Abstract
Transforming growth factor β (TGF-β) and related growth factors are secreted pleiotropic factors that play critical roles in embryogenesis and adult tissue homeostasis by regulating cell proliferation, differentiation, death, and migration. The TGF-β family members signal via heteromeric complexes of type I and type II receptors, which activate members of the Smad family of signal transducers. The main attribute of the TGF-β signaling pathway is context-dependence. Depending on the concentration and type of ligand, target tissue, and developmental stage, TGF-β family members transmit distinct signals. Deregulation of TGF-β signaling contributes to developmental defects and human diseases. More than a decade of studies have revealed the framework by which TGF-βs encode a context-dependent signal, which includes various positive and negative modifiers of the principal elements of the signaling pathway, the receptors, and the Smad proteins. In this review, we first introduce some basic components of the TGF-β signaling pathways and their actions, and then discuss posttranslational modifications and modulatory partners that modify the outcome of the signaling and contribute to its context-dependence, including small noncoding RNAs.
The Smad-dependent TGF-β signaling pathway is involved in embryogenesis and tissue homeostasis. Key components of the pathway can be modified (e.g., via posttranslational modifications) to transmit context-dependent signals.
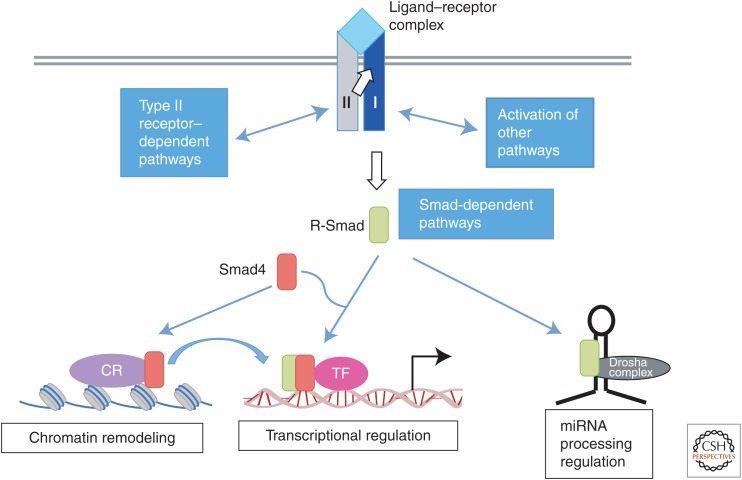
The transforming growth factor β (TGF-β) family of secreted growth and differentiation factors comprises more than 30 structurally related proteins. These ligands signal through cell-surface receptors, which are dual-specificity kinases, and intracellular Smad signal transducer proteins. On activation of the receptors, Smad proteins are phosphorylated by type I receptor kinase at the two carboxy-terminal serine residues and translocate into the nucleus to regulate gene expression (Fig. 1). In addition to Smad-dependent signaling, TGF-β receptors also activate several signaling pathways that are collectively called Smad-independent signaling or non-Smad signaling (Fig. 1) (Moustakas and Heldin 2009; Massagué 2012). Both Smad-dependent and Smad-independent signaling pathways are finely tuned to generate cell-type-specific or context-dependent signals through cross talk with other signaling pathways (Fig. 1). This review focuses on the Smad signaling pathway, its modes of regulation, and the Smad-mediated control of gene expression.
Figure 1.
Transforming growth factor β (TGF-β) receptors and signal transducers. TGF-β family ligands, shown in light blue, transmit signals by assembling a heterotetrameric receptor complex with two type I receptors, shown in dark blue, and two type II receptors, shown in gray. Upon ligand binding, signaling is transmitted by a cytoplasmic kinase domain of type I receptors by phosphorylating receptor-regulated Smad proteins (R-Smad proteins, green box). This is considered as the “Smad signaling pathway.” Additionally, the receptor complex can activate “non-Smad signaling pathways” through type II receptor– and type I receptor–interacting proteins. TGF-β and bone morphogenetic protein (BMP) receptors can also activate mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K) pathways. Activated R-Smad proteins form a complex with the common-Smad, Smad4 (co-Smad, shown in red box), and, as a complex, translocate to the nucleus, where they regulate transcription of target genes together with cofactors (pink circle). R-Smads also form a complex with chromatin remodeling proteins (CR, purple circle) that recognizes certain histone modifications and promotes formation of active chromatin, which is a prerequisite for transcriptional activation by R-Smad/co-Smad complexes. Additionally, R-Smad proteins can participate in microRNA (miRNA) processing by the Drosha microprocessor complex (black circle) for the biogenesis of a subset of primary transcripts of miRNA (pri-miRNAs).
BASIC COMPONENTS OF THE TGF-β/SMAD SIGNALING PATHWAY
All TGF-βs and TGF-β-related family of secreted factors bind and activate heteromeric cell-surface complexes of receptors that are classified as type I and type II based on their sequence similarities. Both types of receptor contain a cytoplasmic kinase domain that has both serine/threonine kinase activity and tyrosine kinase activity and are, hence, classified as dual-specificity kinases (Fig. 1) (ten Dijke and Heldin 2006). Below we summarize the various steps of receptors activation and the different regulatory factors that are critical modifiers of the signal received by the cytoplasmic transducers, the Smad proteins.
Upon binding to the dimeric ligands, two type I and two type II receptors assemble into heteromeric complexes that allow the type II receptors, which are constitutively active kinases, to phosphorylate the juxtamembrane regions of the cytoplasmic domains of the type I receptors, activating the type I receptor kinases (Moustakas and Heldin 2009; Massagué 2012; Xu et al. 2012; Weiss and Attisano 2013). Subsequently, the type I receptor kinases phosphorylate two serine (Ser) residues in the Ser–Ser–X–Ser sequence (known as “SSXS motif”) at the carboxy-terminal end of the receptor-regulated Smads (R-Smads). This event activates the R-Smads and enables the formation of heteromeric complexes between two R-Smads and one common-Smad (co-Smad), Smad4, and their translocation to the nucleus. Seven type I and five type II receptors exist in humans; based on the R-Smads that they phosphorylate, type I receptors can be further divided into two subgroups, those that activate Smad2 and Smad3 in response to TGF-β-like proteins, and those that activate Smad1, Smad5, and Smad8 in response to bone morphogenetic proteins (BMPs). Their specificities are determined by the L45 loop of the type I receptors and the L3 loop of Smads (Feng and Derynck 1997; Chen et al. 1998; Lo et al. 1998). In addition, the inhibitory Smads (I-Smads), Smad6 and Smad7, antagonize the signaling mediated by R-Smads and co-Smad. TGF-βs, in particular TGF-β1 and TGF-β3, appear to bind with the high affinity type II receptor, which then recruits the lower affinity type I receptor, whereas BMPs bind both type I and type II receptor with equal affinity and greater flexibility (Groppe et al. 2008; Huang et al. 2011).
The duration and intensity of the signals transmitted to the Smad proteins depend on the abundance and availability of ligands and their inhibitors, such as extracellular ligand-trapping proteins (e.g., noggin and Gremlin1, which trap BMP ligands) or antagonistic ligands (e.g., Lefty, which inhibits nodal binding to receptors) (Moustakas and Heldin 2009; Massagué 2012; Weiss and Attisano 2013). They also depend on the level of expression and cell-surface localization of type I and type II receptors, and on the posttranslational modifications of the receptors that modulate their kinase activities and substrate recognition.
RECEPTORS: ACTIVATION AND REGULATION OF THEIR ACTIVITIES
Receptor complex formation is essential for TGF-β signaling initiation. Upon ligand binding, TGF-β receptors form a hetero-oligomer, most likely containing two type I and two type II receptor molecules (Wrana et al. 1992, 1994; Yamashita et al. 1994; Massagué 1998; Massagué and Chen 2000; Feng and Derynck 2005). Biochemical and immunofluorescence copatching studies revealed that both type I (TβRI) and type II (TβRII) TGF-β receptors form ligand-independent homomeric complexes, and the binding of TGF-β to preformed homomeric TβRII leads to the formation of a heterotetrameric TβRI–TβRII complex (Chen and Derynck 1994; Henis et al. 1994; Gilboa et al. 1998). However, single-molecule imaging studies showed that, when receptors were expressed at amounts close to the endogenous levels, most TβRI and TβRII molecules are monomers, and TGF-β treatment causes dimerization of TβRII and then recruitment of TβRI, forming a heterotetrameric TβRI–TβRII complex (Zhang et al. 2009, 2010). As these studies are mainly based on ectopic expression systems due to lack of good antibodies for endogenous receptors, these discrepancies could be a result of the different expression levels of the receptors. It will be important to investigate this issue with other approaches, such as clustered regularly interspaced short palindromic repeat (CRISPR)-mediated knockin of tags to follow endogenous proteins. Although the formation of a multimeric complex between TβRI and TβRII is thought to be required for TGF-β signaling under physiological conditions, a study using a synthetic TGF-β3 dimer, consisting of one wild-type and one receptor-binding-deficient mutant, showed that the each pair of TβRI:TβRII heterodimers is sufficient for signaling (Huang et al. 2011).
Receptor Activation
The activities of type I and type II receptors are controlled by phosphorylation at multiple residues (Table 1). TβRII is thought to be constitutively active (Lin and Wang 1992; Lin et al. 1992), and its activity is influenced by phosphorylation. For instance, autophosphorylation at Ser213 and Ser409 is essential for signaling, whereas Ser416 phosphorylation exerts an inhibitory effect (Luo and Lodish 1997). In addition, TβRII can autophosphorylate tyrosine (Tyr) residues Tyr259, Tyr336, and Tyr424, and substitution of these three residues with phenylalanine blocks the receptor kinase activity (Lawler et al. 1997). Thus, TβRII, as well as TβRI, is a dual specificity kinase that can phosphorylate both serine/threonine and tyrosine residues (Lee et al. 2007). Additional phosphorylation sites have been identified in TβRII (Souchelnytskyi et al. 1996); however, the responsible kinase(s) and functional significance of this phosphorylation are yet to be elucidated.
Table 1.
Posttranslational modifications of transforming growth factor β (TGF-β) receptors
| Receptors | Sites | Mediators | Other regulators | Functions | References | |
|---|---|---|---|---|---|---|
| Phosphorylation | TβRI | GS domain | TβRII | TβRI activation | Wrana et al. 1994; Wieser et al. 1995 | |
| S165 | TβRII | Required for TGF-β-mediated apoptosis, but attenuating growth inhibition and extracellular matrix (ECM) production | Souchelnytskyi et al. 1996 | |||
| Tyrosine | Unknown | Extracellular signal-regulated kinase (ERK) activation | Lee et al. 2007 | |||
| TβRII | S213, S409 | Autophosphorylation | TβRII activation | Luo and Lodish 1997 | ||
| S416 | Autophosphorylation | TβRII inhibition | Luo and Lodish 1997 | |||
| Y259, Y336, Y424 | Autophosphorylation | TβRII activation | Lawler et al. 1997 | |||
| Dephosphorylation | TβRI | Protein phosphatase 1 | Smad7, SARA, GADD34 | Inhibition of TGF-β signaling | Bennett and Alphey 2002; Shi et al. 2004 | |
| Ubiquitylation | TβRI | Smurf1/2, WWP1, NEDD4-2 | Smad7 | Receptor degradation | Kavsak et al. 2000; Ebisawa et al. 2001; Komuro et al. 2004; Kuratomi et al. 2005 | |
| TβRII | Unknown | Receptor degradation | Atfi et al. 2007; Zuo et al. 2013 | |||
| BMPRI, BMPRII | Smurf1 | Smad7 | Receptor degradation | Murakami et al. 2010 | ||
| BMPRII | Itch | Durrington et al. 2010 | ||||
| Deubiquitylation | TβRI | UCH37 | Smad7 | Receptor stabilization | Wicks et al. 2005 | |
| USP4 | Receptor stabilization | Zhang et al. 2012 | ||||
| USP15 | TRAF4 | Receptor stabilization | Zhang et al. 2013a | |||
| USP11 | Smad7? | Receptor stabilization | Al-Salihi et al. 2012 | |||
| Sumoylation | TβRI | K389 | Unknown | Receptor activation | Kang et al. 2008 | |
| Neddylation | TβRII | K556, K567 | c-Cbl | Receptor stabilization | Zuo et al. 2013 |
GADD34, Growth arrest and DNA damage protein; SARA, Smad anchor for receptor activation; TRAF4, tumor necrosis factor receptor-associated factor 4; USP, ubiquitin-specific protease.
TGF-β ligand binding brings the type I receptor to the type II receptor at the cell surface and promotes phosphorylation of TβRI by TβRII, which is essential for TβRI activation (Cárcamo et al. 1995). Major sites of TβRI phosphorylation by TβRII are serine and threonine residues in a region that precedes the kinase domain and is enriched in glycine and serine residues, hence its name “GS domain” (Wrana et al. 1994). Phosphorylation of any four of the five serine or threonine residues in the GS domain (TTSGSGSG) appears sufficient for TβRI activation and signal transduction (Wieser et al. 1995). A similar mechanism involving GS domain phosphorylation activates other TGF-β family type I receptors, including those for activins and BMPs (Willis et al. 1996; Massagué 1998). TGF-β can also induce tyrosine phosphorylation of TβRI by yet-to-be-identified kinases, which contributes to TGF-β-induced extracellular signal-regulated kinase (ERK) activation (Lee et al. 2007). As phosphorylation is necessary for TβRI activation, dephosphorylation should turn off its activity. Indeed, protein phosphatase 1 (PP1) has been shown to dephosphorylate TβRI and antagonize TGF-β signaling (Bennett and Alphey 2002; Shi et al. 2004), an effect that is mediated by the membrane-associated protein Smad anchor for receptor activation (SARA) and by recruitment of the catalytic subunit of PP1 (PP1c) to the receptor (Bennett and Alphey 2002). The I-Smad Smad7 interacts with GADD34 (growth arrest and DNA damage-inducible protein 34), a regulatory subunit of the PP1 holoenzyme, within the receptor complex, and, thus, cooperates with SARA to enforce the phosphatase activity of PP1 toward TβRI (Shi et al. 2004). The regulatory subunit Bα of PP2A (Ser/Thr protein phosphatase 2A) has also been shown to interact with TβRI through its WD40 domain in response to TGF-β treatment and enhance TGF-β-induced growth inhibition, presumably via the kinase p70S6K (p70 S6 kinase) (Griswold-Prenner et al. 1998; Petritsch et al. 2000), but it is unclear whether PP2A modulates receptor phosphorylation. No phosphatases have been identified for TβRII so far.
Regulation of Receptor Activity by Other Posttranslational Modifications
In addition to phosphorylation, the receptor activity is regulated by a variety of posttranslational modifications (Table 1) (Kang et al. 2009; Huang and Chen 2012; Xu et al. 2012). Both TβRI and TβRII are polyubiquitylated, leading to receptor degradation (Atfi et al. 2007; Kang et al. 2009; Imamura et al. 2013; Zuo et al. 2013). Little is known about TβRII ubiquitylation (Fukasawa et al. 2010), although much attention has been devoted to TβRI ubiquitylation. Several E3 ubiquitin ligases, such as HECT (homologous with E6-associated protein carboxyl terminus) domain-containing Smurf1 (Smad-specific E3 ubiquitin protein ligase 1), Smurf2, WWP1 (WW domain containing E3 ubiquitin protein ligase 1, also known as TGIF-interacting ubiquitin ligase 1 or Tiul1), and NEDD4-2 (neural precursor cell expressed, developmentally down-regulated protein 4-2, also known as NEDD4L), have been shown to mediate TβRI ubiquitylation, all by interaction with Smad7 as an adaptor to TβRI (Kavsak et al. 2000; Ebisawa et al. 2001; Komuro et al. 2004; Kuratomi et al. 2005).
Like phosphorylation, ubiquitylation is a reversible process. Several deubiquitylating enzymes have been reported to remove ubiquitin from TβRI, leading to receptor stabilization and enhanced TGF-β signaling, including UCH37 (ubiquitin carboxy-terminal hydrolase 37), USP4 (ubiquitin-specific protease 4), USP11, and USP15 (Wicks et al. 2005; Al-Salihi et al. 2012; Zhang et al. 2012, 2013a). As both UCH37 and USP11 require Smad7 to bind TβRI, a question to be addressed is how Smad7 regulates the level of TβRI ubiquitylation by recruiting both E3 ubiquitin ligases and deubiquitylating enzymes (Wicks et al. 2005; Al-Salihi et al. 2012). Additional regulators could be involved; for instance, Hsp90 (heat shock protein 90) interacts with and stabilizes both TβRI and TβRII by blocking Smurf2-mediated ubiquitylation (Wrighton et al. 2008).
Sumoylation is a ubiquitylation-like posttranslational modification, regulating protein activity and subcellular localization (Flotho and Melchior 2013). TβRI, but not other type I receptors, can be sumoylated (Kang et al. 2008). The sumoylation at Lys389 in the kinase domain of TβRI is important for TGF-β signaling, but its mediator is unknown. As TβRI sumoylation is induced by TGF-β and requires the kinase activities of TβRI and TβRII (Kang et al. 2008), receptor phosphorylation may control this process.
TβRII can be “neddylated” (i.e., linked to the ubiquitin-like protein NEDD8) (Zuo et al. 2013), a modification that regulates protein activity, subcellular localization, and stability (Rabut and Peter 2008; Watson et al. 2011). TβRII neddylation is mediated by the proto-oncogene c-Cbl, an E3 ligase for both ubiquitin and NEDD8 (Thien and Langdon 2001; Oved et al. 2006), and stabilizes TβRII by antagonizing its ubiquitylation and lipid raft-mediated endocytosis (Zuo et al. 2013).
