Supplemental Digital Content is available in the text.
Keywords: blood pressure, cost analysis, health policy, hypertension, pregnancy, randomized controlled trials
Abstract
The CHIPS randomized controlled trial (Control of Hypertension in Pregnancy Study) found no difference in the primary perinatal or secondary maternal outcomes between planned “less tight” (target diastolic 100 mm Hg) and “tight” (target diastolic 85 mm Hg) blood pressure management strategies among women with chronic or gestational hypertension. This study examined which of these management strategies is more or less costly from a third-party payer perspective. A total of 981 women with singleton pregnancies and nonsevere, nonproteinuric chronic or gestational hypertension were randomized at 14 to 33 weeks to less tight or tight control. Resources used were collected from 94 centers in 15 countries and costed as if the trial took place in each of 3 Canadian provinces as a cost-sensitivity analysis. Eleven hospital ward and 24 health service costs were obtained from a similar trial and provincial government health insurance schedules of medical benefits. The mean total cost per woman–infant dyad was higher in less tight versus tight control, but the difference in mean total cost (DM) was not statistically significant in any province: Ontario ($30 191.62 versus $24 469.06; DM $5723, 95% confidence interval, −$296 to $12 272; P=0.0725); British Columbia ($30 593.69 versus $24 776.51; DM $5817; 95% confidence interval, −$385 to $12 349; P=0.0725); or Alberta ($31 510.72 versus $25 510.49; DM $6000.23; 95% confidence interval, −$154 to $12 781; P=0.0637). Tight control may benefit women without increasing risk to neonates (as shown in the main CHIPS trial), without additional (and possibly lower) cost to the healthcare system.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01192412.
Hypertension in pregnancy is common, occurring in ≤10% of all pregnancies with ≤20% of those women having preeclampsia. Treatment approaches of “less tight” and “tight” control of chronic or gestational hypertension can be found in international guidelines for management of hypertension in pregnancy.1
To understand the effects of these contrasting approaches, we undertook an open, multicenter international randomized controlled trial. The study is described elsewhere,2 but briefly, 987 consenting women were enrolled between March 26, 2009 and August 2, 2012 at centers confirmed to have capacity to provide the necessary maternal and neonatal care. Eligible women were between 14 weeks 0 days and 33 weeks 6 days of gestation and had a live fetus, nonproteinuric chronic or gestational hypertension, an office diastolic blood pressure (BP) of 90 to 105 mm Hg (or 85–105 mm Hg if antihypertensive medication was being taken), and had no exclusion criteria, including a systolic BP of ≥160 mm Hg systolic or proteinuria. Women were randomized to less tight BP control (target diastolic BP of 100 mm Hg) or tight control (target diastolic BP of 85 mm Hg) until delivery, with labetalol as the drug of first choice. The primary composite outcome (pregnancy loss or high-level neonatal care for more than 48 hours) was similar between groups (adjusted odds ratio [aOR], 1.02; 95% confidence interval [CI], 0.77–1.35). The secondary outcome of serious maternal complications ≤6 weeks postpartum or until hospital discharge was not significantly different (aOR, 1.74; 95% CI, 0.79–3.84). At the prespecified 99.9% significance level for the secondary analysis of maternal outcomes adjusted for stratification factors, 40.6% of women in the less tight group experienced postrandomization severe hypertension compared with 27.5% in the tight control group (aOR, 1.80; 95% CI, 1.34–2.38). At the 95%, but not at the prespecified 99.9%, significance level, 4.3% of less tight compared with 1.6% of tight women had thrombocytopenia (platelet count <100×109; aOR, 2.63; 95% CI, 1.15–6.05), and 4.3% compared with 1.8%, respectively, had elevated aspartate aminotransferase or alanine aminotransferase levels with symptoms (aOR, 2.33; 95% CI, 1.05–5.16), indicating a positive impact for women in the tight control group. The current analysis was planned to determine the cost to the healthcare system related to less tight compared with tight BP control of pregnancy hypertension to inform resource allocation decisions and policy.
