Abstract
Psychiatric disorders continue to be among the most challenging disorders to diagnose and treat because there is no single genetic or anatomical locus that is causative for the disease. Current treatments are often blunt tools used to ameliorate the most severe symptoms, at the risk of disrupting functional neural systems. There is a critical need to develop new therapeutic strategies that can target circumscribed functional or anatomical domains of pathology. Adult hippocampal neurogenesis may be one such domain. Here, we review the evidence suggesting that adult hippocampal neurogenesis plays a role in emotional regulation and forms of learning and memory that include temporal and spatial memory encoding and context discrimination, and that its dysregulation is associated with psychiatric disorders, such as affective disorders, schizophrenia, and drug addiction. Further, adult neurogenesis has proven to be an effective model to investigate basic processes of neuronal development and converging evidence suggests that aberrant neural development may be an etiological factor, even in late-onset diseases. Constitutive neurogenesis in the hippocampus of the mature brain reflects large-scale plasticity unique to this region and could be a potential hub for modulation of a subset of cognitive and affective behaviors that are affected by multiple psychiatric disorders.
The dysregulation of adult hippocampal neurogenesis is associated with depression, schizophrenia, and addiction. Adult neurogenesis may therefore serve as a therapeutic target and as a model system for drug development.
Adult neurogenesis is the process of continuously generating new neurons that can functionally integrate into the adult mammalian brain throughout life and a unique form of structural and functional plasticity in the hippocampus, a brain region key to learning, memory and mood regulation (Ming and Song 2011). The subgranular zone (SGZ) of the dentate gyrus in the hippocampus is one of two discrete “neurogenic” regions in the adult mammalian brain that was initially identified using tritiated thymidine labeling of proliferating cells in rodents (Altman and Das 1965; Kempermann and Gage 1999). Importantly, adult neurogenesis is also present in the human hippocampus, illustrating the intrinsic capacity of the human central nervous system to support newborn neurons and the possibility of exploiting this phenomenon for repair of dysregulated neural systems or recovery from injury (Eriksson et al. 1998; Sahay and Hen 2007; Kernie and Parent 2010). Adult neurogenesis recapitulates the developmental process of embryonic neurogenesis, from proliferation and fate specification of neural progenitors, to differentiation, migration, axonal and dendritic development, and, finally, synaptic integration of newborn neurons (Ming and Song 2005; Braun and Jessberger 2013). This unique form of neural development has attracted much interest not only as a model system to investigate brain development, but also to understand hippocampal functions because of the role of newborn granule neurons in several cognitive functions, including spatial learning and retention, memory retrieval, forgetting, and clearance of memory traces (Kitabatake et al. 2007; Deng et al. 2010; Christian et al. 2014). Impaired adult neurogenesis, on the other hand, has been associated with cognitive impairments that are often present in patients with psychiatric disorders such as major depression, schizophrenia, anxiety disorders, and addictive behaviors (Christian et al. 2014).
Psychiatric disorders account for an estimated 13% of the global disease burden and are among the most challenging diseases to treat. These disorders typically manifest with a diverse array of symptoms, have complex genetic risk associations, and poorly understood etiology. Currently, most available treatments are designed to ameliorate symptoms, rather than address the underlying pathology. Moving forward, it is imperative that we develop rational therapeutics based on a better understanding of the etiology and pathogenesis of the diseases. Different from neurodegenerative diseases that primarily affect specific cell types in the nervous system, psychiatric disorders affect widely distributed neural systems and cellular subtypes. However, cumulative evidence strongly suggests that structural and functional abnormalities in the hippocampus are associated with many mental disorders, indicating that this circumscribed region may be an area that is highly vulnerable to pathology relevant to these disorders and a specific target for new therapeutics.
Does impaired adult hippocampus neurogenesis directly contribute to the pathogenesis of psychiatric disorders? Extensive studies have now shown correlative changes in adult hippocampal neurogenesis under various pathophysiological conditions, such as aging, epilepsy, stroke, neurodegenerative disorders, and psychiatric disorders (Parent 2003; Sahay and Hen 2007; Kempermann et al. 2008; Winner et al. 2011). Whether these changes represent adaptive responses to various pathophysiological conditions, part of the pathophysiology that contributes to the disease, or both, remains a key question for the field. Interestingly, for most psychiatric disorders, such as major depression, schizophrenia, anxiety disorders, and addiction, a decrease in cell proliferation within the dentate gyrus and reduced hippocampal volume have been reported, which are associated with impaired hippocampus-dependent functions, including working memory, context-dependent memory and recognition memory, and spatial pattern separation (Bremner 1999; Lie et al. 2004; Mirescu and Gould 2006; Reif et al. 2007; Revest et al. 2009; Morris et al. 2010; Nixon et al. 2010). For example, a significant subpopulation of patients with major depression has consistently shown reduced hippocampal volume and cognitive deficits (Sheline et al. 1996; Bremner 1999; Campbell and Macqueen 2004; Savitz and Drevets 2009; Kempton et al. 2011). Volumetric magnetic resonance imaging (MRI) studies have further shown that the degree of hippocampal volume reduction correlates with total duration of major depression (Kempton et al. 2011). The diagnosis of posttraumatic stress disorder (PTSD) has also been associated with hippocampal volume reduction and deficits in declarative memory function (Bremner 1999, 2006; Villarreal and King 2001). In addition, a number of studies have indicated that the hippocampus is smaller in juvenile and adult patients with schizophrenia (Pfefferbaum and Marsh 1995; Csernansky et al. 1998; Nelson et al. 1998; Wright et al. 2000; Heckers 2001; Mattai et al. 2011). Hippocampal volume reduction represents a loss of gray matter, which could lead to memory deficits and impaired cognitive functions (Goldman and Mitchell 2004). Intriguingly, these structural and functional changes of the hippocampus in patients with psychiatric disorders can be attenuated or even reversed by treating patients with antidepressants, antipsychotics, or increasing physical exercise, many of which are known to have a profound impact on hippocampal neurogenesis (Sala et al. 2004; Dhikav and Anand 2007; Kempermann et al. 2010; Erickson et al. 2014).
These studies and others raise the possibility that dysfunction of adult neurogenesis itself may play a causal role in psychiatric symptomatology and that adult neurogenesis could serve as a novel therapeutic target, particularly in light of provocative findings related to neurogenesis-mediated effects of antidepressants as described below. Thus, understanding endogenous mechanisms that regulate adult neurogenesis may give us critical insight into effector systems of successful treatments for psychiatric disorders and facilitate the development of more specific therapeutic strategies. Here, we review roles of adult hippocampal neurogenesis in three major psychiatric disorders, major depression, schizophrenia, and drug addiction, and discuss adult hippocampal neurogenesis as both a therapeutic target and a model system for drug development.
DEPRESSION
Major depression, or major depressive disorder, is a psychiatric disorder characterized by episodes of all-encompassing low mood accompanied by low self-esteem, decreased ability to concentrate, loss of interest or pleasure in normally enjoyable activities and recurrent thoughts of suicide. The “adult neurogenesis hypothesis” of major depression was the first proposed link between adult neurogenesis and a psychiatric disorder (Jacobs et al. 2000). Jacobs and colleagues proposed that a stress-induced reduction in hippocampal neurogenesis is an important causal factor contributing to episodes of depression. Since the original proposal, numerous studies have either argued in favor of or against the hypothesis, which has evolved to postulate that decreased generation of new neurons in the hippocampus contributes to the pathogenesis of depression, and enhancing adult hippocampal neurogenesis is necessary for successful antidepressant treatments (Duman 2004; Sahay and Hen 2007). This hypothesis is supported by the finding that antidepressants stimulate production of newborn neurons in the adult brain, which can partially repair structural abnormalities in the hippocampus of depressed patients (Sheline et al. 2003; Bremner and Vermetten 2004; Perera et al. 2008). Similarly, promoting adult hippocampal neurogenesis is sufficient to mimic antidepressant action (Santarelli et al. 2003; Mirescu and Gould 2006; Reif et al. 2007; Sahay and Hen 2007). Still, some findings do not fit into this idea, including the fact that ablation of neurogenesis by factors other than stress, such as irradiation, fails to lead a “depressive-like” state in animals (Airan et al. 2007) and that both neurogenesis-dependent and neurogenesis-independent antidepressant actions have been observed (Sahay and Hen 2007; David et al. 2009). Although there is no clear functional theory that explains how newborn neurons in the adult hippocampus contribute to the induction or recurrence of depression, antidepressant-induced increases in adult neurogenesis has nevertheless been consistently shown across species, including primates and humans, by multiple laboratories and for multiple treatments, and has been shown to affect local circuit activity in the dentate gyrus (Airan et al. 2007). In this section, three main lines of evidence linking adult hippocampal neurogenesis and depression will be discussed, including postmortem and structural imaging analyses of patients, roles of adult hippocampal neurogenesis in cognitive and affective functions, and interaction of antidepressant treatments with adult neurogenesis.
Clinical Observations of Patients with Major Depression
The observation that patients with chronic depression have smaller hippocampi, as compared with an age- and sex-matched group of healthy individuals, was the first evidence suggesting a correlation between depression and abnormal hippocampal structure (Sheline et al. 1996). Meta-analyses from numerous MRI studies have since confirmed this observation (Videbech and Ravnkilde 2004; Geuze et al. 2005; McKinnon et al. 2009). This same correlation holds true even for late-onset depression (Sawyer et al. 2012; Sexton et al. 2012). Postmortem observations have also confirmed similar reductions in hippocampal volume (Rajkowska et al. 1999), but it is not yet known whether a decrease in adult neurogenesis is a contributing factor and to what extent. Using proliferation markers, such as Ki-67 or MCM2, a few postmortem studies have reported lower levels of cell proliferation in the hippocampus of patients with major depression compared with healthy controls, but the reduction in hippocampal volume could confound this interpretation in the absence of stereological analyses and results have been contradictory (Reif et al. 2006; Boldrini et al. 2009; Lucassen et al. 2010). The Lucassen group showed a reduction of MCM2+ cells and the Boldrini group also observed a decrease of Ki-67+ cells, whereas Reif group observed no change in Ki-67+ cells in the adult hippocampus of patients with depression. But cell proliferation is not the only parameter that could affect adult neurogenesis. Changes in the number and properties of neural stem cells or the survival rate of new neurons would also affect the net outcome of adult hippocampal neurogenesis. Further studies are needed to clarify the connection between the level of adult neurogenesis and hippocampal volume change.
