Abstract
It has been suggested that intra-individual variability (IIV) in performance on attention and other cognitive tasks might be a cognitive endophenotype in individuals with ADHD. Despite robust IIV findings in behavioral data, only sparse data exist on how what type of brain dysfunction underlies variable response times. In this study, we asked whether ADHD IIV in reaction time on a commonly-used test of attention might be related to variation in hemodynamic responses (HRs) observed trial-to-trial. Based on previous studies linking IIV to regions within the “default mode” network (DMN), we predicted that adolescents with ADHD would have higher HR variability in the DMN compared with controls, and this in turn would be related to behavioral IIV. We also explored the influence of social anxiety on HR variability in ADHD as means to test whether higher arousal associated with high trait anxiety would affect the neural abnormalities. We assessed single-trial variability of HRs, estimated from fMRI event-related responses elicited during an auditory oddball paradigm in adolescents with ADHD and healthy controls (11–18 years old; N = 46). Adolescents with ADHD had higher HR variability compared with controls in anterior regions of the DMN. This effect was specific to ADHD and not associated with traits of age, IQ and anxiety. However, an ADHD effect of higher HR variability also appeared in a basal ganglia network, but for these brain regions the relationships of HR variability and social anxiety levels were more complex. Performance IIV correlated significantly with variability of HRs in both networks. These results suggest that assessment of trial-to-trial HR variability in ADHD provides information beyond that detectable through analysis of behavioral data and average brain activation levels, revealing specific neural correlates of a possible ADHD IIV endophenotype.
Keywords: ADHD, fMRI, Single-trial variability, Intraindividual variability, Reaction time, Anxiety
Highlights
-
•
We studied if the behavioral variability in ADHD is also found on a neuronal level.
-
•
Independent component analysis was combined with BOLD amplitude variability.
-
•
Adolescents with ADHD had higher amplitude variability than healthy controls.
-
•
Higher amplitude variability was shown in an anterior default mode network.
-
•
Social anxiety in ADHD associated with high amplitude variability in the striatum
1. Introduction
Children and adolescents diagnosed with Attention-Deficit/Hyperactivity Disorder (ADHD; American Psychiatric Association, 1994, American Psychiatric Association, 2013) show greater performance variability in reaction time (RT) on attention and cognitive control tests compared with typically developing children (Buzy et al., 2009, Klein et al., 2006, Kuntsi et al., 2001, Vaurio et al., 2009). Meta-analytic reviews find that RT variability is present in ADHD at all ages, has a genetic basis (Bellgrove et al., 2005, Kuntsi and Klein, 2012), is attenuated by psychostimulant medications (Spencer et al., 2009), is unrelated to response speed (Karalunas et al., 2014, Kofler et al., 2013) and predicts with real-world ADHD behaviors (Antonini et al., 2013), thus representing a stable feature of the disorder. It has been suggested that this characteristic intra-individual variability (IIV) in behavioral performance might be a cognitive endophenotype for ADHD (Castellanos et al., 2005, Castellanos and Tannock, 2002, Kuntsi and Klein, 2012, Tamm et al., 2012). As such, study of neurobiological factors underlying IIV in ADHD could lead to improved understanding of the disorder and its causes.
It has been suggested that spontaneous brain activity comprising low frequency fluctuations of the default mode network (DMN) interrupts with task-positive brain activation in children with ADHD, possibly underlying attentional lapses and leading to high IIVRT in ADHD (Sonuga-Barke and Castellanos, 2007). Only a handful of studies have begun to investigate the neural correlates of IIV to ask if this DMN spontaneous activation account might explain the IIV behavioral performance profile. Attentional lapses observed in RT measurements have been linked to low frequency fluctuations in the DMN that interfere with task-related cognitive processing (Broyd et al., 2009, Eichele et al., 2008, Weissman et al., 2006). An abnormal DMN function is also linked to developmental immaturity (Tam et al., 2014). However, despite IIV being a robust finding in ADHD behavioral data, only sparse data exists on how IIV manifests in measures of ADHD brain activation. Variable RT has been associated with average levels of brain activity in medial prefrontal cortex (mPFC) regions, which form a key region of the anterior DMN (Fassbender et al., 2009, Rubia et al., 2007). In ADHD, greater IIV also has been linked by post hoc correlation analyses to lower activation during Go/No-Go tasks in frontoparietal brain regions (Suskauer et al., 2008) and less white matter integrity in cingulum bundle and frontostriatal tracts (Lin et al., 2014), as well as smaller amplitudes in the cingulo-opercular system at errors (Plessen et al., 2016).
A potentially more meaningful approach for understanding brain function underlying IIV in ADHD would be to estimate hemodynamic responses (HRs) on a single-trial basis to evaluate IIV in the brain's response to each event of interest (e.g., Eichele et al., 2008). In this way, it would be possible to link trial-to-trial behavioral variability so often observed in previous ADHD studies to trial-to-trial hemodynamic variability. For instance, behavioral IIV was previously found to be positively associated with HR amplitude variability over trials (Danielmeier et al., 2011, Eichele et al., 2008) in the anterior DMN (van Maanen et al., 2011). To the best of our knowledge, hemodynamic amplitude variability over task trials has not been studied in children with ADHD. Therefore, the present study re-examines fMRI data from adolescents' boys with DSM-IV/DSM-V (American Psychiatric Association, 1994, American Psychiatric Association, 2013) Combined-subtype ADHD performing an auditory oddball attention task (Stevens et al., 2007). Oddball performance requires participants to detect and respond to infrequent (oddball) stimuli (i.e., target processing) (Rubia et al., 2007) requiring attention allocation (Stevens et al., 2007) and the ability to stay vigilant (Tamm et al., 2006). These are attention-related abilities previously associated with IIV (Kuntsi et al., 2001, Rubia et al., 2007, Stevens et al., 2007). A study using the analogous estimation of single-trial variability in event-related EEG data found that adolescents with ADHD had higher amplitude variability over trials relative to healthy controls for the P3 component (i.e., related to target processing on oddball tasks) (Lazzaro et al., 1997). We assessed event-related HR amplitude variability over individual target trials as in previous reports (Eichele et al., 2008). We hypothesized that ADHD-diagnosed adolescents would show higher HR amplitude variability over trials in the anterior DMN than a comparison group of typically developing adolescents without ADHD. We also expected that the level of single-trial HRs variability in these brain regions would be associated with behavioral performance IIV.
