ABSTRACT
A lack of immunocompetent-small-primate models has been an obstacle for developing hepatitis C virus (HCV) vaccines and affordable antiviral drugs. In this study, HCV/GB virus B (GBV-B) chimeric virus carrying the major nonstructural proteins NS2 to NS4A (HCV NS2 to -4A chimera) was produced and used to infect common marmosets, since HCV NS2 to NS4A proteins are critical proteases and major antigens. Seven marmosets were inoculated intrahepatically with HCV NS2 to -4A chimera RNA for primary infection or intravenously injected with chimera-containing serum for passage infection. Three animals used as controls were injected with phosphate-buffered saline (PBS) or GBV-B, respectively. Six of seven HCV NS2 to -4A chimera-infected marmosets exhibited consistent viremia and one showed transient viremia during the course of follow-up detection. All six infected animals with persistent circulating viremia presented characteristics typical of viral hepatitis, including viral RNA and proteins in hepatocytes and histopathological changes in liver tissue. Viremia was consistently detected for 5 to 54 weeks of follow-up. FK506 immunosuppression facilitated the establishment of persistent chimera infection in marmosets. An animal with chimera infection spontaneously cleared the virus in blood 7 weeks following the first inoculation, but viral-RNA persistence, low-level viral protein, and mild necroinflammation remained in liver tissue. The specific antibody and T-cell response to HCV NS3 in this viremia-resolved marmoset was boosted by rechallenging, but no viremia was detected during 57 weeks of follow-up. The chimera-infected marmosets described can be used as a suitable small-primate animal model for studying novel antiviral drugs and T-cell-based vaccines against HCV infection.
IMPORTANCE HCV infection causes approximately 70% of chronic hepatitis and is frequently associated with primary liver cancer globally. Chimpanzees have been used as a reliable primate model for HCV infection, but ethical considerations have restricted their utility in biomedical research. GB virus B (GBV-B) is a flavivirus related to HCV. It can infect common marmosets, a New World small primate, and induces viral hepatitis similar to HCV infection in humans. To minimize differences between GBV-B and HCV, we generated HCV NS2 to -4A/GBV-B chimeric viruses and established a chimera-infected marmoset model. HCV NS2 to -4A chimera-infected marmosets provide a small-animal model for evaluating novel antiviral drugs targeting HCV NS3-NS4A protease and T-cell-based HCV vaccines.
INTRODUCTION
Hepatitis C virus (HCV) infection is a global health threat that causes chronic hepatitis and is associated with 78% of primary hepatocellular carcinoma (1). Currently, limitations of small-primate models hamper the development of HCV vaccines and affordable antiviral drugs. Chimpanzees have been used as a uniquely reliable animal for HCV infection in past decades (2), significantly contributing to defining the infection natural history, pathogenesis, immune response, and rechallenge of HCV (3–6). However, the utility of chimpanzees has been more and more restricted by ethical concerns, and though rare, the use of this primate model in medical studies is extremely costly (2). The nonprimate animal models simulating HCV infection might potentially be mimicked with rodent hepacivirus (RHV)-infected rats (7, 8), canine hepacivirus (CHV)-infected dogs (9), and equine hepacivirus (EHCV) (nonprimate hepacivirus [NPHV])-infected horses (10). HCV infection in immunocompetent mice was reported in genetically humanized mouse CD81 and occludin (OCLN) (11, 12). However, the differences in infection courses and immune responses fundamentally separate these mice from HCV-infected patients.
Common marmosets (Callithrix jacchus), one of the New World small primates, are susceptible to GB virus B (GBV-B), a flavivirus phylogenetically related to HCV (13). Marmosets infected with GBV-B developed typical viral hepatitis similar to human hepatitis C (14, 15) and were used as an animal model for validating HCV inhibitors (16). In order to explore a particular gene function, a short piece of HCV 5′ noncoding region (NCR), p7, or HVR1 sequence was integrated into the GBV-B genome to develop an HCV/GBV-B chimera-infected marmoset or tamarin (another small monkey) (17–20). Common marmosets have the advantage of carrying immunological markers similar to those of humans, which makes this small monkey highly attractive for biomedical studies (21). To minimize the differences between GBV-B and HCV, two HCV/GBV-B chimeras carrying the full-length structural proteins (core [C], E1, E2, and p7) or envelope proteins (E1, E2, and p7) of HCV were previously reported (22). Successfully infected marmosets developed typical viral hepatitis and raised specific immune responses (22). To enlarge the panel of HCV structural-protein chimeras, an HCV/GBV-B chimera sharing the major nonstructural proteins NS2, NS3, and NS4A of HCV (HCV NS2 to -4A chimera) was produced and characterized in the present study. It could be used in a small-primate animal model for developing and evaluating antiviral drugs and vaccines against HCV infection.