Role of Adult Hippocampal Neurogenesis in Cognitive and Affective Functions
Since the discovery that the dentate gyrus is one of two regions of the adult mammalian brain to generate new neurons constitutively and robustly, this region has been the focus of many investigations to understand functional consequences of this phenomenon. Recent studies have focused on behaviors associated with the dentate gyrus, with specific emphasis on functions ascribed to distinct anatomical regions. Based on anatomical studies of efferent connectivity and behavioral reports, the hippocampus is often divided into two functional domains that span the longitudinal axis. Dorsal hippocampus is thought to contribute to spatial memory formation and the associative encoding of discrete stimuli. Behavioral analyses using different approaches for ablation of adult hippocampal neurogenesis have suggested a connection between neurogenesis and cognitive functions, such as spatial and temporal encoding and contextual discrimination (Aimone et al. 2014). Ventral hippocampus has been associated with modulation of affective behaviors and emotionally salient memories (Kheirbek and Hen 2011; Kheirbek et al. 2013; Tannenholz et al. 2014; Wu and Hen 2014). The relationship between adult hippocampal neurogenesis and mood regulation is complex. After the initial study reporting that adult hippocampal neurogenesis is required for the effectiveness of antidepressants fluoxetine and imipramine in certain depressive-like and anxiety-like behaviors in certain mouse species (Santarelli et al. 2003), subsequent studies have shown variable degrees of dependence on adult neurogenesis for established antidepressant treatment efficacy (Sahay and Hen 2007). Enhancement of adult hippocampal neurogenesis via exercise, pharmacological, or genetic manipulations elicits anxiolytic and antidepressant effects on behaviors (Jang et al. 2013b; Wu and Hen 2014; Hill et al. 2015), suggesting the sufficiency for an increase in adult hippocampal neurogenesis to reduce depressive-like behavior. Interestingly, several lines of evidence also suggest a role of adult hippocampal neurogenesis in stress adaptation via regulation of the hypothalamic–pituitary–adrenal (HPA) axis (Schloesser et al. 2009; Snyder et al. 2011; Surget et al. 2011) and this function might affect depressive- or anxiety-like behavior in an indirect manner. On the other hand, a recent study by Hill and colleagues (2015) reports that increasing adult hippocampal neurogenesis produces antidepressant-like behaviors independent of HPA axis. It is also important to consider the hippocampus as both an integrated structure, as well as comprised of different functional domains, and that the dorsal hippocampus can also be indirectly involved in the regulation of emotion, as supported by the negative correlation between performance on a pattern separation task and the Depression Anxiety Stress Scale (Shelton and Kirwan 2013). Further studies are warranted to elucidate the functional domains of dorsal versus ventral hippocampus, their interaction, and how adult hippocampal neurogenesis contributes to the totality of hippocampal functions.
It is still challenging to even measure the extent of adult neurogenesis in humans, much less to study its functional role in human cognition and emotion. Nonetheless, there are some links to suggest the relationship between human neurogenesis and cognition and emotion. Patients with major depression show various hippocampal-dependent core symptoms related to cognitive and affective functions, including impaired working and declarative memory and learning deficits (Hasler et al. 2004). There are also negative correlations of pathological conditions with both cognitive function and levels of neurogenesis (Raber et al. 2004; Rola et al. 2004; Lupien et al. 2007; Bishop et al. 2010). Age-related declines in adult human hippocampal neurogenesis do not appear as robust as in rodents, but the correlation between relative rates of decline and cognitive impairment have yet to be determined. A confirmation of direct or indirect links between human adult hippocampal neurogenesis and pathoetiology will require the development of novel imaging technologies allowing tracing of neurogenesis in patients.
Role of Adult Neurogenesis in Mediating Antidepressant Action
Correlations between the efficacy of antidepressant treatments and the extent of adult neurogenesis have been a driving force to pursue the “neurogenesis model” of depression (Jacobs et al. 2000). The hypothesis that adult hippocampal neurogenesis plays a critical role in affective behavior and responsiveness to exogenous antidepressants gained early support from studies in which dividing cells were targeted for ablation. In a pioneering study, Rene Hen and colleagues reported that inhibition of hippocampal neurogenesis by X-irradiation resulted in the striking finding that the effects of fluoxetine on anxiety-like behavior were abolished (Santarelli et al. 2003). Given these provocative results, many studies attempted to replicate these findings, and the cumulative evidence now suggests that this effect depends on the strain of mice, as well as the type of antidepressant (Surget et al. 2008; David et al. 2009, 2010). For example, behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the 5-HT1A receptor, suggesting that antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), can lead to antidepressant-like effects via distinct mechanisms in different mouse strains (Holick et al. 2008). Genetic manipulations of pathways that regulate neurogenesis have also been shown to interact with antidepressant treatment. For example, conditional deletion of receptor tropomyosin-related kinase B (TrkB), the cognate receptor for brain-derived neurotrophic factor (BDNF), results in decreased adult hippocampal neurogenesis, increased anxiety-like behavior, and abolishes the action of antidepressants (Bergami et al. 2009; Revest et al. 2009). Although adult neurogenesis appears to be necessary for the beneficial effects of some antidepressants, suppression of neurogenesis alone does not result in a depressive phenotype, suggesting that a reduction in newborn neurons is not sufficient to cause depression (Airan et al. 2007; David et al. 2009). Conversely, physical exercise can increase performance in many cognitive tasks and may also restore some degree of hippocampal function in psychiatric patients or in healthy but aging individuals by promoting adult neurogenesis (van Praag et al. 1999a,b, 2005; Creer et al. 2010; Lugert et al. 2010).
Effects of Antidepressant Treatment on Adult Neurogenesis
In parallel, one of the most intriguing insights into the role of adult hippocampal neurogenesis in depression comes from the observation that many antidepressant treatments lead to concomitant beneficial effects on behavior and adult hippocampal neurogenesis. A large number of studies have found that antidepressants dramatically enhance adult hippocampal neurogenesis in species ranging from rodents to nonhuman primates to humans (Fig. 1) (Malberg et al. 2000; Malberg and Duman 2003; Santarelli et al. 2003; Encinas et al. 2006; Perera et al. 2007; Boldrini et al. 2009; Pinnock et al. 2009). For example, SSRI or tricyclic antidepressant (TCA)-treated patient groups with major depression had more NESTIN+ neural progenitor cells and Ki-67+ dividing cells compared with untreated patients or control groups. Surprisingly, the untreated patient group had 50% fewer dividing cells than controls (Boldrini et al. 2009). Although these results are consistent with studies in animal models showing a correlation between levels of neurogenesis and affective behavior, they are based on a relatively small sample size. Replications with larger samples are warranted for further conclusion. Table 1 summarizes the studies of antidepressant treatments in animal models in the context of adult hippocampal neurogenesis and behavior. The most widely used antidepressants are SSRIs, which increase the action of serotonin at the synapse (Cipriani et al. 2005; Deshauer et al. 2008). Fluoxetine, an SSRI, enhances adult hippocampal neurogenesis (Malberg and Duman 2003; Encinas et al. 2006; Pinnock et al. 2009; Jang et al. 2013b). Classical TCAs, such as desipramine and imipramine, also up-regulate adult hippocampal neurogenesis (Malberg et al. 2000; Santarelli et al. 2003). Unlike most antidepressants, tianeptine is an atypical antidepressant that is a selective serotonin reuptake enhancer but also regulates AMPA and NMDA receptor activity. Tianeptine treatment ameliorates depressive-like behavioral effects following chronic stress in tree shrews and increases adult neurogenesis (Czeh et al. 2001; Fuchs et al. 2002).
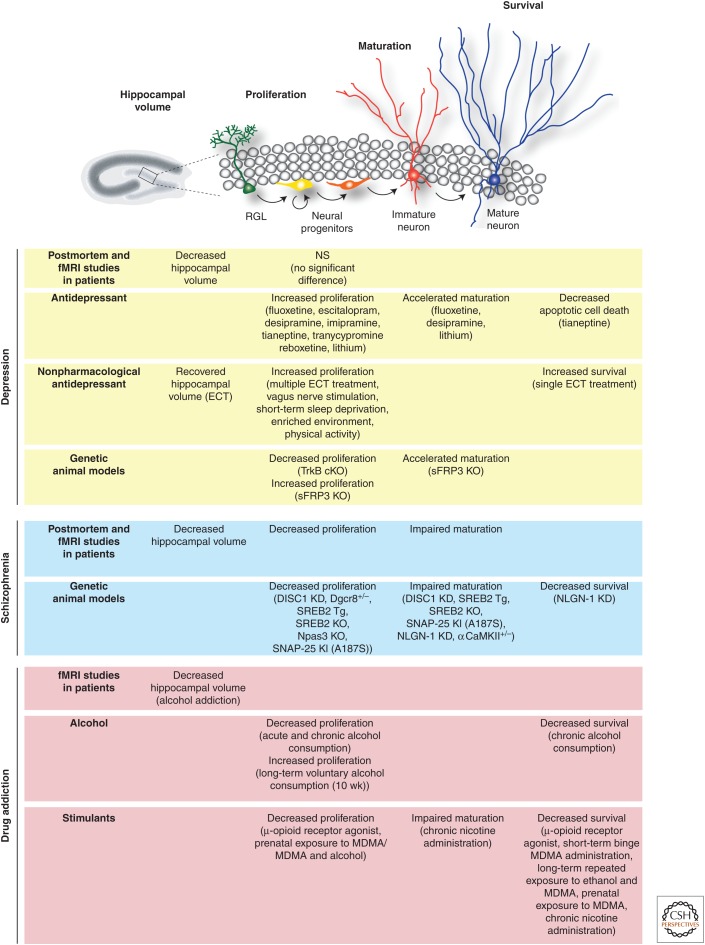
Figure 1.
Adult hippocampal neurogenesis and psychiatric disorders. Shown at the top is a schematic illustration of stages showing specific stages of adult hippocampal neurogenesis. Shown at the bottom is a summary of effect of three different classes of psychiatric disorders on hippocampal volume and adult hippocampal neurogenesis, including depression, schizophrenia or drug addiction. Note that multiple developmental stages during adult neurogenesis are shown to be affected. RGL, Radial glia-like cells; ECT, electroconvulsive therapy; KO, knockout; KD, knockdown; MDMA, 3,4-methylenedioxymethamphetamine.
Table 1.
Studies of antidepressant treatments in animal models
| Antidepressant treatment | Classification | Animal model and experimental approach | Effects on adult hippocampal neurogenesis | Effects on behavior | References |
|---|---|---|---|---|---|
| Fluoxetine | Serotonin-selective reuptake inhibitor | BrdU labeling in adult rat hippocampus, learned helplessness test | Increased cell proliferation and neuronal maturation | Decreased learned helplessness behavior induced by inescapable shock | Malberg et al. 2000; Malberg and Duman 2003 |
| Fluoxetine | Serotonin-selective reuptake inhibitor | BrdU labeling in 129/Sv mouse brain, NSF test | Increased cell proliferation and neuronal maturation | A decreased latency to feed in NSF test | Santarelli et al. 2003 |
| Fluoxetine | Serotonin-selective reuptake inhibitor | BrdU labeling in nestin-CFPnuc mouse | Increased proliferation of progenitors, but not quiescent neural progenitors | NS | Encinas et al. 2006 |
| Escitalopram | Serotonin-selective reuptake inhibitor | BrdU labeling in adult rat hippocampus | Increased cell proliferation with escitalopram treatment under mild stress condition | Recovery from anhedonic-like behavior induced by the mild stress | Jayatissa et al. 2006 |
| Desipramine | Tricyclic antidepressant | Adult rat or 129/Sv mouse brain, learned helplessness test, NSF test | Increased cell proliferation and neuronal maturation | Decreased learned helplessness behavior, a decreased latency to feed in NSF test | Santarelli et al. 2003; Chen et al. 2006 |
| Imipramine | Tricyclic antidepressant | BrdU labeling in adult rat or 129/Sv mouse brain, NSF test | Increased cell proliferation | A decreased latency to feed in NSF test | Santarelli et al. 2003; Kitamura et al. 2011 |
| Tianeptine | Tricyclic antidepressant; selective serotonin reuptake enhancer | BrdU labeling in stressed tree shrews dentate gyrus | Increased cell proliferation | NS | Czeh et al. 2001; Fuchs 2002 |
| Tranylcypromine | Monomine oxidase inhibitor | BrdU labeling in adult rat hippocampus | Increased cell proliferation | NS | Malberg et al. 2000 |
| Reboxetine | Nerepinephrine-selective reuptake inhibitor | BrdU labeling in adult rat hippocampus | Increased cell proliferation | NS | Malberg et al. 2000 |
| Lithium | Mood stabilizer | BruU labeling in adult mouse hippocampus | Increased proliferation, neuronal maturation and glial maturation | Decreased depressive like behavior | Chen et al. 2000 |
BrdU, Bromodeoxyuridine; NSF, novelty-suppressed feeding; NS, not studied.
