ABSTRACT
Inhalation of infected brain homogenate results in transepithelial transport of prions across the nasal mucosa of hamsters, some of which occurs rapidly in relatively large amounts between cells (A. E. Kincaid, K. F. Hudson, M. W. Richey, and J. C. Bartz, J. Virol 86:12731–12740, 2012, doi:http://dx.doi.org/10.1128/JVI.01930-12). Bulk transepithelial transport in the nasal cavity has not been studied to date. In the present study, we characterized the frequency, size, and specificity of the intercellular spaces that mediate the bulk transport of inhaled prions between cells of mice or hamsters following extranasal inoculation with mock-infected brain homogenate, different strains of prion-infected brain homogenate, or brain homogenate mixed with India ink. Infected or mock-infected inoculum was identified within lymphatic vessels of the lamina propria and in spaces of >5 μm between a small number of cells of the nasal mucosa in >90% of animals from 5 to 60 min after inhalation. The width of the spaces between cells, the amount of the inoculum within the lumen of lymphatic vessels, and the timing of the transport indicate that this type of transport was taking place through preexisting spaces in the nasal cavity that were orders of magnitude wider than what is normally reported for paracellular transport. The indiscriminate rapid bulk transport of brain homogenate in the nasal cavity results in immediate entry into nasal cavity lymphatics following inhalation. This novel mechanism may underlie the recent report of the early detection of prions in blood following inhalation and has implications for horizontal prion transmission.
IMPORTANCE The results of these studies demonstrate that the nasal mucosa of mice and hamsters is not an absolute anatomical barrier to inhaled prion-infected or uninfected brain homogenate. Relatively large amounts of infected and uninfected brain homogenate rapidly cross the nasal mucosa and enter the lumen of lymphatic vessels following inhalation. These bulk transepithelial transport events were relatively rare but present in >90% of animals 5 to 60 min following inhalation. This novel mechanism of bulk transepithelial transport was seen in experimental and control hamsters and mice, indicating that it was not species specific or in response to prion exposure. The indiscriminate bulk intercellular transport of inhaled pathogens across the nasal mucosa followed by entry into the lymphatic system may be a mechanism that underlies the entry and spread of other toxins and pathogens in olfactory system-driven animals.
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs) are a group of fatal neurological disorders affecting a variety of animal species, including humans. TSEs have relatively long incubation periods, and although they are rare in humans, they are relatively common in some animal populations. The TSEs affecting animals include scrapie in sheep and goats and chronic wasting disease (CWD) in deer, elk, and moose. The infectious agent is thought to consist largely if not entirely of PrPSc, a misfolded conformation of the naturally occurring prion protein (PrP), designated PrPC (1–6).
Specific details of the transmission of these diseases are not known, but there is evidence for the horizontal transmission of PrPSc that has been excreted into the environment (7, 8). The natural route(s) of entry for the infectious agent in the animal TSEs is not known, but there is experimental evidence for both oral (9–13) and nasal (14–19) routes of infection. Whether the infectious agent is ingested or inhaled, it must cross an epithelium to enter the body, where prion conversion occurs prior to the onset of disease. Inhalation of prions into the nasal cavity (NC) is a more efficient route of infection than the per os route (14, 18, 19). The transport of prions across the nasal mucosa occurs within minutes of inhalation (20) and does not involve transport by olfactory receptor neurons (15, 20). Transport of inhaled prions across the nasal mucosa was demonstrated by the detection of hyper (HY) strain transmissible mink encephalopathy (TME)-infected brain homogenate (BH) within the lumen of lymphatic vessels in the lamina propria of the nasal cavity and involved both M cell transport and transport between epithelial cells of the NC (20). While there have been previous reports of M cell transport of prions in vitro (21–25) and in vivo (26–28), the in vivo bulk transport of HY TME-infected inoculum between cells of the NC was unexpected, as this mechanism of transepithelial transport had not been reported previously. The purpose of the following study was to characterize this novel mechanism of transepithelial transport by determining (i) whether this was a species- or prion strain-specific process, (ii) the frequency of these bulk intercellular transport events, and (iii) and the size of the spaces between the cells that mediate this transport.
MATERIALS AND METHODS
Animal care.
All procedures involving animals were approved by the Creighton University Institutional Animal Care and Use Committee and were done in accordance with the Guide for the Care and Use of Laboratory Animals (29). Adult male golden Syrian hamsters from Harlan Sprague-Dawley (Indianapolis, IN) and C57BL/6 mice from The Jackson Laboratory (Bar Harbor, ME) were housed in standard cages with ad libitum access to water and food.
Brain homogenate and control solutions.
India ink (colloidal carbon in water), Dulbecco's phosphate-buffered saline (DPBS), or BH (10%, wt/vol, in DPBS) from either an HY TME-infected hamster (containing 109.3 intracerebral [i.c.] 50% lethal doses [LD50]/g), a Drowsy (DY) strain TME-infected hamster (containing 107.8 i.c. LD50/g), a strain RML-infected mouse (containing 109.3 i.c. LD50/g), an uninfected hamster, or an uninfected mouse was used.
Animal inoculations.
Extranasal (e.n.) inoculations were performed as described previously (14, 20). Animals were anesthetized with either isoflurane (Patterson Veterinary, Kansas City, MO) or ketamine (120 mg/kg of body weight for hamsters; 80 mg/kg for mice; Patterson Veterinary, Kansas City, MO) and xylazine (10 mg/kg for hamsters and mice; Patterson Veterinary, Kansas City, MO) and placed in the supine position, and the inoculum was applied just inferior to each nostril, where it was immediately inhaled into the nasal cavity. The e.n. placement avoided injury to the nasal mucosa. The animals began moving freely within 1 to 2 min following isoflurane anesthesia and within 10 to 15 min following ketamine-xylazine anesthesia.