Regulation of Receptors by Their Interacting Proteins
Numerous proteins have been reported to interact with TGF-β receptors (Table 2), many of them as negative regulators (reviewed in Kang et al. 2009; Lönn et al. 2009). The best-characterized protein is the I-Smad, Smad7, which associates with activated type I receptors (Hayashi et al. 1997; Nakao et al. 1997). Smad7 antagonizes TGF-β signaling through multiple mechanisms, including interfering with R-Smad recruitment, promoting receptor dephosphorylation, recruiting E3 ubiquitin ligases to induce receptor degradation, and blocking the functional Smad complex from interacting with DNA in the nucleus (reviewed in Yan and Chen 2011). The pseudoreceptor BAMBI (BMP and activin membrane-bound inhibitor) inhibits TGF-β family signaling by directly forming a complex with TGF-β, activin, and/or BMP receptors to generate an inactive receptor complex (Onichtchouk et al. 1999; Sekiya et al. 2004a), or by binding and enforcing the inhibitory effect of Smad7 on TGF-β signaling (Yan et al. 2009). BAMBI expression is induced by Wnt/β-catenin signaling and repressed by Toll-like receptor 4 (TLR4) signaling, thus allowing for cross talk of these pathways with TGF-β signaling (Sekiya et al. 2004b; Seki et al. 2007). Serine-threonine kinase receptor-associated protein (STRAP), a WD domain-containing protein, can interact with both TβRI and TβRII (Datta et al. 1998) and promote Smad7 binding to the activated TβRI, leading to inhibition of TGF-β signaling (Datta et al. 1998; Datta and Moses 2000). Tollip, which contains both ubiquitin-associated domains and an endosome-targeting domain, can interact with both Smad7 and ubiquitylated TβRI to promote TβRI degradation via the endocytic pathway (Zhu et al. 2012). The salt-inducible kinase (SIK), a TGF-β-inducible gene, was reported to cooperate with Smad7 to induce degradation of the activated TβRI, creating another negative feedback regulation of TGF-β signaling (Kowanetz et al. 2008). In addition, the basal activities of the TGF-β family type I receptors are controlled by FKBP12 (Wang et al. 1996; Chen et al. 1997; Huse et al. 1999; Spiekerkoetter et al. 2013), possibly by preventing the spontaneous formation of type I and type II receptor complex (Chen et al. 1997) or by forming a complex with Smad7 and Smurf1 and promoting ubiquitylation and degradation of type I receptors (Yamaguchi et al. 2006). Two WD-repeat proteins that regulate translation initiation factors (eIFs), eIF2α and eIF3 (also called TRIP-1 for TGF-β receptor-interacting protein-1), associate with and are phosphorylated by TβRII. Both of them exert an inhibitory effect on TGF-β signaling (Chen et al. 1995; Choy and Derynck 1998; McGonigle et al. 2002).
Table 2.
Transforming growth factor β (TGF-β)-receptor-interacting proteins
| Interacting partners |
Functions | References |
|---|---|---|
| TβRI | ||
| 14-3-3ɛ | Enhance TGF-β signaling | McGonigle et al. 2001 |
| BAMBI | Interfere with receptor activation and Smad phosphorylation | Onichtchouk et al. 1999; Yan et al. 2009 |
| Caveolin-1 | Promote TβRI degradation | Razani et al. 2001; Nohe et al. 2005; Hartung et al. 2006 |
| c-Ski | Block Smad2 release from TβRI | Ferrand et al. 2010 |
| Dab2 | Enhance the clathrin-mediated endocytosis of TβRI and inhibit TGF-β-induced JNK activation | Shapira et al. 2014 |
| Dapper2 | Target receptors for lysosomal degradation | Zhang et al. 2004; Su et al. 2007 |
| DRAK2 | Interfere with the recruitment of Smad2 and 3 to TβRI | Yang et al. 2012 |
| Endofin | Facilitate Smad activation | Chen et al. 2007 |
| FKBP12 | Block receptor complex formation and inhibit the basal TGF-β signaling | Chen et al. 1997 |
| FKBP12 | Attenuate TβRI internalization | Yao et al. 2000 |
| Hsp90 | Stabilize both TβRI and TβRII by blocking Smurf2-mediated ubiquitylation | Wrighton et al. 2008 |
| Hrs/Hgs | Facilitate activation of Smad2 and Smad3 | Miura et al. 2000 |
| PICK1 | Enhance its ubiquitylation and degradation in lipid rafts/caveolae | Zhao et al. 2012 |
| Regulatory subunit Bα of PP2A | Enhance TGF-β-induced growth inhibition | Griswold-Prenner et al. 1998; Petritsch et al. 2000 |
| SARA | Recruit the catalytic subunit of PP1 to TβRI to inactivate the receptor | Bennett and Alphey 2002 |
| SARA | Facilitate activation of Smad2 and Smad3 | Tsukazaki et al. 1998 |
| ShcA | Promote the caveolar localization of TβRI, attenuate Smad3 signaling and activate Erk MAP kinase | Lee et al. 2007; Muthusamy et al. 2015 |
| Smad7-UCH37 | Induce TβRI deubiquitylation and stabilization | Wicks et al. 2005 |
| Smad7-GADD34 | Recruit PP1 to TβRI to inactivate the receptor | Shi et al. 2004 |
| Smad7-NEDD4-2 | Induce TβRI ubiquitylation and degradation | Kuratomi et al. 2005 |
| Smad7-SIK | Induce degradation of the activated TβRI | Kowanetz et al. 2008 |
| Smad7-Smurf1/2 | Induce TβRI ubiquitylation and degradation | Kavsak et al. 2000; Ebisawa et al. 2001 |
| Smad7-USP11 | Induce TβRI deubiquitylation and stabilization | Al-Salihi et al. 2012 |
| Smad7-WWP1 | Induce TβRI ubiquitylation and degradation | Komuro et al. 2004 |
| STRAP | Promote Smad7 binding to the activated TβRI, leading to inhibition of TGF-β signaling | Datta and Moses 2000 |
| Tollip | Interact with both Smad7 and ubiquitylated TβRI and promote TβRI degradation | Zhu et al. 2012 |
| TRAF4-USP15 | Induce TβRI deubiquitylation and stabilization | Zhang et al. 2013a |
| TRAF6 | Mediate TβRI cleavage by TACE and γ-secretase | Gudey et al. 2014 |
| TRAF6 | Mediate p38 MAP kinase activation | Yamashita et al. 2008 |
| TSC-22 | Stabilize TβRI by impairing the association of Smad7/Smurfs to the receptor | Yan et al. 2011 |
| VEPH1 | Block Smad2 release from TβRI | Shathasivam et al. 2015 |
| TβRII | ||
| ADAM12 | Block TβRII internalization into caveolin1-positive vesicles and stabilize receptor | Atfi et al. 2007 |
| c-Cbl | Induce TβRII neddylation and stabilization | Zuo et al. 2013 |
| elF2α | Inhibit TGF-β signaling | McGonigle et al. 2002 |
| elF3/TRIP-1 | Inhibit TGF-β signaling | Chen et al. 1995; Choy and Derynck 1998 |
| Par6 | Mediate TGF-β-induced EMT | Ozdamar et al. 2005 |
ADAM12, A disintegrin and metalloproteinase 12; BAMBI, BMP and activin membrane-bound inhibitor; Endofin, endosome-associated FYVE-domain protein; GADD34, growth arrest and DNA damage protein; EMT, epithelial to mesenchymal transition; PP1, protein phosphatase 1, PP2A: protein phosphatase 2A; SARA, Smad anchor for receptor activation; SIK, salt-inducible kinase; STRAP, serine-threonine kinase receptor-associated protein; TRAF, tumor necrosis factor receptor-associated factor; TRIP-1, TGF-β receptor-interacting protein-1; TSC-22, TGF-β-stimulated clone 22; USP, ubiquitin-specific protease; WWP1, WW domain-containing protein 1; TACE, tumor necrosis factor-α converting enzyme.
In contrast to the above-mentioned proteins that negatively regulate receptor activity, a few proteins that facilitate TGF-β signaling have been identified. TSC-22 (TGF-β-stimulated clone 22) is a TGF-β target that suppresses cell proliferation and promotes differentiation (Kawamata et al. 2004). It may promote TGF-β signaling by interacting with Smad4 and enhancing the transcriptional activities of Smad proteins (Choi et al. 2005) or by interacting with TβRI and Smad7 in a mutually exclusive manner to impair the association of Smad7 and Smurfs with TβRI, thereby preventing receptor degradation (Yan et al. 2011). 14-3-3ɛ has also been shown to interact with TβRI and enhance TGF-β signaling (McGonigle et al. 2001), but the underlying mechanism for this enhancement is unclear.
Proteolytic Cleavage of TβRI
Many cell-surface receptors are proteolytically cleaved to release their extracellular or intracellular fragments. Two groups have reported proteolytic cleavage of TβRI. Liu et al. (2009) found that on Erk mitogen-activated protein kinase (MAPK) activation, the metalloproteinase tumor necrosis factor-α converting enzyme (TACE, also known as ADAM17) cleaves TβRI, but not TβRII. This cleavage decreases the cell-surface level of TβRI and down-regulates TGF-β-mediated Smad3 activation, antiproliferation and epithelial-mesenchymal transition. It has also been reported that, following TACE-mediated cleavage of TβRI at Gly120 and Leu121, the released intracellular fragment translocates to the nucleus, interacts with the p300 acetyltransferase, activates invasion-related genes and promotes TGF-β-mediated invasiveness of cancer cells (Mu et al. 2011). The binding of tumor necrosis factor receptor-associated factor 6 (TRAF6) to TβRI is required for this process as TRAF6 recruits protein kinase C ζ (PKC-ζ), which regulates the subcellular localization of TβRI and promotes TACE-mediated TβRI cleavage (Mu et al. 2011). Defining the physiological role of the cleaved TβRI intracellular fragment requires further investigation. TACE can also cleave the transmembrane protein vasorin to release a soluble form that binds and inhibits TGF-β (Malapeira et al. 2011). TACE expression can be induced by TGF-β (Lu et al. 2011). In addition, genetic polymorphism analysis revealed that TACE functions in angiogenesis by acting as a modifier of TGF-β signaling in mice and humans (Kawasaki et al. 2014). These observations add multiple layers of complexity to the TACE/TGF-β relationship and shed light on the critical role of TACE as a modifier of the TGF-β signaling pathway. TRAF6 can also elicit TβRI proteolytic cleavage by recruiting presenilin 1, a catalytic subunit of the γ-secretase complex (Gudey et al. 2014). γ-Secretase can cleave numerous transmembrane proteins, including amyloid protein precursor and Notch (De Strooper et al. 2012). The presenilin 1–mediated cleavage occurs between Val129 and Ile130 in the transmembrane domain of TβRI after the TACE cleavage and is promoted by TGF-β stimulation (Gudey et al. 2014). Like the TACE-cleaved TβRI intracellular fragment, the presenilin 1–released fragment stimulates the expression of cell-invasion-related genes, such as Snai1 (encoding Snail) and Jag1 (Jagged1) (Gudey et al. 2014). A recent study reported that TRAF6-mediated TβRI cleavage is regulated by the scaffold protein Ran-binding protein M (RanBPM), which interacts with TβRI and prevents its association with TRAF6 and its subsequent cleavage and nuclear accumulation of the intracellular fragment (Zhang et al. 2014). As this fragment was observed in the nuclei of malignant tumors (Mu et al. 2011; Gudey et al. 2014), these results underscore the role of the finely regulated proteolytic cleavage of TβRI in tumorigenesis.
THE SMAD FAMILY OF SIGNALING MEDIATORS
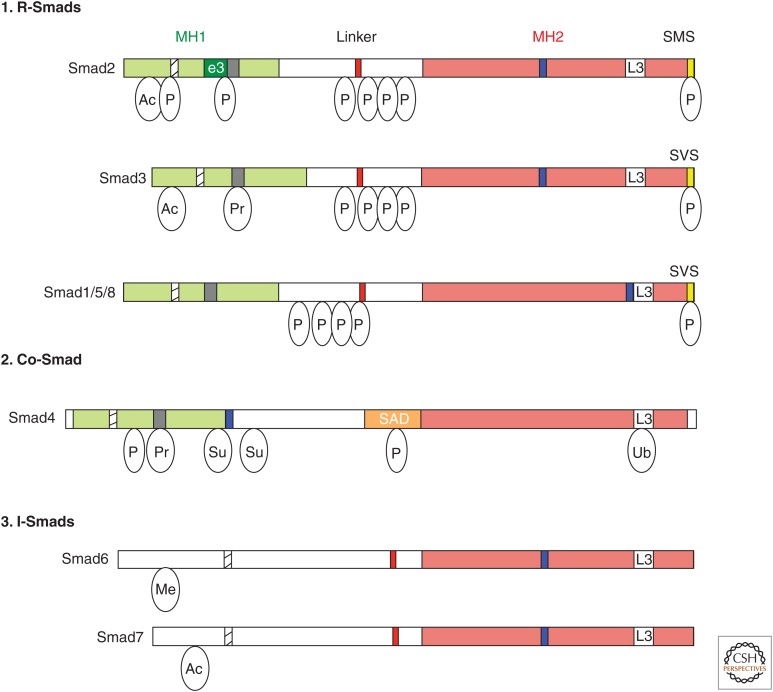
TGF-β family ligands exhibit context-dependent activities mainly by regulating gene expression through receptor-mediated activation of Smad proteins. As critical mediators of TGF-β signaling, various modes of regulation feed into the Smad proteins to modulate signal intensity, duration, and specificity (Massagué 2012). Both R-Smad and co-Smad proteins comprise two highly conserved domains known as Mad homology 1 (MH1) and MH2 domains (Fig. 2). The amino-terminal MH1 domain contains nuclear localization signals and a β-hairpin structure that is critical for DNA binding (Fig. 2) and association with a subset of microRNA (miRNA) primary transcripts (pri-miRNAs). The carboxyl MH2 domain encompasses the L3 loop structure that specifies the interaction of the R-Smads with type I receptors (Lo et al. 1998), leading to the phosphorylation of the carboxy-terminal SSXS motif of R-Smads by the type I receptor kinases. The MH2 domain of Smad4 contains an L3 loop (Lo et al. 1998), but lacks the carboxy-terminal SSXS motif, and is not phosphorylated by the type I receptor (Fig. 2). The type I receptor–mediated phosphorylation of the carboxy end of R-Smads triggers its association with the MH2 domain of Smad4. The heteromeric Smad complex, which is a trimer of two R-Smads and one Smad4, translocates to the nucleus and binds to DNA through the MH1 domain (Massagué 2012). In the nucleus, the MH2 domain interacts with various nuclear factors and controls gene expression by modulating transcription or the epigenetic landscape (Moustakas and Heldin 2009; Massagué 2012; Weiss and Attisano 2013).
Figure 2.
The Smad family. Schematic representations of the eight human Smad proteins divided into (1) receptor-regulated Smads (R-Smads), (2) common-Smad (co-Smad), and (3) inhibitory-Smads (I-Smads). The conserved amino-terminal Mad-homology 1 (MH1) and carboxy-terminal MH2 domains are indicated as green and red boxes, respectively. Highlighted are the nuclear localization signal (NLS, hatched box), the unique insert in Smad2-MH1 domain, which corresponds to exon 3 (e3, dark green box), the β-hairpin in the MH1 domain that binds DNA or the stem region of a subset of primary microRNAs (pri-miRNAs) (black box), the proline-tyrosine (PPXY) motif (red box) in the linker domain that is recognized by the WW domain of Smurf family proteins, the Smad activation domain (SAD, orange box) at the linker-MH2 border of Smad4, the nuclear export signal (NES, blue box), and the L3 loop of the MH2 domain (white box). The carboxy-terminal serine residues in the SXS motif that is phosphorylated by the type I receptor kinases are shown in the yellow box. Relative locations of different posttranslational modifications identified in Smad proteins are indicated. Ac, Acetylation; Ub, ubiquitylation; Pr, poly(ADP)ribosylation; Su, sumoylation; P, Ser or Thr phosphorylation.
The MH1 and MH2 domains are connected by a linker region, which is not conserved among Smad proteins. The linker regions of R-Smads contain multiple serine and threonine residues that are phosphorylated by kinases, such as cyclin-dependent kinases (CDKs), MAPKs, and glycogen synthase kinase 3 (GSK3) (Massagué 2012). Phosphorylation of R-Smads in the linker region controls their nuclear residency and creates docking sites for positive and negative modulators of nuclear R-Smad (Massagué 2012). Smad linker phosphorylation is discussed in detail below (Fig. 2).
The third class of Smad proteins represents the I-Smads, which antagonize the TGF-β signaling pathway by R-Smads and co-Smad (Fig. 2). Unlike R-Smads, I-Smads, which include Smad6 and Smad7, lack an MH1 domain and the SSXS motif, but retain a conserved MH2 domain and negatively regulate signaling (Fig. 2). Both TGF-β- and BMP-specific Smad complexes induce the expression of I-Smads to form a negative feedback loop. I-Smads antagonize the Smad signaling pathway at multiple levels by (1) associating with the type I receptor, (2) recruiting Smurf1 or Smurf2 E3 ubiquitin ligases, (3) binding the receptor-phosphorylated R-Smads and interfering with the association with co-Smad, or (4) interacting with DNA and nuclear Smad complexes. Smad7 acts as a general inhibitor of all TGF-βs, whereas Smad6 preferentially blocks BMP signaling (Moustakas and Heldin 2009; Massagué 2012; Weiss and Attisano 2013).