Neurogenesis-induced structural and functional changes in the hippocampus could arise from increased proliferation and/or increased survival of newborn cells. The effects of antidepressants on specific stages of neurogenesis have been investigated in detail and revealed several potential mechanisms of action (Fig. 1) (David et al. 2010). For example, fluoxetine increases the rate of symmetric divisions of amplifying neural progenitors (Encinas et al. 2006). In addition, it accelerates maturation of immature neurons after chronic treatment, as shown by enhanced dendritic arborization and complexity (David et al. 2010). On the other hand, tianeptine decreases apoptotic cell death in the dentate gyrus (Lucassen et al. 2004).
Strikingly, nonpharmacological antidepressant treatments have also been shown to up-regulate adult hippocampal neurogenesis. Electroconvulsive therapy (ECT), vagus nerve stimulation, short-term sleep deprivation, environmental enrichment, and physical activity represent considerably different modes of therapy for major depression, all of which have been shown to enhance adult hippocampal neurogenesis and ameliorate depressive-like behavior (Madsen et al. 2000; Hellsten et al. 2002; Segi-Nishida et al. 2008; Ma et al. 2009). For example, a single ECT treatment in rodent models, similar to what is used clinically for the treatment of major depression and schizophrenia, significantly increases the survival rate among newborn neurons for up to 2 months, whereas multiple treatments also increase proliferation of neural progenitors in the adult dentate gyrus (Madsen et al. 2000; Malberg et al. 2000; Wang et al. 2000; Segi-Nishida et al. 2008). Recent studies have identified secreted frizzled-related protein 3 (sFRP3), a Wnt signaling inhibitor that regulates activity-induced adult neural progenitor proliferation and differentiation, as a molecular mediator of multiple antidepressant treatments in rodent models and may correlate to response time to antidepressants in depression patients (Jang et al. 2013a,b). Collectively, these studies suggest that reversal of stress or depression-induced structural and cellular changes in the hippocampus is one mechanism for the therapeutic effect of antidepressant treatments.
Animal Models of Depression
Many animal models rely on different forms of stress to induce depressive-like behavior. Chronic or acute stress episodes have been identified as an etiological factor and trigger for the onset of anxiety and affective disorders in patients and shown to negatively regulate adult hippocampal neurogenesis in rodent models (Gould et al. 1997; Santarelli et al. 2003; Surget et al. 2008). Moreover, physical stressors such as inescapable foot shock (Malberg and Duman 2003) and repeated restraint (Pham et al. 2003) have been used to induce depressive-like behaviors in animal models and also have a negative impact on adult neurogenesis (Fig. 1). For example, chronic psychosocial stress via a social defeat protocol reduces cell proliferation and survival of newborn granule neurons (Czeh et al. 2002).
Although it is estimated that major depression is moderately inheritable (40%–50%), the development of genetic animal models for major depression has been limited (Levinson 2006). Increased levels of corticosterone, a glucocorticoid hormone secreted by the adrenal gland in response to stress, have been consistently observed in patients with major depression and in animals subjected to chronic stress. Adult neurogenesis in the dentate gyrus is known to be sensitive to glucocorticoid levels (Gould and Tanapat 1999; Mirescu and Gould 2006; Mirescu et al. 2006). In heterozygous knockout mice for the glucocorticoid receptor, adult neurogenesis was inhibited (Kronenberg et al. 2009). In addition, mice treated chronically with corticosterone show anxiety-related and depressive-like behaviors (David et al. 2009). Genetic mutations of serotonin receptor 1A (5-HT1A) in humans that correlate with lowered expression levels of 5-HT1A are risk factors for depression and also reduced responsiveness to antidepressants (Le Francois et al. 2008). Comparatively, in 5-HT1A knockout mice, the effect of fluoxetine on behavior, as well as neurogenesis, was abolished (Radley and Jacobs 2002). Thus far, many animal models appear to share many genetic and environmental risk factors for anxiety or depression-like behaviors, and the potential for mechanistic overlap.
Converging evidence that includes neuroanatomical and neurochemical abnormalities in the hippocampus of patients with depression, behavioral studies implicating the hippocampus in cognitive and affective behavior, dependence of antidepressant efficacy on neurogenesis in some cases, and modulation of adult neurogenesis by distinct classes of clinically effective antidepressants, supports the adult neurogenesis hypothesis of depression, at least in part. However, it remains to be determined whether impaired adult hippocampal neurogenesis is a causal factor underlying the relevant pathology in depression. In addition, considerable work is still required to understand how newborn neurons could mediate the action of antidepressants at the molecular, cellular, or system levels.
SCHIZOPHRENIA
Schizophrenia is a complex genetic disorder that presents with variable cognitive deficits and affective symptoms. Patients typically show a subset of positive symptoms, which include delusions, hallucinations, disorganized speech and behavior, and/or negative symptoms, which include an inability to express emotion, cognitive deficits, memory impairments, and a lack of motivation, interest, and empathy.
Neurodevelopmental Model of Schizophrenia
The neurodevelopmental model of schizophrenia holds that schizophrenia is the behavioral outcome of neural dysregulation that begins long before the onset of clinical symptoms (Weinberger 1987; Cardno et al. 1999; Singh et al. 2004). This hypothesis has been supported by diverse lines of research. First, structural changes have been documented in the brains of children with prodromal symptoms (Rapoport et al. 2012), many of which parallel neuroanatomical studies of schizophrenic patients that have shown structural abnormalities including enlarged lateral and third ventricles and decreased volumes of gray and white matter (Lawrie and Abukmeil 1998; Wright et al. 2000). These decreases occur predominantly in the hippocampus (Nelson et al. 1998), thalamus (Konick and Friedman 2001), and frontal lobes (Davidson and Heinrichs 2003). Second, microarray gene expression studies have linked alterations in gene expression in postmortem patient tissue to presynaptic function, signaling, myelination, and migration, many of which are integral stages in neural development. However, it should be noted that most of these results are from studies with relatively small sample sizes and were not controlled for pharmacological treatment history (Kumarasinghe et al. 2012). The strongest evidence to date supporting the neurodevelopmental hypothesis comes from recent linkage and genome-wide analysis studies that have identified a large number of susceptibility genes for schizophrenia that are known to function in neural development (Rapoport et al. 2012).
The idea that abnormal adult hippocampal neurogenesis might be involved in the symptomatology or pathogenesis of schizophrenia is based on postmortem and functional imaging studies, as well as clinical features of schizophrenia. Postmortem samples from patients with schizophrenia show decreased expression of the cell proliferation marker, Ki-67 in the hippocampus (Fig. 1) (Reif et al. 2006). Correlated with this observation, reduced hippocampal volume, using meta-analysis MRI, has been reported in patients (Steen et al. 2006). In addition to changes to cell proliferation, impaired maturation of adult-born dentate granule cells in patients with schizophrenia has also been reported (Walton et al. 2012). Interestingly, several studies show a correlation between amelioration of the behavioral and cognitive symptoms of schizophrenia and normalization of hippocampal volume, thus linking the anatomical and cognitive behavioral manifestations of this disorder to the hippocampus (Sapolsky 2000; Sala et al. 2004; Dhikav and Anand 2007). Impaired adult hippocampal neurogenesis, as a distinct form of dysregulated neurodevelopment, might contribute to the structural changes and hippocampal-dependent affective and cognitive symptoms.
Animal Models of Schizophrenia and Adult Neurogenesis
Modeling schizophrenia in animals is an approximation of specific facets of the disorder, including behavioral abnormalities that resemble a subset of symptoms or neural mechanisms that may contribute to the etiology. Although there have been several studies demonstrating an association between a particular genetic manipulation and behavioral effects that appear to be related to symptoms of schizophrenia, a one-to-one relationship between a single gene and a specific behavior is unlikely. It is largely unknown whether the risk genes identified in genome-wide association studies interact or converge on common signaling pathways. There is, however, an increased appreciation for the idea that deficits in shared signaling cascades may contribute to comorbid endophenotypes. Exploring the functional role of schizophrenia risk genes in neurodevelopment and adult neurogenesis and identifying the underlying molecular mechanisms has therefore been an important approach to understand the etiology of psychiatric disorders. Table 2 summarizes the studies of schizophrenia susceptibility genes in animal models in the context of adult hippocampal neurogenesis and behavior.
Table 2.
Studies of schizophrenia susceptibility genes in animal models
| Gene | Animal model and experimental approach | Effects on adult hippocampal neurogenesis | Effects on behavior | References | Genetic study references |
|---|---|---|---|---|---|
| DISC1 | Retrovirus-mediated knockdown of DISC1 in newborn neurons | Enhanced dendritic outgrowth, soma hypertrophy, mispositioning of cell body, accelerated excitability, and synaptogenesis in hippocampal newborn neurons | NS | Duan et al. 2007 | Chubb et al. 2008 |
| DISC1 | Retrovirus-mediated knockdown of DISC1 in newborn neurons | Mistargeting of axonal mossy fibers, failure of maturation of presynaptic output | NS | Faulkner et al. 2008 | Chubb et al. 2008 |
| DISC1 | Lentivirus-mediated knockdown of DISC1 | Decreased cell proliferation | Hyperlocomotion in a novel environment, depressive-like behavior (forced swim test) | Mao et al. 2009 | Chubb et al. 2008 |
| DISC1 | Retrovirus-mediated knockdown of DISC1 in newborn neurons | NS | Cognitive (object place recognition test, Morris water maze test), and affective deficits (elevated place maze, forced swim test) rescued by rapamycin treatment | Zhou et al. 2013 | Chubb et al. 2008 |
| Dgcr8 (22q11.2 deletion) | Dgcr8+/−mice | Reduced cell proliferation and neurogenesis, reduced neural progenitor markers decrease, DCX decrease, rescued by insulin-like growth factor (IGF)-2 | Defects in spatial working memory (Y-maze test, Morris water maze test) and depressive-like behavior (forced swim test) rescued by IGF-2 | Stark et al. 2008; Ouchi et al. 2013 | Pulver et al. 1994; Murphy et al. 1999 |
| SREB2/GBR85 | SREB2 Tg, SREB2KO | Negatively regulates hippocampal adult neurogenesis and dendritic growth | Deficits in spatial learning and memory (spatial pattern separation test, delayed in spontaneous alteration, Y-maze) | Chen et al. 2012 | Matsumoto et al. 2008 |
| FEZ1 | Retrovirus-mediated KD of FEZ1 in hippocampus/FEZ1KO | Enhanced dendritic outgrowth, soma hypertrophy | Hyperactivity, enhanced behavioral responses to the psychostimulants MK-801 | Sakae et al. 2008; Kang et al. 2011 | Yamada et al. 2004 |
| NPAS3 | Npas3 KO | Reduce cell proliferation in Npas3KO | Hyperactivity, subtle gait deficits, impaired PPI, defects in cognitive memory, altered anxiety-like behavior | Brunskill et al. 2005; Pieper et al. 2005 | Kamnasaran et al. 2003; Pickard et al. 2005, 2009; Huang et al. 2010 |
| SNAP-25 | SNAP-25 KI (Ala 187Ser) | Decreased neurogenesis immaturity of dentate granule cells | Working memory deficit (spontaneous forced alteration task, T-maze) | Ohira et al. 2013 | Lewis et al. 2003 |
| αCaMKII | αCaMKII+/− | Increased DG, but decreased number of mature neurons in DG, immaturity in morphological and physiological features of DG neurons | Severe working memory deficit and an exaggerated infradian rhythm | Yamasaki et al. 2008 | Xing et al. 2002 |
NS, Not studied; KO, knockout; KD, knockdown; DG, dentate gyrus.