The first group of tissue sections was collected from hamsters used in a previous study (20); these animals had been e.n. inoculated with 50 to 200 μl of HY TME-infected BH as reported previously (n = 7) (20). NCs with good morphology were selected, and serial sections were collected in those parts of the NC where bulk intercellular transport events had been noted. The serial sections were processed immunohistochemically (IHC) to determine the location and dimensions of the spaces between cells where the inoculum was located. The next group of tissue sections was taken from hamsters that were anesthetized with isoflurane and e.n. inoculated with HY TME-infected BH mixed with India ink (n = 4; 50 μl colloid carbon in water mixed with 50 μl infected BH; 100 μl total), mock-infected BH mixed with India ink (n = 2; 50 μl ink mixed with 50 μl mock-infected BH), or mock-infected BH alone (n = 1); collectively, these animals served as a control for immunohistochemical processing effects. A subset of these animals (n = 5; Table 1) were immersion fixed following inhalation of BH to examine the effects of transcardial perfusion on tissue integrity and bulk transepithelial transport. To control for the effects of gas anesthesia on the integrity of the nasal mucosa and to determine if this type of transepithelial transport was strain specific, some hamsters were anesthetized i.p. with ketamine and xylazine prior to e.n. inoculation of 100 μl HY (n = 4) or DY (n = 2) TME-infected or mock-infected (n = 2) BH. To determine if this specific mode of transepithelial transport was species specific, mice were e.n. inoculated with 20 μl of RML-infected (n = 6) or mock-infected (n = 4) BH.
TABLE 1.
Rapid transepithelial transport of prions, ink, and BH
| Species | Anesthesia | Survival time (min) | Inhalanta | No. of animals with inhalant-positive structures/no. of animals examined |
|
|---|---|---|---|---|---|
| Bulk intercellular transport | Lymphatic positive | ||||
| Hamster | Isoflurane | 5 | HY | 3/3 | 3/3 |
| Hamster | Isoflurane | 10 | HYb | 4/4 | 4/4 |
| Hamster | Isoflurane | 10 | Mockc | 1/1 | 1/1 |
| Hamster | Isoflurane | 10 | Mock + ink | 2/2 | 2/2 |
| Hamster | Isoflurane | 10 | HY + inkb | 3/4 | 4/4 |
| Hamster | Isoflurane | 60 | HY | 2/2 | 2/2 |
| Hamster | Ketamine | 15 | Mock | 2/2 | 2/2 |
| Hamster | Ketamine | 15 | HY | 4/4 | 4/4 |
| Hamster | Ketamine | 15 | DY | 2/2 | 2/2 |
| Mouse | Ketamine | 5 | Mock | 1/2 | 0/2 |
| Mouse | Ketamine | 5 | RML | 3/3 | 3/3 |
| Mouse | Ketamine | 30 | Mock | 2/2 | 2/2 |
| Mouse | Ketamine | 30 | RML | 3/3 | 3/3 |
| Total | 32/34 | 32/34 | |||
Between 20 and 200 μl of the indicated inhalant was delivered extranasally.
Two animals were immersion fixed.
This animal was immersion fixed.
Tissue collection.
Following e.n. inoculations and survival periods of 5 to 60 min, animals were reanesthetized with isoflurane and transcardially perfused with 50 ml of 0.01 M DPBS followed by 50 to 75 ml of McLean's paraformaldehyde-lysine-periodate (PLP) fixative (Table 1). The animal skulls were removed, placed in PLP fixative at room temperature overnight, and then decalcified for 2 weeks at room temperature; the solution was changed after 1 week (decalcifying solution; Richard-Allan Scientific, Kalamazoo, MI). The nasal cavity, consisting of the region between the posterior edge of the incisors and the anterior edge of the seventh palatal ridge, a length of about 12 mm, was excised. This block was cut and divided into three 4-mm coronal slices and placed in a cassette for embedment in paraffin. The paraffin tissue sections (7 μm, 10 μm, or 30 μm) were cut through the entire extent of the NC using a standard Leica rotary microtome and mounted on glass slides (Superfrost Plus; Fisher).
Histology.
Six hamsters were not inoculated and were fixed using PLP fixative by either transcardial perfusion or by immersion to serve as additional negative controls and to aid in the identification of glandular ducts that open into the airspace of the NC and lymphatic vessels in the lamina propria of the hamster nasal cavity (Table 1). Tissue sections from these untreated animals was cleared, dehydrated, and stained with periodic acid-Schiff (PAS) or hematoxylin and eosin (H&E). Pairs of tissue sections not further than 112 μm apart through the entire rostral-caudal extent of the hamster nasal cavity were examined using an Olympus BX 40 light microscope.
IHC.
Immunohistochemical (IHC) processing of tissue sections was carried out to identify the brain homogenate in the NC of hamsters following inhalation. IHC was targeted to identify either the presence of glial fibrillary acid protein (GFAP), a prominent component of BH, or the disease-associated form of the prion protein (PrPd). These procedures were performed as reported previously on tissue sections through the entire extent of the NC (14, 20). Detection of GFAP does not require an antigen retrieval step and results in a better morphology of the NC; thus, it was used for detection of BH when the presence of PrPd was not required. For the IHC detection of PrPd, tissue sections were deparaffinized and subjected to antigen retrieval in formic acid (95%) for 10 min at room temperature. The following steps were carried out at room temperature; incubations were separated by 3 rinses in 0.05% (vol/vol) Tween in Tris-buffered saline (TTBS). Endogenous peroxidase and nonspecific antibody binding was inhibited by incubating the tissue sections in 0.3% H2O2-methanol for 20 min followed by an incubation for 30 min in 10% normal horse serum in TTBS. The avidin-biotin (ABC) method was used to visualize the prion protein. Incubation in an anti-prion protein monoclonal antibody (clone 3F4; 1:600; Chemicon, Temecula, CA) for 2 h was followed by incubation in biotinylated horse anti-mouse immunoglobulin antibody (1:600; Vector Laboratories, Burlingame, CA) for 30 min. The sections were placed in ABC solution (1:200; Vector Laboratories, Burlingame, CA) for 15 to 20 min and then reacted in filtered 0.05% diaminobenzidine tetrachloride (Sigma, St. Louis, MO) with 0.0015% H2O2. The tissue sections were counterstained with hematoxylin and dehydrated using graded alcohol solutions, and a coverslip was placed and sealed with Cytoseal-XYL (Richard Allan Scientific, Kalamazoo, MI). GFAP IHC was done in a similar manner with the following differences: no antigen retrieval step was required, blocking was carried out using normal goat serum, the primary antibody was rabbit anti-GFAP (1:800; Dako, Carpinteria, CA), and the secondary antibody was biotinylated goat anti-rabbit immunoglobulin (1:800; Vector Laboratories, Burlingame, CA). Some tissue sections were processed in an identical manner but with either the primary or the secondary antibodies omitted or by use of the same concentration of a mouse immunoglobulin G isotype control (Abcam, Cambridge, MA) in place of the primary antibody. The tissue sections were examined using an Olympus BX 40 light microscope and photographed using an Olympus XC50 digital camera and analysis software (Soft Imaging Systems, Lakewood, CO). When additional morphological detail was required, adjacent tissue sections were stained with H&E or PAS. The tissue sections were examined either as part of a group of serial sections extending through 300 μm of NC (every tissue section was processed and examined) or as pairs of tissue sections from the NC not further than 56 μm apart. Photomicrographs of each example of transepithelial transport of BH between epithelial cells of the nasal mucosa and each example of BH located within lymphatic vessels of the lamina propria of the NC were taken. To estimate the frequency of occurrence of transepithelial transport events in each animal in this study, the number of examples of BH extending greater than halfway through the width of the nasal mucosa was recorded, and this number was divided by the total number of tissue sections processed and analyzed.