Methods
Ethics approval for the trial and cost analysis was obtained from the trial coordinating center (University of British Columbia Clinical Research Ethics Board, H08-00882) and at each recruiting center. Informed consent was obtained from each woman before enrollment. The cost analysis was undertaken from the perspective of a third-party payer (eg, Ministry of Health) as if all the study participants had received care in each of 3 Canadian provinces (ie, Ontario, British Columbia, and Alberta) to provide a sensitivity analysis of different price structures on the robustness of cost outcomes and to enhance generalizability. The study included 3 provinces that represented 63% (21.84 million people) of Canada’s population3 and 62.3% of total public sector health expenditure in Canada in 2013,4 and share similar provincial government-funded healthcare systems, including funding structures for nurses and hospitals and fee-for-service or salaries for physicians. Outcomes included the difference in mean cost between tight and less tight groups of: the total cost of 24 services in each province, total cost of 11 hospital ward durations, and overall total cost of all services and ward durations in each province. All costs are presented in 2013 Canadian dollars, the year that the last recruits delivered and study data collection ended.
Resource Use
Using case report forms for mothers and babies, information was collected on healthcare utilization from randomization until maternal primary hospital discharge after birth and infant primary hospital discharge home. Table 1 lists the 24 services and 11 hospital ward stays that were considered to assess cost.
Table 1.
Healthcare Resources Consumed by Study Groups in Trial
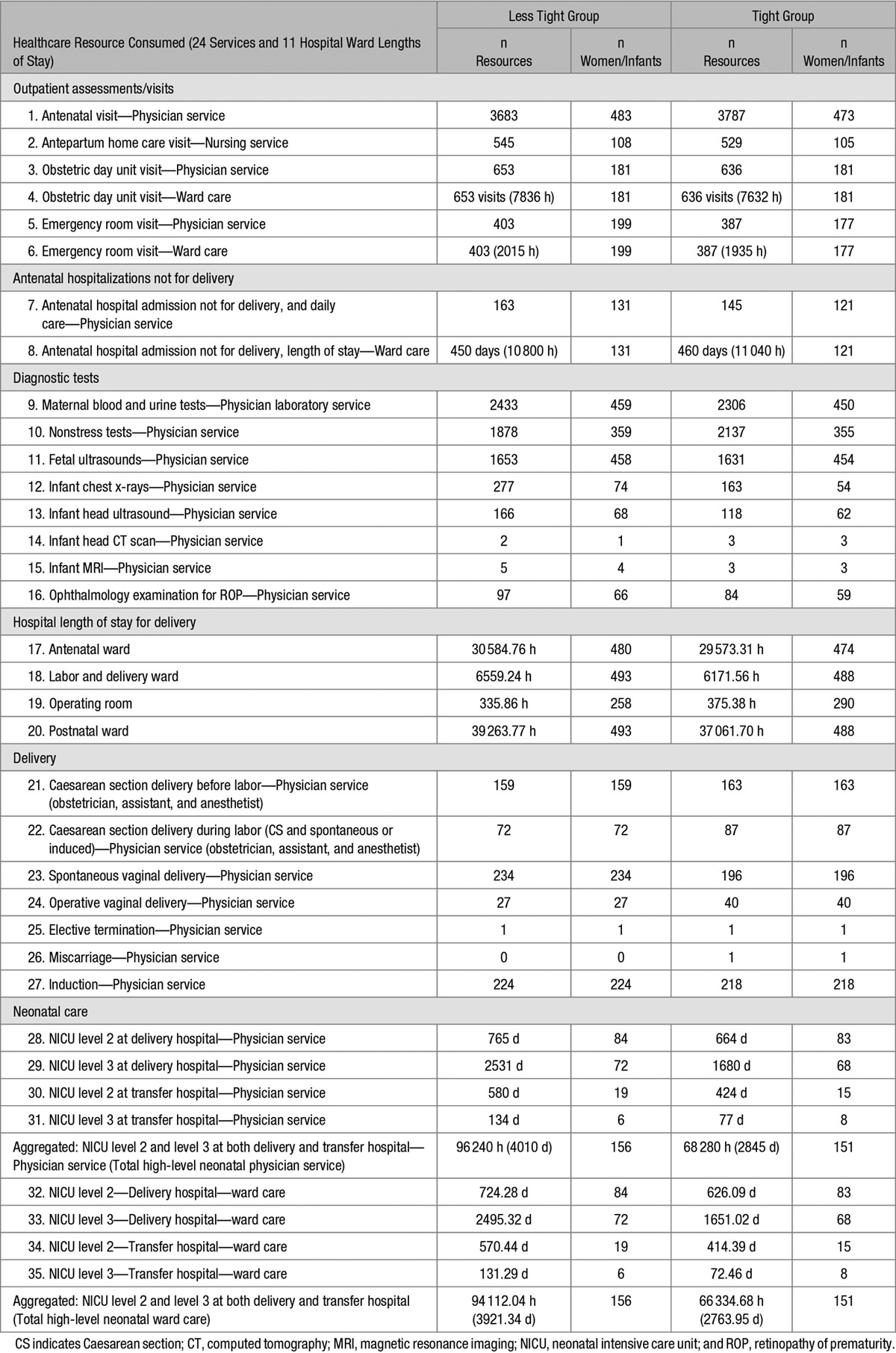