MATERIALS AND METHODS
Animal approval and study design.
The use of common marmosets for this study was approved by the authorities of the Forest Bureaus, Guangdong and Tianjin, China, governments (Yuelinhu [2010]90 and Yuelinhu [2011]404), respectively. Ethical approval for the marmoset experimentation and sample collection was also obtained from the Southern Medical University (SMU) Animal Care and Use Committee at Nanfang Hospital, SMU, Guangzhou, China (permit number SYXK [Yue] 2010-0056). All animal care and experimental procedures (NFYYLASOP-037) were in accordance with national and institutional policies for animal health and well-being. The studies were approved by the Nanfang Hospital Animal Ethics Committee (certificate no. NFYY-2010-38).
Ten adult common marmosets, juvenile males with an average weight of 350 g, were imported from Tianjin Medical University and individually bred in the Laboratory Animal Research Center of Nanfang Hospital, Guangzhou, China. All the marmosets tested negative for GBV-B, HCV, and hepatitis B virus (HBV) by reverse-transcribed quantitative PCR (RT-qPCR) and qPCR before inoculation (22). Seven animals were used as the experimental group and three animals as the control group (Table 1). Injection and sample collection were performed following the guide for laboratory animals. Animal surgery was performed under appropriate anesthesia to minimize animal suffering.
TABLE 1.
Groups of marmosets infected with the HCV NS2 to -4A/GBV-B chimera
| Group | Animal | Inoculation | Follow-up (wk) |
|---|---|---|---|
| HCV NS2 to -4A/GBV-B chimera | |||
| P0, intrahepatically | M5-P0 | HCV NS2 to -4A chimeric RNA | 18 |
| M14-P0 | HCV NS2 to -4A chimeric RNA | 17 | |
| P1, intravenously | M8-P1 | Serum from M5-P0 at wk 1 | 12 |
| M22-P1 | Serum from M23-P0 at wk 13, 23 | 5 | |
| M25-P1 | Serum from M23-P0 at wk 34, 38, 39, 43, 50; rechallenged with P1 serum from M24-P1 at wk 5, 19, 20, 26 | 34 + 57 | |
| FK506-treated P0 or P1 infection | M23-P0-FK506 | RNA | 54 |
| M24-P1-FK506 | Serum from M23-P0 at wk 4, 5, 8 | 50 | |
| Control | |||
| P0 | M2-P0 | PBS buffer | 26 |
| P0 | M4-P0 | GBV-B RNA | 23 |
| P1 | M9-P1 | GBV-B serum of M4-P0 | 29 |
Construction of the HCV NS2 to -4A/GBV-B chimeric genome.
The nonstructural genes of HCV NS2, NS3, and NS4A (NS2 to -4A) were isolated from an HCV (strain Z14, genotype 1b)-infected blood donor in China (GenBank accession number JN870283). The HCV NS2 to -4A/GBV-B chimeric genome (GenBank accession number KF285485) was constructed by replacing the corresponding GBV-B elements with HCV NS2 to -4A genes in the backbone of an infectious cDNA clone (pGBB) containing the full-length GBV-B genome (13). Plasmid DNA of the HCV NS2 to -4A chimeric genome was linearized by SacI digestion and transcribed into the infectious HCV NS2 to -4A chimeric RNA under the direction of the T7 promoter in vitro using the T7 Megascript kit (Ambion, Applied Biosystems, Austin, TX, USA). The intact HCV NS2 to -4A chimeric RNA was examined with 5′ and 3′ terminus sequences by RT-qPCR or RT-nested PCR, respectively, before intrahepatic injection.
Marmoset inoculation and follow-up sampling.
Eight immunocompetent and two FK506-treated immunosuppressed marmosets were used for primary or passage infections as previously described (Table 1) (22). Primary infection (P0) of marmosets was carried out with 300 μl of 500 μg HCV NS2 to -4A chimeric RNA diluted in Dulbecco phosphate-buffered saline (DPBS) by intrahepatic injection at two sites. Passage infection (P1) marmosets were intravenously injected in the femoral vein with P0 serum containing 2 × 104 viral-RNA copies. Blood samples (0.6 to 1 ml) were collected at 1 or 2 weeks postinoculation.
RT-qPCR and RT-nested PCR.