Furthermore, we wanted to explore the effect of self-reported social anxiety on HR variability in ADHD. Higher symptom levels of anxiety are typically co-occurring with ADHD (Chavira et al., 2004), which in adolescent years, often constitute in social anxiety (Caouette and Guyer, 2014, Dell'Osso et al., 2003). Thus, anxiety is a significant modulator of arousal — affecting the ability to adjust energetic level to task requirements (Eysenck et al., 2007) and possibly affecting the DMN activation (Broyd et al., 2009). Both ventro- and dorsomedial PFC regions that constitute part of the DMN are believed to have a key role in the pathophysiology of social anxiety (Blackford et al., 2014, Damsa et al., 2009, Evans et al., 2009, Peterson et al., 2014). Therefore, examining anxiety is a useful way to further test neurobiological modulation of IIV in ADHD, which previous studies linking IIV RT to neurobiological dysfunction have not done. Because these analyses are exploratory, they are included as follow-up analyses for effects found for the primary IIV ADHD analyses.
2. Materials and methods
2.1. Participants
In the present study 23 boys with ADHD and 23 typically developing boys, 11–18 years of age, were recruited via physician referral and community advertisements (Stevens et al., 2007). The two groups were matched on age and demographic characteristics. Experienced clinical staff conducted a diagnostic evaluation with the Schedule for Affective Disorders and Schizophrenia for School-Age Children — Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997). The criteria for children to be included in the sample were no other mental health disorder except for ADHD, no history of formal learning disability, and no significant medical conditions. Further, the healthy control group was matched to the ADHD group on handedness, age, socioeconomic status (Hollingshead and Redlich, 1958), self-report scores for depression (Beck et al., 1996) and anxiety (March et al., 1997), and estimated intelligence (Wilkinson, 1993). The boys with ADHD had not taken medication for at least 24 h before the time of fMRI. Descriptive information of the sample and t-test comparison results is shown in Table 1. For further details about characteristics of the sample, see Stevens et al. (2007).
Table 1.
Descriptive information about the sample.
| Variables | ADHD |
HC |
Analysis |
||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | |
| Age | 14.65 | 1.85 | 15.13 | 1.94 | 0.86 |
| Matrix Reasoning | 25.81 | 6.19 | 27.45 | 3.36 | 0.65 |
| Target mean RT | 388.07 | 72.23 | 440.77 | 104.39 | 1.99a |
| Target IIV RT | 136.06 | 63.11 | 113.80 | 53.86 | − 1.29 |
| Target hits | 46.87 | 2.97 | 45.96 | 3.20 | − 1.00 |
| Target percentiles | 0.98 | 0.06 | 0.96 | 0.07 | − 0.98 |
| Social anxiety subscoresR | − 0.06 | 1.10 | 0.06 | 0.83 | 0.42 |
Note. RT = reaction time; IIV = intra-individual variability. R = residual scores.
p < 0.06.
2.2. fMRI task and procedure: oddball task
Participants performed two runs of an auditory three-stimulus oddball task (Kiehl et al., 2001, Kiehl et al., 2005). The oddball task consisted of 24 target tone stimuli, 24 novel sound stimuli, and 196 non-target stimuli. The standard stimulus was a 1000 Hz tone (probability = 0.80), the target stimulus was a 1500 Hz tone (probability = 0.10), and the novel stimuli (probability = 0.10) consisted of nonrepeating random digital noises (e.g., tone sweeps, whistles). Each stimulus was presented for 200 ms with a pseudorandom stimulus onset asynchrony ranging from 1000 to 3000 ms (mean = 1500). Moreover, three to five standard tones were followed by either target or novel tones. The children were instructed to make a right index finger button response quickly and accurately for every target tone, but not for other stimuli. All the children in the present sample reported that they could hear the stimuli and discriminate the stimuli from the background scanner noise. The events of stimuli presentation and the behavioral responses (hits or false alarms within 1250 ms) were recorded and monitored online on a separate computer. In the present study, only the behavioral responses to the target tones, and the hemodynamic responses following the presentation of the target tones and the behavioral responses, were included in the statistical analyses. We calculated the standard deviation for each adolescent based on the single-trials of target RT to generate a measure of performance IIV. See Table 1 for the mean (e.g., reaction time, number of hits, and percentile of target hits) and single-trial variability scores for each diagnostic group in relation to the target processing on the oddball task.
2.3. Symptoms of social anxiety
The social anxiety level was measured with the Multidimensional Anxiety Scale for Children (MASC). The MASC comprises 39 items covering four subscales: a) physical symptoms (tense/restless and somatic/autonomic), b) harm avoidance (anxious coping and perfectionism), c) social anxiety (humiliation/rejection and public performance fears), and d) separation anxiety (March et al., 1997). The subscales of MASC represent the symptom constellations of anxiety disorders/symptoms in DSM-IV (American Psychiatric Association, 1994) and are designed to include items not overlapping with symptom criteria in ADHD or major depressive disorders (March et al., 1997). For the current study, we isolated the unique effect of social anxiety by estimating a residual score for social anxiety from a linear regression analysis that accounted for variance explained by the three other MASC subscores. None of the adolescents in the present study met criteria for any anxiety disorder on the K-SADS-PL (Kaufman et al., 1997) and there were no significant differences in level of social anxiety symptoms between the adolescents with ADHD and healthy controls (see Table 1). Forty-four of the 46 adolescents filled in the MASC questionnaire and the missing values for two of the healthy controls were replaced with the sample mean scores.
2.4. WASI Matrix Reasoning
The Matrix Reasoning subtest from the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) was used to estimate intellectual function. The Matrix Reasoning is one of the subtests in the Wechsler intelligence battery that children with ADHD typically perform normally on a group level and thus is not sensitive to types of cognitive dysfunction commonly found in ADHD (Mayes and Calhoun, 2006) (see Table 1 for the mean scores for each diagnostic group). For three of the healthy controls and for two of the children with ADHD there were missing values on the subtest of Matrix Reasoning, which were replaced with the sample mean score.