ACTIVATION OF Smads BY THE RECEPTOR COMPLEXES
Upon ligand binding, TβRIi-mediated phosphorylation of the GS domain of TβRI induces a conformational change that activates the TβRI kinase and enhances the binding affinity of the receptor for Smad2 and Smad3 (Huse et al. 1999, 2001), by creating a binding site for a highly basic surface patch in the MH2 domain of R-Smads (Wu et al. 2000). Phosphorylation of the last two serine residues (SXS) at the carboxy end of R-Smads (Abdollah et al. 1997; Kretzschmar et al. 1997; Liu et al. 1997; Souchelnytskyi et al. 1997) by the type I receptor enables the association of R-Smads with co-Smad (Smad4) and, consequently, the interaction of the Smad complex with other factors to regulate gene expression in the nucleus (Shi and Massagué 2003; Feng and Derynck 2005).
Presentation of Smads to Receptors
Several receptor- or Smad-interacting proteins regulate the activation of Smad2 and Smad3. The FYVE domain-containing protein SARA has been described earlier. It promotes the activation of Smad2 and Smad3 by binding and recruiting to the receptor, and facilitating phosphorylation by the receptor kinase (Tsukazaki et al. 1998; Wu et al. 2000). The FYVE domain associates with phosphatidylinositol-3-phosphate, which is enriched in the early endosome (Schink et al. 2013), where SARA facilitates TGF-β/Smad signaling (Itoh et al. 2002) with the help of the promyelocytic leukemia (PML) tumor suppressor (Lin et al. 2004). Another FYVE domain-containing protein, hepatic growth factor-regulated tyrosine kinase substrate (Hrs/Hgs), has also been shown to interact with Smad2, and may play a role similar to SARA to promote TGF-β/activin signaling (Miura et al. 2000). Transmembrane prostate androgen-induced (TMEPAI) interacts with Smad2 and Smad3 and competes with SARA for Smad binding, thereby attenuating Smad activation (Watanabe et al. 2010). As TMEPAI expression is induced by TGF-β stimulation, it generates a negative feedback loop to control TGF-β signaling (Watanabe et al. 2010). Like TMEPAI, its homolog C18 ORF1 can also interfere with the interaction of Smad2 and Smad3 with SARA and, thus, attenuates Smad recruitment to TβRI (Nakano et al. 2014). Similarly, ERBIN (ERBB2/HER2-interacting protein), a SARA-interacting protein, inhibits TGF-β signaling by competing with SARA for the association with Smad2 and Smad3 and inhibiting their activation (Sflomos et al. 2011). Although all of these studies support the role of SARA in promoting TGF-β signaling, it was reported that silencing SARA expression using small interfering RNA (siRNA) has no effect on TGF-β signaling in HeLa cells (Bakkebo et al. 2012), which might be caused by incomplete down-regulation of SARA expression and/or a redundant functions of Hrs/Hgs. Nonetheless, more genetic evidence, such as targeted inactivation of SARA expression, is required to clarify the role of SARA in TGF-β signaling. Endofin (endosome-associated FYVE-domain protein), a FYVE domain-containing and early endosome-localized protein that shares sequence similarity with SARA (Seet and Hong 2001) can enhance TGF-β signaling by facilitating Smad4 recruitment to the activated Smad2 and Smad3 (Chen et al. 2007). Endofin also acts as an anchor for BMP-specific R-Smad in the context of BMP receptor-dependent activation, analogous to the function of SARA in TGF-β receptor signaling (Shi et al. 2007; Goh et al. 2015). The molecular mechanism underlying endofin’s multiple activities and the regulation of each activity need to be resolved in the future.
It was originally proposed that Axin, a negative regulator of Wnt signaling, interacts with Smad3 and facilitates its activation by TGF-β under conditions, in which Smad3 is expressed at higher than normal levels (Furuhashi et al. 2001), but it was later found that Axin reduces the level of Smad3 protein by promoting ubiquitylation and degradation (Guo et al. 2008). Furthermore, Axin acts as a scaffold protein to bring together Smad7 and the E3 ubiquitin ligase RNF111 (RING finger protein 111, also known as Arkadia) to promote Smad7 degradation (Liu et al. 2006). Therefore, the regulatory role of Axin in TGF-β signaling is pleiotropic and likely context-dependent. Dok-1 (Docking protein 1, also known as p62), a RasGAP (Ras GTPase-activating protein)-binding protein, has been reported to interact with Smad3 and with both the type I and type II activin receptors, therefore acting as an adaptor to bridge the activin receptors with Smad proteins to promote B-cell apoptosis (Yamakawa et al. 2002). DRAK2, which is a death-associated protein kinase (DAPK) family member and was identified by mass spectrometry-based proteomic screen of TβRI-associated proteins, is induced by TGF-β and antagonizes TGF-β/Smad signaling by interfering with the recruitment of Smad2 and 3 to TβRI (Yang et al. 2012). Ventricular zone expressed PH domain-containing 1 (VEPH1), the human ortholog of Drosophila Melted, however, blocks TGF-β by impeding Smad2 release from TβRI (Shathasivam et al. 2015). Similarly, c-Ski, which disrupts the functional R-Smad–Smad4 complex or represses Smad transcriptional activity (Deheuninck and Luo 2009), can also impair the activation and subsequent nuclear translocation of Smad2 by inducing its stable interaction with TβRI (Ferrand et al. 2010).
Several proteins have been indicated to balance TGF-β-induced Smad signaling against non-Smad signaling. In addition to its regulatory function in modulating the trafficking of TβRII from the early endosome to the recycling endosome (Penheiter et al. 2010), the adaptor protein Disabled-2 (Dab2) can associate with and facilitate the activation of Smad2 and Smad3 by TGF-β (Hocevar et al. 2001). Dab2 also interacts with TβRI, enhances its clathrin-mediated endocytosis, and inhibits TGF-β-induced JNK (c-Jun amino-terminal kinase) activation (Shapira et al. 2014). In contrast, the adaptor protein ShcA (also known as Shc1) can sequester TβRI in caveolin-1-positive compartments and promote Erk and Akt signaling while attenuating Smad3 signaling (Muthusamy et al. 2015). These results are consistent with the requirement of lipid raft localization of TGF-β receptors for TGF-β-mediated MAPK activation (Zuo and Chen 2009). The membrane compartmentalization of TβRI is also regulated by PICK1 (protein interacting with carboxykinase 1) that enhances the TβRI interaction with caveolin-1 and promotes the caveolae-mediated internalization of TβRI, enhancing its degradation (Zhao et al. 2012). Similarly, the localization of BMP receptors in distinct membrane domains also modulates BMP signaling. BMP receptors mediate BMP-induced Smad1 and Smad5 phosphorylation in non-raft membrane regions, whereas their localization in lipid rafts is required for BMP-stimulated expression of alkaline phosphatase (Hartung et al. 2006). Several other receptor-interacting proteins link TGF-β receptors to non-Smad signaling, such as TRAF6 in TGF-β-mediated activation of MAPKs and Par6 in TGF-β-induced epithelial-mesenchymal transition (Table 2).
Specificity of Smad Activation by the Receptors
Two major Smad pathways are activated in response to TGF-β family proteins. In response to TGF-β and TGF-β/activin-like proteins, Smad2 and Smad3 are specifically phosphorylated at their carboxy-terminal tails by the type I receptors ACVR1B/ActRIB/ALK-4, TβRI/ALK-5, and ACVR1C/ALK-7, whereas in response to BMPs and related proteins Smad1, Smad5, and Smad8 are activated by the ACVRL1/ALK-1, ACVR1/ALK-2, BMPRIA/ALK-3, and BMPRIB/ALK-6 receptors (Massagué and Chen 2000; Feng and Derynck 2005). Considering that the receptors and R-Smads are highly conserved, great attention has been given to how the specific activation of R-Smads is achieved. Detailed functional mapping of the regions in the TβRI/ALK-5 receptor identified a critical role of the L45 loop between its kinase subdomains IV and V in specifying TGF-β responses (Feng and Derynck 1997). Swapping the L45 loop sequence between the TβRI/ALK-5 and BMPRIB/ALK-6 receptors can switch the signaling specificity in Smad activation and transcriptional responses (Chen et al. 1998; Persson et al. 1998). The L45 loop is not required for the kinase activity of the type I receptors, but determines the specificity of the Smad interaction (Chen et al. 1998; Yu et al. 2002; Itoh et al. 2003). Similarly, the search for the regions in Smad2 that is important for the interaction with TβRI/ALK-5 identified the L3 loop in the MH2 domain (Lo et al. 1998). The exchange of two amino acid residues in the L3 loop sequence of human Smad1 (His425 and Asp428) and human Smad2 (Arg427, Thr430) can switch the specific receptor-Smad interaction and Smad activation. The L45 loop of the type I receptors functionally interacts with the L3 loop of R-Smads (Chen et al. 1998; Wu et al. 2000). Interestingly, although the L45 loop of TβRI/ALK-5 is essential for Smad signaling, it is not important for TGF-β-induced activation of the MAP kinases p38 and JNK (Yu et al. 2002; Itoh et al. 2003).
NUCLEOCYTOPLASMIC SHUTTLING AND INTRACELLULAR MOVEMENT OF Smads
Regardless of the presence or absence of ligands, Smads constantly shuttle between the cytoplasm and the nucleus. Receptor-mediated phosphorylation and association with Smad4 retain R-Smads in the nucleus, where they function as transcription factors and miRNA regulators. Therefore, nuclear transport offers another level of regulation in the control of Smad activity (Reguly and Wrana 2003; Xu and Massagué 2004; Hill 2009). Smad proteins can be transported into the nucleus via importin-mediated or nuclear pore protein-mediated mechanisms. Although Smad1, Smad2, Smad3, and Smad4 contain Lys-rich nuclear localization signal (NLS)-like motifs in the MH1 domains (Fig. 1), their modes of nuclear import are distinct. Through their NLS-like motifs, Smad3 and Smad4 interact with and are transported by importin-β and importin-α, respectively (Xiao et al. 2000, 2003b; Kurisaki et al. 2001). Smad2, Smad3, and Smad4 can also be imported into the nucleus by directly interacting with the nuclear pore proteins Nucleoporin 153 (Nup153) and Nup214 (Xu et al. 2002, 2003). In addition, importin 7 and 8 and their Drosophila ortholog Msk may also mediate nuclear import of Smad1, Smad2, and Smad3 (Xu et al. 2007).
The nuclear export of Smad4 is mediated by exportin 1 (also known as CRM1), as apparent by the nuclear accumulation of Smad4 in the presence of the exportin 1 inhibitor leptomycin B, even in the absence of ligand (Pierreux et al. 2000; Watanabe et al. 2000), and requires a nuclear export signal (NES) in the linker region of Smad4 (Fig. 2) (Watanabe et al. 2000). Smad1 uses a similar mechanism for nuclear export and two NES sequences have been identified (Xiao et al. 2001, 2003a). Although these two NES sequences are conserved in Smad2 and Smad3, they do not mediate the nuclear export of Smad2 and Smad3. The nuclear export of Smad3 is instead mediated by exportin 4 and a Ran GTPase (Kurisaki et al. 2006). RanBP3, which is known as a cofactor of exportin 1 (Lindsay et al. 2001), preferentially recognizes dephosphorylated Smad2 and Smad3 and exports them from the nucleus (Dai et al. 2009). Exportin 4 and RanBP3 do not display extensive sequence similarity, and it is unknown whether they share the same export machinery. In a similar manner, RanBP3L recognizes dephosphorylated Smad1, Smad5, and Smad8 and mediates their nuclear export in a Ran-dependent fashion (Chen et al. 2015).
Several models have been proposed for ligand-induced Smad nuclear accumulation. One of them is the cytoplasmic retention model, which is supported by several lines of evidence. Smad proteins are retained in the cytoplasm in the absence of ligands, whereas receptor-mediated phosphorylation favors their stay in the nucleus by enhancing their interaction with nuclear factors. SARA, for example, can sequester inactive Smad2 and Smad3 in the cytoplasm (Xu et al. 2000). Akt/PKB (protein kinase B) can also directly interact with Smad3 to block its phosphorylation and nuclear accumulation, and this effect is independent of the kinase activity of Akt/PKB (Conery et al. 2004; Remy et al. 2004). After Smads enter the nucleus, they can be retained in the nucleus through their association with DNA and nuclear proteins, such as Fast1/FoxH1 (Xu et al. 2002) and TAZ (Varelas et al. 2008). In the case of Smad3, receptor-mediated phosphorylation can increase its interaction with importin β1 and promote nuclear import (Kurisaki et al. 2001). The nuclear accumulation of Smad4 may depend on its interaction with nuclear R-Smads. For instance, phospho-Smad3 can block Smad4 interaction with exportin 1, therefore promoting nuclear accumulation of Smad4 (Chen et al. 2005). Dephosphorylation of R-Smads by phosphatases, such as PPM1A, may promote their nuclear export (Lin et al. 2006).
The nucleocytoplasmic shuttling of Smads is not only regulated by TGF-β family ligands, but also by other signaling events. The phosphorylation of the linker region of R-Smads by Erk MAPK (Kretzschmar et al. 1997), CDKs (Matsuura et al. 2004; Wang et al. 2009), and GSK3β (Fuentealba et al. 2007; Millet et al. 2009) has been shown to inhibit Smad nuclear accumulation. Thus, intracellular signals are integrated to control the subcellular localization of Smad proteins and finely tune TGF-β signaling (Schmierer et al. 2008).
Smad intracellular trafficking between the cell membrane and the nucleus is also under the control of microtubules and associated motor proteins, such as kinesin (Batut et al. 2007). Dynein light chain protein km23-1 (DYNLRB1) plays a role in Smad movement toward the nucleus after activation by the receptor kinases (Jin et al. 2009). These studies imply that Smad movement within the cell is an active and directed process but not passive diffusion.
POSTTRANSLATIONAL MODIFICATIONS OF SMADS AND MODULATION OF THEIR ACTIVITY
In addition to the two critical regulatory steps that lead to Smad activity (i.e., phosphorylation of the SSXS motif and nucleocytoplasmic trafficking), Smad proteins are subject to various posttranslational modifications that occur upon specific stress or growth factor stimulation. In the section below, we summarize well-described posttranslational modifications found in Smad proteins.
Ubiquitylation and Sumoylation
E3 ubiquitin ligases are recruited to Smad proteins to promote ubiquitin-mediated degradation of Smads and Smad partners. Smurfs and other HECT domain E3 ligases contain WW domains that interact with conserved PPXY (Pro–Pro–X–Tyr) motifs in the linker region of R-Smads and I-Smads (Fig. 2). Both R-Smads and I-Smads recruit HECT domain E3 ligases to target either their own degradation or that of binding partners. I-Smads recruit Smurfs to the receptors and trigger their ubiquitin-mediated degradation (Murakami et al. 2003; Ogunjimi et al. 2005). Upon TGF-β stimulation, R-Smads recruit Smurfs and mediate degradation of the transcriptional corepressor SnoN (also known as Ski-like), which in turn promotes transcriptional regulation by the Smad complex (Deheuninck and Luo 2009). Smurf1 causes degradation of BMP-regulated R-Smads and inhibits BMP signaling (Zhu et al. 1999). Smurf1 also modulates the inhibitory activity and the stability of Smad7 and, hence, the TGF-β signaling output (Zhu et al. 1999; Suzuki et al. 2002). Smurf2 is also known to mediate target degradation of the TGF-β-regulated R-Smads (Lin et al. 2000; Zhang et al. 2001). The HECT domain E3 ligase NEDD4-2 causes degradation of Smad2 (Kuratomi et al. 2005) and Smad4 (Morén et al. 2005).
Members of the RING-finger class of E3 ligases also mediate degradation of Smads or Smad partners. RNF111/Arkadia induces Smad7 ubiquitylation and degradation to promote nodal signaling. Upon TGF-β treatment, RNF111 interacts with R-Smads, mediates degradation of the corepressors Ski and SnoN, and facilitates transcriptional regulation by the Smad complex (Levy et al. 2007; Nagano et al. 2007; Le Scolan et al. 2008). The RING finger E3 ligase complex SCF (Skp1, Cullin1, and Fbw1a)/ROC mediates TGF-β-dependent degradation of Smad3 and Smad4 and termination of TGF-β signaling (Fukuchi et al. 2001; Wan et al. 2004), whereas the U-Box-dependent E3 ligase STUB1 (STIP1 homology and U-Box containing protein 1, also known as CHIP) negatively regulates the BMP-regulated R-Smads and Smad-mediated signaling (Li et al. 2004). Another RING finger E3 ligase, anaphase-promoting complex (APC), interacts with Smad3, mediates ubiquitin-dependent degradation of SnoN, and promotes TGF-β signaling (Stroschein et al. 2001).