Disrupted-in-schizophrenia 1 (DISC1) is one of the most extensively studied susceptibility genes for schizophrenia and has proven to be a useful model for how to investigate complex genetic disorders starting with a single known risk factor. DISC1 was originally identified in a large Scottish pedigree in which a translocation in this locus cosegregates with mental disorders such as schizophrenia and major depression (Millar et al. 2000; Blackwood et al. 2001; Taylor et al. 2003). DISC1 is highly expressed in the hippocampus, both during development and in adulthood, suggesting it has a functional role in both neurodevelopment and adult neurogenesis (Austin et al. 2004; Schurov et al. 2004). Behavioral assays of several genetic DISC1 mouse models, such as single nucleotide mutations or overexpression of truncated DISC1 resulting in altered DISC1 expression, revealed schizophrenic-like or depressive-like behaviors (Thomson et al. 2013). DISC1 has been shown to regulate precursor cell proliferation during adult neurogenesis through the GSK-3β/β-catenin pathway, thus linking DISC1 to Wnt signaling (Mao et al. 2009). Furthermore, several studies have described a critical cell-autonomous role for DISC1 in regulating the tempo of newborn neuron maturation in the adult hippocampus. Retrovirus-mediated expression of short hairpin RNA (shRNA) against DISC1, specifically in newborn granule neurons, results in enhanced dendritic outgrowth, enlarged soma size, mispositioning of the cell body, ectopic dendrites, aberrant connectivity of axonal mossy fibers, and arrested maturation of presynaptic output (Duan et al. 2007; Faulkner et al. 2008; Kang et al. 2011). DISC1-dependent regulation of neurodevelopment is modulated through the AKT/mammalian target of rapamycin (mTOR) pathway. DISC1 directly interacts with KIAA1212 (girdin) and suppresses the activation of AKT (Kim et al. 2009). Pharmacological inhibition of the AKT downstream effector mTOR, via rapamycin, largely rescues the cellular defects caused by DISC1 deficiency. Interestingly, depolarizing γ-aminobutyric acid (GABA) signaling has been identified to regulate dendritic development of immature neurons via the AKT-mTOR pathway and is subject to DISC1 regulation. Release of neurotransmitters, such as glutamate and GABA, can serve as an indicator of general circuitry activity. Therefore, this study showed an interaction between local neural activity and a schizophrenia susceptibility gene, a gene-environment interaction in the regulation of neuronal development in the mammalian brain (Kim et al. 2012). Intriguingly, hippocampal-dependent cognitive and affective deficits in mice resulting from DISC1 deficiency, specifically in newborn granule neurons of the hippocampus, were partially rescued by rapamycin (Zhou et al. 2013). Taken together, these studies suggest that the mental disorder susceptibility gene DISC1 regulates discrete components of neurogenesis and neuronal development through distinct signaling pathways, which is consistent with the neurodevelopmental hypothesis. Understanding the biological role of key players in this pathway may reveal novel therapeutic targets to modulate neurogenesis and relevant behaviors, which could be effective even in adulthood.
Another prominent risk gene for schizophrenia, NPAS3 (neuronal PAS domain-containing protein 3), was identified through linkage and association studies (Kamnasaran et al. 2003; Pickard et al. 2005, 2009; Huang et al. 2010). NPAS3 encodes a transcription factor of the hHLH (basic helix–loop–helix)-PAS family and is mainly expressed in the developing central nervous system and the adult brain (Brunskill et al. 1999). Npas3 knockout mice showed reduced cell proliferation in the adult hippocampus, conferring a potential inability to appropriately remodel neural connections in hippocampal circuitry in response to environmental stimuli or psychological challenges (Pieper et al. 2005). Moreover, these mice have shown schizophrenia-like behaviors, such as hyperactivity, impaired prepulse inhibition, deficits in memory formation, and anxiety-like behavior (Brunskill et al. 2005; Pieper et al. 2005).
Many genes encoding proteins that are involved in synaptic function have been shown to be susceptibility genes for schizophrenia. One such gene is synaptosomal-associated protein, 25 kDa (SNAP-25). SNAP-25 is a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein, which plays a critical role in regulating synaptic vesicle exocytosis (Chapman 2002; Jahn et al. 2003; Sudhof 2004; Corradini et al. 2009). Human genetic studies have identified the association between SNAP-25 and neurological disorders, including schizophrenia, attention-deficit/hyperactivity disorder (ADHD), and epilepsy. Moreover, SNAP-25 was identified as one of the top 42 candidate genes for schizophrenia in a genome-wide meta-analysis (Lewis et al. 2003). In SNAP-25 mutant knock-in mice (Ala187Ser), adult hippocampal neurogenesis was suppressed. Furthermore, significant numbers of dentate gyrus granule cells were histologically and electrophysiologically immature, more than would be predicted based on proliferation rates and the tempo of normal development, and these mice showed severe impairments in working memory (Ohira et al. 2013). Neurexins (NRXNs) and neuroligins (NLGNs) are families of synaptic proteins, which are known to be risk factors for schizophrenia (Novak et al. 2009; Rujescu et al. 2009; Ikeda et al. 2010; Gauthier et al. 2011; Mozhui et al. 2011; Sun et al. 2011; Yue et al. 2011). These proteins are synaptic cell-adhesion molecules involved in various neuronal processes, including differentiation, maturation, stabilization, and plasticity of both inhibitory and excitatory synapses (Bang and Owczarek 2013). Although the functional role of NRXNs in adult hippocampal neurogenesis is unknown, one study has shown that neuroligin-1 plays a critical role in determining the morphology and survival rate of newborn granule neurons in the hippocampus (Schnell et al. 2014).
Similar to SNAP-25 mutant mice, a disproportionate number of immature granule cells have also been observed in other animal models of schizophrenia, such as heterozygous calmodulin-dependent protein kinase II (αCaMKII) knockout mice (Yamasaki et al. 2008) and Schnurri-2 knockout mice (Takao et al. 2013). Importantly, αCaMKII knockout mice and Shn-2 knockout mice also show schizophrenic-like behaviors including working memory deficits and hyperlocomotive activity. These animal studies thus suggest a link between disrupted maturation processes of adult-born cells and hippocampal dysfunction.
DiGeorge syndrome chromosomal critical region 8 (DGCR8), is a gene within 22q11.2 and the encoded protein is a component of the microprocessor complex involved in microRNA biogenesis (Wang et al. 2007). The 22q11.2 microdeletion is the most commonly occurring copy number variation (CVN) (Botto et al. 2003) and is a well-established risk factor for schizophrenia (Pulver et al. 1994; Murphy et al. 1999). Heterozygote Dgcr8 knockout mice (Dgcr8+/−) show a significant decrease in the proliferation of adult hippocampal neural progenitor cells and corresponding deficits in spatial working memory (Stark et al. 2008; Ouchi et al. 2013). Interestingly, rescue of decreased proliferation in adult hippocampus by administration of insulin-like growth factor (IGF)-2 was sufficient to reverse the behavioral defects in hippocampal-dependent learning, suggesting a potential role of defective adult hippocampal neurogenesis in mediating deficits in learning observed in schizophrenia patients. These studies suggest that enhancement of IGF-2 signaling and adult neurogenesis could serve as a potential therapeutic strategy for the treatment of cognitive deficits associated with schizophrenia.
In summary, impaired adult hippocampal neurogenesis and arrested growth of developing neurons appear to be consistent across multiple animal models of schizophrenia (Fig. 1) and have also been observed in patients with schizophrenia (Walton et al. 2012), often linked to a particular genetic risk factor. Together, these studies suggest a critical role for a functionally mature dentate gyrus in cognitive function and a potential contribution of dysregulated adult neurogenesis in the pathogenesis of schizophrenia.
DRUG ABUSE AND ADDICTION
Drug addiction involves the acquisition and maintenance of a drug-taking habit, which often becomes a recurrent problem wherein affected individuals can spend weeks, months, or years abstaining from substance abuse, and yet remain highly susceptible to relapse and drug-seeking behavior. Hippocampal modulation of drug addiction has received increasing attention because of the putative role of the hippocampus in mediating associative memories of drug-related behavior that can trigger a relapse on re-exposure to similar contexts. It is well established that dopaminergic reward circuitry in the brain, that is, dopaminergic projections from the midbrain ventral tegmental area (VTA) to the nucleus accumbens (NAc) and frontal cortex, plays an important role in drug addiction by enhancing dopamine efflux in the NAc, either directly or indirectly (Koob et al. 1992; Spanagel and Weiss 1999). The hippocampus, on the other hand, is positioned to influence brain reward pathways by receiving inputs from both the NAc and VTA and establishing a feedback loop through efferent projections to the NAc (Gasbarri et al. 1991, 1994; Floresco et al. 2001; Bannerman et al. 2004). These neuroanatomical observations suggesting that the hippocampus interacts with reward circuitry were further substantiated by functional studies showing that manipulations of hippocampal subfields alter the firing of VTA dopaminergic cells and dopaminergic responses in NAc (Mitchell et al. 2000; Floresco et al. 2001; Charara and Grace 2003; Won et al. 2003; Zornoza et al. 2005a,b). The recent discovery that addictive drugs alter adult hippocampal neurogenesis in animal models has added new insight into the neurobiology of drug addiction. The most intriguing findings are the negative correlations between the level of hippocampal neurogenesis and drug-taking or drug-seeking behaviors. Manipulations that enhance adult hippocampal neurogenesis, such as exposure to enriched environments, chronic treatment with antidepressants, or exercise, are associated with reduced drug-taking and a lower rate of relapse in animal models (Kanarek et al. 1995; Baker et al. 2001; Green et al. 2002; Stairs et al. 2006; Smith et al. 2008). On the other hand, conditions that decrease neurogenesis in humans, including schizophrenia or stress (Mirescu and Gould 2006; Reif et al. 2006), are associated with increased likelihood of drug addiction (Erb et al. 1996; Chambers and Self 2002; Covington and Miczek 2005).
