Hypotheses. 1978;4(6):581–590. doi: 10.1016/0306-9877(78)90048-8. [DOI] [PubMed] [Google Scholar]
- Schott B.H., Niklas C., Kaufmann J., Bodammer N.C., Machts J., Schütze H., Düzel E. Fiber density between rhinal cortex and activated ventrolateral prefrontal regions predicts episodic memory performance in humans. PNAS. 2011;108(13):5408–5413. doi: 10.1073/pnas.1013287108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott B.H., Voss M., Wagner B., Wüstenberg T., Düzel E., Behr J. Fronto-limbic novelty processing in acute psychosis: disrupted relationship with memory performance and potential implications for delusions. Front. Behav. Neurosci. 2015;9(144):1–13. doi: 10.3389/fnbeh.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Butovsky O., Brück W., Hanisch U.K. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29(2):68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Seal M.L., Yücel M., Fornito A., Wood S.J., Harrison B.J., Walterfang M., Pell G.S., Pantelis C. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr. Res. 2008;101(1–3):106–110. doi: 10.1016/j.schres.2007.12.489. [DOI] [PubMed] [Google Scholar]
- Selemon L.D., Kleinman J.E., Herman M.M., Goldman-Rakic P.S. Smaller frontal gray matter volume in postmortem schizophrenic brains. Am. J. Psychiatry. 2002;159(12):1983–1991. doi: 10.1176/appi.ajp.159.12.1983. [DOI] [PubMed] [Google Scholar]
- Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Streit W.J. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29(9):506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Syková E., Nicholson C. Diffusion in brain extracellular space. Physiol. Rev. 2008;88(4):1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K., Yamasue H., Abe O., Yamada H., Inoue H., Suga M., Sekita K., Sasaki H., Rogers M., Aoki S., Kasai K. Disrupted integrity of the fornix is associated with impaired memory organization in schizophrenia. Schizophr. Res. 2008;103(1–3):52–61. doi: 10.1016/j.schres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Thaker G.K., Buchanan R., Kirkpatrick B., Alphs L.D., Chase T.N., Carpenter W.T. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch. Gen. Psychiatry. 1992;49(7):522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- Tang J., Liao Y., Zhou B., Tan C., Liu T., Hao W., Hu D., Chen X. Abnormal anterior cingulum integrity in first episode, early-onset schizophrenia: a diffusion tensor imaging study. Brain Res. 2010;1343:199–205. doi: 10.1016/j.brainres.2010.04.083. [DOI] [PubMed] [Google Scholar]
- Uranova N.A., Vostrikov V.M., Vikhreva O.V., Zimina I.S., Kolomeets N.S., Orlovskaya D.D. The role of oligodendrocyte pathology in schizophrenia. Int. J. Neuropsychopharmacol. 2007;10(4):537–545. doi: 10.1017/S1461145707007626. [DOI] [PubMed] [Google Scholar]
- Wang X., Cusick M.F., Wang Y., Sun P., Libbey J.E., Trinkaus K., Fujinami R.S., Song S.K. Diffusion basis spectrum imaging detects and distinguishes coexisting subclinical inflammation, demyelination and axonal injury in experimental autoimmune encephalomyelitis mice. NMR Biomed. 2014;27(7):843–852. doi: 10.1002/nbm.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford T.J., Kubicki M., Pelavin P.E., Lucia D., Schneiderman J.S., Pantelis C., McCarley R.W., Shenton M.E. Cingulum bundle integrity associated with delusions of control in schizophrenia: preliminary evidence from diffusion tensor tractography. Schizophr. Res. 2014;161(1):36–41. doi: 10.1016/j.schres.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford T.J., Lee S.W., Oh J.S., de Luis-Garcia R., Savadjiev P., Alvarado J.L., Westin C.F., Niznikiewicz M., Nestor P.G., McCarley R.W., Kubicki M., Shenton M.E. Localized abnormalities in the cingulum bundle in patients with schizophrenia: a Diffusion Tensor tractography study. Neuroimage Clin. 2014;5:93–99. doi: 10.1016/j.nicl.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]