The total maternal hospital length of stay was available, but not by hospital ward type, which varies substantially in cost; therefore, the operating room duration and labor and delivery ward duration before and after the actual birth time were estimated for each woman using the mean ward duration by parity and delivery mode observed from a trial of women who had an external cephalic version for breech presentation.5 Women who received magnesium sulfate before or during delivery had their postnatal labor and delivery ward duration estimate increased to 24 hours after delivery for monitoring purposes according to Canadian guidelines. All women were assumed to have had a labor and delivery room duration, including those who went in to the operating room during which time their bed was held. Time in hospital after hospital admission (but before estimated labor and delivery ward admission) was ascribed to the antenatal ward, and time after estimated labor and delivery discharge to actual hospital discharge was ascribed to the postnatal ward, totaling the actual maternal hospital length of stay.
Infants admitted to high-level neonatal care did not have their actual level of care indicated, given the variability in definition of intermediate and intensive care across hospitals. For costing purposes, cases of high-level neonatal care were categorized masked to treatment allocation into level 3 or level 2 neonatal ward care. Level 3 neonatal intensive care was attributed to babies with characteristics consistent with provincial admission guidelines and billing definitions, as follows: birth at <32 weeks, birth weight <1500 g, receipt of any positive pressure ventilation (continuous positive airway pressure or endotracheal intubation), or any of ten serious neonatal morbidities (ie, patent ductus arteriosus, early-onset sepsis within the first 48 hours of life, bronchopulmonary dysplasia, retinopathy of prematurity stage >2, intraventricular hemorrhage, cystic periventricular leukomalacia, hypoxic-ischemic encephalopathy, necrotizing enterocolitis, laparotomy, or thoracotomy). All other high-level neonatal care was regarded as level 2 intermediate care. Any neonatal hospital transfer was assumed to be for the purposes of the other type of care (ie, level 3 transfer to level 2 and level 2 transfer to level 3).