RESULTS
Brain homogenate passes through spaces between a small number of mucosal cells that line the NC of almost all animals following inhalation.
A small amount of inoculum was placed inferior to the nostrils and immediately inhaled into the NC (Table 1). At 5 to 60 min postinhalation (p.i.), regularly spaced adjacent pairs of tissue sections through the entire extent of the NC of the animals were processed for hematoxylin and eosin (H&E) staining, periodic acid-Schiff (PAS) staining, and detection of the disease-associated form of the prion protein (PrPd) or the glial fibrillary acidic protein (GFAP) and examined with a light microscope. Relatively large amounts of BH were detected between the respiratory epithelial (RE), olfactory epithelial (OE), and follicle-associated epithelial (FAE) cells that line the NC in 32 of 34 hamsters and mice (Table 1). In addition, BH was identified within the lumen of lymphatic vessels of the lamina propria in 32 of 34 animals; 30 of the 34 animals had examples of transport of BH between cells and BH within the lumen of the lymphatics in the lamina propria (Table 1). There was no transepithelial transport between the stratified squamous epithelial cells that are restricted to the vestibule of the NC, but otherwise, there was no obvious pattern or spatial preference for the location of transport across the NC mucosa. Transepithelial transport between the OE and RE cells that line the nasal septum and the turbinates in the main chamber of the NC and between the RE and FAE cells that line the floor of the NC was observed. This type of transport was recorded for 3% of tissue sections examined from each animal (average; range, 0 to 13%; Table 2). There was an average of 8.7 transport events per animal (range, 0 to 44 transport events per animal) in the tissue sections that were examined (average number of sections examined, 285; range, 120 to 630). There did not appear to be a correlation between the volume of inoculum inhaled and the number of transepithelial transport events or between the survival time p.i. and the number of transepithelial transport events (Table 2). The inoculum was not identified within the ducts or the acini of the serous and mucus glands located within the NC in any of the experimental or control animals, and there were no examples of transepithelial transport in tissue sections from uninoculated animals that were immunohistochemically processed for either PrPd or GFAP IHC (data not shown).
TABLE 2.
Estimate of bulk intercellular transport events
| Species | Anesthesia | Survival time (min) | Inhalant | Animal no. | No. of tissue sections analyzed | No. of bulk intercellular events | % tissue sections with transporta |
|---|---|---|---|---|---|---|---|
| Hamster | Isoflurane | 5 | HY | 1 | 540 | 21 | 3.9 |
| Hamster | Isoflurane | 5 | HY | 2 | 435 | 31 | 7.1 |
| Hamster | Isoflurane | 5 | HY | 3 | 312 | 7 | 2.2 |
| Hamster | Isoflurane | 10 | HY | 1 | 266 | 6 | 2.3 |
| Hamster | Isoflurane | 10 | HY | 2b | 178 | 10 | 5.6 |
| Hamster | Isoflurane | 10 | HY | 3c | 120 | 5 | 4.2 |
| Hamster | Isoflurane | 10 | HY | 4c | 150 | 9 | 6.0 |
| Hamster | Isoflurane | 10 | Mock | 1c | 175 | 2 | 1.1 |
| Hamster | Isoflurane | 10 | Mock + ink | 1 | 236 | 3 | 1.3 |
| Hamster | Isoflurane | 10 | Mock + ink | 2 | 236 | 1 | 0.4 |
| Hamster | Isoflurane | 10 | HY + ink | 1 | 270 | 3 | 1.1 |
| Hamster | Isoflurane | 10 | HY + ink | 2 | 384 | 8 | 2.1 |
| Hamster | Isoflurane | 10 | HY + ink | 3c | 240 | 24 | 10 |
| Hamster | Isoflurane | 10 | HY + ink | 4c | 220 | 0 | 0 |
| Hamster | Isoflurane | 60 | HY | 1 | 630 | 14 | 2.2 |
| Hamster | Isoflurane | 60 | HY | 2 | 528 | 4 | 0.8 |
| Hamster | Ketamine | 15 | Mock | 1 | 248 | 8 | 3.2 |
| Hamster | Ketamine | 15 | Mock | 2 | 176 | 4 | 2.3 |
| Hamster | Ketamine | 15 | HY | 1 | 348 | 10 | 2.9 |
| Hamster | Ketamine | 15 | HY | 2 | 328 | 5 | 1.5 |
| Hamster | Ketamine | 15 | HY | 3 | 336 | 4 | 1.2 |
| Hamster | Ketamine | 15 | HY | 4 | 240 | 2 | 0.8 |
| Hamster | Ketamine | 15 | DY | 1 | 248 | 8 | 3.2 |
| Hamster | Ketamine | 15 | DY | 2 | 340 | 44 | 12.9 |
| Mouse | Ketamine | 5 | Mock | 1 | 276 | 0 | 0 |
| Mouse | Ketamine | 5 | Mock | 2 | 216 | 3 | 1.4 |
| Mouse | Ketamine | 5 | RML | 1 | 258 | 7 | 2.7 |
| Mouse | Ketamine | 5 | RML | 2 | 294 | 6 | 2.0 |
| Mouse | Ketamine | 5 | RML | 3 | 240 | 10 | 4.2 |
| Mouse | Ketamine | 30 | Mock | 1 | 228 | 3 | 1.3 |
| Mouse | Ketamine | 30 | Mock | 2 | 270 | 5 | 1.9 |
| Mouse | Ketamine | 30 | RML | 1 | 258 | 19 | 7.4 |
| Mouse | Ketamine | 30 | RML | 2 | 228 | 6 | 2.6 |
| Mouse | Ketamine | 30 | RML | 3 | 234 | 3 | 1.3 |
| Avg | 285 | 8.7 | 3.0 |
An estimate based on the number of transport events divided by the number of sections processed and analyzed. Note that some bulk intercellular transport events were present on multiple tissue sections and some tissue sections had multiple bulk intercellular transport events.