2.5. MR parameters
MR Imaging was performed with a 3.0 T Siemens Allegra system at the Olin Neuropsychiatry Research Center at the Institute of Living/Hartford Hospital in Hartford, CN (USA). Functional image volumes were collected in axial orientation to the anterior commissure-posterior commissure line using a gradient-echo sequence sensitive to the blood-oxygen-level-dependent (BOLD) signal (repetition time = 1500 ms, echo time = 28 ms, flip angle = 65°, field of view = 24 × 24 cm, 64 × 64 matrix, 3.4 × 3.4 mm in plane resolution, 5 mm slice thickness, 30 slices) effectively covering the entire brain (150 mm) in 1.5 s. The two runs each consisted of 255 volumes, including a 9 s rest period at the beginning that was collected to allow T1 effects to stabilize. These initial six images were not included in subsequent analyses.
2.6. Image processing
Functional images were reconstructed offline, and each run was separately realigned using INRIAlign (Freire and Mangin, 2001, Freire et al., 2002) as implemented in the Statistical Parametric Mapping software (SPM2, Wellcome Department of Cognitive Neurology, London). A mean functional image volume was constructed for each participant for each session from the realigned image volumes and was used to determine parameters for spatial normalization into standardized Montreal Neurological Institute space. These normalization parameters were then applied to the corresponding functional image volumes. Normalized images were smoothed with a 12 mm full width at half maximum Gaussian filter.
2.7. Independent component analysis (ICA)
Analyzing temporal dynamics of successive trials with standard fMRI analysis procedures is challenging and we therefore performed a group-level ICA with subsequent extraction of single-trial estimates through deconvolution and regression as described in Eichele et al. (2008). The ICA was performed in order to functionally segregate individual or small groups of brain regions with unique time courses (Abou-Elseoud et al., 2010, Kiviniemi et al., 2009). The preprocessed data were then decomposed into 70 components using a high model order group ICA with the GIFT toolbox (http://mialab.mrn.org/software/gift), where the spatial ICA maps are generated with the infomax algorithm (Bell and Sejnowski, 1995; see Eichele et al., 2008). Participants-specific time-courses were estimated using regression of participant data sets into group components (Erhardt et al., 2011). 12 of the 70 components showed hemodynamic responses following target stimuli onsets across the entire group of participants, where 6 of those components were discarded due to artifacts representing signal from large vessels, ventricles, motion, and susceptibility. For the 6 remaining components, random-effects t-statistics of their maps were conducted, adjusted for multiple comparisons at 5% false-positive discovery rate and cluster extent of at least 20 contiguous voxels. Single-trial estimates were calculated (e.g. means and standard deviations for each adolescent). The average of these amplitude differences were analogous to simple activation from conventional fMRI timeseries GLM analysis. The standard deviation of single-trial HR estimates served as primary dependent measure across trials intra-individually, where larger standard deviations reflect greater trial-to-trial HR variability in hemodynamic response. The single-trial estimates of amplitude were the mean across trials intra-individually.
2.8. Group analyses
The statistical analyses of our group differences were performed with SPSS, version 22. Multivariate Analyses of Covariance (MANCOVAs) were run to test for between-group effects (ADHD versus healthy controls) in the single-trial HR variability and amplitudes for the ICs controlling for the effect of age. This multivariate approach adjusts the family error rate when testing multiple IC dependent variables (Field, 2009, Tabachnick and Fidell, 2007). Our analyses took the following stepwise procedure: a) If the multivariate test for the between-group analyses on either the single-trial variability or the single-trial amplitude for the ICs was significant, then we conducted follow-up univariate Analyses of Covariances (ANCOVAs), reporting on specific ICs that showed significant study group differences. Further, for any significant ANCOVA between-group effect, we also explored alternative ANCOVA models that b) controlled for the effect of general abilities (IQ) and target mean RT (Kuntsi and Klein, 2012) in addition to the inclusion of age as a covariate, and c) additionally controlled for the possible modulating effect of the MASC social anxiety symptom subscores. The follow-up ANCOVAs were done both to ensure the validity of the primary findings as well as to assess if there might be relationships between hemodynamic IIV and participant individual differences (e.g., age, IQ, social anxiety). Finally, to link any single-trial HR variability group differences to participants' task performance, the strength of relationships between HR variability and performance IIV were quantified using Pearson partial correlation (controlling for age, IQ, and target mean RT) in the whole sample and separately for each study group.
In all analyses, data outliers were defined by plus/minus of 3 standard deviations from the sample mean and were replaced with a score of 2 plus/minus standard deviations from the sample mean (Field, 2005). One adolescent with ADHD had a HRs IIV score above 3 standard deviations on IC 2, and HRs mean amplitude scores above 3 standard deviations on ICs 1 and 2.
3. Results
3.1. fMRI data independent component (IC) maps
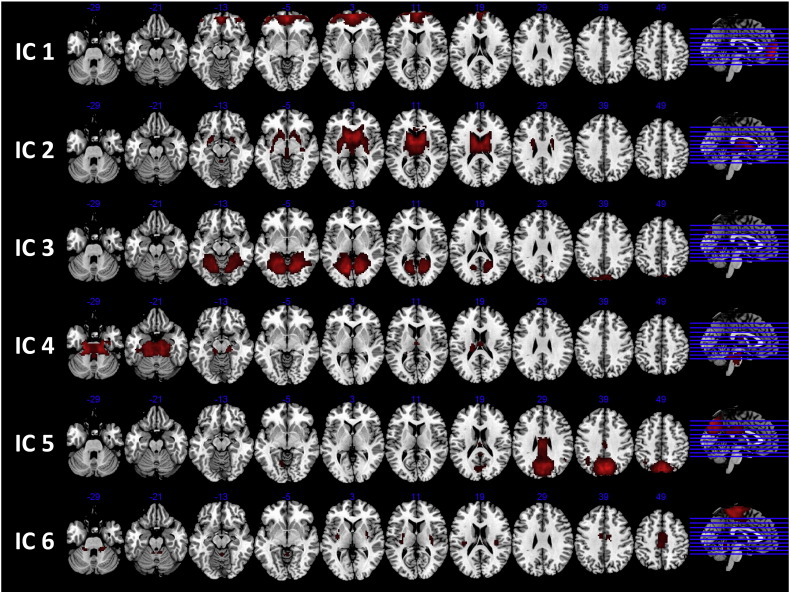
IC 1 comprised an intensity peak in the left hemisphere of the medial orbitofrontal cortex (OFC; see Fig. 1 and Supplemental Table 1) with extended involvement of the same region in the right hemisphere, and bilateral involvement of the ACC, and the middle OFC. The OFC is essential in motivational and reward processing and lesions in OFC associate with less impulse control in humans (Bush, 2010). Further, the mPFC and bilateral middle frontal lobe are typically hypoactive in children with ADHD (Dickstein et al., 2006). The activation pattern in IC 1 overlaps with PFC activation in an anterior DMN (Allen et al., 2011) and fitted our a priori hypothesis for an intensity peak in the anterior DMN (Fassbender et al., 2009, Rubia et al., 2007).