Unit Costs for Physician Services and Wards
Physician services were costed using the provincial government health insurance plan schedule of medical benefits applicable to 2013 for Ontario, British Columbia, and Alberta.6–8 Healthcare billing experts and clinicians in each province assisted with the identification and interpretation of appropriate billing codes. All services involved a flat fee or a time-dependent fee component, usually to a maximum amount, and a time of day and day of week premium (ie, for evening, night, and weekend calls). Accordingly, the fees for some services varied for each woman in the trial and were applied individually at the record level. An average unit cost per patient was calculated to generally illustrate the unit costs applied in each province (Table S1). Hospital ward durations were costed using unit costs by ward type and delivery mode determined for an economic analysis for a similar perinatal study in 2002, the TBT (Term Breech Trial),9 that were updated to 2013 using the Canadian Consumer Price Index healthcare commodity group of 20.5%.10 Ward unit costs were not province specific, but were derived in the TBT from reports from 4 teaching hospitals and 3 community hospitals in each of the included provinces (ie, Ontario, British Columbia, and Alberta) combined into 1 median unit cost per ward type per hour (Table S2). Further definitions and assumptions for physician services and ward durations are included in Table S3. Table S4 summarizes the methods used to derive hospital ward unit costs and their application in this study.
Data Analysis
Costing involved multiplying the actual amount of resources consumed by each participant as collected in the trial by their respective unit costs to determine the total cost of each of 24 services and each of 11 ward stays. The total cost of all 24 services, the total cost of all 11 wards, and the overall total cost of all services and wards were calculated using each province’s unit costs for less tight and tight BP management groups. All results were analyzed according to the intention-to-treat approach that included 493 women in the less tight group and 488 women in the tight group, and excluded 6 women who withdrew or were lost to follow-up in CHIPS (Control of Hypertension in Pregnancy Study). The total per-participant costs for mothers and infants in each arm of the trial were not assumed to be normally distributed. As such, the standard errors (SEs) of the difference in the means between groups and 95% CIs of the differences in the means were estimated using bootstrap methods and P values were estimated using permutation in “R” statistical software version 3.2.2.11 For each province, CIs and P values were estimated for all services, all wards, and for all services and all wards together. To demonstrate which specific services or wards were cost-drivers between less tight and tight control, we determined the absolute difference in mean cost between study groups for each service and ward, ranked the cost differences in descending order of magnitude, and for the top 5 cost-drivers where parameters were similarly estimated, we tested their significance assuming no distribution and using permutation, and found their 95% CIs using boot-strapping.
Results
Primary/transfer hospital ward durations were costed to a maximum of 305 days after which no women or babies remained in hospital. Women and infants in the less tight group relative to the tight group consumed less of 7 services and spent less time on 2 wards (antenatal physician visits, nonstress tests, antenatal hospitalization not for delivery ward time, Caesarean section delivery before labor, Caesarean section delivery during labor, operative vaginal deliveries, miscarriages, operating room time, and infant head CT scans), but consumed more of 16 services and spent more time on 9 wards (antepartum home care visits with a nurse, obstetric day unit visits and ward time, emergency room visits and ward time, antenatal hospital admissions not for delivery, maternal blood and urine tests, fetal ultrasounds, infant chest x-rays, infant head ultrasounds and MRIs, ophthalmology exams for retinopathy of prematurity, longer lengths of stay in the antenatal ward, labor and delivery ward, postnatal ward, more spontaneous vaginal deliveries, inductions for labor, and more physician care and longer length of stay in level 2 and level 3 neonatal care at delivery and transfer hospitals). Both groups had the same number of elective terminations (Table 1). Although the less tight (compared with the tight) group had a similar number of overall admissions to high-level neonatal care level 2 or 3 (156 versus 151 neonates), the total length of stay of less tight group neonates was substantially longer (total of 3921.34 days versus 2763.96 days, or 41.8% more days), especially in level 3 neonatal intensive care at delivery hospitals, an important difference with associated clinical implications, regardless of cost.
The mean cost in each group represents the total cost incurred for each service or ward consumed by women in that group divided by the total number of women (493 or 488) in the study group, whether or not they incurred the service or ward (Table S5). Overall, there was no significant difference in the mean cost of all services and wards between less tight and tight control groups in each province (Table 2), with similar direction and magnitude of effect. Women in the less tight control group incurred costs that were close to $6000 dollars more than women in the tight control, but the difference was not statistically significant.