Alcohol has been one of the most intensively studied drugs in the field of addiction. Generally, there is a negative correlation between alcohol consumption and the level of adult hippocampal neurogenesis, but the effects of alcohol on neurogenesis vary greatly with dosage, intake patterns and duration of exposure (Fig. 1). In an early study, acute alcohol consumption was shown to reduce cell proliferation in the dentate gyrus (Nixon and Crews 2002). Interestingly, this suppression was partially compensated for by a burst of proliferation after withdrawal, suggesting homeostatic maintenance of neurogenesis levels over the short term (Nixon and Crews 2004). Exercise in a running wheel also counteracts the inhibitory effect of alcohol on neural stem-cell proliferation (Crews et al. 2004). Chronic consumption of moderate doses of alcohol, on the other hand, has a negative effect on the survival of newborn granule neurons in mice, with no alteration in the proliferation rate of neural progenitors. This increase in the cell death of newborn neurons is prevented by administration of the antioxidant ebselen, suggesting a possible oxidative mechanism for toxicity in hippocampal cells (Herrera et al. 2003). In mice, long-term voluntary alcohol self-administration for 10 weeks in a two-bottle free-choice test enhanced proliferation in the dentate gyrus, with new cells surviving and differentiating normally (Aberg et al. 2005). Chronic alcohol treatment over 11 months in adolescent macaque monkeys, on the other hand, resulted in long-lasting reduction in number of proliferating neural stem cells in dentate gyrus (Taffe et al. 2010). Moreover, in the abstinence state following withdrawal from chronic alcohol consumption, depressive-like behavior and reduced numbers of neural progenitors and immature neurons were observed in the dentate gyrus of adult mice and both structural and behavioral phenotypes are alleviated by the antidepressant desipramine (Stevenson et al. 2009). These studies suggest that hippocampal neurogenesis might mediate the comorbidity of depressive symptoms associated with alcohol dependence and provide a potential explanation for the clinical observation that hippocampal volume is reduced in alcoholic patients (Bengochea and Gonzalo 1990; Sullivan et al. 1995; Agartz et al. 1999). Chronic alcohol treatment is also associated with long-lasting changes in synaptic plasticity and learning and memory deficits. Thus, the hippocampus may be important for some shared cognitive features of addiction, depression, and schizophrenia (Walker and Freund 1971; Santin et al. 2000; Roberto et al. 2002).
Stimulants are psychoactive drugs that have been used to temporarily enhance mental and/or physical performance. They are used clinically to treat certain conditions, but they are also used recreationally and are a common drug of abuse. Opiates were the first psychostimulants associated with adult neurogenesis and in vivo exposure of μ-opioid receptor agonists in rodent models leads to reduced progenitor proliferation and deficits in the maturation and survival of new neurons in the hippocampus (Eisch et al. 2000). Likewise, cocaine abuse is also negatively associated with some facets of adult hippocampal neurogenesis. Both short- and long-term treatment of cocaine reduces proliferation in the dentate gyrus, but the effect on survival and dendritic maturation is not always consistent, possibly caused by the use of different animal strains and various drug administration protocols (Eisch 2002; Yamaguchi et al. 2004; Dominguez-Escriba et al. 2006; Noonan et al. 2008; Mustroph et al. 2011). Negative effects of cocaine on cell proliferation were concomitant with impairments in working memory, even during abstinence from high dosage cocaine self-administration (Sudai et al. 2011). Ablation of adult hippocampal neurogenesis using X-ray irradiation results in an increase in cocaine self-administration and drug-seeking behaviors, suggesting that impaired neurogenesis leads to increased susceptibility to drug addiction (Noonan et al. 2010). Enhancing adult hippocampal neurogenesis has been suggested to protect against cocaine-primed relapse (Deschaux et al. 2014). These studies indicate that recalibrating baseline levels of neurogenesis following chronic cocaine administration may reduce susceptibility to relapse. MDMA (ecstasy) is a drug with hallucinogenic properties. Short-term binge MDMA administration in rats has been shown to decrease the survival of neural progenitor cells without affecting the proliferation rate (Hernandez-Rabaza et al. 2006). Long-term repeated exposure to ethanol and MDMA together leads to prominent cognitive impairments associated with neuronal loss and microgliosis in the dentate gyrus of adolescent rats, implying that simultaneous use of alcohol and MDMA may pose serious health risks (Hernandez-Rabaza et al. 2010). Simultaneous use of MDMA and alcohol during pregnancy in rats resulted in long-lasting effects on pups over the course of development and into adulthood. Rats with prenatal exposure to MDMA and alcohol had decreased proliferation, reduced adult neurogenesis and showed significant abnormalities in cognitive function and exploratory activity (Canales and Ferrer-Donato 2014). Similarly, in utero exposure to MDMA reduces proliferation and survival of newborn neurons in the adult mouse hippocampus (Cho et al. 2008). Together, these studies suggest that there may be irreversible adverse effects of MDMA exposure during neural development. Nicotine is a stimulant drug used widely around the world. The impact of nicotine on adult neurogenesis appears to be more complicated. A recent study reported that ZY-1, a nicotinic analog, shows a positive effect on cell proliferation and migration of hippocampal neural stem cells in vitro (He et al. 2013), whereas in vivo chronic nicotine administration decreases the survival and maturation of new neurons, without affecting cell proliferation, in the adult rodent hippocampus (Abrous et al. 2002; Wei et al. 2012).
Collectively, most addictive drugs, especially when administered chronically, decrease the proliferation or survival rate of neural progenitors, leading to global deficits in hippocampal plasticity (Fig. 1). However, the role of neurogenesis in addiction has not yet been studied in sufficient detail. The studies performed so far have been primarily descriptive and correlative. Future studies are needed to address the functional interaction between addiction and adult neurogenesis, including the effects and underlying mechanisms of drugs on adult hippocampal neurogenesis and the extent to which adult neurogenesis mediates addiction and drug abuse.
ADULT NEUROGENESIS AND DRUG DEVELOPMENT
Psychiatric disorders are usually comprised of a diverse array of symptoms. The treatments that have proven effective for major psychiatric diseases include psychotherapy (such as cognitive, behavior, and group therapy) (Hofmann and Smits 2008; Rathod et al. 2008), brain-stimulation treatments (such as ECT, transcranial magnetic stimulation, vagus nerve stimulation, and deep brain stimulation) (George et al. 2000; Mayberg et al. 2005; Fink and Taylor 2007; O’Reardon et al. 2007), and psychopharmacology (drugs such as lithium, haloperidol, TCAs, atypical antipsychotics, SSRIs, and memantine). However, because of a lack of knowledge regarding the underlying genetic and neurobiological mechanisms of psychiatric disease, we are mostly treating symptoms with blunt approaches that can have many off-target effects on well-functioning neural systems. In addition, only ∼65% of patients with mood disorders respond adequately to available medications (Al-Harbi 2012). As for schizophrenia, existing treatments are usually targeted toward the subset of symptoms that is the most disruptive and fail to ameliorate all categories of symptoms. Furthermore, currently available medications are difficult to tolerate and can cause serious side effects, and patients with schizophrenia may be particularly prone to noncompliance (Miyamoto et al. 2005). For example, clozapine is an effective medication that treats psychotic symptoms, hallucinations, and breaks with reality, but can frequently cause agranulocytosis (Wahlbeck et al. 1999; Chakos et al. 2001; Tuunainen et al. 2002), a life-threatening side effect in which the immune system is seriously impaired. Therefore, there is an urgent need for developing new strategies for prevention and treatment of psychiatric disorders. Increasing evidence supports that dysfunction of adult hippocampal neurogenesis itself plays a causal role in psychiatric symptomatology, thus raising the hope that manipulation of this endogenous phenomenon can be a target for the amelioration or prevention of some aspects of psychiatric illness, with fewer side effects.
Adult Neurogenesis as a Potential Therapeutic Target
Identification of pathological lesions in specific brain regions or degeneration of specific cell types has contributed greatly to the rapid progress in understanding the etiology of some neurological disorders and the subsequent development of targeted treatments. A large body of evidence now suggests that impaired adult neurogenesis in the hippocampus is associated with various mental disorders, including major depression, schizophrenia, mood, and anxiety disorders as well as addictive behaviors. Importantly, decreased adult neurogenesis correlates with reduced cognitive and affective functions, a common symptom in patients and a frequent phenotype in animal models of these diseases, and often coincides with a decrease in cell proliferation in the dentate gyrus and reduced hippocampal volume (Bremner 1999; Lie et al. 2004; Mirescu and Gould 2006; Reif et al. 2007; Revest et al. 2009; Morris et al. 2010; Nixon et al. 2010; Noonan et al. 2010). As adult hippocampal neurogenesis is directly linked to the action of antidepressants, it has been suggested that adult neurogenesis could be a target for treatment of depression (Banasr et al. 2006; Dranovsky and Hen 2006; Drew and Hen 2007; Sahay and Hen 2007). Using strategies to mimic physiological or environmental neurogenic stimuli may thus facilitate the development of novel therapeutics.
Based on the findings that hippocampal neurogenesis is down-regulated under pathological psychiatric conditions in patients and animal models, and up-regulated by antidepressant drugs and other antidepressant treatments (Table 1), a neurogenic hypothesis of depression emerged, proposing that neurogenesis in the adult hippocampus may involve mood control and is necessary for some of the behavioral effects of antidepressants. Further studies on animal models of psychiatric disease provided direct evidence to support the hypothesis that restoration of adult hippocampal neurogenesis can rescue some cognitive impairments. For example, work performed by the McKnight group discovered that the proneurogenic chemical P7C3 leads to enhanced cognitive capacity in aged rats on administration (Pieper et al. 2010). Importantly, treatment with P7C3 elicits antidepressant efficacy in mice by increasing hippocampal neurogenesis (Walker et al. 2015). In addition, a study on fragile-X syndrome showed that, although ablation of fragile-X mental retardation protein (FMRP) in neural progenitor cells leads to reduced hippocampal neurogenesis in vitro and in vivo, resulting in significantly impaired hippocampus-dependent learning in mice, restoration of adult neurogenesis via FMRP expression in neural progenitor cells (NPCs) rescues these learning deficits (Guo et al. 2011). Furthermore, DISC1 knockdown specifically in adult-born dentate gyrus neurons has been shown to result in increased mTOR signaling and pronounced cognitive and affective deficits. Importantly, suppression of mTOR signaling with treatment of the Food and Drug Administration (FDA)-approved inhibitor rapamycin can rescue the behavioral deficits (Zhou et al. 2013). These studies provide evidence that dysregulated adult neurogenesis may contribute to the cognitive impairments seen in psychiatric diseases, and that the cognitive deficits in animal models can be at least partially rescued by restoration of neurogenesis in the adult brain, suggesting that adult neurogenesis is a viable therapeutic target.