Viral RNA was extracted from sera of infected marmosets using the High Pure Viral Nucleic Acid kit (Roche Diagnostic GmbH, Mannheim, Germany). Two sets of RT-qPCR with primers targeting the GBV-B 5′ NCR (23) and HCV NS3 regions were used for detecting and quantifying HCV NS2 to -4A chimera viremia of the infected marmosets. The primers and probe specific for HCV NS3 were HCVNS-QF (5′-GGTTTCTACCGCAACACAATCTT-3′), HCVNS-QR (5′-CGCCATGGTAGACAGTCCAA-3′), and HCVNS-QP (5′-Cy5-CCTGGCAACCTGCGTCAACGG-BHQ2-3′), respectively.
Viremia detected by RT-qPCR in HCV NS2 to -4A chimera-infected marmosets was further identified by RT-nested PCR with primers specific for HCV NS2 of chimeric virus (outer NS-F1, 5′-TAGAGCCGAGGCGCACTTGCATGTGTG-3′; outer NS-R1, 5′-TGAGATGGTCATAAACGTACGTGCCTGTCAGTGTG-3′; inner NS-F2, 5′-ATGCCATCATCCTCCTCACGTGCGCGG-3′; and inner NS-R2, 5′-TCCGCACCAACATGCATGCACGGATGAG-3′). The amplified DNA products (chimeric viral genome nucleotides [nt] 2826 to 2993) were sequenced commercially (Invitrogen, Shanghai, China).
Sequencing analysis.
HCV NS2 to -4A/GBV-B chimeric RNA was extracted from 300 μl of plasma (40,000 copies/ml) from marmoset M22-P1 or 300 mg liver tissue (266.2 copies/mg) from marmoset M24-P1-FK506 as described previously (22). Viral cDNA was synthesized with random primers and sequenced commercially by next-generation sequencing (Huada Gene Company, Shenzhen, China). Nucleotide substitution or mutation of the HCV NS2 to -4A chimera from the infected animals was analyzed by reads of the sequence (approximately 150 bp/each) in comparison with the parental sequence.
FISH.
A 189-bp HCV NS3 cDNA fragment was used as a specific fluorescent in situ hybridization (FISH) probe for detection of the HCV NS2 to -4A chimeric genome (nucleotide positions 4297 to 4483; GenBank accession number KF285485) in liver tissue of marmosets. The probes were labeled with fluorescein isothiocyanate (FITC) using the FISH kit (Biosense, Guangzhou, China). The sections of liver tissues were fixed in 4% paraformaldehyde, and detection was performed by hybridization with the FITC-labeled HCV NS3 probe at 42°C overnight. The hepatocyte nucleus was counterstained with DAPI (4′,6-diamidino-2-phenylindole).
IHC.
The formalin-fixed, paraffin-embedded liver tissues were tested for HCV NS2 to -4A protein expression by immunohistochemical (IHC) staining as described previously (22). Primary antibodies to HCV NS3 (clone 3E5) and to both HCV and GBV-B NS3 conserved epitopes (clone 2E12) were available in the laboratory (24). Antibodies to HCV core (clone C7-50) and alpha smooth muscle actin (α-SMA) (clone 1A4) were purchased from Abcam (Cambridge, United Kingdom). A liver biopsy specimen from an HCV-infected patient was obtained from the Department of Infectious Diseases of Nanfang Hospital, Guangzhou, China. The male patient was 50 years old, carried a 1.2 × 103-IU/ml HCV RNA load in serum, and was anti-HCV antibody positive (signal-to-cutoff [S/CO] ratio = 6.7) but negative for five serological markers of hepatitis B, including normal alanine aminotransferase (ALT)/aspartate transaminase (AST) levels (13/14 U/liter). The scoring was evaluated according to the intensity of staining and the frequency of stained cells (25).
Histopathological examination.
The liver tissues of marmosets were examined with hematoxylin and eosin (H&E) staining and Masson's trichrome staining as described previously (22). The necrosis and inflammation were graded on a scale of 0 to 18, while fibrosis was scored on a scale of 0 to 6 according to the modified HAI system (26, 27). The histological score for each marmoset was calculated as the average of scores obtained with 3 to 5 sections per animal.
Biochemical tests.
Serum ALT and AST levels were measured in units per liter with kits from Biosino Biotechnology and Science Inc. (Beijing, China) on a Hitachi 7170S full-automatic biochemical analyzer (Hitachi Ltd., Tokyo, Japan).
Enzyme immunoassay.
Antibody to the HCV NS2 to -4A chimera in sera of infected marmosets was tested by enzyme-linked immunosorbent assay (ELISA) with a commercial recombinant HCV-1b NS3 protein (HCV-247; ProSpec-Tany TechnoGene Ltd., Ness Ziona, Israel). Antibody reactivity was expressed as the S/CO ratio. The cutoff was calculated as the mean of three replicates plus 3 standard deviations (SD).