Fig. 1.
An overview of the activation of brain areas in the six ICs.
Note. One-sample t-test, False Discovery Rate, < 0.05, 20 voxels.
IC 2 showed the main peak of intensity in the left hemisphere of the caudate, with extended intensity of activation in bilateral basal ganglia (i.e., striatum: caudate and putamen, and pallidum) and thalamus. The striatum is working as a node in parallel networks involved in attention and motivation/reward processing, connecting with the mPFC and middle PFC, networks compromised in children with ADHD (Bush, 2010). Moreover, the thalamus mediates the interaction between attention and arousal in humans (Portas et al., 1998) and shown to be hypoactivate in children with ADHD relative to in healthy controls (Dickstein et al., 2006). IC 2 reveals a network important in attentional control and arousal, attention functions central in vigilance (Sergeant, 2005).
IC 3 showed peaks of activation foci in the left hemisphere of the lingual and in the right hemisphere of the cuneus, with extended activation to right lingual and left cuneus. Further, the activation extended to bilateral activation in the fusiform gyrus, the parahippocampal and the hippocampal areas, and in the cerebellum. This mainly occipital peak of activation overlaps with visual networks (Allen et al., 2011) and with networks revealed in schizophrenic patients performing an auditory oddball task (Kim et al., 2009).
IC 4 represented peaks of activation in the left hemisphere of thalamus and cerebellum, with extended activation to the right hemisphere of thalamus and cerebellum, and the brainstem. The subcortical region of the thalamus and cerebellum are recognized as nodes within parallel networks of attention (Bush, 2011), nodes that are shown to be hypoactive in children with ADHD (Dickstein et al., 2006). Further, the thalamus is mediating the interaction between attention and arousal in humans (Portas et al., 1998) together with the reticular activating system in the brainstem (Bush, 2011). Though, measuring hemodynamic activity in the regions of thalamus, cerebellum, and brain stem are associated with challenges because of the high influence of “artifacts and pulsatile motion” (Bush, 2010). While acknowledging the limitation, the activity pattern in IC 4 will be referred to as a sub-cortical network.
IC 5 was composed of main peaks of activation foci in the left precuneus and left lingual, and in the right superior temporal gyrus. The activation extended to the right hemisphere of the precuneus and angular, to the left inferior parietal cortex, and to bilateral activation in the mid and post cingulum areas. This network overlaps with a posterior default-mode network (Allen et al., 2011).
IC 6 represented a sensorimotor network (Allen et al., 2011, Smith et al., 2009) because of activation revealed in the motor association areas of frontal/parietal lobules.
3.2. Group analyses
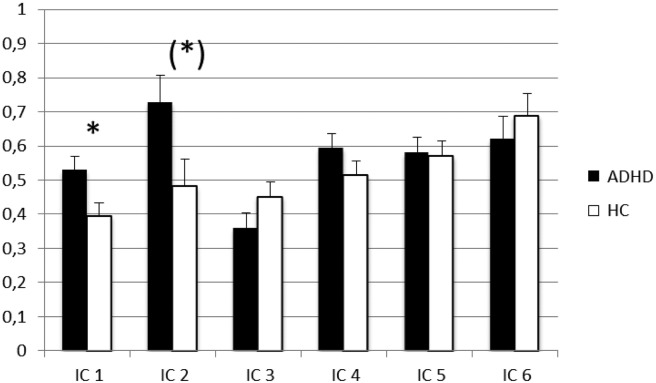
3.2.1. Between-groups comparison of amplitude variability of HRs estimates
MANCOVAs showed a multivariate main effect of the study group on HR variability over trials, Wilk's Lambda = 0.718; F(6, 38) = 2.48, p = 0.04, ηρ2 = 0.28, whereas no significant multivariate main effect appeared for the HR amplitude over trials. Age was included as a covariate in these analyses and showed no significant multivariate effect.
In the follow-up univariate ANCOVAs (controlling for the effect of age) conducted to better describe the multivariate effect, there was a significant main effect of ADHD diagnosis on the single-trial variability showed in IC 1 and IC 2 (see Table 2 for F and p values, and Fig. 2 for estimated marginal means). As hypothesized, adolescents with ADHD showed a higher mean score of single-trial variability in IC 1 (anterior DMN) relative to healthy controls; however, they also showed a higher mean score of HR variability in IC 2 (striatum). Age showed a statistical trend to covary with HR variability in IC 2 (F(1, 43) = 3.84, p = 0.057, ηρ2 = 0.08). When the effects of IQ and target mean RT were considered in addition to age, the ADHD versus non-ADHD difference on single-trial variability in IC 1 remained significant (F(1, 41) = 5.20, p = 0.028, ηρ2 = 0.11). However, the group differences for IC 2 was not longer significant, as variance related to single trial HR variability was significant related to both age (F(1, 41) = 7.28; p = 0.01, ηρ2 = 0.15) and IQ (F(1, 41) = 7.31; p = 0.01, ηρ2 = 0.15).
Table 2.