Table 2.
Analysis of Difference in Mean Costs of All Services and Wards, All Services, and All Wards by Study Group
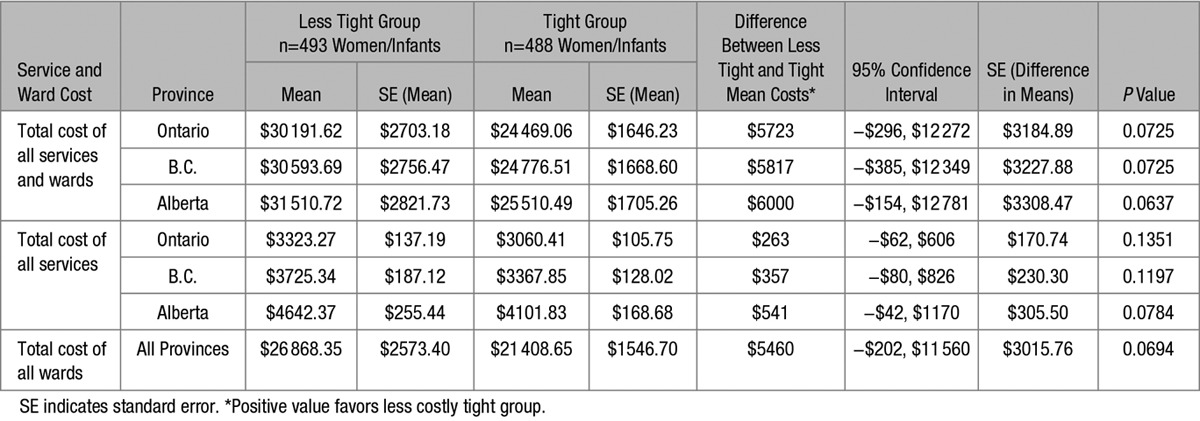
Table S6 shows the top 5 items with the greatest difference in mean cost between the study groups. All were related to neonatal intensive care. The top 4 cost-drivers were neonatal care ward costs at the delivering and transfer hospitals. In the less tight group, high-level neonatal intensive care ward costs represented the majority of overall costs: at 56.8% of overall cost per woman/infant dyad in Ontario, 56.1% in British Columbia, and 54.5% in Alberta. There was no statistically significant difference between the study groups in any of the top 5 cost drivers.
Discussion
Main Findings
There has been much debate about the best approach to the management of hypertension.2 The CHIPS trial showed that tight BP control is not harmful to the baby, and consistent with literature from other clinical trials, was beneficial to the mother by decreasing the development of severe hypertension.12 To our knowledge, the analysis reported here is the first to compare the cost implications of less tight versus tight BP control strategies for pregnancy hypertension. The study found that the mean cost per woman–infant dyad managed by a policy of less tight (versus tight) BP control is not significantly different with regard to overall services and hospital ward costs incurred from as early as from 14 weeks of gestation up to 305 days after delivery (ie, last neonatal primary hospital discharge date recorded), using costs obtained from Ontario, British Columbia, or Alberta, Canada.
Although costs in each province were almost $6000 higher for the less tight group in which infants spent more time in high-level neonatal intensive care wards, the result did not reach statistical significance and may reflect a lack of statistical power.13,14
The upper and lower limits of the 95% CI can be examined to determine whether they would exclude a minimally important (cost) difference (MID), like that used for noninferiority trials.15,16 If the MID is either outside or included within both CI boundaries, then neither treatment strategy is significantly different in cost and the decision threshold to change policy is not reached. However, if an MID is included on one side of the 95% CI boundaries but not on the other, the decision threshold is reached for that treatment if adopted, and would be associated with cost savings per patient to at least the level of the MID: similar savings would not be experienced if the alternate treatment approach was adopted. In our study, a decision maker might determine, for example, that a difference in mean cost per patient of ±$500 is an MID threshold above which a less tight or tight policy would be attractive to implement. Using outcomes for British Columbia in our study, the total mean cost per patient was $30 593.69 for less tight and $24 776.51 for tight groups, with a difference in mean costs of $5817, and CI of −$385 cost savings up to a $12 349 cost increase, for a less tight patient (Table 2). In this example, with an MID of $500, we could exclude a cost savings with a policy of less tight control. Tight control may be substantially cheaper with a marked reduction in neonatal intensive care unit days.