Adult Neurogenesis as a Platform for Understanding Psychiatric Disorders
Although the causes of many severe mental disorders are still poorly understood, cumulative evidence suggests a role for neurodevelopmental dysregulation, even in late-onset disorders. Both genetic and environmental factors play a role in disease onset and progression. Recent advances in genome-wide sequencing technology and analyses have identified a large number of susceptibility genes for psychiatric disorders, but a major challenge is to understand the role of these genes in neural development and function and how they contribute to causally relevant pathology (Abdolmaleky et al. 2005; O’Donovan et al. 2009; Kleinman et al. 2011). Adult hippocampal neurogenesis provides an attractive cellular model to understand the mechanisms mediating the etiopathophysiology of mental disorders with several unique advantages (Christian et al. 2010). First, adult neurogenesis recapitulates each step of neuronal development, including proliferation, cell fate specification, migration, axonal and dendritic development, and synapse formation. Second, the time course of these developmental events is significantly prolonged in the adult brain compared with the developing brain, which allows for increased temporal resolution in the analysis of each developmental step and is thus more amenable to dissecting the cellular and molecular mechanisms regulating each stage. Third, adult hippocampal neurogenesis is not only regulated by intrinsic factors, but is also affected by external stimuli, such as neuronal activity, environmental factors, and psychotropic drugs. Thus, the system provides a unique platform to study the interactions between genes and environment. Finally, transgenic or retroviral techniques allow for inducible and reversible manipulation of genes and proteins of interest in specific neuronal or stem-cell populations, allowing the opportunity to investigate how susceptibility genes contribute to the pathology underlying psychiatric disorders. Thus, adult neurogenesis may not only play a role in psychiatric symptomatology, but is also useful for investigating the developmental mechanisms that contribute to psychiatric diseases. The utility of adult neurogenesis as a model to explore the genetic basis of neurodevelopmental disorders is constrained by the number of identified genetic risk factors. For some disorders, including schizophrenia and autism spectrum disorders, numerous risk factors have been reported, although the biological functions of most of the identified genetic variants in neurodevelopment are still unknown. For other psychiatric disorders, including depression and addiction, we know much less about the genetic risk factors and thus it is much more difficult at the moment to capitalize on the potential of this system to explore the consequences of disease-relevant genetic mutations on neuronal development. But exploiting this cellular model through targeted genetic or environmental perturbations should further our understanding of the molecular basis of neuronal development to diagnose, treat, and potentially prevent some of the most debilitating psychiatric illnesses.
CLOSING REMARKS
The discovery of neurogenesis and neural stem cells in the adult mammalian hippocampus has enhanced our understanding of the plasticity of the mature brain and provided a unique model to explore neuronal development and the integration of newly born cells into existing neural circuitry. It has also raised questions regarding the functional contribution of these cells in established neural circuits. As the role of adult hippocampal neurogenesis in cognitive and affective functions is slowly being revealed, studies are now focusing on how dysregulation of neurogenesis in the adult brain is related to the pathogenesis and progression of mental disorders. Although much work remains to be performed in this regard, a wealth of provocative data support that intact neurogenesis promotes cognitive function, prenatal or adult exposure to detrimental factors impair adult neurogenesis and hippocampal function, disparate antidepressant treatments enhance neurogenesis and require neurogenesis to be effective, and many psychiatric risk genes affect both perinatal brain development and adult neurogenesis. The contribution of these two phases of neurogenesis to psychiatric diseases are not known, especially in patients, and begs for further investigation. As a model system to investigate neuronal development, adult neurogenesis is invaluable in our attempt to identify key signaling pathways and cellular processes affected by risk genes, which can pave the way to a deeper understanding of the biology underlying psychiatric disorders. As an endogenous phenomenon that modulates affective and cognitive behaviors, we are only beginning to understand how this constitutive reshaping of local hippocampal circuitry impacts neural systems, and how it could be a therapeutic target to restore some functions in patients with psychiatric disorders.
ACKNOWLEDGMENTS
The research in the authors’ laboratories is supported by National Institutes of Health (NIH) Grants (NS048271, MH105128, NS047344, NS0937772), Brain & Behavior Research Foundation (formerly, National Alliance for Research on Schizophrenia and Depression, NARSAD), and Maryland Stem Cell Research Fund (MSCRF).
Footnotes
Editors: Fred H. Gage, Gerd Kempermann, and Hongjun Song
Additional Perspectives on Neurogenesis available at www.cshperspectives.org
REFERENCES
- Abdolmaleky HM, Thiagalingam S, Wilcox M. 2005. Genetics and epigenetics in major psychiatric disorders: Dilemmas, achievements, applications, and future scope. Am J Pharmacogenomics 5: 149–160. [DOI] [PubMed] [Google Scholar]
- Aberg E, Hofstetter CP, Olson L, Brene S. 2005. Moderate ethanol consumption increases hippocampal cell proliferation and neurogenesis in the adult mouse. Int J Neuropsychopharmacol 8: 557–567. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, Piazza PV. 2002. Nicotine self-administration impairs hippocampal plasticity. J Neurosci 22: 3656–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. 1999. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry 56: 356–363. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. 2014. Regulation and function of adult neurogenesis: From genes to cognition. Physiol Rev 94: 991–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. 2007. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317: 819–823. [DOI] [PubMed] [Google Scholar]
- Al-Harbi KS. 2012. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer Adherence 6: 369–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. [DOI] [PubMed] [Google Scholar]
- Austin CP, Ky B, Ma L, Morris JA, Shughrue PJ. 2004. Expression of disrupted-in-schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience 124: 3–10. [DOI] [PubMed] [Google Scholar]
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. 2001. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 155: 18–26. [DOI] [PubMed] [Google Scholar]
- Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. 2006. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry 59: 1087–1096. [DOI] [PubMed] [Google Scholar]
- Bang ML, Owczarek S. 2013. A matter of balance: Role of neurexin and neuroligin at the synapse. Neurochem Res 38: 1174–1189. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. 2004. Regional dissociations within the hippocampus—Memory and anxiety. Neurosci Biobehav Rev 28: 273–283. [DOI] [PubMed] [Google Scholar]
- Bengochea O, Gonzalo LM. 1990. Effect of chronic alcoholism on the human hippocampus. Histol Histopathol 5: 349–357. [PubMed] [Google Scholar]
- Bergami M, Berninger B, Canossa M. 2009. Conditional deletion of TrkB alters adult hippocampal neurogenesis and anxiety-related behavior. Commun Integr Biol 2: 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA. 2010. Neural mechanisms of ageing and cognitive decline. Nature 464: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. 2001. Schizophrenia and affective disorders—Cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: Clinical and P300 findings in a family. Am J Hum Genet 69: 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. 2009. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34: 2376–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O’Leary LA, Wong LY, Elixson EM, et al. 2003. A population-based study of the 22q11.2 deletion: Phenotype, incidence, and contribution to major birth defects in the population. Pediatrics 112: 101–107. [DOI] [PubMed] [Google Scholar]
- Braun SMG, Jessberger S. 2013. Adult neurogenesis in the mammalian brain. Front Biol 8: 295–304. [Google Scholar]
- Bremner JD. 1999. Alterations in brain structure and function associated with post-traumatic stress disorder. Semin Clin Neuropsychiatry 4: 249–255. [DOI] [PubMed] [Google Scholar]
- Bremner JD. 2006. Traumatic stress: Effects on the brain. Dialogues Clin Neurosci 8: 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. 2004. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Ann NY Acad Sci 1032: 154–157. [DOI] [PubMed] [Google Scholar]
- Brunskill EW, Witte DP, Shreiner AB, Potter SS. 1999. Characterization of Npas3, a novel basic helix–loop–helix PAS gene expressed in the developing mouse nervous system. Mech Dev 88: 237–241. [DOI] [PubMed] [Google Scholar]
- Brunskill EW, Ehrman LA, Williams MT, Klanke J, Hammer D, Schaefer TL, Sah R, Dorn GW II, Potter SS, Vorhees CV. 2005. Abnormal neurodevelopment, neurosignaling and behaviour in Npas3-deficient mice. Eur J Neurosci 22: 1265–1276. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. 2004. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 29: 417–426. [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Ferrer-Donato A. 2014. Prenatal exposure to alcohol and 3,4-methylenedioxymethamphetamine (ecstasy) alters adult hippocampal neurogenesis and causes enduring memory deficits. Dev Neurosci 36: 10–17. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham PC, et al. 1999. Heritability estimates for psychotic disorders: The Maudsley twin psychosis series. Arch Gen Psychiatry 56: 162–168. [DOI] [PubMed] [Google Scholar]
- Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. 2001. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: A review and meta-analysis of randomized trials. Am J Psychiatry 158: 518–526. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. 2002. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: An animal model of dual diagnosis schizophrenia. Neuropsychopharmacology 27: 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. 2002. Synaptotagmin: A Ca2+ sensor that triggers exocytosis? Nat Rev Mol Cell Biol 3: 498–508. [DOI] [PubMed] [Google Scholar]
- Charara A, Grace AA. 2003. Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology 28: 1412–1421. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. 2000. Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75: 1729–1734. [DOI] [PubMed] [Google Scholar]
- Chen H, Pandey GN, Dwivedi Y. 2006. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport 17: 863–867. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kogan JH, Gross AK, Zhou Y, Walton NM, Shin R, Heusner CL, Miyake S, Tajinda K, Tamura K, et al. 2012. SREB2/GPR85, a schizophrenia risk factor, negatively regulates hippocampal adult neurogenesis and neurogenesis-dependent learning and memory. Eur J Neurosci 36: 2597–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Rhee GS, Kwack SJ, Chung SY, Kim SY. 2008. Developmental exposure to 3,4-methylenedioxymethamphetamine results in downregulation of neurogenesis in the adult mouse hippocampus. Neuroscience 154: 1034–1041. [DOI] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. 2008. The DISC locus in psychiatric illness. Mol Psychiatry 13: 36–64. [DOI] [PubMed] [Google Scholar]
- Christian K, Song H, Ming GL. 2010. Adult neurogenesis as a cellular model to study schizophrenia. Cell Cycle 9: 636–637. [DOI] [PubMed] [Google Scholar]
- Christian KM, Song H, Ming GL. 2014. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci 37: 243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Brambilla P, Furukawa T, Geddes J, Gregis M, Hotopf M, Malvini L, Barbui C. 2005. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst Rev 7: CD004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini I, Verderio C, Sala M, Wilson MC, Matteoli M. 2009. SNAP-25 in neuropsychiatric disorders. Ann NY Acad Sci 1152: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE 3rd, Miczek KA. 2005. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: Dissociation from corticosterone activation. Psychopharmacology (Berl) 183: 331–340. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. 2010. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci 107: 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. 2004. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol 33: 63–71. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI. 1998. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci 95: 11406–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. 2001. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci 98: 12796–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, Toschi N, Fuchs E, Keck ME. 2002. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 52: 1057–1065. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, et al. 2009. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Wang J, Samuels BA, Rainer Q, David I, Gardier AM, Hen R. 2010. Implications of the functional integration of adult-born hippocampal neurons in anxiety-depression disorders. Neuroscientist 16: 578–591. [DOI] [PubMed] [Google Scholar]