Results from univariate analyses of ADHD versus non-ADHD study group differences on the variability in hemodynamic response amplitude over trials.
|
IC |
Amplitude variability |
||
|---|---|---|---|
| F value | p value | ηρ2 value | |
| IC 1 | 5.87 | 0.02⁎ | 0.12 |
| IC 2 | 4.76 | 0.04⁎ | 0.10 |
| IC 3 | 2.31 | 0.14 | 0.05 |
| IC 4 | 1.81 | 0.19 | 0.04 |
| IC 5 | 0.03 | 0.87 | 0.00 |
| IC 6 | 0.49 | 0.49 | 0.01 |
Note. In all the univariate analyses age was included as a covariate.
p < 0.05.
Fig. 2.
The estimated marginal means of amplitude variability over trials of HRs for the two diagnostic groups from the univariate tests of variance.
Note. * = p < 0.05. Standard errors of the mean are presented in the error bars. In all the univariate analyses age was included as a covariate, where the two significant group effects on IC 1 and IC 2 where further tested for the influence of IQ, target mean RT, and social anxiety subscores. (*) = the significant difference between the diagnostic groups on IC 2 were dependent on which covariates were included in the univariate tests of variance. It appeared significant when only age was included as a covariate, though this significant effect did not remain when including IQ and target mean RT as additional covariates to age, and thereafter appeared significant when the social anxiety subscores were included as a covariate in addition to age, IQ, and target mean RT.
The final follow-up ANCOVAs considered the role of MASC-measured social anxiety subscores as an additional covariate (see Supplementary data). The significant main effect of ADHD diagnosis on the single-trial variability in IC 1 remained significant (F(1, 40) = 5.46, p = 0.025, ηρ2 = 0.12). Further, a significant main effect appeared again for the single-trial HR variability in IC 2 (F(1, 40) = 4.32, p = 0.044, ηρ2 = 0.10). All the covariates except for target mean RT covaried with single-trial variability in IC 2 (social anxiety subscores: F(1, 40) = 4.04; p = 0.05, ηρ2 = 0.09, age: F(1, 40) = 7.59; p = 0.009, ηρ2 = 0.16 and IQ: F(1, 40) = 8.52; p = 0.006, ηρ2 = 0.18).
3.2.2. Partial correlations between single-trial variability of HRs and of target RT
Partial correlation analyses were run to investigate the association between single-trial HR variability and IIV for target RT by controlling for the effect of age, IQ, and mean target RT (see Table 3). In the total sample, significant correlations appeared between HRs variability in IC 1, IC 2, and IC 4 and variability of target RT. In the ADHD group, IIV for target RT was significantly associated with variability of HRs in IC 1 and IC 2, and near significant with HRs variability in IC 4. In the healthy control group variability of HRs in IC 1 and IC 2 did not associate significantly with variability of target RT. Instead, HR variability was inversely associated with target IIV in IC 6 (supplementary motor area) (see Supplemental Table 2 for bivariate correlations among all these terms).
Table 3.
Partial correlations between amplitude variability over trials of HRs and of target RT IIV.
| Group | Behavioral variability | Amplitude variability over trials (IIV HRs) |
|||||
|---|---|---|---|---|---|---|---|
| IC 1 | IC 2 | IC 3 | IC 4 | IC 5 | IC 6 | ||
| Total sample (n = 46) | IIV RTs | 0.35⁎ | 0.36⁎ | − 0.05 | 0.37⁎ | 0.13 | 0.03 |
| ADHD (n = 23) | IIV RTs | 0.46⁎ | 0.51⁎ | 0.02 | .43a | 0.23 | 0.26 |
| HC (n = 23) | IIV RTs | 0.07 | − 0.04 | − 0.09 | 0.23 | − 0.02 | − 0.51⁎ |
Note. HC = healthy controls. IIV = intra-individual variability; RTs = reaction times. In all of the partial correlation analyses the effects of age, IQ, and mean target RT were controlled for.
p < 0.05.
p < 0.06
4. Discussion
The current study sought to determine if behavioral IIV during performance on attention tasks in adolescents with ADHD was linked to HR variability in fMRI-measured brain activity data. The evidence generally supported the conclusion that ADHD behavioral IIV is linked to abnormal HR variability in the anterior parts of the default mode network (IC 1) during oddball task target processing, as predicted. A similar effect of ADHD diagnosis on HR variability also appeared in a basal ganglia network (the striatum). However, this effect had a complex relationship with other factors tested. These single-trial HR variability abnormalities in ADHD were significantly associated with task performance variability in RT for both ICs 1 and 2. For IC 4 (cerebellum/thalamus), hemodynamic and behavioral IIV also were significantly associated in the total sample, but only at a trend level for ADHD and not at all for non-ADHD. The latter is suggestive of a true group difference, but because hemodynamic variability for the regions in IC 4 was not found to differ between ADHD and non-ADHD this IC will not be discussed further. Overall, these results show that ADHD-diagnosed adolescents have variable, inconsistent neural responses to stimuli in some brain regions. This underscores that the attention difficulties so often observed in ADHD may partly result from variability in the neurobiological allocation of attention resources. Such trial-to-trial variability of neural responses is not detectable through analysis of behavioral data and average fMRI-measured activation levels. However, this association suggests the possibility of a causal relationship, most logically that variable neural responses lead to response inconsistency. This hypothesis should be tested in future research.
The current evidence substantiates the proposal that variability of DMN response to target stimuli is related to behavioral IIV in ADHD. This is in accordance with the neurobiological hypothesis of attentional lapses to be explained by the DMN producing low frequency brain oscillations that interfere with task-related cognitive processing (Sonuga-Barke and Castellanos, 2007). In our previous work, we have shown that such interference is often followed by performance errors (Eichele et al., 2008). Previous findings of an association between IIV and increased activation in vmPFC/ACC (Fassbender et al., 2009) were interpreted to support the theory that the ADHD group fails to deactivate the DMN during task processing. Instead, default mode activity might persist or even re-emerge into states of active task processing to impair performance (Sonuga-Barke and Castellanos, 2007). IC 1 in the current study overlaps with an anterior DMN (Allen et al., 2011) and therefore, task-positive activation may have been susceptible to interruption by activity in IC 1 brain regions. However, DMN interference may not be the sole explanatory for ADHD performance inconsistency. There also were indications that both caudate and perhaps also cerebellar activation variability was related to behavioral IIV in ADHD. This is consistent with prior studies that found frontostriatal regions are implicated in behavioral stability (Kuntsi and Klein, 2012) and that behavioral variability in RT is linked to hemodynamic activity in the caudate in typically developing children (Simmonds et al., 2007).