Study Strengths and Limitations
The data come from CHIPS, a large, international, multicenter randomized trial. Resource utilization was measured prospectively and the economic analysis planned, all contributing to minimization of bias in cost assessment between groups. The analysis used mean costs from 3 Canadian provinces with different fee structures related to provincial schedules of medical benefits, especially flat rate fees, premiums for start time of day and day of week, and time-dependent fees. The direction and magnitude in the difference in mean cost of overall services and wards in each of the 3 provinces were similar, all favoring tight control as less expensive. The purpose of conducting the analysis in each province was to demonstrate the independence of findings from the effect of jurisdiction and price structures, similar to a sensitivity analysis. Any specific cost differences between provinces are ultimately less important than the fact that the interventions were costed in 3 different healthcare jurisdictions with different funding and price structures, yet produced a similar magnitude and direction in results, which supports the validity of the findings. There were no major differences between provinces, which supports the generalizability in this context.
Our study is limited by the application of 2002 TBT ward unit costs scaled for health commodity inflation in Canada to 2013 trial data: true ward unit costs incurred by hospitals in each province in 2013 may be different. The TBT combined ward type unit cost data for each province, so we could not report province-specific total ward costs. TBT ward unit costs were derived for women with breech pregnancies, their infants and costs of related complications, which may underestimate these costs for hypertensive pregnancies. However, hypertension-specific care was accounted for, such as more time allocated on the labor and delivery ward to women on magnesium sulfate to capture the enhanced nursing care required. CHIPS women may have had different mean lengths of stay on the labor and delivery ward and in the operating room for Caesareans than those in the EECV2 (Early External Cephalic Version 2) trial from which costs were estimated, although these durations are not likely to be substantially different in the overall findings, and total hospital length of stay in CHIPS was preserved. Whereas CHIPS women may have received more interventions than EECV2 women, this approach is conservative and has been applied equally to both the study groups. The neonatal intensive care level 3 ward unit cost of $107.00/h from the TBT may be higher for CHIPS neonates who may require a higher intensity of hospital care, related to nursing staff time, hospital service consumables used, and associated ward costs. The time spent in any high-level care was preserved, but we were unable to standardize a definition of levels 2 and 3 neonatal care across jurisdictions: categorization of high-level neonatal care into levels 2 and 3 care cases was performed according to an algorithm that may not have reflected the true level, intensity and cost of care received. Although every attempt was made to ensure the correct billing codes in each province were applied to the physician service resources consumed in CHIPS, actual billing practice of individual physicians vary by individual practice (such as the use of after-hours codes), time of day, and day of week that care was provided. The trial collected data from 94 centers in 15 countries, with centers selected to have similar facilities, conditions and medical practices to fulfill the CHIPS Protocol as in Canada. However, the resources consumed in the trial may differ from that which would have been consumed in the actual Canadian context, although any potential differences cannot be easily ascertained. Nonetheless, the application of any estimates was applied to both groups equally. As for all clinical efficacy scenarios, our results apply to the 3 provinces mentioned because we used their unit prices. No subgroup analysis was done with Canadian study participants only.