In addition to promoting the proteosomal degradation of Smad proteins or their partners, monoubiquitylation can modulate Smad activities. In the MH2 domain of Smad3, ablation of lysine residues that are monoubiquitylated by Smurf2 has no impact on protein stability but inhibits Smad3 signaling (Tang et al. 2011). Conversely, the deubiquitylating enzyme USP15 reverses this modification and restores responsiveness to TGF-β (Inui et al. 2011). The RING ubiquitin ligase TRIM33 (tripartite motif containing 33, also known as TIF1γ or ectodermin) monoubiquitylates Smad4 in the MH2 domain and efficiently inhibits both TGF-β and BMP signaling, presumably by disrupting the R-Smad/Smad4 complex and promoting Smad4 translocation to the cytoplasm (Dupont et al. 2005). The deubiquitylating enzyme USP9X (also known as FAM) reverts the effects of TRIM33 on Smad4 and restores TGF-β signaling (Dupont et al. 2009). Ubiquitylation of Smad4 by TRIM33 is regulated by association of the PHD finger-bromo domain of TRIM33 with unmodified histone H3 tails (Agricola et al. 2011). TRIM33 also competes with Smad4 for binding to phosphorylated Smad2 and Smad3 and, thus, mediates TGF-β-induced and Smad4-independent responses, such as erythroid differentiation in hematopoiesis (He et al. 2006). TRIM33 forms a complex with activated R-Smads, binds to the promoter region of nodal target genes characterized by H3K9 (histone H3 Lys9) trimethylation and H3K18 (histone H3 Lys18) acetylation, and displaces the chromatin-compacting factor chromobox homolog 3 (CBX3, also known as HP1γ) (Xi et al. 2011). This process is a prerequisite for the transcriptional activation of nodal target genes by the Smad complex during differentiation of embryonic stem cells (Xi et al. 2011). Future studies must assess how general the requirement of TRIM33 is for Smad-dependent gene regulation, and what determines whether TRIM33 activates or represses target genes.
Similar to ubiquitylation, sumoylation is a multistep posttranslational modification in which SUMO (a small ubiquitin like modifier protein) is attached to the target protein. Sumoylation of Smad4 by the E2 SUMO ligase Ubc9 and the PIAS (protein inhibitor of activated STAT-1) family members of the E3 SUMO ligases has been implicated in the increased level of nuclear Smad4 and the activation of both TGF-β and BMP signaling (Lee et al. 2003; Lin et al. 2003; Shimada et al. 2008). However, sumoylation of Smad4 can also suppress TGF-β and BMP signaling by repressing the transcriptional activity of Smad4 (Long et al. 2004; Yukita et al. 2012).
Acetylation and ADP-Ribosylation
Both R-Smads and Smad7 are substrates of acetyltransferases. Acetylation of Smad2 and Smad3 in the MH1 or MH2 domains appears to promote TGF-β signaling by enhancing the transactivation activity of Smad proteins (Inoue et al. 2007). Acetylation of Smad7 by the histone acetyltransferase p300 protects Smad7 from proteasomal degradation, as the acetylation occurs at the same lysine residues that the E3 ubiquitin ligase Smurf1 would otherwise ubiquitylate, and also increases Smad7 stability and inhibits TGF-β signaling (Grönroos et al. 2002). Smad3 and Smad4 are also subject to poly-ADP ribosylation by poly(ADP-ribose) polymerase-1 (PARP-1), a modification that interferes with Smad DNA binding and, thus, attenuates transcription (Lönn et al. 2010).
Linker Phosphorylation
The linker region between the MH1 and MH2 domains of Smads is rich in serine and proline residues, which provide prime target sites for modulation of Smad signaling in response to growth factors and other signaling cascades. Signaling pathways cross talk exerts great impact during embryogenesis and in homeostatic processes to generate complex context-dependent biological responses (Moustakas and Heldin 2009; Massagué 2012; Weiss and Attisano 2013).
Smad proteins that are actively engaged in transcription can be phosphorylated in the linker region by cyclin C-CDK8 or cyclin T-CDK9 (Alarcon et al. 2009; Gao et al. 2009). This phosphorylation step primes the secondary phosphorylation of the linker region by GSK3, generates binding sites for E3 ubiquitin ligases, such as Smurf1 and NEDD4-2, and targets Smad proteins for degradation (Alarcon et al. 2009; Gao et al. 2009). Linker phosphorylation by CDK8 or CDK9 also triggers the recruitment of Yes-associated protein (YAP), a signal transducer of the Hippo pathway (Alarcon et al. 2009), or Pin1 (peptidylprolyl cis/trans isomerase) (Nakano et al. 2009; Matsuura et al. 2010; Aragon et al. 2011; Shen et al. 2012; Ueberham et al. 2014), which modulate the nuclear activity of Smads.
Dephosphorylation of Smads
Following the activating phosphorylation of R-Smad proteins at their carboxyl terminus and inhibitory phosphorylation in the linker region, different phosphatases can reverse the phosphorylation to control the duration and intensity of the Smad signal. Both linker phosphorylation and receptor-mediated carboxy-terminal phosphorylation of R-Smad proteins can be reversed by small carboxy-terminal domain phosphatases (SCP) 1, 2, and 3 (Sapkota et al. 2006; Bruce et al. 2012). The effects of SCP1, 2, and 3 on TGF-β-regulated and BMP-regulated Smads are different, as SCP1, 2, and 3 undo the phosphorylation of the linker but not of the carboxy terminal of Smad2 and Smad3, and thus enhance TGF-β signaling (Sapkota et al. 2006). On the contrary, SCP1, 2, and 3 dephosphorylate both the linker and carboxyl terminus of Smad1, resulting in an overall inhibitory effect on BMP signaling (Sapkota et al. 2006). SCP1, 2, and 3 are, thus, regulatory molecules that exert opposing controls on the TGF-β and BMP pathways (Sapkota et al. 2006). It is still unclear, however, how the activities of SCP1, 2, or 3 are regulated and coordinated with the phosphorylation step.
The Bβ subunit of PP2A interacts with the BMP receptors and mediates Smad1 dephosphorylation, mainly in the linker region, leading to amplification of BMP signaling (Bengtsson et al. 2009). The Bα subunit of PP2A, however, interacts with the TGF-β receptor and modulates TGF-β signaling (Griswold-Prenner et al. 1998; Petritsch et al. 2000). PPM1A, the prototype of metal ion-dependent protein phosphatases, also known as PP2C, is the only phosphatase shown to dephosphorylate Smad2 and Smad3 at their carboxy-terminal SXS motif (Lin et al. 2006), whereas several phosphatases can mediate carboxy-terminal dephosphorylation of Smad1. In addition to SCPs (Sapkota et al. 2006), which dephosphorylate the carboxyl terminus of Smad1 to terminate BMP signaling (Duan et al. 2006; Zhao et al. 2014), PPM1H has also been reported to interact with and dephosphorylate activated Smad1 (Shen et al. 2014). An RNA interference screen identified pyruvate dehydrogenase phosphatase (PDP) as a phosphatase for Mad, the homolog of Smad1 and Smad5 in Drosophila, that inactivates decapentaplegic (Dpp)/Mad signaling (Chen et al. 2006). Myotubularin-related protein 4 (MTMR4), a FYVE domain-containing dual-specificity protein phosphatase, can dephosphorylate Smad1, Smad2, and Smad3 in the early endosome and block their nuclear accumulation, functioning as a general negative regulator for both TGF-β and BMP signaling (Yu et al. 2010, 2013). Further studies are required to elucidate the mechanism that regulates the activity of phosphatases, which are critical determinants of the duration and intensity of the Smad signal.
REGULATION OF TARGET GENES BY Smads AND THEIR PARTNERS
Once located in the nucleus, Smad complexes can directly bind DNA and modulate transcription. Smad complexes bind with low affinity to a DNA sequence known as either the “Smad-binding element” (SBE) (i.e., GTCT or AGAC) or a GC-rich sequence, and require DNA binding transcription factors as partners to increase specificity and DNA binding affinity. Many DNA binding partners of Smads are tissue-specific transcription factors and, thus, are essential in mediating context-dependent gene regulation. A variety of DNA binding partners of the Smad complex has been identified, including chromatin modifiers, such as histone acetyltransferases (HATs) and histone deacetylases (HDACs), or DNA cytosine-5-methyltransferase 3A (DNMT3A), which removes repressive DNA methylation and activates transcription in a TGF-β-inducible manner (Thillainadesan et al. 2012).
DNA binding partners are often prime recipients of cross talk input from other signaling pathways. For example, Wnt cooperates with BMP and TGF-β through co-occupancy of Smad target enhancers by Wnt-activated lymphoid enhancer-binding factor 1 (LEF1, also known as TCF1α) and transcription factor 7-like 2 (TCF7L2) transcription factors (Labbé et al. 2000, 2007; Nakano et al. 2010). Also, the interaction between Smad proteins and FoxO factors provides an integration point of the Akt and TGF-β pathways (Seoane et al. 2004; Naka et al. 2010). Cross talk can also be achieved at the level of Smad target genes. For example, in epithelial cells, the TGF-β-activated Smad complex stimulates the expression of ATF3 (activating transcription factor 3) and Snail1, which then cooperates with Smads to repress ID1 and CDH1, encoding E-cadherin, and mediates epithelial to mesenchymal transition (Kang et al. 2003; Vincent et al. 2009).
REGULATION OF miRNA EXPRESSION BY SMADS
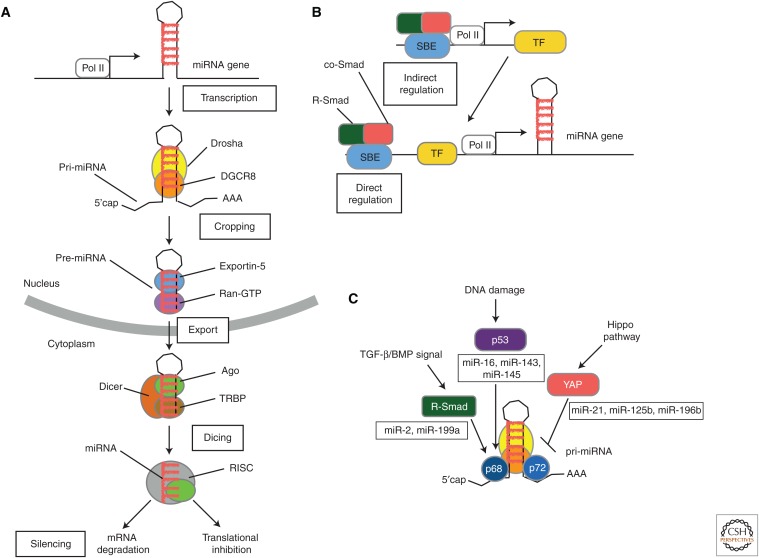
miRNAs are small (∼22 nucleotides) noncoding RNAs that associate with a partially complementary sequence often found in the 3′-untranslated region (UTR) of target mRNAs, and repress their expression either by promoting mRNA degradation or inhibiting translation (Siomi and Siomi 2010; Ha and Kim 2014). The biosynthesis of miRNAs begins with transcription of miRNA genes by RNA polymerase II to generate long primary transcripts known as pri-miRNAs. These contain one or more stable stem-loop structures that will give rise to mature miRNA sequence(s) after two sequential cleavage steps by the RNase III enzymes Drosha and Dicer (Fig. 3A) (Ha and Kim 2014). Gene regulation by miRNAs is an integral component of the control of gene expression exerted by the TGF-β family of ligands (Blahna and Hata 2012, 2013). Because a single miRNA is capable of modulating more than 100 mRNAs simultaneously (Lim et al. 2005; Blahna and Hata 2012), regulation of even a few miRNAs can affect the expression of hundreds of genes and contribute to tissue- and time-controlled biological outcomes mediated by TGF-β family ligands. In general, TGF-β signaling modulates miRNA expression both transcriptionally, via DNA binding activity of Smad complexes, and posttranscriptionally, via the RNA binding activity of R-Smads, as summarized below.
Figure 3.
MicroRNA (miRNA) biogenesis pathway and its regulation by Smad proteins. (A) The miRNA biogenesis pathway. The biogenesis of a miRNA is a stepwise process that includes (1) transcription of a primary transcript (pri-miRNA), (2) nuclear cropping to produce the precursor-miRNA (pre-miRNA), (3) export to the cytoplasm, and (4) cytoplasmic cropping to a double-stranded (ds) miRNA precursor. miRNA genes are generally transcribed by RNA polymerase II (Pol II) as long, 5′-capped and 3′-polyadenylated transcripts (pri-miRNAs) that are processed by the RNase III enzyme, Drosha, in the microprocessor complex to generate hairpin-loop RNAs, known as pre-miRNAs. Pre-miRNAs are recognized by the exportin 5 (Xpo5)/Ran-GTP transporter and exported to the cytoplasm, where another enzyme of the RNase III family, Dicer, catalyzes secondary processing (“dicing”) to produce miRNA/miRNA* duplexes. Dicer, TRBP, and Argonaute (Ago) proteins mediate the processing of pre-miRNAs and the assembly of the RNA-induced silencing complex (RISC) in mammalian cells. Ago proteins associate with Dicer in both the cropping and RISC assembly steps (Hata and Lieberman 2015). (From Hata and Lieberman 2015; adapted, with permission, from the authors.) (B) Transcription of miRNA genes can be regulated by TGF-β and BMP signaling pathways either by a direct binding of Smad complex (R-Smad/co-Smad) to Smad-binding element (SBE) in the promoter regions of miRNA genes or by transcriptional regulation of other transcription factors, which, in turn, modulate the transcription of miRNA genes. (C) Pri-miRNA to pre-miRNA processing of a subset of miRNAs catalyzed by Drosha and its cofactors p68 and p72 is positively regulated by transcription factors, such as R-Smad and p53, and negatively regulated by YAP, a signal transducer of the Hippo pathway. Specific binding of R-Smads to a dsRNA sequence motif located in the pre-miRNA provides specificity of this regulation. TF, Transcription factor. (From Hata and Lieberman 2015; adapted, with permission, from the authors.)
Transcriptional Regulation of miRNA Expression by Smads
Because the promoter structure of genes encoding miRNAs closely resembles that of protein coding genes (Corcoran et al. 2009), Smad complexes control the transcription of miRNA genes by binding to SBEs in their promoter (Figs. 2,3B). In addition, Smads can indirectly modulate miRNA levels through activation of transcription factors that regulate the miRNA promoter activity (Fig. 3B). For instance, during epithelial to mesenchymal transition, the miR-200 family of miRNAs is repressed by TGF-β through induction of the transcriptional repressors ZEB1 (also known as δEF1) and ZEB2 (also known as SIP1) (Gregory et al. 2008). These factors directly bind an E-box proximal promoter element and repress the transcription of miR-200, and are reciprocally targeted by miR-200 (Burk et al. 2008; Korpal et al. 2008).
Posttranscriptional Regulation of miRNA Biogenesis by Smads
Besides acting as transcriptional regulators, R-Smad proteins promote the processing of a subset of miRNAs and rapidly enhance their expression on ligand stimulation (Figs. 2,3C) (Davis et al. 2008, 2010; Blahna and Hata 2012). In the first processing step, the RNase III enzyme Drosha in complex with cofactors DGCR8 (DeGeorge critical region 8, also known as Pasha), DEAD-box RNA helicases p68 (DDX5), and p72 (DDX17) cleave the pri-miRNAs to generate precursor miRNAs (pre-miRNAs) in the nucleus (Figs. 2,3C) (Blahna and Hata 2012). Both TGF-β- and BMP-regulated R-Smad proteins interact with p68 in the nucleus and facilitate the processing of pri-miRNAs by Drosha (Davis et al. 2008). The carboxyl-terminus phosphorylation by the receptor kinase is required for nuclear translocation of R-Smads but dispensable for the regulation of Drosha activity (Davis et al. 2008). Furthermore, unlike the transcriptional control by R-Smads that require co-Smad, R-Smads are capable of modulating the processing activity of Drosha in the absence of co-Smad (Davis et al. 2008), possibly explaining instances of Smad4-independent gene regulation (Bardeesy et al. 2006). In addition to their interaction with p68, Smads also directly associate with a 5-nucleotide double-stranded RNA (dsRNA) sequence motif that closely resembles the SBE and is enclosed within the mature miRNA sequence. This RNA motif specifies the pri-miRNAs that are regulated by R-Smads (Davis et al. 2010). miRNA-mediated gene regulation by Smad proteins can play a central role under stresses, such as hypoxia, during which transcription is compromised. Similarly to Smads but in response to different stimuli, other transcription factors, such as p53 (Fukuda et al. 2007; Suzuki et al. 2009) and YAP (Mori et al. 2014), associate with the Drosha microprocessor complex and modulate processing of a set of pri-miRNAs. Thus, R-Smads are not unique in terms of modulating gene expression through two mechanisms: regulation of transcription via DNA binding and miRNA biogenesis via RNA binding.
CONTROL OF TGF-β SIGNALING PATHWAY MEDIATORS BY miRNAS
It has been estimated that the translation of more than 30% of the coding genes is regulated by miRNAs. Molecules of the TGF-β signaling pathway are no exceptions. Protein expression of ligands, receptors, and Smads is under the control of miRNAs: one more regulatory layer for TGF-β signaling (Blahna and Hata 2012). Deregulation of miRNA expression, therefore, can lead to aberrant activity of TGF-β signaling and contribute to the pathogenesis of various disorders, including tumorigenesis (Blahna and Hata 2012). miRNAs often act in a tissue-specific manner, because of either tissue-specific expression of miRNA and targets, or tissue-specific variation of the length of the 3′-UTR of target mRNAs (Blahna and Hata 2012). These constraints contribute to limited expression of many genes, including those controlled by TGF-β. Furthermore, miRNAs whose expression is regulated by TGF-β family pathways (in Table 3) often target mRNAs encoding mediators of the TGF-β signaling pathway (in Table 4), indicating a regulatory feedback loop between miRNAs and targets.