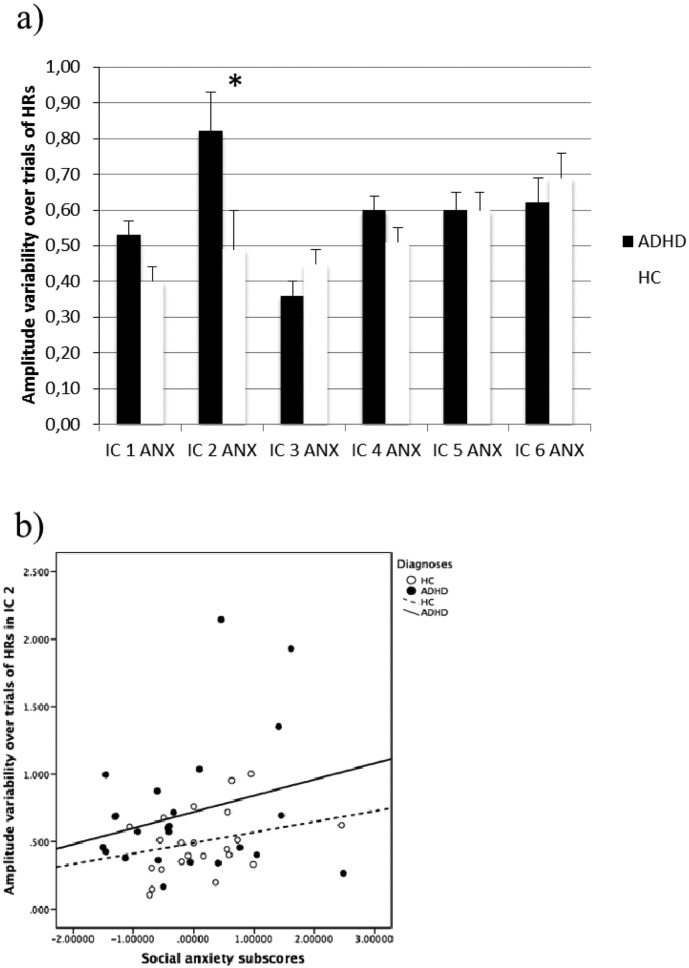
Alternatively, impairments in state regulation, arousal, or learning/motivational processes may give rise to behavioral IIV in ADHD (Halperin and Schulz, 2006, Halperin et al., 2008, Sagvolden et al., 2005, Sergeant, 2005). Our explorative focus on the effect of trait anxiety levels was motivated by an interest in beginning inquiry into whether “bottom-up” processes known to increase the level of arousal, would enhance or lower vigilance, in either protecting against or worsening behavioral IIV or HR variability. Interestingly, our supplement analyses found that social anxiety levels were associated with the HR variability in IC 2. However, the pattern of ANCOVA results suggested complex relationship between anxiety and age and IQ individual differences that also were related to IC 2 HR variability (see Supplementary data for a full description of these analyses, relevant background reading and brief interpretative observations of this relationship). These preliminary indications support the promise of future studies that specifically attempt to understand how trait anxiety might influence both behavioral and neural response variability.
In conclusion, the current study has contributed to our understanding of which brain regions are associated with IIV in adolescents with ADHD, thereby increasing the understanding of causal factors for the suggested IIV endophenotype of ADHD. We found that in adolescents with ADHD, hemodynamic activity in relation to sustained attention performance is more variable in neural networks known to be compromised in individuals diagnosed with ADHD, in particular, anterior parts of the DMN. This finding complements already existing evidence on variability in attention performance associated with ADHD. It also supports the notion that IIV is a possible cognitive endophenotype of ADHD by showing the variable attention processing not just to be evident in behavioral IIV, but also in a neurobiological IIV specific to ADHD. If anterior DMN variability represents a key abnormality related to this proposed endophenotype, one would expect it to be observed on other ongoing, repetitive performance-based cognitive tasks, such as Go/No-Go or Stop Signal Reaction Time tasks or continuous performance tasks. This can be readily tested in any number of already-existing ADHD fMRI datasets whose primary results already have been published. Interestingly, it is possible that greater HR variability could partially account for diminished ADHD responsiveness so ubiquitously found in previous ADHD fMRI studies. Indeed, such studies rely on GLM-measured fit between canonical models of the typical HR; deviations from such an ideal model would be reflected as diminished fit, i.e., lower “activation.” Therefore, it might be instructive to re-evaluate some of the key findings of frontostriatal and frontocerebellar dysfunction (Cortese et al., 2012) to see if hemodynamic amplitude and shape variability might be independent influences on ADHD brain dysfunction. In contrast to these generally informative findings, exploration of the influence of anxiety on HR variability yielded mixed results. We showed that the influence of bottom-up effects of self-reported symptoms of anxiety might relate to attention and arousal problems caused by an ADHD diagnosis in adolescents. Trait anxiety seemed to be empirically linked to variability in brain activation during attention. Thus, in re-analyzing originally published data, such as in the current study, the originally preprocessing of data may be of other standards than that typically applied nowadays. In our case that means the BOLD time series were originally preprocessed with the software of SPM2. This may affect the size of the smoothing kernels used, which is typically of smaller size today than 12 mm. The main risk of using the 12 mm smoothing kernel is that a small isolated brain region might be overlooked by high-order ICA and not be one of the components detected for further analysis. However, ICA identify voxels showing comparable (i.e., functionally connected) time courses, and as such issues involved with smoothing kernel size are not as relevant compared with doing inferential statistic testing under Random Field Theory (e.g., conventional SPM analyses). ICA typically identifies the same types and configuration of components across different datasets. In this way, we have kept the originally preprocessing of data so that the re-analyzed data can be compared with the originally published ones, at the same time as we have used a whole-brain analysis that makes the results comparable with results from data preprocessed in accordance with the standards of today.