- Davidson LL, Heinrichs RW. 2003. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: A meta-analysis. Psychiatry Res 122: 69–87. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. 2010. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschaux O, Vendruscolo LF, Schlosburg JE, Diaz-Aguilar L, Yuan CJ, Sobieraj JC, George O, Koob GF, Mandyam CD. 2014. Hippocampal neurogenesis protects against cocaine-primed relapse. Addict Biol 19: 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshauer D, Moher D, Fergusson D, Moher E, Sampson M, Grimshaw J. 2008. Selective serotonin reuptake inhibitors for unipolar depression: A systematic review of classic long-term randomized controlled trials. CMAJ 178: 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhikav V, Anand KS. 2007. Is hippocampal atrophy a future drug target? Med Hypotheses 68: 1300–1306. [DOI] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, Canales JJ. 2006. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci 24: 586–594. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. 2006. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol Psychiatry 59: 1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Hen R. 2007. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets 6: 205–218. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, et al. 2007. Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130: 1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. 2004. Neural plasticity: Consequences of stress and actions of antidepressant treatment. Dialogues Clin Neurosci 6: 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ. 2002. Adult neurogenesis: Implications for psychiatry. Prog Brain Res 138: 315–342. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. 2000. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci 97: 7579–7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. 2006. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci 103: 8233–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. 1996. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 128: 408–412. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. 1998. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM. 2014. Physical activity, fitness, and gray matter volume. Neurobiol Aging 35: S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, et al. 2008. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci 105: 14157–14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Taylor MA. 2007. Electroconvulsive therapy: Evidence and challenges. JAMA 298: 330–332. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. 2001. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 21: 4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Czeh B, Michaelis T, de Biurrun G, Watanabe T, Frahm J. 2002. Synaptic plasticity and tianeptine: Structural regulation. Eur Psychiatry 17: 311–317. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Campana E, Pacitti C, Hajdu F, Tombol T. 1991. Organization of the projections from the ventral tegmental area of Tsai to the hippocampal formation in the rat. J Hirnforsch 32: 429–437. [PubMed] [Google Scholar]
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. 1994. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: A combined retrograde tracing and immunohistochemical study. Brain Res 668: 71–79. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, Lapointe M, Spiegelman D, Noreau A, Lafreniere RG, et al. 2011. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum Genet 130: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, Lisanby S, Burt T, Goldman J, Ballenger JC. 2000. Vagus nerve stimulation: A new tool for brain research and therapy. Biol Psychiatry 47: 287–295. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. 2005. MR-based in vivo hippocampal volumetrics. 2: Findings in neuropsychiatric disorders. Mol Psychiatry 10: 160–184. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Mitchell CP. 2004. What is the functional significance of hippocampal pathology in schizophrenia? Schizophr Bull 30: 367–392. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. 1999. Stress and hippocampal neurogenesis. Biol Psychiatry 46: 1472–1479. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. 1997. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17: 2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. 2002. Environmental enrichment decreases intravenous amphetamine self-administration in rats: Dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 162: 373–378. [DOI] [PubMed] [Google Scholar]
- Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, Murthy AC, Goggin SL, Eisch AJ, Oostra BA, et al. 2011. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat Med 17: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. 2004. Discovering endophenotypes for major depression. Neuropsychopharmacology 29: 1765–1781. [DOI] [PubMed] [Google Scholar]
- He N, Wang Z, Wang Y, Shen H, Yin M. 2013. ZY-1, a novel nicotinic analog, promotes proliferation and migration of adult hippocampal neural stem/progenitor cells. Cell Mol Neurobiol 33: 1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. 2001. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11: 520–528. [DOI] [PubMed] [Google Scholar]
- Hellsten J, Wennstrom M, Mohapel P, Ekdahl CT, Bengzon J, Tingstrom A. 2002. Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosterone treatment. Eur J Neurosci 16: 283–290. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Dominguez-Escriba L, Barcia JA, Rosel JF, Romero FJ, Garcia-Verdugo JM, Canales JJ. 2006. Binge administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) impairs the survival of neural precursors in adult rat dentate gyrus. Neuropharmacology 51: 967–973. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Navarro-Mora G, Velazquez-Sanchez C, Ferragud A, Marin MP, Garcia-Verdugo JM, Renau-Piqueras J, Canales JJ. 2010. Neurotoxicity and persistent cognitive deficits induced by combined MDMA and alcohol exposure in adolescent rats. Addict Biol 15: 413–423. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. 2003. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci 100: 7919–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R. 2015. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA. 2008. Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry 69: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. 2008. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology 33: 406–417. [DOI] [PubMed] [Google Scholar]
- Huang J, Perlis RH, Lee PH, Rush AJ, Fava M, Sachs GS, Lieberman J, Hamilton SP, Sullivan P, Sklar P, et al. 2010. Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am J Psychiatry 167: 1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Aleksic B, Kirov G, Kinoshita Y, Yamanouchi Y, Kitajima T, Kawashima K, Okochi T, Kishi T, Zaharieva I, et al. 2010. Copy number variation in schizophrenia in the Japanese population. Biol Psychiatry 67: 283–286. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH. 2000. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol Psychiatry 5: 262–269. [DOI] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC. 2003. Membrane fusion. Cell 112: 519–533. [DOI] [PubMed] [Google Scholar]
- Jang MH, Bonaguidi MA, Kitabatake Y, Sun J, Song J, Kang E, Jun H, Zhong C, Su Y, Guo JU, et al. 2013a. Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Kitabatake Y, Kang E, Jun H, Pletnikov MV, Christian KM, Hen R, Lucae S, Binder EB, Song H, et al. 2013b. Secreted frizzled-related protein 3 (sFRP3) regulates antidepressant responses in mice and humans. Mol Psychiatry 18: 957–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O. 2006. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 31: 2395–2404. [DOI] [PubMed] [Google Scholar]
- Kamnasaran D, Muir WJ, Ferguson-Smith MA, Cox DW. 2003. Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J Med Genet 40: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek RB, Marks-Kaufman R, D’Anci KE, Przypek J. 1995. Exercise attenuates oral intake of amphetamine in rats. Pharmacol Biochem Behav 51: 725–729. [DOI] [PubMed] [Google Scholar]
- Kang E, Burdick KE, Kim JY, Duan X, Guo JU, Sailor KA, Jung DE, Ganesan S, Choi S, Pradhan D, et al. 2011. Interaction between FEZ1 and DISC1 in regulation of neuronal development and risk for schizophrenia. Neuron 72: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. 1999. New nerve cells for the adult brain. Sci Am 280: 48–53. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. 2008. Exercise attenuates oral intake of amphetamine in rats. Curr Opin Psychiatry 21: 290–295. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA. 2010. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci 4: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. 2011. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68: 675–690. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. 2010. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis 37: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Hen R. 2011. Dorsal vs ventral hippocampal neurogenesis: Implications for cognition and mood. Neuropsychopharmacology 36: 373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R. 2013. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77: 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. 2009. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 63: 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, Lu B, Rujescu D, St Clair D, et al. 2012. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 148: 1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabatake Y, Sailor KA, Ming GL, Song H. 2007. Adult neurogenesis and hippocampal memory function: New cells, more plasticity, new memories? Neurosurg Clin N Am 18: 105–113, x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Doi M, Kuwatsuka K, Onoue Y, Miyazaki I, Shinomiya K, Koyama T, Sendo T, Kawasaki H, Asanuma M, et al. 2011. Chronic treatment with imipramine and lithium increases cell proliferation in the hippocampus in adrenocorticotropic hormone-treated rats. Biol Pharm Bull 34: 77–81. [DOI] [PubMed] [Google Scholar]
- Kleinman JE, Law AJ, Lipska BK, Hyde TM, Ellis JK, Harrison PJ, Weinberger DR. 2011. Genetic neuropathology of schizophrenia: New approaches to an old question and new uses for postmortem human brains. Biol Psychiatry 69: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konick LC, Friedman L. 2001. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry 49: 28–38. [DOI] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. 1992. Neural substrates of opiate withdrawal. Trends Neurosci 15: 186–191. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Kirste I, Inta D, Chourbaji S, Heuser I, Endres M, Gass P. 2009. Reduced hippocampal neurogenesis in the GR+/− genetic mouse model of depression. Eur Arch Psychiatry Clin Neurosci 259: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasinghe N, Tooney PA, Schall U. 2012. Finding the needle in the haystack: A review of microarray gene expression research into schizophrenia. Aust N Z J Psychiatry 46: 598–610. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Abukmeil SS. 1998. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry 172: 110–120. [DOI] [PubMed] [Google Scholar]
- Le Francois B, Czesak M, Steubl D, Albert PR. 2008. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology 55: 977–985. [DOI] [PubMed] [Google Scholar]
- Levinson DF. 2006. The genetics of depression: A review. Biol Psychiatry 60: 84–92. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, et al. 2003. Genome scan meta-analysis of schizophrenia and bipolar disorder. Part II: Schizophrenia. Am J Hum Genet 73: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. 2004. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol 44: 399–421. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Fuchs E, Czeh B. 2004. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biol Psychiatry 55: 789–796. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Stumpel MW, Wang Q, Aronica E. 2010. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 58: 940–949. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. 2010. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6: 445–456. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. 2007. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn 65: 209–237. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. 2009. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323: 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. 2000. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry 47: 1043–1049. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. 2003. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology 28: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. 2000. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. 2009. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell 136: 1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattai A, Hosanagar A, Weisinger B, Greenstein D, Stidd R, Clasen L, Lalonde F, Rapoport J, Gogtay N. 2011. Hippocampal volume development in healthy siblings of childhood-onset schizophrenia patients. Am J Psychiatry 168: 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Straub RE, Marenco S, Nicodemus KK, Matsumoto S, Fujikawa A, Miyoshi S, Shobo M, Takahashi S, Yarimizu J, et al. 2008. The evolutionarily conserved G protein-coupled receptor SREB2/GPR85 influences brain size, behavior, and vulnerability to schizophrenia. Proc Natl Acad Sci 105: 6133–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. 2005. Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. 2009. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 34: 41–54. [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, et al. 2000. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 9: 1415–1423. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. 2005. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28: 223–250. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. 2011. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 70: 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E. 2006. Stress and adult neurogenesis. Hippocampus 16: 233–238. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. 2006. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci 103: 19170–19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SN, Yee BK, Feldon J, Gray JA, Rawlins JN. 2000. Activation of the retrohippocampal region in the rat causes dopamine release in the nucleus accumbens: Disruption by fornix section. Eur J Pharmacol 407: 131–138. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. 2005. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10: 79–104. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. 2010. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus 20: 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhui K, Wang X, Chen J, Mulligan MK, Li Z, Ingles J, Chen X, Lu L, Williams RW. 2011. Genetic regulation of Nrxn1 expression: An integrative cross-species analysis of schizophrenia candidate genes. Transl Psychiatry 1: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. 1999. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 56: 940–945. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Stobaugh DJ, Miller DS, DeYoung EK, Rhodes JS. 2011. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci 34: 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. 1998. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta-analytic study. Arch Gen Psychiatry 55: 433–440. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. 2002. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem 83: 1087–1093. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. 2004. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci 24: 9714–9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, Kelso ML. 2010. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol 44: 39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. 2008. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci 28: 2516–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. 2010. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci 30: 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak G, Boukhadra J, Shaikh SA, Kennedy JL, Le Foll B. 2009. Association of a polymorphism in the NRXN3 gene with the degree of smoking in schizophrenia: A preliminary study. World J Biol Psychiatry 10: 929–935. [DOI] [PubMed] [Google Scholar]