The CHIPS trial, as with many randomized controlled trials, was powered for the primary study outcome and not the cost analysis, which typically requires larger sample sizes because of large variances and positively skewed distributions of cost, as observed in this analysis. It is possible that a larger sample size may have confirmed the observed trend toward a higher mean cost per woman in the less tight group, driven by the longer infant length of stay in high-level neonatal intensive care units. More than 55% of the cost of care was attributed to neonatal costs, which was the top cost-driver in each of the 3 provinces, raising the possibility that differences seen in the primary study may be real.
Perspectives
The mean cost for women/infants under a less tight treatment approach to pregnancy hypertension is not different to those under a tight control approach. However, based on our findings, there is little chance that less tight control is cheaper; and in the main CHIPS Study, less tight control was implicated in significantly more morbidity among women. Thus, a treatment approach of tight control of pregnancy hypertension may have clinical benefit for women, with no increased risk to neonates and without additional (and possibly lower) cost to the healthcare system.
Acknowledgments
This article is dedicated to the memory of our dear friend and colleague, Dr Andrée Gruslin. We thank all of the women who participated in the CHIPS trial (Control of Hypertension in Pregnancy Study), and members of the CHIPS Collaborative Group (online-only Data Supplement).
Sources of Funding
This economic research and corresponding randomized controlled trial (the CHIPS trial [Control of Hypertension in Pregnancy Study]) was supported by a grant (MCT 87522) from the Canadian Institutes of Health Research (CIHR) and is registered with Current Controlled Trials (ISRCTN71416914) and ClinicalTrials.gov (NCT01192412). CIHR had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosures
All authors have completed a Conflict of Interest Disclosure Questionnaire and declare that (1) none of the authors have received support from any company for the submitted work; (2) none of the authors have relationships with a company that might have an interest in the submitted work in the previous 3 years; (3) P. von Dadelszen received consulting fees and in-kind research support from Alere International related to preeclampsia and fetal growth restriction through the provision of Triage PlGF cartridges; and no other authors have nonfinancial interests that may be relevant to the submitted work. No author had any financial or personal relationships with other people or organizations that could inappropriately influence (bias) their work on this project. P. von Dadelszen reports personal fees and other from Alere International, outside the submitted work. J. Menzies reports grants, personal fees and nonfinancial support from Canadian Institutes of Health Research (CIHR, a Canadian federal government granting agency), during the conduct of the study. M. Helewa reports grants from CIHR-Factors associated with inadequate prenatal care, grants from CIHR-Reducing inequities in access to and use of prenatal care in the Winnipeg Health Region through Health System improvement, grants from CIHR-Quality of prenatal care questionnaire development, grants from CIHR-Effect of Folic Acid supplementation in pregnancy on preeclampsia Folic Acid Clinical Trial (FACT), outside the submitted work. L.A. Magee reports grants from CIHR, during the conduct of the study; personal fees from Bill & Melinda Gates Foundation, outside the submitted work.
Statement on Ethics Approval and Informed Consent From Participants
Ethics approval for the CHIPS trial (Control of Hypertension in Pregnancy Study) and cost analysis was obtained from the trial coordinating center (University of British Columbia Clinical Research Ethics Board, H08-00882) and at each of the recruiting centers. Informed consent was obtained from each woman participating in the trial before enrollment.
Supplementary Material
Footnotes
A list of all Members of the CHIPS Trial Collaborative Group is given in the online-only Data Supplement.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.116.07466/-/DC1.
Novelty and Significance
What Is New?
This study presents a cost analysis of less tight versus tight blood pressure management strategies for women with chronic or gestational hypertension in a large, multicenter randomized controlled trial.
What Is Relevant?
The CHIPS trial (Control of Hypertension in Pregnancy Study) found no difference in pregnancy loss or high-level neonatal care >48 hours when comparing a less tight (target 100 mm Hg) and tight (target 85 mm Hg) approach to blood pressure management during pregnancy. However, women in the CHIPS study experienced significantly less morbidity with a tight approach.
No significant difference was found in mean total cost of all services and wards per woman–infant dyad between less tight versus tight strategies.