All tissue sections were cut at 7 μm, except for those from this animal, which were cut at 10 μm.
An immersion-fixed animal.
Bulk transepithelial transport of the inoculum occurred in relatively large spaces between cells and was identified using several tissue processing techniques in both immersion- and transcardial perfusion-fixed animals.
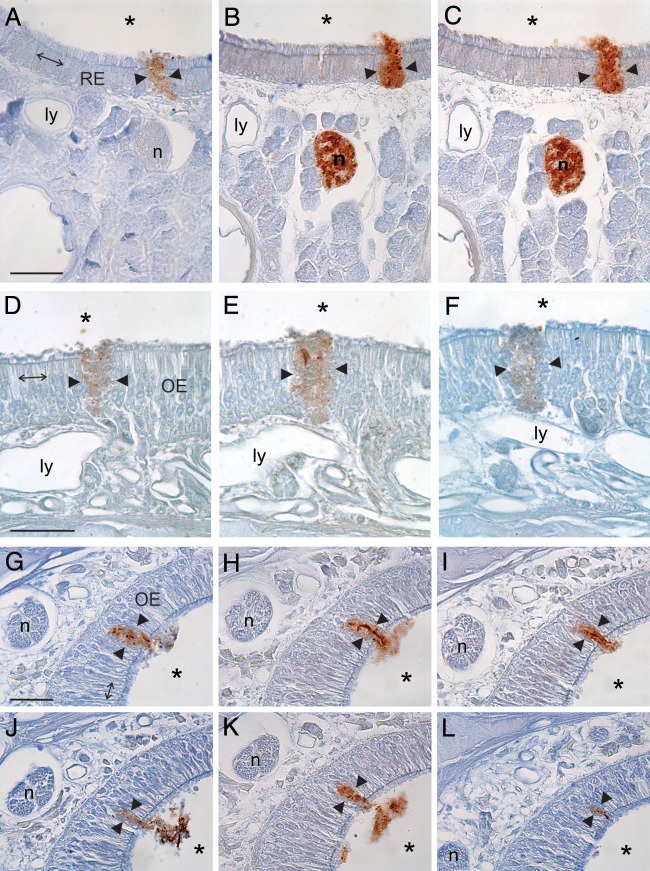
Examination of 10 to 30 serial tissue sections showed that the inhaled inoculum was located between cells and not within cells. This was determined by identifying examples of BH located between cells of the nasal mucosa that were present on multiple adjacent tissue sections from the same animal. Confirmation that BH was continuous from one adjacent section to the next was made by using fiducial landmarks, such as blood vessels, lymphatic vessels, and nerve fascicles (Fig. 1). Focusing through the entire thickness of the tissue sections (7 μm or 10 μm) demonstrated that the inoculum was located extracellularly and not within the cytoplasm of adjacent cells. The width of the inoculum crossing the nasal mucosa in coronal sections was similar to or greater than the width of 1 to 4 epithelial cells that lined the nasal cavity in any one section (Fig. 1). Examination of the serial sections showed that the inoculum located between mucosal cells was present in 2 to 10 adjacent serial tissue sections; thus, the transepithelial transport extended between 10 μm and 100 μm in the rostral-caudal dimension (Fig. 1). The identification of BH between cells of serial tissue sections taken from multiple different animals using both GFAP and PrPd IHC essentially rules out the possibility that the transport was due to a cutting artifact, tissue processing, or tissue shrinkage, since it is unlikely that the same artifact would be present in exactly the same location on multiple tissue sections from multiple slides.
FIG 1.
Serial tissue sections demonstrate inhaled inoculum passing between epithelial cells (between arrowheads) that line the NC in animals that survived from 5 to 60 min p.i. (A to C) Serial 7-μm tissue sections taken from an animal that inhaled 100 μl of infected BH and perfused at 5 min p.i. and processed for PrPd (A) or GFAP (B, C) IHC. Note that the BH can be seen spanning the width of the respiratory epithelium (RE), extending from the airspace of the NC (asterisks) to the lamina propria that contains lymphatic vessels (ly) and nerve fascicles (n), which served as fiducial markers. (D to F) Serial 7-μm tissue sections taken from an animal that inhaled 100 μl of infected BH and survived to 60 min p.i. and processed for GFAP IHC. In this case, the inhaled BH extended from the airspace to the lamina propria between cells of the olfactory epithelium (OE). Note the proximity of the BH to a lymphatic vessel, which served as a fiducial marker. (G to L) Six of 10 serial tissue sections of OE that demonstrated a continuous transport event that extended over 100 μm in the rostral-caudal dimension. These sections were taken from an animal that inhaled 100 μl of infected BH and survived for 10 min p.i. The 10-μm tissue sections were processed for PrPd (G, J, L) or GFAP (H, I, K) IHC. Note the nerve fascicle (n), which served as a fiducial marker, in the 6 tissue sections. Arrows in panels A, D, and G, the width of four epithelial cells in these sections to demonstrate that the width of the space occupied by the inoculum is between 1 and 4 cells. Bars = 25 μm.