ELISpot assay.
Twenty-nine HCV NS3 peptides (P1 to P29 16-mer with 7-mer overlap spanning 268 amino acids [aa] of HCV NS3 between aa 1192 and 1459) were commercially synthesized by the Chinese Peptide Company (Hangzhou, Zhejiang, China) (24). The peptides (4 μg/ml each) were pooled in PBS. The specific T-cell response of peripheral blood mononuclear cells (PBMCs) from marmosets was measured by stimulating them with the pooled peptides in an enzyme-linked immunosorbent spot (ELISpot) assay as described previously (22). A precoated human gamma interferon (IFN-γ) ELISpot Plus plate (Mebtech AB, Sweden) was used in the assay. The negative and positive controls were 1640 medium and phytohemagglutinin (PHA), respectively. The number of spot-forming cells (SFC) was normalized to the number of SFC per 106 PBMCs.
Statistical analysis.
Statistical analysis was conducted with SPSS 19.0 software. The correlation between GBV-B and HCV quantification systems was assessed by Spearman's rho nonparametric correlation analysis. The differences in viral load or antibody reactivity between control and HCV NS2 to -4A chimera-infected marmosets were analyzed with the nonparametric Mann-Whitney U test. A P value of <0.05 was considered statistically significant.
Accession number(s).
The full-length nucleotide sequence of HCV NS2 to -4A/GBV-B chimeric virus has been deposited in GenBank under accession number KF285485.
RESULTS
Generation of the HCV NS2 to -4A chimeric genome.
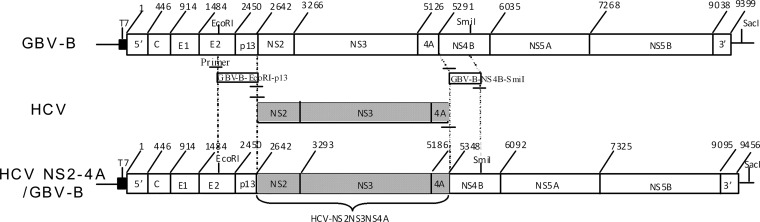
The full-length genes encoding HCV nonstructural proteins NS2 to -4A were isolated from a Chinese blood donor infected with wild-type HCV (strain Z14, genotype 1b; GenBank accession number JN870283). HCV NS2 to -4A genes were inserted into an infectious GBV-B clone (pGBB) by replacing the corresponding genes of GBV-B (Fig. 1) and were designated the HCV NS2 to -4A chimeric genome (GenBank accession number KF285485). The intact RNA from the chimeric genome was obtained by in vitro transcription under the direction of the T7 RNA polymerase promoter. The synthetic RNA was diluted in DPBS before injecting the marmosets.
FIG 1.
Diagram of the HCV NS2 to -4A chimeric genome. The HCV NS2 to -4A cDNA segment was integrated into the GBV-B cDNA genome (pGBB) by substituting the corresponding genes of GBV-B between EcoRI and SmiI restriction sites.
Detection of HCV NS2 to -4A chimera viremia in infected marmosets.
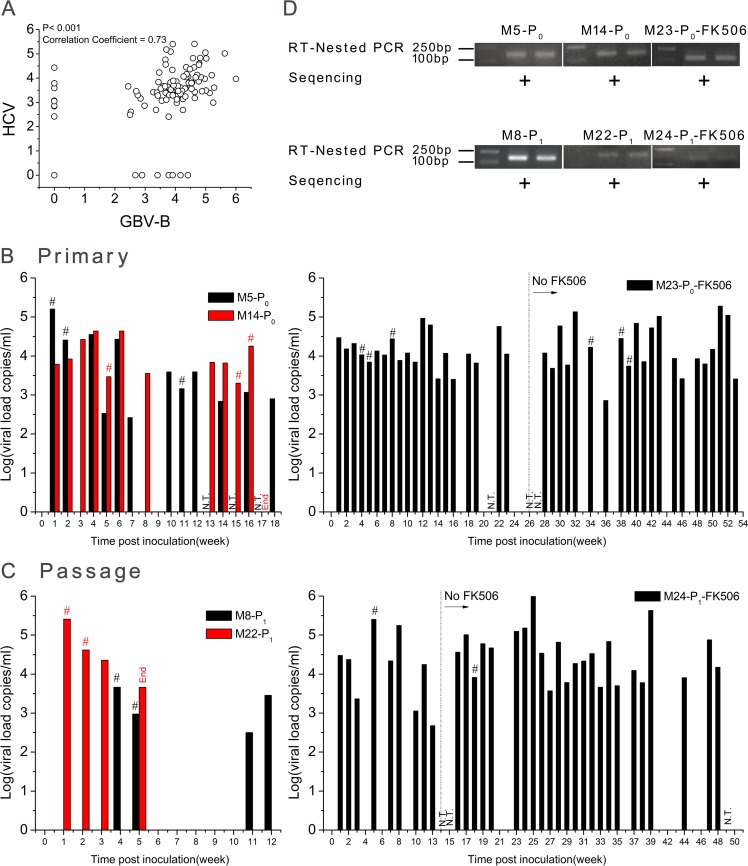
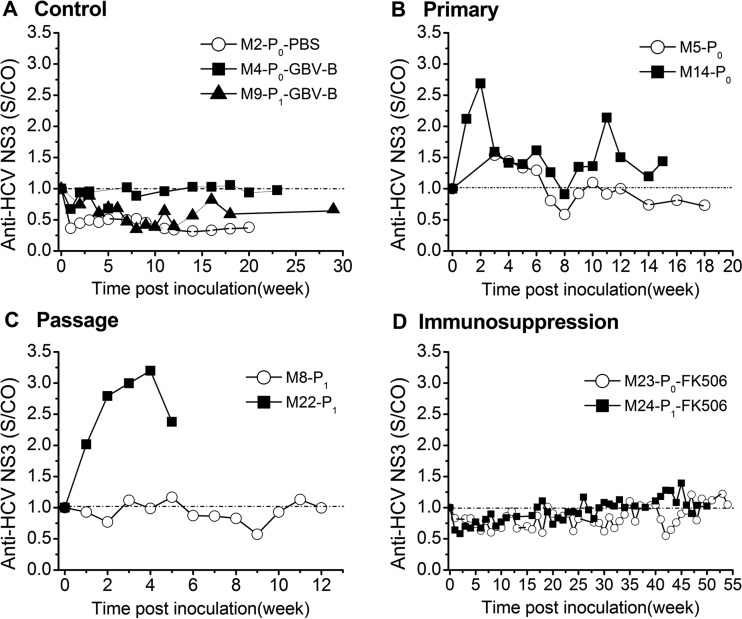