- O’Donovan MC, Craddock NJ, Owen MJ. 2009. Genetics of psychosis; insights from views across the genome. Hum Genet 126: 3–12. [DOI] [PubMed] [Google Scholar]
- Ohira K, Kobayashi K, Toyama K, Nakamura HK, Shoji H, Takao K, Takeuchi R, Yamaguchi S, Kataoka M, Otsuka S, et al. 2013. Synaptosomal-associated protein 25 mutation induces immaturity of the dentate granule cells of adult mice. Mol Brain 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, et al. 2007. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol Psychiatry 62: 1208–1216. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Banno Y, Shimizu Y, Ando S, Hasegawa H, Adachi K, Iwamoto T. 2013. Reduced adult hippocampal neurogenesis and working memory deficits in the Dgcr8-deficient mouse model of 22q11.2 deletion-associated schizophrenia can be rescued by IGF2. J Neurosci 33: 9408–9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM. 2003. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist 9: 261–272. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, et al. 2007. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci 27: 4894–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Park S, Nemirovskaya Y. 2008. Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist 14: 326–338. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Marsh L. 1995. Structural brain imaging in schizophrenia. Clin Neurosci 3: 105–111. [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. 2003. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17: 879–886. [DOI] [PubMed] [Google Scholar]
- Pickard BS, Malloy MP, Porteous DJ, Blackwood DH, Muir WJ. 2005. Disruption of a brain transcription factor, NPAS3, is associated with schizophrenia and learning disability. Am J Med Genet B Neuropsychiatr Genet 136B: 26–32. [DOI] [PubMed] [Google Scholar]
- Pickard BS, Christoforou A, Thomson PA, Fawkes A, Evans KL, Morris SW, Porteous DJ, Blackwood DH, Muir WJ. 2009. Interacting haplotypes at the NPAS3 locus alter risk of schizophrenia and bipolar disorder. Mol Psychiatry 14: 874–884. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, et al. 2005. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci 102: 14052–14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, et al. 2010. Discovery of a proneurogenic, neuroprotective chemical. Cell 142: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock SB, Lazic SE, Wong HT, Wong IH, Herbert J. 2009. Synergistic effects of dehydroepiandrosterone and fluoxetine on proliferation of progenitor cells in the dentate gyrus of the adult male rat. Neuroscience 158: 1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D, et al. 1994. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis 182: 476–478. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. 2004. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 162: 39–47. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. 2002. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res 955: 264–267. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. 1999. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45: 1085–1098. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. 2012. Neurodevelopmental model of schizophrenia: Update 2012. Mol Psychiatry 17: 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod S, Kingdon D, Weiden P, Turkington D. 2008. Cognitive-behavioral therapy for medication-resistant schizophrenia: A review. J Psychiatr Pract 14: 22–33. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. 2006. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 11: 514–522. [DOI] [PubMed] [Google Scholar]
- Reif A, Schmitt A, Fritzen S, Lesch KP. 2007. Neurogenesis and schizophrenia: Dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci 257: 290–299. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. 2009. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry 14: 959–967. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Gruol DL. 2002. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol 87: 2385–2397. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. 2004. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 188: 316–330. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietilainen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, et al. 2009. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet 18: 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. 2007. Adult hippocampal neurogenesis in depression. Nat Neurosci 10: 1110–1115. [DOI] [PubMed] [Google Scholar]
- Sakae N, Yamasaki N, Kitaichi K, Fukuda T, Yamada M, Yoshikawa H, Hiranita T, Tatsumi Y, Kira J, Yamamoto T, et al. 2008. Mice lacking the schizophrenia-associated protein FEZ1 manifest hyperactivity and enhanced responsiveness to psychostimulants. Hum Mol Genet 17: 3191–3203. [DOI] [PubMed] [Google Scholar]
- Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC, Brambilla P. 2004. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol 14: 393–405. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809. [DOI] [PubMed] [Google Scholar]
- Santin LJ, Rubio S, Begega A, Arias JL. 2000. Effects of chronic alcohol consumption on spatial reference and working memory tasks. Alcohol 20: 149–159. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. 2000. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57: 925–935. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. 2009. Bipolar and major depressive disorder: Neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev 33: 699–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC. 2012. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health 16: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K. 2009. Suppression of adult neurogenesis leads to an increased hypothalamo–pituitary–adrenal axis response. Neuroreport 20: 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Long TH, Bensen AL, Washburn EK, Westbrook GL. 2014. Neuroligin-1 knockdown reduces survival of adult-generated newborn hippocampal neurons. Front Neurosci 8: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurov IL, Handford EJ, Brandon NJ, Whiting PJ. 2004. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol Psychiatry 9: 1100–1110. [DOI] [PubMed] [Google Scholar]
- Segi-Nishida E, Warner-Schmidt JL, Duman RS. 2008. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci 105: 11352–11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Le Masurier M, Allan CL, Jenkinson M, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Mackay CE, Ebmeier KP. 2012. Magnetic resonance imaging in late-life depression: Vascular and glucocorticoid cascade hypotheses. Br J Psychiatry 201: 46–51. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. 1996. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci 93: 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. 2003. Untreated depression and hippocampal volume loss. Am J Psychiatry 160: 1516–1518. [DOI] [PubMed] [Google Scholar]
- Shelton DJ, Kirwan CB. 2013. A possible negative influence of depression on the ability to overcome memory interference. Behav Brain Res 256: 20–26. [DOI] [PubMed] [Google Scholar]
- Singh SM, McDonald P, Murphy B, O’Reilly R. 2004. Incidental neurodevelopmental episodes in the etiology of schizophrenia: An expanded model involving epigenetics and development. Clin Genet 65: 435–440. [DOI] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. 2008. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend 98: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. 2011. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476: 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. 1999. The dopamine hypothesis of reward: Past and current status. Trends Neurosci 22: 521–527. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, Bardo MT. 2006. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol 17: 597–604. [DOI] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, et al. 2008. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet 40: 751–760. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. 2006. Brain volume in first-episode schizophrenia: Systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 188: 510–518. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. 2009. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology 34: 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudai E, Croitoru O, Shaldubina A, Abraham L, Gispan I, Flaumenhaft Y, Roth-Deri I, Kinor N, Aharoni S, Ben-Tzion M, et al. 2011. High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict Biol 16: 251–260. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. 2004. The synaptic vesicle cycle. Annu Rev Neurosci 27: 509–547. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. 1995. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res 19: 110–122. [DOI] [PubMed] [Google Scholar]
- Sun M, Xing G, Yuan L, Gan G, Knight D, With SI, He C, Han J, Zeng X, Fang M, et al. 2011. Neuroligin 2 is required for synapse development and function at the Drosophila neuromuscular junction. J Neurosci 31: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. 2008. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 64: 293–301. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, et al. 2011. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16: 1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. 2010. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci 107: 11104–11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao K, Kobayashi K, Hagihara H, Ohira K, Shoji H, Hattori S, Koshimizu H, Umemori J, Toyama K, Nakamura HK, et al. 2013. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology 38: 1409–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenholz L, Jimenez JC, Kheirbek MA. 2014. Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Front Behav Neurosci 8: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MS, Devon RS, Millar JK, Porteous DJ. 2003. Evolutionary constraints on the disrupted in schizophrenia locus. Genomics 81: 67–77. [DOI] [PubMed] [Google Scholar]
- Thomson PA, Malavasi EL, Grunewald E, Soares DC, Borkowska M, Millar JK. 2013. DISC1 genetics, biology and psychiatric illness. Front Biol (Beijing) 8: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuunainen A, Wahlbeck K, Gilbody S. 2002. Newer atypical antipsychotic medication in comparison to clozapine: A systematic review of randomized trials. Schizophr Res 56: 1–10. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. 1999a. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci 96: 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. 1999b. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25: 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. 2004. Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry 161: 1957–1966. [DOI] [PubMed] [Google Scholar]
- Villarreal G, King CY. 2001. Brain imaging in posttraumatic stress disorder. Semin Clin Neuropsychiatry 6: 131–145. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Cheine M, Essali A, Adams C. 1999. Evidence of clozapine’s effectiveness in schizophrenia: A systematic review and meta-analysis of randomized trials. Am J Psychiatry 156: 990–999. [DOI] [PubMed] [Google Scholar]
- Walker DW, Freund G. 1971. Impairment of shuttle box avoidance learning following prolonged alcohol consumption in rats. Physiol Behav 7: 773–778. [DOI] [PubMed] [Google Scholar]
- Walker AK, Rivera PD, Wang Q, Chuang JC, Tran S, Osborne-Lawrence S, Estill SJ, Starwalt R, Huntington P, Morlock L, et al. 2015. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatry 20: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Zhou Y, Kogan JH, Shin R, Webster M, Gross AK, Heusner CL, Chen Q, Miyake S, Tajinda K, et al. 2012. Detection of an immature dentate gyrus feature in human schizophrenia/bipolar patients. Transl Psychiatry 2: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. 2000. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol 42: 248–257. [PubMed] [Google Scholar]
- Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. 2007. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet 39: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Belal C, Tu W, Chigurupati S, Ameli NJ, Lu Y, Chan SL. 2012. Chronic nicotine administration impairs activation of cyclic AMP-response element binding protein and survival of newborn cells in the dentate gyrus. Stem Cells Dev 21: 411–422. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. 1987. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44: 660–669. [DOI] [PubMed] [Google Scholar]
- Winner B, Kohl Z, Gage FH. 2011. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci 33: 1139–1151. [DOI] [PubMed] [Google Scholar]
- Won M, Minabe Y, Tani K, Suzuki K, Kawai M, Sekine Y, Ashby CR Jr, Takei N, Mori N. 2003. The effects of dentate granule cell destruction on behavioral activity and Fos protein expression induced by systemic MDMA in rats. Neurosci Res 46: 153–160. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. 2000. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157: 16–25. [DOI] [PubMed] [Google Scholar]
- Wu MV, Hen R. 2014. Functional dissociation of adult-born neurons along the dorsoventral axis of the dentate gyrus. Hippocampus 24: 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G, Russell S, Hough C, O'Grady J, Zhang L, Yang S, Zhang LX, Post R. 2002. Decreased prefrontal CaMKII α mRNA in bipolar illness. Neuroreport 13: 501–505. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nakamura K, Minabe Y, Iwayama-Shigeno Y, Takao H, Toyota T, Hattori E, Takei N, Sekine Y, Suzuki K, et al. 2004. Association analysis of FEZ1 variants with schizophrenia in Japanese cohorts. Biol Psychiatry 56: 683–690. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. 2004. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann NY Acad Sci 1025: 351–362. [DOI] [PubMed] [Google Scholar]
- Yamasaki N, Maekawa M, Kobayashi K, Kajii Y, Maeda J, Soma M, Takao K, Tanda K, Ohira K, Toyama K, et al. 2008. α-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Yang Y, Zhang Y, Lu T, Hu X, Wang L, Ruan Y, Lv L, Zhang D. 2011. A case-control association study of NRXN1 polymorphisms with schizophrenia in Chinese Han population. Behav Brain Funct 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Li W, Huang S, Song J, Kim JY, Tian X, Kang E, Sano Y, Liu C, Balaji J, et al. 2013. mTOR Inhibition ameliorates cognitive and affective deficits caused by Disc1 knockdown in adult-born dentate granule neurons. Neuron 77: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornoza T, Cano-Cebrian MJ, Martinez-Garcia F, Polache A, Granero L. 2005a. Hippocampal dopamine receptors modulate cFos expression in the rat nucleus accumbens evoked by chemical stimulation of the ventral hippocampus. Neuropharmacology 49: 1067–1076. [DOI] [PubMed] [Google Scholar]
- Zornoza T, Cano-Cebrian MJ, Miquel M, Aragon C, Polache A, Granero L. 2005b. Hippocampal dopamine receptors modulate the motor activation and the increase in dopamine levels in the rat nucleus accumbens evoked by chemical stimulation of the ventral hippocampus. Neuropsychopharmacology 30: 843–852. [DOI] [PubMed] [Google Scholar]