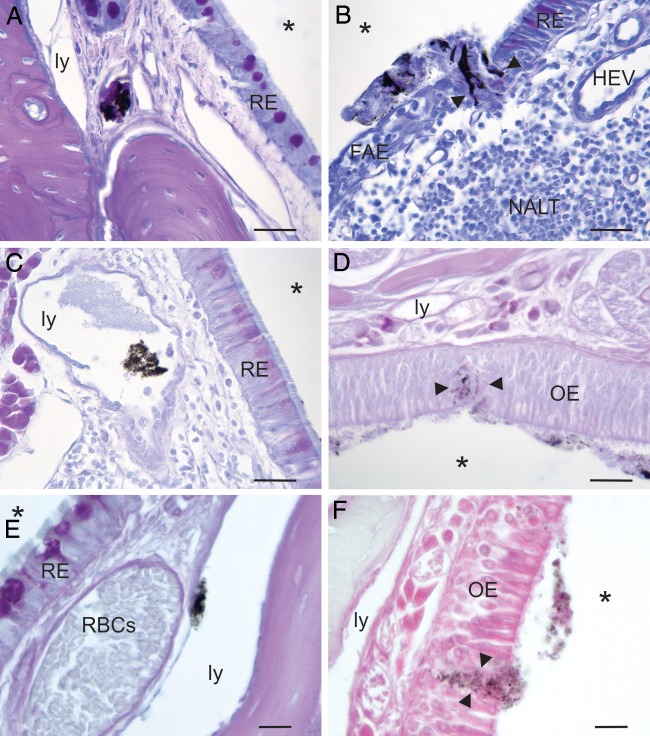
BH was also identified to pass between epithelial cells of the nasal mucosa and within lymphatic vessels of the lamina propria in the NCs of animals that inhaled infected and mock-infected BH mixed with India ink (Table 1; Fig. 2). The India ink-BH mixture was visible without the need for immunohistochemical processing to identify BH, as in the assay whose results are shown in Fig. 1; thus, tissue sections from these animals were not subject to immunohistochemical processing, and the observed transport was not an artifact of staining. BH was noted within the lumen of lymphatic vessels in all animals (n = 5), and bulk transport was noted in 4 of the 5 animals that were immersion fixed, indicating that the appearance of the inoculum between the nasal mucosal cells and within the lymphatic vessels was not due to an increase in vascular pressure that may have been generated during transcardial perfusion (Fig. 2E and F).
FIG 2.
Identification of India ink mixed with infected and uninfected BH within the lumen of lymphatic vessels (ly) in the lamina propria (A, C, E) or between cells (between arrowheads) that line the NC (B, D, F) at 10 to 15 min p.i. in perfusion-fixed and immersion-fixed animals. Ink mixed with BH obviated the need for immunohistochemical processing of the tissue sections; thus, the appearance of BH between cells or in the lymphatics was not caused by immunohistochemical processing. (A and B) Tissue sections from an animal inoculated with mock-infected BH mixed with India ink. Note that the BH-ink mixture almost filled the lumen of a lymphatic vessel in panel A (the adjacent lymphatic does not contain BH-ink). The BH-ink is located between cells in a space that spans the width of the nasal mucosa from the surface to the underlying lamina propria at the junction of the follicle-associated epithelium (FAE) that overlies the nasal-associated lymphoid tissue (NALT) and the respiratory epithelium (RE) in panel B. HEV, a high endothelial venule associated with the nasal cavity-associated lymphoid tissue. (C and D) Tissue sections from an animal inoculated with HY TME-infected BH mixed with India ink. The BH-ink is located within the lumen of a lymphatic deep from the RE in panel C and is located in a space between cells of the olfactory epithelium (OE) in panel D. (E and F) Tissue sections from an immersion-fixed animal inoculated with HY TME-infected BH mixed with India ink. The presence of bulk transport in these animals demonstrates that the spaces between cells were not due to the effects of transcardial perfusion. Note the presence of red blood cells (RBCs) in the blood vessel in panel E, indicating that this animal was not transcardially perfused. *, airspace of NC. Bars = 20 μm (A to D) and 10 μm (E and F).
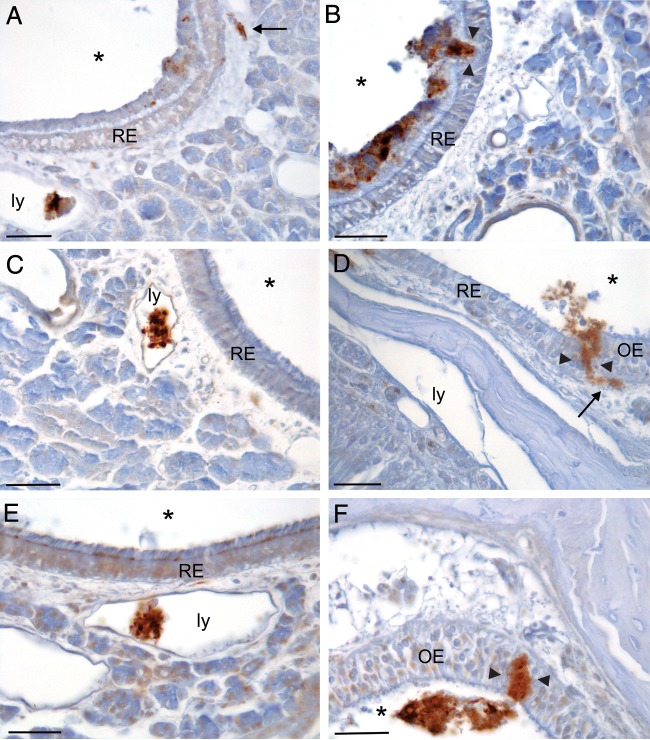
Bulk transport between cells in the NC was not affected by gas anesthesia and was not a prion strain- or species-specific event.