5. Conclusions
We observed significant differences between adolescents with ADHD and healthy controls in level of IIV HRs in an anterior DMN that correlated with behavioral IIV. Additionally, our findings revealed that the adolescents with ADHD also had higher IIV HRs in a basal ganglia network. This effect of ADHD was thus related to other traits such as the age, IQ level, and social anxiety symptoms of the adolescents. These results suggests that assessment of trial-to trial HR variability in ADHD provides information beyond what is detectable through analyses of averaged behavioral and brain activation levels.
Acknowledgement
We thank the children and parents who participated in this study. This work was supported in part by NIMH grant K23MH070036 and R01MH080956 (principal investigator, Dr. Stevens), the Holton and Yanner Trusts, a grant from Hartford Hospital, and by a post-doc grant to Dr. Sørensen from the Western Norway Health Authority, Norway (911460).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.08.007.
Appendix A. Supplementary data
The following are the supplementary data related to this article.
Supplementary material.
Supplemental Figure.
a) Exploring the effect of social anxiety on the estimated marginal means of amplitude variability over trials for the two diagnostic groups from the univariate tests of variance. b) Scatterplot showing the relationship between amplitude variability over trials in IC 2 and the social anxiety subscores for each of the diagnostic groups.
References
- Abou-Elseoud A., Starck T., Remes J., Nikkinen J., Tervonen O., Kiviniemi V. The effect of model order selection in group PICA. Hum. Brain Mapp. 2010;31(8):1207–1216. doi: 10.1002/hbm.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F.…Calhoun V.D. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fourth ed. American Psychiatric Association; Washigton DC: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Association; Washington DC: 2013. Diagnostic and Statistical Manual of Mental Health Disorders. [Google Scholar]
- Antonini T.N., Narad M.E., Langberg J.M., Epstein J.N. Behavioral correlates of reaction time variability in children with and without ADHD. Neuropsychology. 2013;27(2):201–209. doi: 10.1037/a0032071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Harcourt Assessment; San Antonio, TX: 1996. Beck Depression Inventory-II (BDI-II) [Google Scholar]
- Bell A.J., Sejnowski T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bellgrove M.A., Hawi Z., Kirley A., Gill M., Robertson I.H. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43(13):1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Blackford J.U., Clauss J.A., Avery S.N., Cowan R.L., Benningfield M.M., VanDerKlok R.M. Amygdala-cingulate intrinsic connectivity is associated with degree of social inhibition. Biol. Psychol. 2014;99:15–25. doi: 10.1016/j.biopsycho.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd S.J., Demanuele C., Debener S., Helps S.K., James C.J., Sonuga-Barke E.J. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35(1):278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69(12):1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzy W.M., Medoff D.R., Schweitzer J.B. Intra-individual variability among children with ADHD on a working memory task: an ex-Gaussian approach. Child Neuropsychol. 2009;15(5):441–459. doi: 10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caouette J.D., Guyer A.E. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Dev. Cogn. Neurosci. 2014;8:65–76. doi: 10.1016/j.dcn.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Sonuga-Barke E.J., Scheres A., Di Martino A., Hyde C., Walters J.R. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol. Psychiatry. 2005;57(11):1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavira D.A., Stein M.B., Bailey K., Stein M.T. Comorbidity of generalized social anxiety disorder and depression in a pediatric primary care sample. J. Affect. Disord. 2004;80(2–3):163–171. doi: 10.1016/S0165-0327(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Cortese S., Azoulay R., Castellanos F.X., Chalard F., Lecendreux M., Chechin D.…Konofal E. Brain iron levels in attention-deficit/hyperactivity disorder: a pilot MRI study. World J. Biol. Psychiatry. 2012;13(3):223–231. doi: 10.3109/15622975.2011.570376. [DOI] [PubMed] [Google Scholar]
- Damsa C., Kosel M., Moussally J. Current status of brain imaging in anxiety disorders. Curr. Opin. Psychiatry. 2009;22(1):96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- Danielmeier C., Eichele T., Forstmann B.U., Tittgemeyer M., Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J. Neurosci. 2011;31(5):1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Osso L., Rucci P., Ducci F., Ciapparelli A., Vivarelli L., Carlini M.…Cassano G.B. Social anxiety spectrum. Eur. Arch. Psychiatry Clin. Neurosci. 2003;253(6):286–291. doi: 10.1007/s00406-003-0442-5. [DOI] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J. Child Psychol. Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Eichele T., Debener S., Calhoun V.D., Specht K., Engel A.K., Hugdahl K.…Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105(16):6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt E.B., Rachakonda S., Bedrick E.J., Allen E.A., Adali T., Calhoun V.D. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum. Brain Mapp. 2011;32(12):2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K.C., Simon N.M., Dougherty D.D., Hoge E.A., Worthington J.J., Chow C.…Rauch S.L. A PET study of tiagabine treatment implicates ventral medial prefrontal cortex in generalized social anxiety disorder. Neuropsychopharmacology. 2009;34(2):390–398. doi: 10.1038/npp.2008.69. [DOI] [PubMed] [Google Scholar]
- Eysenck M.W., Derakshan N., Santos R., Calvo M.G. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fassbender C., Zhang H., Buzy W.M., Cortes C.R., Mizuiri D., Beckett L., Schweitzer J.B. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A.P. second ed. Sage Publications; London; Thousand Oaks, Calif.: 2005. Discovering Statistics Using SPSS. [Google Scholar]
- Field A.P. third ed. SAGE Publications; Los Angeles: 2009. Discovering Statistics Using SPSS. [Google Scholar]
- Freire L., Mangin J.F. Motion correction algorithms may create spurious brain activations in the absence of subject motion. NeuroImage. 2001;14(3):709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L., Roche A., Mangin J.F. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans. Med. Imaging. 2002;21(5):470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Halperin J.M., Schulz K.P. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol. Bull. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Halperin J.M., Trampush J.W., Miller C.J., Marks D.J., Newcorn J.H. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J. Child Psychol. Psychiatry. 2008;49(9):958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A.D.B., Redlich F.C. John Wiley & Sons; New York: 1958. Social Class and Mental Illness: A Community Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas S.L., Geurts H.M., Konrad K., Bender S., Nigg J.T. Annual research review: reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J. Child Psychol. Psychiatry. 2014;55(6):685–710. doi: 10.1111/jcpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P.…Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kiehl K.A., Laurens K.R., Duty T.L., Forster B.B., Liddle P.F. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38(1):133–142. [PubMed] [Google Scholar]