Table 3.
List of miRNAs whose expression is regulated by the TGF-β family of ligands
| miRNA | Ligand | References |
|---|---|---|
| let-7b, let-7c, miR-19b, miR-221, miR-222 | Activin A | Tsai et al. 2010 |
| miR-17 ∼ 92 cluster | TGF-β | Luo et al. 2014 |
| miR-21 | TGF-β | Zhong et al. 2011 |
| BMP-4 | Ahmed et al. 2011 | |
| BMP-6 | Du et al. 2009 | |
| miR-22 | BMP-2 | Berenguer et al. 2013 |
| miR-23a cluster | TGF-β | Huang et al. 2008 |
| miR-24-1, miR-31 | BMP-2 | Sun et al. 2009; Dunworth et al. 2014 |
| miR-30b/c | BMP-2 | Balderman et al. 2012 |
| miR-96 | BMP-4 | Kim et al. 2014 |
| miR-140-5p, miR-455-3p | TGF-β | Swingler et al. 2012 |
| miR-141, miR-200a | BMP-2 | Itoh et al. 2009 |
| miR-143/145 | TGF-β and BMP-4 | Davis-Dusenbery et al. 2011 |
| Activin A | Blumensatt et al. 2013 | |
| miR-181a | BMP-2 | Dunworth et al. 2014 |
| miR-181b | Activin, TGF-β | Wang et al. 2010a; Neel and Lebrun 2013 |
| miR-181c/d, miR-341 ∼ 3072 cluster | TGF-β | Redshaw et al. 2013 |
| miR-192 | BMP-6 | Hu et al. 2013 |
| TGF-β | Sun et al. 2011 | |
| miR-200 family | TGF-β | Gregory et al. 2008 |
| BMP-7 | Samavarchi-Tehrani et al. 2010 | |
| miR-206 | BMP-2 | Sato et al. 2009 |
| Nodal | Liu et al. 2013 | |
| miR-302/367 | BMP-4 | Lipchina et al. 2011 |
This list contains both direct and indirect transcriptional targets of Smad complexes.
Table 4.
List of miRNAs and their targets in the TGF-β family of signaling pathways
| miRNA | miRNA targets | References |
|---|---|---|
| let-7 | ACVR1B/ALK4 | Colas et al. 2012 |
| miR-14, miR-200c, miR-203 | Noggin, Bmper (Crossveinless-2) | Cao et al. 2013 |
| miR-15, miR-16 | ACVR2A | Martello et al. 2007 |
| miR-17 ∼ 92 cluster | TβRII, Smad2, Smad4 | Li et al. 2012 |
| BMPR2 | Luo et al. 2014 | |
| miR-18 | Smad2 | Colas et al. 2012 |
| miR-20a | BMP2 | Tiago et al. 2014 |
| miR-21 | Smad3 | Kim et al. 2009 |
| miR-22 | BMP6, BMP7 | Long et al. 2013 |
| miR-23a | Smad2, Smad3, Smad4 | Huang et al. 2008 |
| miR-23b cluster | Smad3, Smad4, Smad5 | Rogler et al. 2009 |
| miR-24 | ACVR1B/ALK4 | Wang et al. 2008 |
| miR-26a | Smad4 | Liang et al. 2014 |
| miR-27a | Smad2 | Bao et al. 2014 |
| miR-30 | Smad1 | Wu et al. 2012 |
| miR-34a | INHBB (Activin B) | Tu et al. 2014 |
| miR-92 | Noggin3 | Ning et al. 2013 |
| miR-98 | ACVR1B/ALK4 | Siragam et al. 2012 |
| miR-106b | TGFβR2 | Wang et al. 2010b |
| miR-130a | ACVR1/ALK2 | Zumbrennen-Bullough et al. 2014 |
| miR-134 | CHRDL1 | Gaughwin et al. 2011 |
| miR-135 | Smad5 | Li et al. 2008 |
| miR-140-5p | BMP2 | Hwang et al. 2014 |
| TβRI | Yang et al. 2013 | |
| miR-141, 192, 194, 215, 200c | ACVR2B | Senanayake et al. 2012 |
| miR-145 | Smad2, Smad3 | Kim et al. 2011 |
| ACVR1B/ALK4 | Yan et al. 2012 | |
| miR-146a | Smad2, Smad3 | Cheung et al. 2014 |
| Smad4 | Lv et al. 2014 | |
| miR-148a | ACVR1/ALK2 | Song et al. 2012 |
| miR-155 | Smad2 | Xiao et al. 2009 |
| Smad5 | Rai et al. 2010 | |
| Smad1 | Yin et al. 2010 | |
| miR-181a | ACVR2A | Zhang et al. 2013b |
| miR-195 | ACVR2A | Bai et al. 2012 |
| miR-199a-5p | ACVR1B/ALK4 | Lin et al. 2014 |
| miR-199-3p | Smad1 | Lin et al. 2009 |
| miR-204-5p | Smad4 | Wang et al. 2013 |
| miR-210 | ACVR1B/ALK4 | Mizuno et al. 2009 |
| miR-224 | Smad4 | Yao et al. 2010 |
| miR-302 | TOB2, DAZAP2, and SLAIN1 | Lipchina et al. 2011 |
| miR-370 | TβRII | Lo et al. 2012 |
| miR-376c | TβRI, ACVR1C/ALK7 | Fu et al. 2013 |
| miR-378 | TGF-β1 | Nagalingam et al. 2014 |
| Nodal | Luo et al. 2012 | |
| miR-455-3p | ACVR2B, Smad2 | Swingler et al. 2012 |
| miR-656 | BMPR1A/ALK3 | Guo et al. 2014 |
CONCLUDING REMARKS
About two decades have elapsed since the discovery of the receptors and signal transducers of the TGF-β family ligands. Despite a detailed understanding of the signaling principles and the effectors of its regulation, the TGF-β pathway remains rather mysterious as it is still unclear how ligands can transmit context- and concentration-dependent signals through a deceptively simple signaling pathway. The mere number of different regulatory proteins that modulate the TGF-β signaling pathway at different steps underscores the complex mode by which a specific biological outcome is generated. A major challenge at the current stage is to elucidate the logic that integrate these various regulatory inputs to explain the multifunctional nature of the TGF-β pathway during embryogenesis and in the maintenance of homeostasis. Understanding the precise nature of context-dependent signal transduction has tremendous medical relevance to numerous pathological conditions and developmental defects linked to deregulation of the TGF-β signaling pathways.
ACKNOWLEDGMENTS
We give special thanks to Dr. G. Lagna for critical reading and editing of the text, and all past and present members of our laboratories for numerous contributions. We apologize to all colleagues whose work could not be cited because of space restrictions. Work in the authors’ laboratories is funded by grants from the National Natural Science Foundation of China (NSFC) (31330049, 91519310) and the 973 Program (2013CB933700) (to Y.-G.C.); and the National Heart, Lung, and Blood Institute (NHLBI), American Heart Association, and Fondation LeDucq (to A.H.). The authors declare no financial interest.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
- Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. 1997. TβRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2–Smad4 complex formation and signaling. J Biol Chem 272: 27678–27685. [DOI] [PubMed] [Google Scholar]
- Agricola E, Randall RA, Gaarenstroom T, Dupont S, Hill CS. 2011. Recruitment of TIF1γ to chromatin via its PHD finger-bromodomain activates its ubiquitin ligase and transcriptional repressor activities. Mol Cell 43: 85–96. [DOI] [PubMed] [Google Scholar]
- Ahmed MI, Mardaryev AN, Lewis CJ, Sharov AA, Botchkareva NV. 2011. MicroRNA-21 is an important downstream component of BMP signalling in epidermal keratinocytes. J Cell Sci 124: 3399–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, et al. 2009. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell 139: 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salihi MA, Herhaus L, Macartney T, Sapkota GP. 2012. USP11 augments TGFβ signalling by deubiquitylating ALK5. Open Biol 2: 120063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon E, Goerner N, Zaromytidou AI, Xi Q, Escobedo A, Massagué J, Macias MJ. 2011. A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev 25: 1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atfi A, Dumont E, Colland F, Bonnier D, L’Helgoualc’h A, Prunier C, Ferrand N, Clement B, Wewer UM, Theret N. 2007. The disintegrin and metalloproteinase ADAM12 contributes to TGF-β signaling through interaction with the type II receptor. J Cell Biol 178: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Yang W, Yang HX, Liao Q, Ye G, Fu G, Ji L, Xu P, Wang H, Li YX, et al. 2012. Downregulated miR-195 detected in preeclamptic placenta affects trophoblast cell invasion via modulating ActRIIA expression. PLoS ONE 7: e38875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkebo M, Huse K, Hilden VI, Forfang L, Myklebust JH, Smeland EB, Oksvold MP. 2012. SARA is dispensable for functional TGF-β signaling. FEBS Lett 586: 3367–3372. [DOI] [PubMed] [Google Scholar]
- Balderman JA, Lee HY, Mahoney CE, Handy DE, White K, Annis S, Lebeche D, Hajjar RJ, Loscalzo J, Leopold JA. 2012. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. J Am Heart Assoc 1: e003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Chen Z, Guo Y, Feng Y, Li Z, Han W, Wang J, Zhao W, Jiao Y, Li K, et al. 2014. Tumor suppressor microRNA-27a in colorectal carcinogenesis and progression by targeting SGPP1 and Smad2. PLoS ONE 9: e105991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, et al. 2006. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 20: 3130–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batut J, Howell M, Hill CS. 2007. Kinesin-mediated transport of Smad2 is required for signaling in response to TGF-β ligands. Dev Cell 12: 261–274. [DOI] [PubMed] [Google Scholar]
- Bengtsson L, Schwappacher R, Roth M, Boergermann JH, Hassel S, Knaus P. 2009. PP2A regulates BMP signalling by interacting with BMP receptor complexes and by dephosphorylating both the C-terminus and the linker region of Smad1. J Cell Sci 122: 1248–1257. [DOI] [PubMed] [Google Scholar]
- Bennett D, Alphey L. 2002. PP1 binds Sara and negatively regulates Dpp signaling in Drosophila melanogaster. Nat Genet 31: 419–423. [DOI] [PubMed] [Google Scholar]
- Berenguer J, Herrera A, Vuolo L, Torroba B, Llorens F, Sumoy L, Pons S. 2013. MicroRNA 22 regulates cell cycle length in cerebellar granular neuron precursors. Mol Cell Biol 33: 2706–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blahna MT, Hata A. 2012. Smad-mediated regulation of microRNA biosynthesis. FEBS Lett 586: 1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blahna MT, Hata A. 2013. Regulation of miRNA biogenesis as an integrated component of growth factor signaling. Curr Opin Cell Biol 25: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumensatt M, Greulich S, Herzfeld de Wiza D, Mueller H, Maxhera B, Rabelink MJ, Hoeben RC, Akhyari P, Al-Hasani H, Ruige JB, et al. 2013. Activin A impairs insulin action in cardiomyocytes via up-regulation of miR-143. Cardiovasc Res 100: 201–210. [DOI] [PubMed] [Google Scholar]
- Bruce DL, Macartney T, Yong W, Shou W, Sapkota GP. 2012. Protein phosphatase 5 modulates SMAD3 function in the transforming growth factor-β pathway. Cell Signal 24: 1999–2006. [DOI] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. 2008. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9: 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Jheon A, Li X, Sun Z, Wang J, Florez S, Zhang Z, McManus MT, Klein OD, Amendt BA. 2013. The Pitx2: miR-200c/141:noggin pathway regulates Bmp signaling and ameloblast differentiation. Development 140: 3348–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárcamo J, Zentella A, Massagué J. 1995. Disruption of transforming growth factor β signaling by a mutation that prevents transphosphorylation within the receptor complex. Mol Cell Biol 15: 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Derynck R. 1994. Homomeric interactions between type II transforming growth factor-β receptors. J Biol Chem 269: 22868–22874. [PubMed] [Google Scholar]
- Chen RH, Miettinen PJ, Maruoka EM, Choy L, Derynck R. 1995. A WD-domain protein that is associated with and phosphorylated by the type II TGF-β receptor. Nature 377: 548–552. [DOI] [PubMed] [Google Scholar]
- Chen YG, Liu F, Massagué J. 1997. Mechanism of TGF-β receptor inhibition by FKBP12. EMBO J 16: 3866–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Hata A, Lo RS, Wotton D, Shi Y, Pavletich N, Massagué J. 1998. Determinants of specificity in TGF-β signal transduction. Genes Dev 12: 2144–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HB, Rud JG, Lin K, Xu L. 2005. Nuclear targeting of transforming growth factor-β-activated Smad complexes. J Biol Chem 280: 21329–21336. [DOI] [PubMed] [Google Scholar]
- Chen HB, Shen J, Ip YT, Xu L. 2006. Identification of phosphatases for Smad in the BMP/DPP pathway. Genes Dev 20: 648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Wang Z, Ma J, Zhang L, Lu Z. 2007. Endofin, a FYVE domain protein, interacts with Smad4 and facilitates transforming growth factor-β signaling. J Biol Chem 282: 9688–9695. [DOI] [PubMed] [Google Scholar]
- Chen F, Lin X, Xu P, Zhang Z, Chen Y, Wang C, Han J, Zhao B, Xiao M, Feng XH. 2015. Nuclear export of Smads by RanBP3L regulates bone morphogenetic protein signaling and mesenchymal stem cell differentiation. Mol Cell Biol 35: 1700–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KS, Sposito N, Stumpf PS, Wilson DI, Sanchez-Elsner T, Oreffo RO. 2014. MicroRNA-146a regulates human foetal femur derived skeletal stem cell differentiation by down-regulating SMAD2 and SMAD3. PLoS ONE 9: e98063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Moon JH, Ahn YW, Ahn JH, Kim DU, Han TH. 2005. Tsc-22 enhances TGF-β signaling by associating with Smad4 and induces erythroid cell differentiation. Mol Cell Biochem 271: 23–28. [DOI] [PubMed] [Google Scholar]
- Choy L, Derynck R. 1998. The type II transforming growth factor (TGF)-β receptor-interacting protein TRIP-1 acts as a modulator of the TGF-β response. J Biol Chem 273: 31455–31462. [DOI] [PubMed] [Google Scholar]
- Colas AR, McKeithan WL, Cunningham TJ, Bushway PJ, Garmire LX, Duester G, Subramaniam S, Mercola M. 2012. Whole-genome microRNA screening identifies let-7 and mir-18 as regulators of germ layer formation during early embryogenesis. Genes Dev 26: 2567–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conery AR, Cao Y, Thompson EA, Townsend CM Jr, Ko TC, Luo K. 2004. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-β induced apoptosis. Nat Cell Biol 6: 366–372. [DOI] [PubMed] [Google Scholar]
- Corcoran DL, Pandit KV, Gordon B, Bhattacharjee A, Kaminski N, Benos PV. 2009. Features of mammalian microRNA promoters emerge from polymerase II chromatin immunoprecipitation data. PLoS ONE 4: e5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai F, Lin X, Chang C, Feng XH. 2009. Nuclear export of Smad2 and Smad3 by RanBP3 facilitates termination of TGF-β signaling. Dev Cell 16: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta PK, Moses HL. 2000. STRAP and Smad7 synergize in the inhibition of transforming growth factorβ signaling. Mol Cell Biol 20: 3157–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta PK, Chytil A, Gorska AE, Moses HL. 1998. Identification of STRAP, a novel WD domain protein in transforming growth factor-β signaling. J Biol Chem 273: 34671–34674. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. 2008. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. 2010. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 39: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A. 2011. Down-regulation of Krüppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-β and bone morphogenetic protein 4. J Biol Chem 286: 28097–28110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deheuninck J, Luo K. 2009. Ski and SnoN, potent negative regulators of TGF-β signaling. Cell Res 19: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Iwatsubo T, Wolfe MS. 2012. Presenilins and γ-secretase: Structure, function, and role in Alzheimer disease. Cold Spring Harb Perspect Med 2: a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Yang S, An D, Hu F, Yuan W, Zhai C, Zhu T. 2009. BMP-6 inhibits microRNA-21 expression in breast cancer through repressing δEF1 and AP-1. Cell Res 19: 487–496. [DOI] [PubMed] [Google Scholar]
- Duan X, Liang YY, Feng XH, Lin X. 2006. Protein serine/threonine phosphatase PPM1A dephosphorylates Smad1 in the bone morphogenetic protein signaling pathway. J Biol Chem 281: 36526–36532. [DOI] [PubMed] [Google Scholar]
- Dunworth WP, Cardona-Costa J, Bozkulak EC, Kim JD, Meadows S, Fischer JC, Wang Y, Cleaver O, Qyang Y, Ober EA, et al. 2014. Bone morphogenetic protein 2 signaling negatively modulates lymphatic development in vertebrate embryos. Circ Res 114: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Zacchigna L, Cordenonsi M, Soligo S, Adorno M, Rugge M, Piccolo S. 2005. Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell 121: 87–99. [DOI] [PubMed] [Google Scholar]
- Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M, Martello G, Stinchfield MJ, Soligo S, Morsut L, et al. 2009. FAM/USP9x, a deubiquitinating enzyme essential for TGF-β signaling, controls Smad4 monoubiquitination. Cell 136: 123–135. [DOI] [PubMed] [Google Scholar]
- Durrington HJ, Upton PD, Hoer S, Boname J, Dunmore BJ, Yang J, Crilley TK, Butler LM, Blackbourn DJ, Nash GB, et al. 2010. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II. J Biol Chem 285: 37641–37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. 2001. Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J Biol Chem 276: 12477–12480. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. 1997. A kinase subdomain of transforming growth factor-β (TGF-β) type I receptor determines the TGF-β intracellular signaling specificity. EMBO J 16: 3912–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. 2005. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693. [DOI] [PubMed] [Google Scholar]
- Ferrand N, Atfi A, Prunier C. 2010. The oncoprotein c-ski functions as a direct antagonist of the transforming growth factor-β type I receptor. Cancer Res 70: 8457–8466. [DOI] [PubMed] [Google Scholar]
- Flotho A, Melchior F. 2013. Sumoylation: A regulatory protein modification in health and disease. Annu Rev Biochem 82: 357–385. [DOI] [PubMed] [Google Scholar]