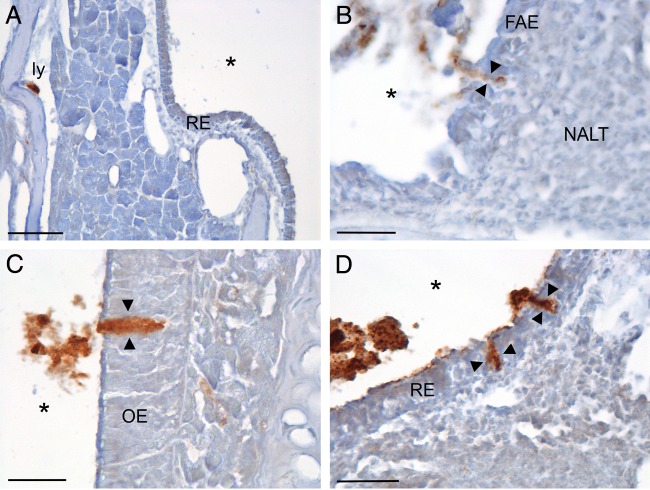
Drowsy (DY)-, hyper (HY)-, or mock-infected BH was identified to be passing between the epithelial cells that line the nasal cavity in each hamster anesthetized intraperitoneally (i.p.) with ketamine-xylazine, indicating that transport was not due to damage or to an increased permeability of the nasal mucosa caused by the inhalation of isoflurane (Table 1; Fig. 3). In addition, the transepithelial transport of DY-infected BH demonstrated that this is not a strain-specific event (Table 1; Fig. 3). The inoculum was identified to pass between the epithelial cells that line the nasal cavity and within lymphatic vessels of the lamina propria in the NCs of 9 of 10 mice inoculated extranasally with RML- or mock-infected BH, demonstrating that this type of transport is not restricted to hamsters (Table 1; Fig. 4).
FIG 3.
The transepithelial transport of inhaled BH was not due to the use of gas anesthesia and was not a strain-specific phenomenon, as shown on the tissue sections shown here processed for GFAP IHC from animals anesthetized with ketamine with a 15-min survival. (A and B) Images from an animal inoculated with mock-infected BH; note the inoculum in the lumen of lymphatic vessels (ly) and deep in the epithelium (arrow) in the lamina propria in panel A and crossing the respiratory epithelium (RE; between arrowheads) of the nasal cavity in panel B. (C and D) Images from an animal inoculated with HY TME-infected BH; note BH in a lymphatic vessel in panel C and crossing the mucosa at the junction of the RE and olfactory epithelium (OE; between arrowheads) in panel D. (E and F) Tissue sections from an animal inoculated with DY TME-infected BH showing the inoculum in a lymphatic in panel E and crossing the OE between the arrowheads in panel F. Note the relatively large amount of BH located within the lymphatic vessels in panels A, C, and E. *, airspace of NC; arrows, BH in the lamina propria of NC. Bars = 25 μm.
FIG 4.
Transepithelial transport of inhaled BH across the epithelium of the nasal cavity occurred in mice within minutes of inhalation. A primary antibody against GFAP was used to identify BH in tissue sections in all panels. (A) Image from an animal that inhaled RML-infected BH; note the BH within the lumen of lymphatic vessels (ly) adjacent to bone in the lamina propria of the NC within 5 min p.i. (B) Image from an animal obtained at 30 min p.i. of mock-infected BH; note BH between cells of the follicle-associated epithelium (FAE; between the arrowheads) adjacent to the nasal-associated lymphoid tissue (NALT). (C) Image from an animal inoculated with RML-infected BH; note BH crossing the olfactory epithelium (OE; between arrowheads) at 5 min after inhalation. (D) Image from an animal that inhaled RML-infected BH demonstrating BH crossing the respiratory epithelium (RE; between the arrowheads) at 30 min p.i. *, airspace of the nasal cavity. Bars = 50 μm (A) and 25 μm (B to D).
DISCUSSION
This is the first report of the bulk transport of inhaled BH between cells of the nasal mucosa. The size range of paracellular transport of molecules across epithelial linings is usually reported to be in the angstrom range (for reviews, see references 30 and 31), which is 3 to 4 orders of magnitude less than the 5 to 20 μm that is reported here for the medial-lateral dimension of the transport and even less than the 10- to 100-μm range observed in the rostral-caudal dimension. The relatively large dimensions of the BH, both infected and uninfected, located between the cells of the nasal mucosa and the observation that transepithelial transport occurred within minutes of inhalation indicate that this mechanism of transport is not what is typically considered to be paracellular transport and is not a response to the presence of infectious prions in the BH. Given that the transepithelial transport reported here was occurring between cells as early as 5 to 10 min after inhalation, it was likely to be occurring across existing breaks in the epithelial lining of the rodent NC that were present at the time of inhalation, rather than traversing spaces that were created as a result of the presence of the inoculum on the nasal mucosa. The results of the experiments reported here indicate that this type of transport was not the result of perfusion fixation, tissue sectioning, or immunohistochemical processing and was not unique to hamsters or restricted to a single strain of prions.
While this type of transport was seen in almost all of the animals used in this study, the frequency of occurrence of this type of transepithelial transport event in the NC of a single animal was relatively rare, as evidenced by the low percentage of tissue sections where it was observed (Table 2). However, the low rate of occurrence of these transport events was offset by the relatively large amount of inhaled matter that passed between the NC mucosal cells (Fig. 2B, D, and F, 3B, D, and F, and 4B to D) and entered lymphatic vessels in the lamina propria (Fig. 2A, C, and E, 3A, C, and E, and 4A); thus, this mechanism of transepithelial transport may contribute to the relative efficiency of this route of prion infection (14, 18, 19).
The transepithelial transport of prions followed by entry into adjacent lymphatic vessels reported here is consistent with the detection in a previous study of inoculum-associated prions in the lumen of submucosal lymphatics within minutes of inoculation of scrapie brain homogenate into sheep gut loops (32). The immediate spread of prions into the lymphatics following exposure to either the nasal cavity or gut could result in a systemic spread of the infectious agent in the blood, as the lymph drains into the subclavian veins. The rapid spread of prions into the blood following inhalation and oral inoculation has been demonstrated by real-time quaking-induced seeding activity in the blood of hamsters and deer within 15 to 30 min of exposure (33). The exposure of prions to either the NC or gut appears to result in a rapid prionemia that is similar to that seen after direct intravenous inoculation (33). The rapid transport of prions into lymphatics located in the lamina propria of the NC following inhalation reported here is consistent with these findings.