A total of 10 common marmosets were inoculated by intrahepatic injection of viral RNA or intravenous injection of virus-containing serum (Table 1), including 3 animals (M2-P0-PBS, M4-P0-GBV-B, and M9-P1-GBV-B) as negative and positive controls. Follow-up blood samples were collected for 5 to 54 weeks from each injected animal at 1- or 2-week intervals. Viremia was quantified by two sets of RT-qPCR targeting both HCV NS2 to -4A and GBV-B. The two types of viral load were significantly correlated (P < 0.001; correlation coefficient = 0.73) (Fig. 2A).
FIG 2.
Detection of HCV NS2 to -4A chimera viremia in infected marmosets. (A) Correlation of viral loads tested with two sets of quantitative assays for GBV-B and HCV (P < 0.0001; correlation coefficient, 0.73). (B) RT-qPCR detection of primary infection (P0). Naive marmosets M5-P0, M14-P0, and M23-P0-FK506 were intrahepatically injected with 500 μg chimeric RNA at two separate liver sites. (C) RT-qPCR detection of passage infection (P1). Marmosets M8-P1, M22-P1, and M24-P1-FK506 were intravenously injected with the P0 serum containing 2 × 104 chimeric RNA copies. (D) RT-nested PCR amplification and sequencing of HCV NS3 amplicons. The RT-qPCR specifically targeted both HCV and GBV-B sequences, and the viral load is presented in a merged pattern. The viral loads were statistically different between immunocompetent and immunosuppressed marmosets with the nonparametric Mann-Whitney U test (P = 0.002 to 0.0008), but no difference was observed between the periods of FK506 administration and discontinuation in the course of infection of immunosuppressed marmosets (P = 0.876 to 0.392). N.T., sample not tested by RT-qPCR; #, viremia sample was confirmed by RT-nested PCR and further sequenced; +, sequence was identical to that of the original chimera.
To establish primary infection (P0), a single solution of 500 μg HCV NS2 to -4A chimeric RNA was inoculated into three marmosets (M5-P0, M14-P0, and M23-P0-FK506) by intrahepatic injection (Table 1). Primary chimera viremia (P0) was persistently detected in M5-P0, M14-P0, and FK506-treated M23-P0 (Fig. 2B). The two types of RT-qPCR resulted in consistent patterns of viral loads ranging from 2.6 × 102 to 1.9 × 105 copies/ml (median, 1.1 × 104 copies/ml). The viral load in the immunosuppressed M23-P0-FK506 was significantly (2-fold) higher than in the immunocompetent M5-P0 and M14-P0 (P = 0.02) but was not statistically different between animals consistently treated (weeks 1 to 25) and those with treatment discontinued at week 26 (M23-P0-FK506) (P = 0.876).