Summary
Tight blood pressure control in pregnancy may benefit women without increasing risk to neonates (as shown in the main CHIPS trial), without additional (and possibly lower) cost to the healthcare system.
References
- 1.Gillon TE, Pels A, von Dadelszen P, MacDonell K, Magee LA. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS One. 2014;9:e113715. doi: 10.1371/journal.pone.0113715. doi: 10.1371/journal.pone.0113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 3.Statistics Canada. Ottawa, Canada: 2013. Population by year, by province and territory, CANSIM, table 051-0001. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm. Accessed October 5, 2015. [Google Scholar]
- 4.Canadian Institute for Health Information (CIHI) Ottawa, Canada: 2014. National Health Expenditure Trends, 1975 to 2013, Spending and Health Workforce. https://secure.cihi.ca/estore/productSeries.htm?pc=PCC52. Accessed October 5, 2015. [Google Scholar]
- 5.Ahmed RJ, Gafni A, Hutton EK Early ECV2 Trial Collaborative Group. The Cost Implications in Ontario, Alberta, and British Columbia of Early Versus Delayed External Cephalic Version in the Early External Cephalic Version 2 (EECV2) Trial. J Obstet Gynaecol Can. 2016;38:235–245.e3. doi: 10.1016/j.jogc.2015.12.019. doi: 10.1016/j.jogc.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health and Long Term Care, Government of Ontario. Toronto, Ontario, Canada: 2013. Schedule of Benefits - Physician Services Under the Health Insurance Act, Revised October 1, 2013. [Google Scholar]
- 7.Ministry of Health, Government of British Columbia. Vancouver, British Columbia, Canada: 2013. Medical Services Commission Payment Schedule, Revised November 1, 2013. [Google Scholar]
- 8.Alberta Health, Government of Alberta. Edmonton, Alberta, Canada: 2012. Schedule of Medical Benefits, Health Professional Fees, Medical Benefits Price List, Alberta Health Care Insurance Plan, Revised April 1, 2012 (applicable to 2013 services). [Google Scholar]
- 9.Palencia R, Gafni A, Hannah ME, et al. Term Breech Trial Collaborative Group. The costs of planned cesarean versus planned vaginal birth in the Term Breech Trial. CMAJ. 2006;174:1109–1113. doi: 10.1503/cmaj.050796. doi: 10.1503/cmaj.050796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statistics Canada. CANSIM table 326-0021 - Consumer Price Index (CPI), Canada, 2013 basket, annual, Commodities and commodity groups=Health care. http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=3260021&paSer=&pattern=&stByVal=1&p1=1&p2=37&tabMode=dataTable&csid=. Accessed May 15, 2012.
- 11.R Core Team. Vienna, Austria: 2015. R: A Language and Environment for Statistical Computing. http://www.R-project.org/. Accessed September 1, 2015. [Google Scholar]
- 12.Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014;2:CD002252. doi: 10.1002/14651858.CD002252.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Briggs A. Economic evaluation and clinical trials: size matters. BMJ. 2000;321:1362–1363. doi: 10.1136/bmj.321.7273.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gafni A, Walter SD, Birch S, Sendi P. An opportunity cost approach to sample size calculation in cost-effectiveness analysis. Health Econ. 2008;17:99–107. doi: 10.1002/hec.1244. doi: 10.1002/hec.1244. [DOI] [PubMed] [Google Scholar]
- 15.Treadwell J, Uhl S, Tipton K, Singh S, Santaguida L, Sun X, Berkman N, Viswanathan M, Coleman C, Shamliyan T, Wang S, Ramakrishnan R, Adam Elshaug A. Assessing Equivalence and Non-InferiorityAssessing Equivalence and Noninferiority. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Jun. Report No.: 12-EHC045-EF. [PubMed] [Google Scholar]
- 16.Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis. 2008;66:150–154. [PubMed] [Google Scholar]