- Kiehl K.A., Stevens M.C., Laurens K.R., Pearlson G., Calhoun V.D., Liddle P.F. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. NeuroImage. 2005;25(3):899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Kim D.I., Mathalon D.H., Ford J.M., Mannell M., Turner J.A., Brown G.G.…Calhoun V.D. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr. Bull. 2009;35(1):67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V., Starck T., Remes J., Long X., Nikkinen J., Haapea M.…Tervonen O. Functional segmentation of the brain cortex using high model order group PICA. Hum. Brain Mapp. 2009;30(12):3865–3886. doi: 10.1002/hbm.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Wendling K., Huettner P., Ruder H., Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol. Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kofler M.J., Rapport M.D., Sarver D.E., Raiker J.S., Orban S.A., Friedman L.M., Kolomeyer E.G. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin. Psychol. Rev. 2013;33(6):795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Kuntsi J., Klein C. Intraindividual variability in ADHD and its implications for research of causal links. Curr. Top. Behav. Neurosci. 2012;9:67–91. doi: 10.1007/7854_2011_145. [DOI] [PubMed] [Google Scholar]
- Kuntsi J., Oosterlaan J., Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? J. Child Psychol. Psychiatry. 2001;42(2):199–210. [PubMed] [Google Scholar]
- Lazzaro I., Anderson J., Gordon E., Clarke S., Leong J., Meares R. Single trial variability within the P300 (250–500 ms) processing window in adolescents with attention deficit hyperactivity disorder. Psychiatry Res. 1997;73(1–2):91–101. doi: 10.1016/s0165-1781(97)00107-8. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Gau S.S., Huang-Gu S.L., Shang C.Y., Wu Y.H., Tseng W.Y. Neural substrates of behavioral variability in attention deficit hyperactivity disorder: based on ex-Gaussian reaction time distribution and diffusion spectrum imaging tractography. Psychol. Med. 2014;44(8):1751–1764. doi: 10.1017/S0033291713001955. [DOI] [PubMed] [Google Scholar]
- March J.S., Parker J.D., Sullivan K., Stallings P., Conners C.K. The multidimensional anxiety scale for children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Mayes S.D., Calhoun S.L. WISC-IV and WISC-III profiles in children with ADHD. J. Atten. Disord. 2006;9(3):486–493. doi: 10.1177/1087054705283616. [DOI] [PubMed] [Google Scholar]
- Peterson A., Thome J., Frewen P., Lanius R.A. Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders? Can. J. Psychiatr. 2014;59(6):294–300. doi: 10.1177/070674371405900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen K.J., Allen E.A., Eichele H., van Wageningen H., Hovik M.F., Sorensen L.…Eichele T. Reduced error signalling in medication-naive children with ADHD: associations with behavioural variability and post-error adaptations. J. Psychiatry Neurosci. 2016;41(2):77–87. doi: 10.1503/jpn.140353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas C.M., Rees G., Howseman A.M., Josephs O., Turner R., Frith C.D. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J. Neurosci. 1998;18(21):8979–8989. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Taylor E. Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol. Psychiatry. 2007;62(9):999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Sagvolden T., Johansen E.B., Aase H., Russell V.A. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. discussion 419-368. [DOI] [PubMed] [Google Scholar]
- Sergeant J.A. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol. Psychiatry. 2005;57(11):1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Fotedar S.G., Suskauer S.J., Pekar J.J., Denckla M.B., Mostofsky S.H. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45(9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Smith Y., Raju D., Nanda B., Pare J.F., Galvan A., Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res. Bull. 2009;78(2–3):60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E.J., Castellanos F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31(7):977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Spencer S.V., Hawk L.W., Jr., Richards J.B., Shiels K., Pelham W.E., Jr., Waxmonsky J.G. Stimulant treatment reduces lapses in attention among children with ADHD: the effects of methylphenidate on intra-individual response time distributions. J. Abnorm. Child Psychol. 2009;37(6):805–816. doi: 10.1007/s10802-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Kiehl K.A. An fMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. Am. J. Psychiatry. 2007;164(11):1737–1749. doi: 10.1176/appi.ajp.2007.06050876. [DOI] [PubMed] [Google Scholar]
- Suskauer S.J., Simmonds D.J., Caffo B.S., Denckla M.B., Pekar J.J., Mostofsky S.H. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(10):1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick B.G., Fidell L.S. fifth ed. Pearson/Allyn & Bacon; Boston: 2007. Using Multivariate Statistics. [Google Scholar]
- Tam A., Luedke A.C., Walsh J.J., Fernandez-Ruiz J., Garcia A. Effects of reaction time variability and age on brain activity during Stroop task performance. Brain Imaging Behav. 2014 doi: 10.1007/s11682-014-9323-y. [DOI] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A.L. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am. J. Psychiatry. 2006;163(6):1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- Tamm L., Narad M.E., Antonini T.N., O'Brien K.M., Hawk L.W., Jr., Epstein J.N. Reaction time variability in ADHD: a review. Neurotherapeutics. 2012;9(3):500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maanen L., Brown S.D., Eichele T., Wagenmakers E.J., Ho T., Serences J., Forstmann B.U. Neural correlates of trial-to-trial fluctuations in response caution. J. Neurosci. 2011;31(48):17488–17495. doi: 10.1523/JNEUROSCI.2924-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio R.G., Simmonds D.J., Mostofsky S.H. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47(12):2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, Tex: 1999. Wechsler Abbreviated Scale of Intelligence. WASI. Manual. [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wilkinson G.S. Wide Range; Wilmington, DE: 1993. Wide Range Achievement Test. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.