- Fu G, Ye G, Nadeem L, Ji L, Manchanda T, Wang Y, Zhao Y, Qiao J, Wang YL, Lye S, et al. 2013. MicroRNA-376c impairs transforming growth factor-β and nodal signaling to promote trophoblast cell proliferation and invasion. Hypertension 61: 864–872. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. 2007. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131: 980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa H, Yamamoto T, Fujigaki Y, Misaki T, Ohashi N, Takayama T, Suzuki S, Mugiya S, Oda T, Uchida C, et al. 2010. Reduction of transforming growth factor-β type II receptor is caused by the enhanced ubiquitin-dependent degradation in human renal cell carcinoma. Int J Cancer 127: 1517–1525. [DOI] [PubMed] [Google Scholar]
- Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, Tanaka K, Miyazono K. 2001. Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol Biol Cell 12: 1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Yamagata K, Fujiyama S, Matsumoto T, Koshida I, Yoshimura K, Mihara M, Naitou M, Endoh H, Nakamura T, et al. 2007. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat Cell Bio 9: 604–611. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, Kikuchi A, Miyazono K, Kato M. 2001. Axin facilitates Smad3 activation in the transforming growth factor β signaling pathway. Mol Cell Biol 21: 5132–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Alarcon C, Sapkota G, Rahman S, Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P, Massagué J. 2009. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-β signaling. Mol Cell 36: 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughwin P, Ciesla M, Yang H, Lim B, Brundin P. 2011. Stage-specific modulation of cortical neuronal development by Mμ-miR-134. Cereb Cortex 21: 1857–1869. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Wells RG, Lodish HF, Henis YI. 1998. Oligomeric structure of type I and type II transforming growth factor β receptors: Homodimers form in the ER and persist at the plasma membrane. J Cell Biol 140: 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JB, Wallace DF, Hong W, Subramaniam VN. 2015. Endofin, a novel BMP-SMAD regulator of the iron-regulatory hormone, hepcidin. Sci Rep 5: 13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. 2008. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10: 593–601. [DOI] [PubMed] [Google Scholar]
- Griswold-Prenner I, Kamibayashi C, Maruoka EM, Mumby MC, Derynck R. 1998. Physical and functional interactions between type I transforming growth factor β receptors and Ba, a WD-40 repeat subunit of phosphatase 2A. Mol Cell Biol 18: 6595–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönroos E, Hellman U, Heldin CH, Ericsson J. 2002. Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell 10: 483–493. [DOI] [PubMed] [Google Scholar]
- Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB, Schwarz PM, Wrana JL, Hinck AP. 2008. Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell 29: 157–168. [DOI] [PubMed] [Google Scholar]
- Gudey SK, Sundar R, Mu Y, Wallenius A, Zang G, Bergh A, Heldin CH, Landstrom M. 2014. TRAF6 stimulates the tumor-promoting effects of TGF-β type I receptor through polyubiquitination and activation of presenilin 1. Sci Signal 7: ra2. [DOI] [PubMed] [Google Scholar]
- Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. 2008. Axin and GSK3-β control Smad3 protein stability and modulate TGF-β signaling. Genes Dev 22: 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Jiang Z, Zhang X, Lu D, Ha AD, Sun J, Du W, Wu Z, Hu L, Khadarian K, et al. 2014. miR-656 inhibits glioma tumorigenesis through repression of BMPR1A. Carcinogenesis 35: 1698–1706. [DOI] [PubMed] [Google Scholar]
- Ha M, Kim VN. 2014. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15: 509–524. [DOI] [PubMed] [Google Scholar]
- Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P. 2006. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol 26: 7791–7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A, Lieberman J. 2015. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci Signal 8: re3. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL, et al. 1997. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell 89: 1165–1173. [DOI] [PubMed] [Google Scholar]
- He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massagué J. 2006. Hematopoiesis controlled by distinct TIF1γ and Smad4 branches of the TGF-β pathway. Cell 125: 929–941. [DOI] [PubMed] [Google Scholar]
- Henis YI, Moustakas A, Lin HY, Lodish HF. 1994. The types II and III transforming growth factor-β receptors form homo-oligomers. J Cell Biol 126: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS. 2009. Nucleocytoplasmic shuttling of Smad proteins. Cell Res 19: 36–46. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Smine A, Xu XX, Howe PH. 2001. The adaptor molecule disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J 20: 2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Meng X, Tong Q, Liang L, Xiang R, Zhu T, Yang S. 2013. BMP-6 inhibits cell proliferation by targeting microRNA-192 in breast cancer. Biochim Biophys Acta 1832: 2379–2390. [DOI] [PubMed] [Google Scholar]
- Huang F, Chen YG. 2012. Regulation of TGF-β receptor activity. Cell Biosci 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, He X, Ding J, Liang L, Zhao Y, Zhang Z, Yao X, Pan Z, Zhang P, Li J, et al. 2008. Upregulation of miR-23a∼ 27a∼24 decreases transforming growth factor-β-induced tumor-suppressive activities in human hepatocellular carcinoma cells. Int J Cancer 123: 972–978. [DOI] [PubMed] [Google Scholar]
- Huang T, David L, Mendoza V, Yang Y, Villarreal M, De K, Sun L, Fang X, Lopez-Casillas F, Wrana JL, et al. 2011. TGF-β signalling is mediated by two autonomously functioning TβRI:TβRII pairs. EMBO J 30: 1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Chen YG, Massagué J, Kuriyan J. 1999. Crystal structure of the cytoplasmic domain of the type I TGFβ receptor in complex with FKBP12. Cell 96: 425–436. [DOI] [PubMed] [Google Scholar]
- Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massagué J. 2001. The TGFβ receptor activation process: An inhibitor- to substrate-binding switch. Mol Cell 8: 671–682. [DOI] [PubMed] [Google Scholar]
- Hwang S, Park SK, Lee HY, Kim SW, Lee JS, Choi EK, You D, Kim CS, Suh N. 2014. miR-140–5p suppresses BMP2-mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett 588: 2957–2963. [DOI] [PubMed] [Google Scholar]
- Imamura T, Oshima Y, Hikita A. 2013. Regulation of TGF-β family signalling by ubiquitination and deubiquitination. J Biochem 154: 481–489. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku H, Fukamizu A, Onozaki K, Hayashi H. 2007. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene 26: 500–508. [DOI] [PubMed] [Google Scholar]
- Inui M, Manfrin A, Mamidi A, Martello G, Morsut L, Soligo S, Enzo E, Moro S, Polo S, Dupont S, et al. 2011. USP15 is a deubiquitylating enzyme for receptor-activated SMADs. Nat Cell Biol 13: 1368–1375. [DOI] [PubMed] [Google Scholar]
- Itoh F, Divecha N, Brocks L, Oomen L, Janssen H, Calafat J, Itoh S, Dijke Pt P. 2002. The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-β/Smad signalling. Genes Cells 7: 321–331. [DOI] [PubMed] [Google Scholar]
- Itoh S, Thorikay M, Kowanetz M, Moustakas A, Itoh F, Heldin CH, ten Dijke P. 2003. Elucidation of Smad requirement in transforming growth factor-β type I receptor-induced responses. J Biol Chem 278: 3751–3761. [DOI] [PubMed] [Google Scholar]
- Itoh T, Nozawa Y, Akao Y. 2009. MicroRNA-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J Biol Chem 284: 19272–19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Gao G, Mulder KM. 2009. Requirement of a dynein light chain in TGF-β/Smad3 signaling. J Cell Physiol 221: 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Chen CR, Massagué J. 2003. A self-enabling TGF-β response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell 11: 915–926. [DOI] [PubMed] [Google Scholar]
- Kang JS, Saunier EF, Akhurst RJ, Derynck R. 2008. The type I TGF-β receptor is covalently modified and regulated by sumoylation. Nat Cell Biol 10: 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Liu C, Derynck R. 2009. New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol 19: 385–394. [DOI] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. 2000. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol Cell 6: 1365–1375. [DOI] [PubMed] [Google Scholar]
- Kawamata H, Fujimori T, Imai Y. 2004. TSC-22 (TGF-β stimulated clone-22): A novel molecular target for differentiation-inducing therapy in salivary gland cancer. Curr Cancer Drug Targets 4: 521–529. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Freimuth J, Meyer DS, Lee MM, Tochimoto-Okamoto A, Benzinou M, Clermont FF, Wu G, Roy R, Letteboer TG, et al. 2014. Genetic variants of Adam17 differentially regulate TGF-β signaling to modify vascular pathology in mice and humans. Proc Natl Acad Sci 111: 7723–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hwang SJ, Bae YC, Jung JS. 2009. MiR-21 regulates adipogenic differentiation through the modulation of TGF-β signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 27: 3093–3102. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Oh JS, Shin JY, Lee KD, Sung KW, Nam SJ, Chun KH. 2011. Development of microRNA-145 for therapeutic application in breast cancer. J Control Release 155: 427–434. [DOI] [PubMed] [Google Scholar]
- Kim S, Hata A, Kang H. 2014. Down-regulation of miR-96 by bone morphogenetic protein signaling is critical for vascular smooth muscle cell phenotype modulation. J Cell Biochem 115: 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro A, Imamura T, Saitoh M, Yoshida Y, Yamori T, Miyazono K, Miyazawa K. 2004. Negative regulation of transforming growth factor-β (TGF-β) signaling by WW domain-containing protein 1 (WWP1). Oncogene 23: 6914–6923. [DOI] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y. 2008. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 283: 14910–14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Lönn P, Vanlandewijck M, Kowanetz K, Heldin CH, Moustakas A. 2008. TGF-β induces SIK to negatively regulate type I receptor kinase signaling. J Cell Biol 182: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagué J. 1997. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature 389: 618–622. [DOI] [PubMed] [Google Scholar]
- Kuratomi G, Komuro A, Goto K, Shinozaki M, Miyazawa K, Miyazono K, Imamura T. 2005. NEDD 4–2 (neural precursor cell expressed, developmentally down-regulated 4–2) negatively regulates TGF-β (transforming growth factor-β) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-β type I receptor. Biochem J 386: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisaki A, Kose S, Yoneda Y, Heldin CH, Moustakas A. 2001. Transforming growth factor-β induces nuclear import of Smad3 in an importin-β1 and Ran-dependent manner. Mol Biol Cell 12: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisaki A, Kurisaki K, Kowanetz M, Sugino H, Yoneda Y, Heldin CH, Moustakas A. 2006. The mechanism of nuclear export of Smad3 involves exportin 4 and Ran. Mol Cell Biol 26: 1318–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé E, Letamendia A, Attisano L. 2000. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-β and Wnt pathways. Proc Natl Acad Sci 97: 8358–8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé E, Lock L, Letamendia A, Gorska AE, Gryfe R, Gallinger S, Moses HL, Attisano L. 2007. Transcriptional cooperation between the transforming growth factor-β and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res 67: 75–84. [DOI] [PubMed] [Google Scholar]
- Lawler S, Feng XH, Chen RH, Maruoka EM, Turck CW, Griswold-Prenner I, Derynck R. 1997. The type II transforming growth factor-β receptor autophosphorylates not only on serine and threonine but also on tyrosine residues. J Biol Chem 272: 14850–14859. [DOI] [PubMed] [Google Scholar]
- Lee PS, Chang C, Liu D, Derynck R. 2003. Sumoylation of Smad4, the common Smad mediator of transforming growth factor-β family signaling. J Biol Chem 278: 27853–27863. [DOI] [PubMed] [Google Scholar]
- Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. 2007. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J 26: 3957–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Scolan E, Zhu Q, Wang L, Bandyopadhyay A, Javelaud D, Mauviel A, Sun L, Luo K. 2008. Transforming growth factor-β suppresses the ability of Ski to inhibit tumor metastasis by inducing its degradation. Cancer Res 68: 3277–3285. [DOI] [PubMed] [Google Scholar]
- Levy L, Howell M, Das D, Harkin S, Episkopou V, Hill CS. 2007. Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation. Mol Cell Biol 27: 6068–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xin H, Xu X, Huang M, Zhang X, Chen Y, Zhang S, Fu XY, Chang Z. 2004. CHIP mediates degradation of Smad proteins and potentially regulates Smad-induced transcription. Mol Cell Biol 24: 856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. 2008. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci 105: 13906–13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shi JY, Zhu GQ, Shi B. 2012. MiR-17–92 cluster regulates cell proliferation and collagen synthesis by targeting TGFβ pathway in mouse palatal mesenchymal cells. J Cell Biochem 113: 1235–1244. [DOI] [PubMed] [Google Scholar]
- Liang H, Xu C, Pan Z, Zhang Y, Xu Z, Chen Y, Li T, Li X, Liu Y, Huangfu L, et al. 2014. The antifibrotic effects and mechanisms of microRNA-26a action in idiopathic pulmonary fibrosis. Mol Ther 22: 1122–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. 2005. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433: 769–773. [DOI] [PubMed] [Google Scholar]
- Lin HY, Wang XF. 1992. Expression cloning of TGF-β receptors. Mol Reprod Dev 32: 105–110. [DOI] [PubMed] [Google Scholar]
- Lin HY, Wang XF, Ng-Eaton E, Weinberg RA, Lodish HF. 1992. Expression cloning of the TGF-β type II receptor, a functional transmembrane serine/threonine kinase. Cell 68: 775–785. [DOI] [PubMed] [Google Scholar]
- Lin X, Liang M, Feng XH. 2000. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-β signaling. J Biol Chem 275: 36818–36822. [DOI] [PubMed] [Google Scholar]
- Lin X, Liang M, Liang YY, Brunicardi FC, Melchior F, Feng XH. 2003. Activation of transforming growth factor-β signaling by SUMO-1 modification of tumor suppressor Smad4/DPC4. J Biol Chem 278: 18714–18719. [DOI] [PubMed] [Google Scholar]
- Lin HK, Bergmann S, Pandolfi PP. 2004. Cytoplasmic PML function in TGF-β signalling. Nature 431: 205–211. [DOI] [PubMed] [Google Scholar]
- Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC, et al. 2006. PPM1A functions as a Smad phosphatase to terminate TGF-β signaling. Cell 125: 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EA, Kong L, Bai XH, Luan Y, Liu CJ. 2009. miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J Biol Chem 284: 11326–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HS, Gong JN, Su R, Chen MT, Song L, Shen C, Wang F, Ma YN, Zhao HL, Yu J, et al. 2014. miR-199a-5p inhibits monocyte/macrophage differentiation by targeting the activin A type 1B receptor gene and finally reducing C/EBPα expression. J Leukoc Biol 96: 1023–1035. [DOI] [PubMed] [Google Scholar]
- Lindsay ME, Holaska JM, Welch K, Paschal BM, Macara IG. 2001. Ran-binding protein 3 is a cofactor for Crm1-mediated nuclear protein export. J Cell Biol 153: 1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipchina I, Elkabetz Y, Hafner M, Sheridan R, Mihailovic A, Tuschl T, Sander C, Studer L, Betel D. 2011. Genome-wide identification of microRNA targets in human ES cells reveals a role for miR-302 in modulating BMP response. Genes Dev 25: 2173–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sun Y, Constantinescu SN, Karam E, Weinberg RA, Lodish HF. 1997. Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci 94: 10669–10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan SC, et al. 2006. Axin is a scaffold protein in TGF-β signaling that promotes degradation of Smad7 by Arkadia. EMBO J 25: 1646–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Xu P, Lamouille S, Xu J, Derynck R. 2009. TACE-mediated ectodomain shedding of the type I TGF-β receptor downregulates TGF-β signaling. Mol Cell 35: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma Y, Zhang C, Wei S, Cao Y, Wang Q. 2013. Nodal promotes mir206 expression to control convergence and extension movements during zebrafish gastrulation. J Genet Genomics 40: 515–521. [DOI] [PubMed] [Google Scholar]
- Lo RS, Chen YG, Shi Y, Pavletich NP, Massagué J. 1998. The L3 loop: A structural motif determining specific interactions between SMAD proteins and TGF-β receptors. EMBO J 17: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SS, Hung PS, Chen JH, Tu HF, Fang WL, Chen CY, Chen WT, Gong NR, Wu CW. 2012. Overexpression of miR-370 and downregulation of its novel target TGF-β-RII contribute to the progression of gastric carcinoma. Oncogene 31: 226–237. [DOI] [PubMed] [Google Scholar]