In order to verify the infectivity of the primary HCV NS2 to -4A chimera (P0), sera containing 2 × 104 viral-RNA copies were collected from M5-P0 and M23-P0-FK506 and intravenously injected into 4 naive marmosets, M8-P1, M22-P1, M24-P1-FK506, and M25-P1 (Table 1). Passaged chimera viremia (P1) was detected in blood samples from three of these four animals (M8-P1, M22-P1, and M24-P1-FK506) (Fig. 2C). Marmoset M8-P1 presented low viral loads ranging between 3.2 × 102 and 4.6 × 103 copies/ml at 4, 5, 11, and 12 weeks of follow-up. In contrast, marmoset M22-P1 presented consistent high levels of viral load ranging between 4.6 × 103 and 2.6 × 105 during 5 weeks of follow-up until the animal died. Similarly, persistent and relatively high viral loads ranging between 4.8 × 102 and 1 × 106 copies/ml were detected for up to 48 weeks in the passaged-chimera-infected marmoset, M24-P1-FK506. Such viral loads were significantly (2.65-fold) higher than those observed in the immunocompetent animals M8-P1 and M22-P1 (P = 0.008). No difference in viral load was observed between periods during which FK506 was administered (weeks 1 to 13) or not (weeks 14 to 50) in the same animal (P = 0.392).
In order to confirm the above-mentioned presence of viremia detected by RT-qPCR, serum samples were selected from early (weeks 1 to 8), middle (weeks 11 to 18), and late (weeks 34 to 39) time points of HCV NS2 to -4A chimera-infected marmosets and tested with an RT-nested PCR specific to HCV NS2 (Fig. 2D). The amplicons obtained (168 bp) were sequenced and found to be identical to the corresponding sequence of the HCV NS2 to -4A chimeric construct (Fig. 2D).
Identification of the HCV NS2 to -4A chimeric genome in liver tissues of infected marmosets.
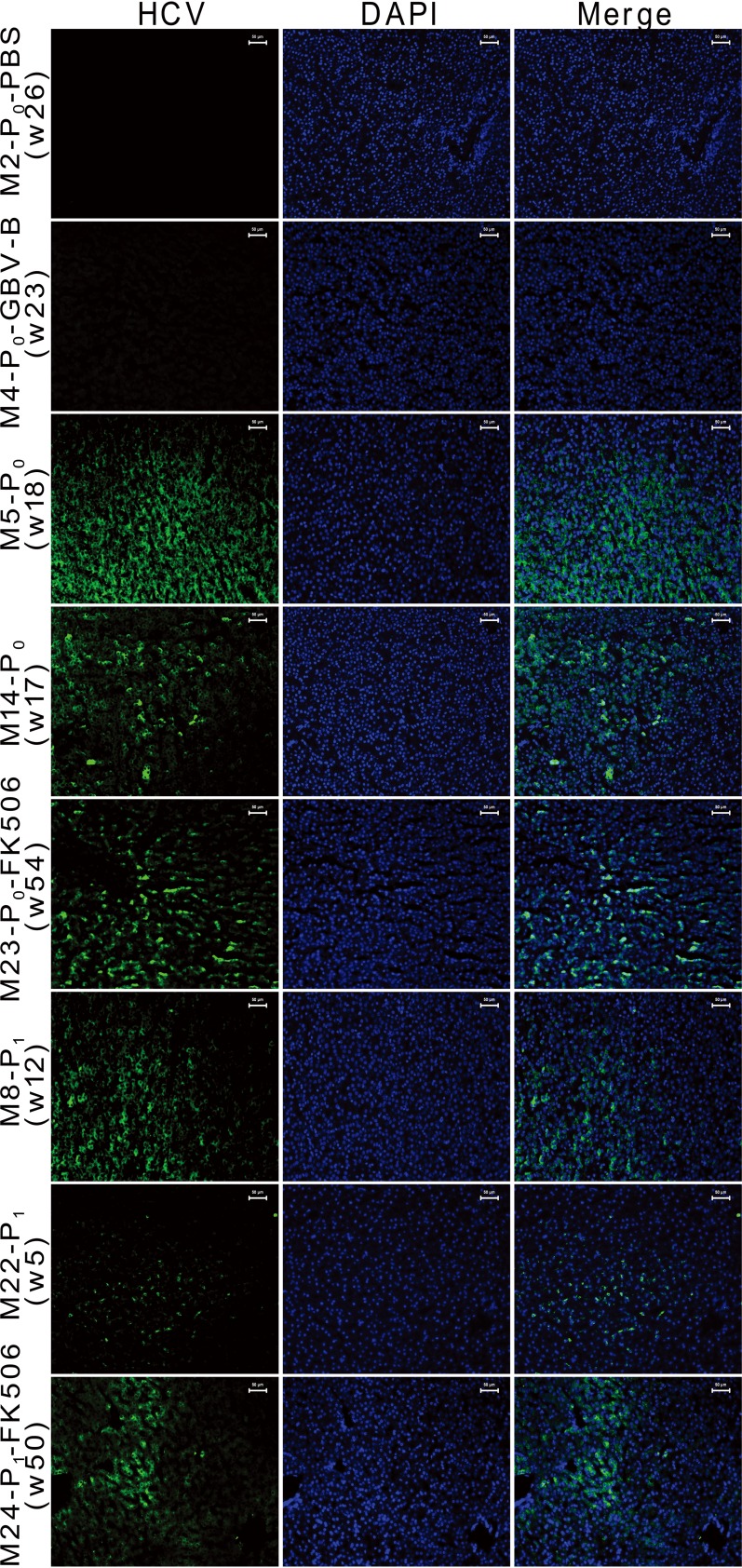
To demonstrate chimera infection and replication in hepatocytes, the liver tissues of HCV NS2 to -4A chimera-infected marmosets and control animals were examined with the specific HCV NS3 probe using FISH (Fig. 3). Positive FISH staining was observed for 20% to 77% of hepatocytes in the liver tissues from primary and passage chimera-infected marmosets (M5-P0, M14-P0, M23-P0-FK506, M8-P1, M22-P1, and M24-P1-FK506), but not from control marmosets M2-P0-PBS and M4-P0-GBV-B.