If this mechanism of bulk transepithelial transport is present in large mammals, there are implications for the intra- and interspecies transmission of the TSEs. Deer, elk, moose, sheep, and cattle use their olfactory systems for reproductive, feeding, and exploratory purposes and thus frequently expose their NCs to environmental materials. There are a number of environmental sources of infectious prions, including decaying carcasses (34), placenta (35, 36), antler velvet (37), blood (38–42), urine (43–46), and feces (47–50), that could be inhaled into the NC and horizontally transmit disease (7, 8) in both captive and free-ranging animal populations. It is worth noting that soil-bound prions remain infectious (19, 51–53) and that the physical size of the intercellular spaces reported here would accommodate the particle size of silt and clay (between 2 and 50 μm), allowing infection from inhaled contaminated soil. Also, the indiscriminate bulk transport of prions following inhalation is consistent with the growing body of work that demonstrates that prions are transported in strain-independent ways (54, 55).
The results of these experiments demonstrate the indiscriminate transport of relatively large amounts of inhaled BH through a small number of spaces between cells that line the NC; the BH rapidly entered lymphatic vessels in the lamina propria. This type of bulk intercellular transport of infectious material that immediately enters lymphatic vessels may be a relevant mechanism for the entry and spread of other inhaled pathogens and toxins in olfactory system-driven animals.
ACKNOWLEDGMENTS
We thank Melissa Clouse, Alberto Lorenzo, Shawn Feilmann, Matt Richey, and Collin Haraden for excellent technical work.
We declare that we have no competing interests.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funding programs had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
A.E.K. and J.C.B. were responsible for the experimental design. All 3 authors contributed to the animal work and tissue collections. A.E.K. carried out the light microscope analysis and prepared the figures. A.E.K. and J.C.B. wrote the manuscript. All authors read and approved the final manuscript.
Funding Statement
This work was funded by the National Institute of Neurological Disorders and Stroke (RO1 NS061994 and RO1 NS061994-03S1 to A. E. Kincaid), Health Future Foundation Discretionary Award number 200600-713131d to A. E. Kincaid, and grant G20RR024001 from the National Center for Research Resources. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Bolton DC, McKinley MP, Prusiner SB. 1982. Identification of a protein that purifies with the scrapie prion. Science 218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 3.Deleault NR, Harris BT, Rees JR, Supattapone S. 2007. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci U S A 104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigurdson CJ, Nilsson KP, Hornemann S, Heikenwalder M, Manco G, Schwarz P, Ott D, Ruicke T, Liberski PP, Julius C, Falsig J, Stitz L, Wuthrich K, Aguzzi A. 2009. De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci U S A 106:304–309. doi: 10.1073/pnas.0810680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, Budka H, Rohwer RG, Baskakov IV. 2010. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol 119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Wang X, Yuan C-G, Ma J. 2010. Generating a prion with bacterially expressed recombinant prion protein. Science 327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MW, Williams ES. 2003. Horizontal prion transmission in mule deer. Nature 425:35–36. doi: 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 8.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hoover EA. 2009. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:e5916. doi: 10.1371/journal.pone.0005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadlow WJ, Kennedy RC, Race RE. 1982. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis 146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 10.Kimberlin RH, Walker CA. 1989. Pathogenesis of scrapie in mice after intragastric infection. Virus Res 12:213–220. doi: 10.1016/0168-1702(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 11.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI, Hoover EA. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol 80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 12.Beekes M, McBride PA. 2000. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci Lett 278:181–184. doi: 10.1016/S0304-3940(99)00934-9. [DOI] [PubMed] [Google Scholar]
- 13.Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen J-M, Lantier F. 2000. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol 81:3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 14.Kincaid AE, Bartz JC. 2007. The nasal cavity is a route for prion infection in hamsters. J Virol 81:4482–4491. doi: 10.1128/JVI.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sbriccoli M, Cardone F, Valanzano A, Lu M, Graziano S, De Pascalis A, Ingrosso L, Zanusso G, Monaco S, Bentivoglio M, Pocchiari M. 2009. Neuroinvasion of the 263K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathol 117:175–184. doi: 10.1007/s00401-008-0474-z. [DOI] [PubMed] [Google Scholar]
- 16.Hamir AN, Kunkle RA, Richt JA, Miller JM, Greenlee JJ. 2008. Experimental transmission of US scrapie agent by nasal, peritoneal, and conjunctival routes to genetically susceptible sheep. Vet Pathol 45:7–11. doi: 10.1354/vp.45-1-7. [DOI] [PubMed] [Google Scholar]
- 17.Denkers ND, Seelig DM, Telling GC, Hoover EA. 2010. Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J Gen Virol 91:1651–1658. doi: 10.1099/vir.0.017335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mathiason CK, Hoover EA. 2013. Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol 87:1890–1892. doi: 10.1128/JVI.02852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols TA, Spraker TR, Rigg TD, Meyerett-Reid C, Hoover C, Michel B, Bian J, Hoover E, Gidlewski T, Balachandran A, O'Rourke K, Telling GC, Bowen R, Zabel MD, VerCauteren KC. 2013. Intranasal inoculation of white-tailed deer (Odocoileus virginianus) with lyophilized chronic wasting disease prion particulate complexed to montmorillonite clay. PLoS One 8:e62455. doi: 10.1371/journal.pone.0062455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kincaid AE, Hudson KF, Richey MW, Bartz JC. 2012. Rapid transepithelial transport of prions following inhalation. J Virol 86:12731–12740. doi: 10.1128/JVI.01930-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heppner FL, Christ AD, Klein MA, Prinz M, Fried M, Kraehenbuhl J-P, Aguzzi A. 2001. Transepithelial prion transport by M cells. Nat Med 7:976–977. doi: 10.1038/nm0901-976. [DOI] [PubMed] [Google Scholar]