- Long J, Wang G, He D, Liu F. 2004. Repression of Smad4 transcriptional activity by SUMO modification. Biochem J 379: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Badal SS, Wang Y, Chang BH, Rodriguez A, Danesh FR. 2013. MicroRNA-22 is a master regulator of bone morphogenetic protein-7/6 homeostasis in the kidney. J Biol Chem 288: 36202–36214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönn P, Morén A, Raja E, Dahl M, Moustakas A. 2009. Regulating the stability of TGF-β receptors and Smads. Cell Res 19: 21–35. [DOI] [PubMed] [Google Scholar]
- Lönn P, van der Heide LP, Dahl M, Hellman U, Heldin CH, Moustakas A. 2010. PARP-1 attenuates Smad-mediated transcription. Mol Cell 40: 521–532. [DOI] [PubMed] [Google Scholar]
- Lu Y, Jiang F, Zheng X, Katakowski M, Buller B, To SS, Chopp M. 2011. TGF-β1 promotes motility and invasiveness of glioma cells through activation of ADAM17. Oncol Rep 25: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Lodish HF. 1997. Positive and negative regulation of type II TGF-β receptor signal transduction by autophosphorylation on multiple serine residues. EMBO J 16: 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Ye G, Nadeem L, Fu G, Yang BB, Honarparvar E, Dunk C, Lye S, Peng C. 2012. MicroRNA-378a-5p promotes trophoblast cell survival, migration and invasion by targeting Nodal. J Cell Sci 125: 3124–3132. [DOI] [PubMed] [Google Scholar]
- Luo T, Cui S, Bian C, Yu X. 2014. Crosstalk between TGF-β/Smad3 and BMP/BMPR2 signaling pathways via miR-17–92 cluster in carotid artery restenosis. Mol Cell Biochem 389: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S, Sun B, Dai C, Shi R, Zhou X, Lv W, Zhong X, Wang R, Ma W. 2014. The downregulation of microRNA-146a modulates TGF-β signaling pathways activity in glioblastoma. Mol Neurobiol 52: 1257–1262. [DOI] [PubMed] [Google Scholar]
- Malapeira J, Esselens C, Bech-Serra JJ, Canals F, Arribas J. 2011. ADAM17 (TACE) regulates TGF-β signaling through the cleavage of vasorin. Oncogene 30: 1912–1922. [DOI] [PubMed] [Google Scholar]
- Martello G, Zacchigna L, Inui M, Montagner M, Adorno M, Mamidi A, Morsut L, Soligo S, Tran U, Dupont S, et al. 2007. MicroRNA control of Nodal signalling. Nature 449: 183–188. [DOI] [PubMed] [Google Scholar]
- Massagué J. 1998. TGF-β signal transduction. Annu Rev Biochem 67: 753–791. [DOI] [PubMed] [Google Scholar]
- Massagué J. 2012. TGF-β signalling in context. Nat Rev Mol Cell Biol 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Chen YG. 2000. Controlling TGF-β signaling. Genes Dev 14: 627–644. [PubMed] [Google Scholar]
- Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. 2004. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430: 226–231. [DOI] [PubMed] [Google Scholar]
- Matsuura I, Chiang KN, Lai CY, He DM, Wang GN, Ramkumar R, Uchida T, Ryo A, Lu KP, Liu F. 2010. Pin1 promotes transforming growth factor-β-induced migration and invasion. J Biol Chem 285: 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle S, Beall MJ, Feeney EL, Pearce EJ. 2001. Conserved role for 14–3-3ɛ downstream of type I TGF-β receptors. FEBS Lett 490: 65–69. [DOI] [PubMed] [Google Scholar]
- McGonigle S, Beall MJ, Pearce EJ. 2002. Eukaryotic initiation factor 2α subunit associates with TGF-β receptors and 14-3-3ɛ and acts as a modulator of the TGF-β response. Biochemistry 41: 579–587. [DOI] [PubMed] [Google Scholar]
- Millet C, Yamashita M, Heller M, Yu LR, Veenstra TD, Zhang YE. 2009. A negative feedback control of transforming growth factor-β signaling by glycogen synthase kinase 3-mediated Smad3 linker phosphorylation at Ser-204. J Biol Chem 284: 19808–19816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Takeshita T, Asao H, Kimura Y, Murata K, Sasaki Y, Hanai JI, Beppu H, Tsukazaki T, Wrana JL, et al. 2000. Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol Cell Biol 20: 9346–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Tokuzawa Y, Ninomiya Y, Yagi K, Yatsuka-Kanesaki Y, Suda T, Fukuda T, Katagiri T, Kondoh Y, Amemiya T, et al. 2009. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett 583: 2263–2268. [DOI] [PubMed] [Google Scholar]
- Morén A, Imamura T, Miyazono K, Heldin CH, Moustakas A. 2005. Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J Biol Chem 280: 22115–22123. [DOI] [PubMed] [Google Scholar]
- Mori M, Triboulet R, Mohseni M, Schlegelmilch K, Shrestha K, Camargo FD, Gregory RI. 2014. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell 156: 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. 2009. The regulation of TGF-β signal transduction. Development 136: 3699–3714. [DOI] [PubMed] [Google Scholar]
- Mu Y, Sundar R, Thakur N, Ekman M, Gudey SK, Yakymovych M, Hermansson A, Dimitriou H, Bengoechea-Alonso MT, Ericsson J, et al. 2011. TRAF6 ubiquitinates TGF-β type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun 2: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. 2003. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell 14: 2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Mathew R, Huang J, Farahani R, Peng H, Olson SC, Etlinger JD. 2010. Smurf1 ubiquitin ligase causes downregulation of BMP receptors and is induced in monocrotaline and hypoxia models of pulmonary arterial hypertension. Exp Biol Med 235: 805–813. [DOI] [PubMed] [Google Scholar]
- Muthusamy BP, Budi EH, Katsuno Y, Lee MK, Smith SM, Mirza AM, Akhurst RJ, Derynck R. 2015. ShcA protects against epithelial-mesenchymal transition through compartmentalized inhibition of TGF-β-induced Smad activation. PLoS Biol 13: e1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalingam RS, Sundaresan NR, Noor M, Gupta MP, Solaro RJ, Gupta M. 2014. Deficiency of cardiomyocyte-specific microRNA-378 contributes to the development of cardiac fibrosis involving a transforming growth factor β1 (TGF-β1)-dependent paracrine mechanism. J Biol Chem 289: 27199–27214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nagano Y, Mavrakis KJ, Lee KL, Fujii T, Koinuma D, Sase H, Yuki K, Isogaya K, Saitoh M, Imamura T, et al. 2007. Arkadia induces degradation of SnoN and c-Ski to enhance transforming growth factor-β signaling. J Biol Chem 282: 20492–20501. [DOI] [PubMed] [Google Scholar]
- Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A. 2010. TGF-β-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 463: 676–680. [DOI] [PubMed] [Google Scholar]
- Nakano A, Koinuma D, Miyazawa K, Uchida T, Saitoh M, Kawabata M, Hanai J, Akiyama H, Abe M, Miyazono K, et al. 2009. Pin1 down-regulates transforming growth factor-β (TGF-β) signaling by inducing degradation of Smad proteins. J Biol Chem 284: 6109–6115. [DOI] [PubMed] [Google Scholar]
- Nakano N, Itoh S, Watanabe Y, Maeyama K, Itoh F, Kato M. 2010. Requirement of TCF7L2 for TGF-β-dependent transcriptional activation of the TMEPAI gene. J Biol Chem 285: 38023–38033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N, Maeyama K, Sakata N, Itoh F, Akatsu R, Nakata M, Katsu Y, Ikeno S, Togawa Y, Vo Nguyen TT, et al. 2014. C18 ORF1, a novel negative regulator of transforming growth factor-β signaling. J Biol Chem 289: 12680–12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, et al. 1997. Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signalling. Nature 389: 631–635. [DOI] [PubMed] [Google Scholar]
- Neel JC, Lebrun JJ. 2013. Activin and TGF-β regulate expression of the microRNA-181 family to promote cell migration and invasion in breast cancer cells. Cell Signal 25: 1556–1566. [DOI] [PubMed] [Google Scholar]
- Ning G, Liu X, Dai M, Meng A, Wang Q. 2013. MicroRNA-92a upholds Bmp signaling by targeting noggin3 during pharyngeal cartilage formation. Dev Cell 24: 283–295. [DOI] [PubMed] [Google Scholar]
- Nohe A, Keating E, Underhill TM, Knaus P, Petersen NO. 2005. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J Cell Sci 118: 643–650. [DOI] [PubMed] [Google Scholar]
- Ogunjimi AA, Briant DJ, Pece-Barbara N, Le Roy C, Di Guglielmo GM, Kavsak P, Rasmussen RK, Seet BT, Sicheri F, Wrana JL. 2005. Regulation of Smurf2 ubiquitin ligase activity by anchoring the E2 to the HECT domain. Mol Cell 19: 297–308. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massagué J, Niehrs C. 1999. Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature 401: 480–485. [DOI] [PubMed] [Google Scholar]
- Oved S, Mosesson Y, Zwang Y, Santonico E, Shtiegman K, Marmor MD, Kochupurakkal BS, Katz M, Lavi S, Cesareni G, et al. 2006. Conjugation to Nedd8 instigates ubiquitylation and down-regulation of activated receptor tyrosine kinases. J Biol Chem 281: 21640–21651. [DOI] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. 2005. Regulation of the polarity protein Par6 by TGF-β receptors controls epithelial cell plasticity. Science 307: 1603–1609. [DOI] [PubMed] [Google Scholar]
- Penheiter SG, Singh RD, Repellin CE, Wilkes MC, Edens M, Howe PH, Pagano RE, Leof EB. 2010. Type II transforming growth factor-β receptor recycling is dependent upon the clathrin adaptor protein Dab2. Mol Biol Cell 21: 4009–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson U, Izumi H, Souchelnytskyi S, Itoh S, Grimsby S, Engstrom U, Heldin CH, Funa K, ten Dijke P. 1998. The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett 434: 83–87. [DOI] [PubMed] [Google Scholar]
- Petritsch C, Beug H, Balmain A, Oft M. 2000. TGF-β inhibits p70 S6 kinase via protein phosphatase 2A to induce G1 arrest. Genes Dev 14: 3093–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierreux CE, Nicolas FJ, Hill CS. 2000. Transforming growth factor β-independent shuttling of Smad4 between the cytoplasm and nucleus. Mol Cell Biol 20: 9041–9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut G, Peter M. 2008. Function and regulation of protein neddylation. “Protein modifications: Beyond the usual suspects” review series. EMBO Rep 9: 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Kim SW, McKeller MR, Dahia PL, Aguiar RC. 2010. Targeting of SMAD5 links microRNA-155 to the TGF-β pathway and lymphomagenesis. Proc Natl Acad Sci 107: 3111–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP. 2001. Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. J Biol Chem 276: 6727–6738. [DOI] [PubMed] [Google Scholar]
- Redshaw N, Camps C, Sharma V, Motallebipour M, Guzman-Ayala M, Oikonomopoulos S, Thymiakou E, Ragoussis J, Episkopou V. 2013. TGF-β/Smad2/3 signaling directly regulates several miRNAs in mouse ES cells and early embryos. PLoS ONE 8: e55186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguly T, Wrana JL. 2003. In or out? The dynamics of Smad nucleocytoplasmic shuttling. Trends Cell Biol 13: 216–220. [DOI] [PubMed] [Google Scholar]
- Remy I, Montmarquette A, Michnick SW. 2004. PKB/Akt modulates TGF-β signalling through a direct interaction with Smad3. Nat Cell Biol 6: 358–365. [DOI] [PubMed] [Google Scholar]
- Rogler CE, Levoci L, Ader T, Massimi A, Tchaikovskaya T, Norel R, Rogler LE. 2009. MicroRNA-23b cluster microRNAs regulate transforming growth factor-β/bone morphogenetic protein signaling and liver stem cell differentiation by targeting Smads. Hepatology 50: 575–584. [DOI] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL. 2010. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7: 64–77. [DOI] [PubMed] [Google Scholar]
- Sapkota G, Knockaert M, Alarcon C, Montalvo E, Brivanlou AH, Massagué J. 2006. Dephosphorylation of the linker regions of Smad1 and Smad2/3 by small C-terminal domain phosphatases has distinct outcomes for bone morphogenetic protein and transforming growth factor-β pathways. J Biol Chem 281: 40412–40419. [DOI] [PubMed] [Google Scholar]
- Sato MM, Nashimoto M, Katagiri T, Yawaka Y, Tamura M. 2009. Bone morphogenetic protein-2 down-regulates miR-206 expression by blocking its maturation process. Biochem Biophys Res Commun 383: 125–129. [DOI] [PubMed] [Google Scholar]
- Schink KO, Raiborg C, Stenmark H. 2013. Phosphatidylinositol 3-phosphate, a lipid that regulates membrane dynamics, protein sorting and cell signalling. BioEssays 35: 900–912. [DOI] [PubMed] [Google Scholar]
- Schmierer B, Tournier AL, Bates PA, Hill CS. 2008. Mathematical modeling identifies Smad nucleocytoplasmic shuttling as a dynamic signal-interpreting system. Proc Natl Acad Sci 105: 6608–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet LF, Hong W. 2001. Endofin, an endosomal FYVE domain protein. J Biol Chem 276: 42445–42454. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. 2007. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med 13: 1324–1332. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Adachi S, Kohu K, Yamada T, Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S, et al. 2004a. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-β signaling, as a target of the β-catenin pathway in colorectal tumor cells. J Biol Chem 279: 6840–6846. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Oda T, Matsuura K, Akiyama T. 2004b. Transcriptional regulation of the TGF-β pseudoreceptor BAMBI by TGF-β signaling. Biochem Biophys Res Commun 320: 680–684. [DOI] [PubMed] [Google Scholar]
- Senanayake U, Das S, Vesely P, Alzoughbi W, Frohlich LF, Chowdhury P, Leuschner I, Hoefler G, Guertl B. 2012. miR-192, miR-194, miR-215, miR-200c and miR-141 are downregulated and their common target ACVR2B is strongly expressed in renal childhood neoplasms. Carcinogenesis 33: 1014–1021. [DOI] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massagué J. 2004. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117: 211–223. [DOI] [PubMed] [Google Scholar]
- Sflomos G, Kostaras E, Panopoulou E, Pappas N, Kyrkou A, Politou AS, Fotsis T, Murphy C. 2011. ERBIN is a new SARA-interacting protein: Competition between SARA and SMAD2 and SMAD3 for binding to ERBIN. J Cell Sci 124: 3209–3222. [DOI] [PubMed] [Google Scholar]
- Shapira KE, Hirschhorn T, Barzilay L, Smorodinsky NI, Henis YI, Ehrlich M. 2014. Dab2 inhibits the cholesterol-dependent activation of JNK by TGF-β. Mol Biol Cell 25: 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shathasivam P, Kollara A, Ringuette MJ, Virtanen C, Wrana JL, Brown TJ. 2015. Human ortholog of Drosophila Melted impedes SMAD2 release from TGF-β receptor I to inhibit TGF-β signaling. Proc Natl Acad Sci 112: 3000–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZJ, Braun RK, Hu J, Xie Q, Chu H, Love RB, Stodola LA, Rosenthal LA, Szakaly RJ, Sorkness RL, et al. 2012. Pin1 protein regulates Smad protein signaling and pulmonary fibrosis. J Biol Chem 287: 23294–23305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Sun C, Zhang Z, Xu N, Duan X, Feng XH, Lin X. 2014. Specific control of BMP signaling and mesenchymal differentiation by cytoplasmic phosphatase PPM1H. Cell Res 24: 724–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massagué J. 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700. [DOI] [PubMed] [Google Scholar]
- Shi W, Sun C, He B, Xiong W, Shi X, Yao D, Cao X. 2004. GADD34-PP1c recruited by Smad7 dephosphorylates TGF-β type I receptor. J Cell Biol 164: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Chang C, Nie S, Xie S, Wan M, Cao X. 2007. Endofin acts as a Smad anchor for receptor activation in BMP signaling. J Cell Sci 120: 1216–1224. [DOI] [PubMed] [Google Scholar]
- Shimada K, Suzuki N, Ono Y, Tanaka K, Maeno M, Ito K. 2008. Ubc9 promotes the stability of Smad4 and the nuclear accumulation of Smad1 in osteoblast-like Saos-2 cells. Bone 42: 886–893. [DOI] [PubMed] [Google Scholar]
- Siomi H, Siomi MC. 2010. Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell 38: 323–332. [DOI] [PubMed] [Google Scholar]
- Siragam V, Rutnam ZJ, Yang W, Fang L, Luo L, Yang X, Li M, Deng Z, Qian J, Peng C, et al. 2012. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget 3: 1370–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Wang Q, Wen J, Liu S, Gao X, Cheng J, Zhang D. 2012. ACVR1, a therapeutic target of fibrodysplasia ossificans progressiva, is negatively regulated by miR-148a. Int J Mol Sci 13: 2063–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchelnytskyi S, ten Dijke P, Miyazono K, Heldin CH. 1996. Phosphorylation of Ser165 in TGF-β type I receptor modulates TGF-β1-induced cellular responses. EMBO J 15: 6231–6240. [PMC free article] [PubMed] [Google Scholar]
- Souchelnytskyi S, Tamaki K, Engstrom U, Wernstedt C, ten Dijke P, Heldin CH. 1997. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-β signaling. J Biol Chem 272: 28107–28115. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, et al. 2013. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 123: 3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Stein JL, van Wijnen AJ, Choi JY, Pratap J, Zaidi SK. 2003. Temporal and spatial parameters of skeletal gene expression: Targeting RUNX factors and their coregulatory proteins to subnuclear domains. Connect Tissue Res 44: 149–153. [PubMed] [Google Scholar]