FIG 3.
FISH detection of HCV NS2 to -4A chimeric RNA in hepatocytes of liver tissues from infected marmosets. The times when liver samples were collected are indicated (w, week). Green indicates hybridization positivity of chimeric RNA specific to the HCV NS3 probe; blue indicates hepatocyte nuclei stained with DAPI.
To evaluate whether the mutations occurred within the HCV NS2 to -4A chimera for replicative fitness in infected marmosets, 56.5% (5,343/9,456 bp) or 53.5% (5,062/9,456 bp) of full-length viral genomic sequence was obtained by deep sequencing from M22-P1 plasma and M24-P1-FK506 liver tissue samples. No common mutation (≥50% reads of sequence) was detected in the consensus sequence, while the nucleotide (amino acid) substitutions occurred twice at T3449A (W1002R), T4934G (S1497A), and C5019T (P1525L) of the HCV NS2 to -4A chimera in 7 to 9 reads of sequence from immunocompetent M22-P1. The frequency of random substitutions was found to be 1/281 or 1/175 at a single site of the nucleotide sequence of the HCV NS2 to -4A chimera from M22-P1 or M24-P1-FK506, respectively.
Measurement of HCV NS3 protein in liver tissues of infected marmosets.
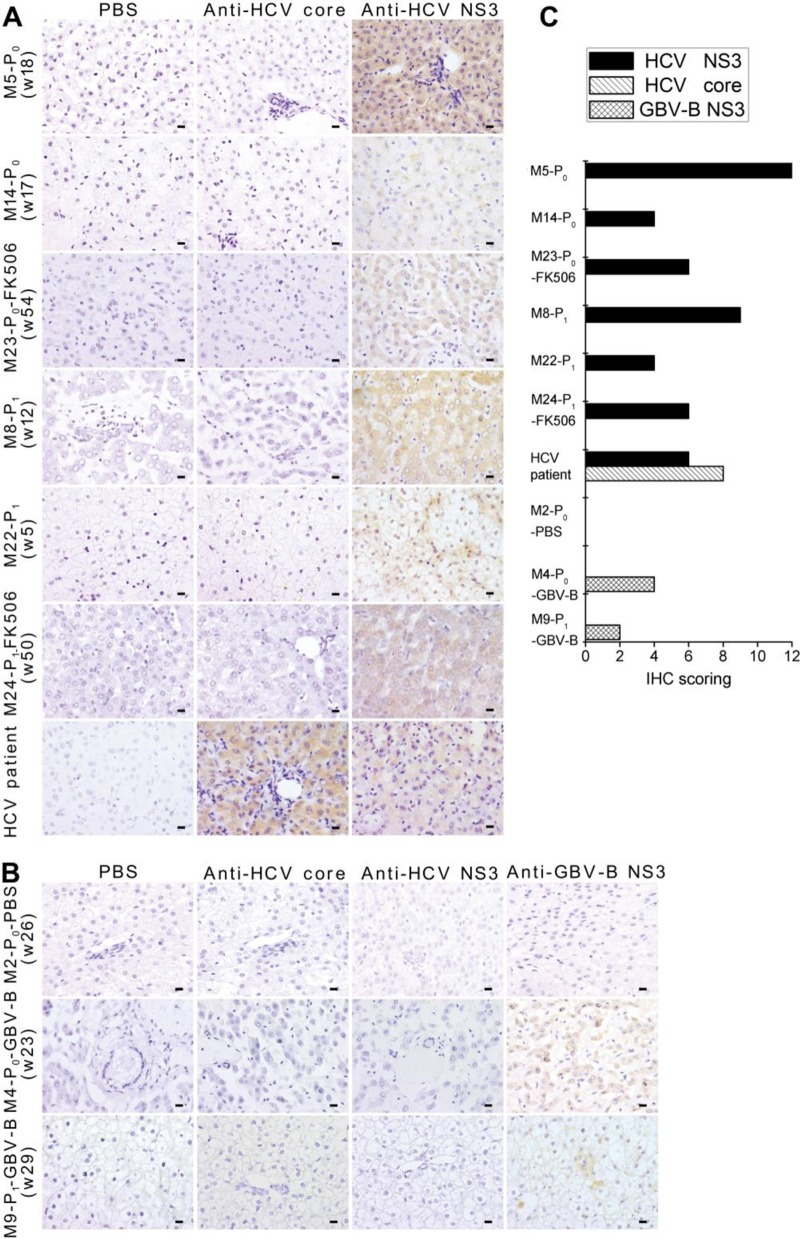
In order to examine the expression of chimeric proteins in hepatocytes, liver tissue from chimera-infected or control marmosets was tested with monoclonal antibodies specific to HCV NS3 or core antigens by IHC staining. In all chimera-infected marmosets, hepatocytes were clearly stained with antibodies to HCV NS3, but not to HCV core (Fig. 4A), while the hepatocytes from an HCV-infected patient showed strong positivity to both HCV NS3 and core antigens. Marmoset M2-P0-PBS, used as a negative control, showed no reactivity, while marmosets M4-P0-GBV-B and M9-P1-GBV-B, used as positive controls, were reactive with monoclonal antibody to GBV-B NS3 epitope (Fig. 4B). The level of viral protein expression in the liver