- 22.Mishra RS, Basu S, Guy Y, Luo X, Zou W-Q, Mishra R, Li R, Chen SG, Gambetti P, Fujioka H, Singh N. 2004. Protease-resistant human prion protein and ferritin are cotransported across Caco-2 epithelial cells: implications for species barrier in prion uptake from the intestine. J Neurosci 24:11280–11290. doi: 10.1523/JNEUROSCI.2864-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morel E, Andrieu T, Casagrande F, Gauczynski S, Weiss S, Grassi J, Rousset M, Dormont D, Chambaz J. 2005. Bovine prion is endocytosed by human enterocytes via the 37 kDa/67 kDa laminin receptor. Am J Pathol 167:1033–1042. doi: 10.1016/S0002-9440(10)61192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazawa K, Kanaya T, Takakura I, Tanaka S, Hondo T, Watanabe H, Rose M, Kitazawa H, Yamaguchi T, Katamine S, Nishida N, Aso H. 2010. Transcytosis of murine-adapted bovine spongiform encephalopathy agents in an in vitro bovine M cell model. J Virol 84:12285–12291. doi: 10.1128/JVI.00969-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunkesula SRB, Luo X, Das D, Singh A, Singh N. 2010. Iron content of ferritin modulates its uptake by intestinal epithelium: implications for co-transport of prions. Mol Brain 3:14. doi: 10.1186/1756-6606-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto M, Furuoka H, Horiuchi M, Noguchi T, Hagiwara K, Muramatsu Y, Tomonaga K, Tsuji M, Ishihara C, Ikuta K, Taniyama H. 2003. Experimental transmission of abnormal prion protein (PrPSc) in the small intestinal epithelial cells of neonatal mice. Vet Pathol 40:723–727. doi: 10.1354/vp.40-6-723. [DOI] [PubMed] [Google Scholar]
- 27.Foster N, Macpherson GG. 2010. Murine cecal patch M cells transport infectious prions in vivo. J Infect Dis 202:1916–1919. doi: 10.1086/657415. [DOI] [PubMed] [Google Scholar]
- 28.Takakura I, Miyazawa K, Kanaya T, Itani W, Watanabe K, Ohwada S, Watanabe H, Hondo T, Rose MT, Mori T, Sakaguchi S, Nishida N, Katamine S, Yamaguchi T, Aso H. 2011. Orally administered prion protein is incorporated by M cells and spreads into lymphoid tissues with macrophages in prion protein knockout mice. Am J Pathol 179:1–9. doi: 10.1016/j.ajpath.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC. [Google Scholar]
- 30.Anderson JM, Van Itallie CM. 2009. Physiology and function of the tight junction. 2009. Cold Spring Harb Perspect Biol 1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. 2011. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffrey M, González L, Espenes A, Press CM, Martin S, Chaplin M, Davis L, Landsverk T, MacAldowie C, Eaton S, McGovern G. 2006. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol 209:4–14. doi: 10.1002/path.1962. [DOI] [PubMed] [Google Scholar]
- 33.Elder AM, Henderson DM, Nalls AV, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. 2015. Immediate and ongoing detection of prions in the blood of hamsters and deer following oral, nasal, or blood inoculations. J Virol 89:7421–7424. doi: 10.1128/JVI.00760-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller MW, Williams ES, Hobbs NT, Wolfe LL. 2004. Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Race R, Jenny A, Sutton D. 1998. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis 178:949–953. [DOI] [PubMed] [Google Scholar]
- 36.Andreoletti O, Lacroux C, Chabert A, Monnereau L, Tabouret G, Lantier F, Berthon P, Eychenne F, Lafond-Benestad S, Elsen J-M, Schelcher F. 2002. PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol 83:2607–2616. doi: 10.1099/0022-1317-83-10-2607. [DOI] [PubMed] [Google Scholar]
- 37.Angers RC, Seward TS, Napier D, Green M, Hoover E, Spraker T, O'Rourke K, Balachandran A, Telling GC. 2009. Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis 15:696–703. doi: 10.3201/eid1505.081458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter N, Foster J, Chong A, McCutcheon S, Parham D, Eaton S, MacKenzie C, Houston F. 2002. Transmission of prion disease by blood transfusion. J Gen Virol 83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- 39.Castilla J, Saa P, Soto C. 2005. Detection of prions in blood. Nat Med 11:982–985. [DOI] [PubMed] [Google Scholar]
- 40.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 41.Houston F, McCutcheon S, Goldmann W, Chong A, Foster J, Siso S, Gonzalez L, Jeffrey M, Hunter N. 2008. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood 112:4739–4745. doi: 10.1182/blood-2008-04-152520. [DOI] [PubMed] [Google Scholar]
- 42.Andreoletti O, Litaise C, Simmons H, Corbiere F, Lugan S, Costes P, Schelcher F, Vilette D, Grassi J, Lacroux C. 2012. Highly efficient prion transmission by blood transfusion. PLoS Pathog 8:e1002782. doi: 10.1371/journal.ppat.1002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, Seifert B, Miele G, Aguzzi A. 2005. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science 310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 44.Murayama Y, Yoshioka M, Okada H, Takata M, Yokoyama T, Mohri S. 2007. Urinary excretion and blood level of prions in scrapie-infected hamsters. J Gen Virol 88:2890–2898. doi: 10.1099/vir.0.82786-0. [DOI] [PubMed] [Google Scholar]
- 45.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA. 2015. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J Virol 89:9338–9347. doi: 10.1128/JVI.01118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Safar JG, Lessard P, Tamguney G, Freyman Y, Deering C, Letessier F, DeArmond SJ, Prusiner SB. 2008. Transmission and detection of prions in feces. J Infect Dis 198:81–89. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maluquer de Motes C, Grassi J, Simon S, Herva ME, Torres JM, Pumarola M, Girones R. 2008. Excretion of BSE and scrapie prions in stools from murine models. Vet Microbiol 131:205–211. doi: 10.1016/j.vetmic.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Kruger D, Thomzig A, Lenz G, Kampf K, McBride P, Beekes M. 2009. Faecal shedding, alimentary clearance and intestinal spread of prions in hamsters fed with scrapie. Vet Res 40:4. doi: 10.1051/vetres:2008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamguney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. 2006. Prions adhere to soil minerals and remain infectious. PLoS Pathog 2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. 2007. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One 2:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saunders SE, Shikiya RA, Langenfeld K, Bartelt-Hunt SL, Bartz JC. 2011. Replication efficiency of soil-bound prions varies with soil type. J Virol 85:5476–5482. doi: 10.1128/JVI.00282-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayers JI, Kincaid AE, Bartz JC. 2009. Prion strain targeting independent of strain-specific neuronal tropism. J Virol 83:81–87. doi: 10.1128/JVI.01745-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langenfeld KA, Shikiya RA, Kincaid AE, Bartz JC. 2016. Incongruity between prion conversion and incubation period following coinfection. J Virol 90:5715–5723. doi: 10.1128/JVI.00409-16. [DOI] [PMC free article] [PubMed] [Google Scholar]