- Stroschein SL, Bonni S, Wrana JL, Luo K. 2001. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev 15: 2822–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Zhang L, Gao X, Meng F, Wen J, Zhou H, Meng A, Chen YG. 2007. The evolutionally conserved activity of Dapper2 in antagonizing TGF-β signaling. FASEB J 21: 682–690. [DOI] [PubMed] [Google Scholar]
- Sun F, Wang J, Pan Q, Yu Y, Zhang Y, Wan Y, Wang J, Li X, Hong A. 2009. Characterization of function and regulation of miR-24-1 and miR-31. Biochem Biophys Res Commun 380: 660–665. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang D, Liu F, Xiang X, Ling G, Xiao L, Liu Y, Zhu X, Zhan M, Yang Y, et al. 2011. Low-dose paclitaxel ameliorates fibrosis in the remnant kidney model by down-regulating miR-192. J Pathol 225: 364–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki C, Murakami G, Fukuchi M, Shimanuki T, Shikauchi Y, Imamura T, Miyazono K. 2002. Smurf1 regulates the inhibitory activity of Smad7 by targeting Smad7 to the plasma membrane. J Biol Chem 277: 39919–39925. [DOI] [PubMed] [Google Scholar]
- Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. 2009. Modulation of microRNA processing by p53 Nature 460: 529–533. [DOI] [PubMed] [Google Scholar]
- Swingler TE, Wheeler G, Carmont V, Elliott HR, Barter MJ, Abu-Elmagd M, Donell ST, Boot-Handford RP, Hajihosseini MK, Munsterberg A, et al. 2012. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum 64: 1909–1919. [DOI] [PubMed] [Google Scholar]
- Tang LY, Yamashita M, Coussens NP, Tang Y, Wang X, Li C, Deng CX, Cheng SY, Zhang YE. 2011. Ablation of Smurf2 reveals an inhibition in TGF-β signalling through multiple mono-ubiquitination of Smad3. EMBO J 30: 4777–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Heldin CH. 2006. The Smad family. In Smad Signal Transduction, Vol. 5 (ed. ten Dijke P, Heldin CH), pp. 1–13. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Thien CB, Langdon WY. 2001. Cbl: Many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol 2: 294–307. [DOI] [PubMed] [Google Scholar]
- Thillainadesan G, Chitilian JM, Isovic M, Ablack JN, Mymryk JS, Tini M, Torchia J. 2012. TGF-β-dependent active demethylation and expression of the p15ink4b tumor suppressor are impaired by the ZNF217/CoREST complex. Mol Cell 46: 636–649. [DOI] [PubMed] [Google Scholar]
- Tiago DM, Marques CL, Roberto VP, Cancela ML, Laize V. 2014. Mir-20a regulates in vitro mineralization and BMP signaling pathway by targeting BMP-2 transcript in fish. Arch Biochem Biophys 543: 23–30. [DOI] [PubMed] [Google Scholar]
- Tsai ZY, Singh S, Yu SL, Kao LP, Chen BZ, Ho BC, Yang PC, Li SS. 2010. Identification of microRNAs regulated by activin A in human embryonic stem cells. J Cell Biochem 109: 93–102. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. 1998. SARA, a FYVE domain protein that recruits Smad2 to the TGF-β receptor. Cell 95: 779–791. [DOI] [PubMed] [Google Scholar]
- Tu F, Pan ZX, Yao Y, Liu HL, Liu SR, Xie Z, Li QF. 2014. miR-34a targets the inhibin βB gene, promoting granulosa cell apoptosis in the porcine ovary. Genet Mol Res 13: 2504–2512. [DOI] [PubMed] [Google Scholar]
- Ueberham U, Rohn S, Ueberham E, Wodischeck S, Hilbrich I, Holzer M, Bruckner MK, Gruschka H, Arendt T. 2014. Pin1 promotes degradation of Smad proteins and their interaction with phosphorylated tau in Alzheimer’s disease. Neuropathol Appl Neurobiol 40: 815–832. [DOI] [PubMed] [Google Scholar]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL. 2008. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10: 837–848. [DOI] [PubMed] [Google Scholar]
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, et al. 2009. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial-mesenchymal transition. Nat Cell Biol 11: 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Tang Y, Tytler EM, Lu C, Jin B, Vickers SM, Yang L, Shi X, Cao X. 2004. Smad4 protein stability is regulated by ubiquitin ligase SCF β-TrCP1. J Biol Chem 279: 14484–14487. [DOI] [PubMed] [Google Scholar]
- Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. 1996. The immunophilin FKBP12 functions as a common inhibitor of the TGFβ family type I receptors. Cell 86: 435–444. [DOI] [PubMed] [Google Scholar]
- Wang Q, Huang Z, Xue H, Jin C, Ju XL, Han JD, Chen YG. 2008. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood 111: 588–595. [DOI] [PubMed] [Google Scholar]
- Wang G, Matsuura I, He D, Liu F. 2009. Transforming growth factor-β-inducible phosphorylation of Smad3. J Biol Chem 284: 9663–9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, Ghoshal K. 2010a. TGF-β-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene 29: 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu J, Zong Y, Xu Y, Deng W, Zhu H, Liu Y, Ma C, Huang L, Zhang L, et al. 2010b. miR-106b aberrantly expressed in a double transgenic mouse model for Alzheimer’s disease targets TGF-β type II receptor. Brain Res 1357: 166–174. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li W, Zang X, Chen N, Liu T, Tsonis PA, Huang Y. 2013. MicroRNA-204-5p regulates epithelial-to-mesenchymal transition during human posterior capsule opacification by targeting SMAD4. Invest Ophthalmol Vis Sci 54: 323–332. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Masuyama N, Fukuda M, Nishida E. 2000. Regulation of intracellular dynamics of Smad4 by its leucine-rich nuclear export signal. EMBO Rep 1: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Itoh S, Goto T, Ohnishi E, Inamitsu M, Itoh F, Satoh K, Wiercinska E, Yang W, Shi L, et al. 2010. TMEPAI, a transmembrane TGF-β-inducible protein, sequesters Smad proteins from active participation in TGF-β signaling. Mol Cell 37: 123–134. [DOI] [PubMed] [Google Scholar]
- Watson IR, Irwin MS, Ohh M. 2011. NEDD8 pathways in cancer, Sine Quibus Non. Cancer Cell 19: 168–176. [DOI] [PubMed] [Google Scholar]
- Weiss A, Attisano L. 2013. The TGF-β superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol 2: 47–63. [DOI] [PubMed] [Google Scholar]
- Wicks SJ, Haros K, Maillard M, Song L, Cohen RE, Dijke PT, Chantry A. 2005. The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGF-β signalling. Oncogene 24: 8080–8084. [DOI] [PubMed] [Google Scholar]
- Wieser R, Wrana JL, Massagué J. 1995. GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. EMBO J 14: 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SA, Zimmerman CM, Li LI, Mathews LS. 1996. Formation and activation by phosphorylation of activin receptor complexes. Mol Endocrinol 10: 367–379. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. 1992. TGF-β signals through a heteromeric protein kinase receptor complex. Cell 71: 1003–1014. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. 1994. Mechanism of activation of the TGF-β receptor. Nature 370: 341–347. [DOI] [PubMed] [Google Scholar]
- Wrighton KH, Lin X, Feng XH. 2008. Critical regulation of TGF-β signaling by Hsp90. Proc Natl Acad Sci 105: 9244–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Chen YG, Ozdamar B, Gyuricza CA, Chong PA, Wrana JL, Massagué J, Shi Y. 2000. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science 287: 92–97. [DOI] [PubMed] [Google Scholar]
- Wu T, Zhou H, Hong Y, Li J, Jiang X, Huang H. 2012. miR-30 family members negatively regulate osteoblast differentiation. J Biol Chem 287: 7503–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Wang Z, Zaromytidou AI, Zhang XH, Chow-Tsang LF, Liu JX, Kim H, Barlas A, Manova-Todorova K, Kaartinen V, et al. 2011. A poised chromatin platform for TGF-β access to master regulators. Cell 147: 1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Liu X, Lodish HF. 2000. Importin β mediates nuclear translocation of Smad 3. J Biol Chem 275: 23425–23428. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Watson N, Rodriguez C, Lodish HF. 2001. Nucleocytoplasmic shuttling of Smad1 conferred by its nuclear localization and nuclear export signals. J Biol Chem 276: 39404–39410. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Brownawell AM, Macara IG, Lodish HF. 2003a. A novel nuclear export signal in Smad1 is essential for its signaling activity. J Biol Chem 278: 34245–34252. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Latek R, Lodish HF. 2003b. An extended bipartite nuclear localization signal in Smad4 is required for its nuclear import and transcriptional activity. Oncogene 22: 1057–1069. [DOI] [PubMed] [Google Scholar]
- Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, Shi Y, Wang F, Wu Y, Tong WD, et al. 2009. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis 200: 916–925. [DOI] [PubMed] [Google Scholar]
- Xu L, Massagué J. 2004. Nucleocytoplasmic shuttling of signal transducers. Nat Rev Mol Cell Biol 5: 209–219. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen YG, Massagué J. 2000. The nuclear import function of Smad2 is masked by SARA and unmasked by TGF-β-dependent phosphorylation. Nat Cell Biol 2: 559–562. [DOI] [PubMed] [Google Scholar]
- Xu L, Kang Y, Col S, Massagué J. 2002. Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGF-β signaling complexes in the cytoplasm and nucleus. Mol Cell 10: 271–282. [DOI] [PubMed] [Google Scholar]
- Xu L, Alarcon C, Col S, Massagué J. 2003. Distinct domain utilization by Smad3 and Smad4 for nucleoporin interaction and nuclear import. J Biol Chem 278: 42569–42577. [DOI] [PubMed] [Google Scholar]
- Xu L, Yao X, Chen X, Lu P, Zhang B, Ip YT. 2007. Msk is required for nuclear import of TGF-β/BMP-activated Smads. J Cell Biol 178: 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Liu J, Derynck R. 2012. Post-translational regulation of TGF-β receptor and Smad signaling. FEBS Lett 586: 1871–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Kurisaki A, Yamakawa N, Minakuchi K, Sugino H. 2006. FKBP12 functions as an adaptor of the Smad7–Smurf1 complex on activin type I receptor. J Mol Endocrinol 36: 569–579. [DOI] [PubMed] [Google Scholar]
- Yamakawa N, Tsuchida K, Sugino H. 2002. The rasGAP-binding protein, Dok-1, mediates activin signaling via serine/threonine kinase receptors. EMBO J 21: 1684–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Franzen P, Miyazono K, Heldin CH. 1994. Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-β. J Biol Chem 269: 20172–20178. [PubMed] [Google Scholar]
- Yamashita M, Fatyol K, Jin CY, Wang XC, Liu ZG, Zhang YE. 2008. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol Cell 31: 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Chen YG. 2011. Smad7: Not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J 434: 1–10. [DOI] [PubMed] [Google Scholar]
- Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning Y, Chen YG. 2009. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-β signaling. J Biol Chem 284: 30097–30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Zhang J, Pan L, Wang P, Xue H, Zhang L, Gao X, Zhao X, Ning Y, Chen YG. 2011. TSC-22 promotes transforming growth factor β-mediated cardiac myofibroblast differentiation by antagonizing Smad7 activity. Mol Cell Biol 31: 3700–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Zhang L, Fang T, Zhang Q, Wu S, Jiang Y, Sun H, Hu Y. 2012. MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett 586: 3263–3270. [DOI] [PubMed] [Google Scholar]
- Yang KM, Kim W, Bae E, Gim J, Weist BM, Jung Y, Hyun JS, Hernandez JB, Leem SH, Park T, et al. 2012. DRAK2 participates in a negative feedback loop to control TGF-β/Smads signaling by binding to type I TGF-β receptor. Cell Rep 2: 1286–1299. [DOI] [PubMed] [Google Scholar]
- Yang H, Fang F, Chang R, Yang L. 2013. MicroRNA-140-5p suppresses tumor growth and metastasis by targeting transforming growth factor β receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology 58: 205–217. [DOI] [PubMed] [Google Scholar]
- Yao D, Dore JJ Jr, Leof EB. 2000. FKBP12 is a negative regulator of transforming growth factor-β receptor internalization. J Biol Chem 275: 13149–13154. [DOI] [PubMed] [Google Scholar]
- Yao G, Yin M, Lian J, Tian H, Liu L, Li X, Sun F. 2010. MicroRNA-224 is involved in transforming growth factor-β-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol 24: 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q, Wang X, Fewell C, Cameron J, Zhu H, Baddoo M, Lin Z, Flemington EK. 2010. MicroRNA miR-155 inhibits bone morphogenetic protein (BMP) signaling and BMP-mediated Epstein–Barr virus reactivation. J Virol 84: 6318–6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Hebert MC, Zhang YE. 2002. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J 21: 3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Pan L, Qin X, Chen H, Xu Y, Chen Y, Tang H. 2010. MTMR4 attenuates transforming growth factor β (TGF-β) signaling by dephosphorylating R-Smads in endosomes. J Biol Chem 285: 8454–8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, He X, Chen YG, Hao Y, Yang S, Wang L, Pan L, Tang H. 2013. Myotubularin-related protein 4 (MTMR4) attenuates BMP/Dpp signaling by dephosphorylation of Smad proteins. J Biol Chem 288: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukita A, Hosoya A, Ito Y, Katagiri T, Asashima M, Nakamura H. 2012. Ubc9 negatively regulates BMP-mediated osteoblastic differentiation in cultured cells. Bone 50: 1092–1099. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Sullivan AJ, van Wijnen AJ, Stein JL, Stein GS, Lian JB. 2002. Integration of Runx and Smad regulatory signals at transcriptionally active subnuclear sites. Proc Natl Acad Sci 99: 8048–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chang C, Gehling DJ, Hemmati-Brivanlou A, Derynck R. 2001. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci 98: 974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou H, Su Y, Sun Z, Zhang H, Zhang Y, Ning Y, Chen YG, Meng A. 2004. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science 306: 114–117. [DOI] [PubMed] [Google Scholar]
- Zhang W, Jiang Y, Wang Q, Ma X, Xiao Z, Zuo W, Fang X, Chen YG. 2009. Single-molecule imaging reveals transforming growth factor-β-induced type II receptor dimerization. Proc Natl Acad Sci 106: 15679–15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yuan J, Yang Y, Xu L, Wang Q, Zuo W, Fang X, Chen YG. 2010. Monomeric type I and type III transforming growth factor-β receptors and their dimerization revealed by single-molecule imaging. Cell Res 20: 1216–1223. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu CX, et al. 2012. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat Cell Biol 14: 717–726. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Garcia de Vinuesa A, de Kruijf EM, Mesker WE, Hui L, Drabsch Y, Li Y, Bauer A, Rousseau A, et al. 2013a. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol Cell 51: 559–572. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sun H, Jiang Y, Ding L, Wu S, Fang T, Yan G, Hu Y. 2013b. MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor IIA. PLoS ONE 8: e59667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma W, Tian S, Fan Z, Ma X, Yang X, Zhao Q, Tan K, Chen H, Chen D, et al. 2014. RanBPM interacts with TβRI, TRAF6 and curbs TGF induced nuclear accumulation of TβRI. Cell Signal 26: 162–172. [DOI] [PubMed] [Google Scholar]
- Zhao B, Wang Q, Du J, Luo S, Xia J, Chen YG. 2012. PICK1 promotes caveolin-dependent degradation of TGF-β type I receptor. Cell Res 22: 1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xiao M, Sun B, Zhang Z, Shen T, Duan X, Yu PB, Feng XH, Lin X. 2014. C-terminal domain (CTD) small phosphatase-like 2 modulates the canonical bone morphogenetic protein (BMP) signaling and mesenchymal differentiation via Smad dephosphorylation. J Biol Chem 289: 26441–26450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. 2011. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 22: 1668–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. 1999. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 400: 687–693. [DOI] [PubMed] [Google Scholar]
- Zhu L, Wang L, Luo X, Zhang Y, Ding Q, Jiang X, Wang X, Pan Y, Chen Y. 2012. Tollip, an intracellular trafficking protein, is a novel modulator of the transforming growth factor-β signaling pathway. J Biol Chem 287: 39653–39663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrennen-Bullough KB, Wu Q, Core AB, Canali S, Chen W, Theurl I, Meynard D, Babitt JL. 2014. MicroRNA-130a is up-regulated in mouse liver by iron deficiency and targets the bone morphogenetic protein (BMP) receptor ALK2 to attenuate BMP signaling and hepcidin transcription. J Biol Chem 289: 23796–23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Chen YG. 2009. Specific activation of mitogen-activated protein kinase by transforming growth factor-β receptors in lipid rafts is required for epithelial cell plasticity. Mol Biol Cell 20: 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Huang F, Chiang YJ, Li M, Du J, Ding Y, Zhang T, Lee HW, Jeong LS, Chen Y, et al. 2013. c-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-β type II receptor. Mol Cell 49: 499–510. [DOI] [PubMed] [Google Scholar]