tissues was graded (Fig. 4C) by the IHC staining scores according to the intensity of staining and the frequency of stained cells (Table 2). Moderate or strong staining was observed in 36% to 88% of hepatocytes from the infected animals (except for M25-P1). The extent of stained hepatocytes by IHC staining was consistent with the cells positive by the FISH method, which indicated HCV NS2 to -4A chimera replication and assembly in the liver cells of infected marmosets.
FIG 4.
Immunohistochemical staining of HCV NS3 protein in liver tissues of infected marmosets. (A) Marmoset liver tissues were stained with anti-HCV NS3 monoclonal antibodies. Anti-HCV core and PBS were used as negative controls. Scale bars, 10 cm. (B) Immunohistochemical staining of liver tissues from PBS- and GBV-B-inoculated control marmosets. Anti-HCV core, anti-HCV NS3, or anti-GBV-B NS3 monoclonal antibodies were used in staining. PBS was used as a negative control. Scale bars, 10 μm. (C) IHC scoring. The score was assigned according to the intensity of staining (no staining, 0; weak staining, 1; moderate staining, 2; strong staining, 3) and the extent of stained cells (0%, 0; 1 to 10%, 1; 11 to 50%, 2; 51 to 80%, 3; 81 to 100%, 4). The final immunoreactive score was determined by multiplying the intensity scores by the extent of positivity scores of stained cells, with a minimum score of 0 and a maximum score of 12.
TABLE 2.
Histopathological and immunohistochemical observations for HCV NS2 to -4A/GBV-B chimera
| Marmoset | Time (wk) | Necroinflammatory gradea |
Fibrosis stageb | IHC stainingc |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-HCV core |
Anti-HCV NS3 |
|||||||||||
| Periportal with/without bridging necrosis | Intralobular degeneration and focal necrosis | Portal inflammation | Total | Intensity | Extent | Final | Intensity | Extent | Final | |||
| M5-P0 | 18 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 3 | 4 | 12 |
| M14-P0 | 17 | 0 | 3 | 1 | 4 | 1 | 0 | 0 | 0 | 2 | 2 | 4 |
| M8-P1 | 12 | 0 | 2 | 1 | 3 | 1 | 0 | 0 | 0 | 3 | 3 | 9 |
| M22- P1 | 5 | 1 | 3 | 3 | 7 | 2 | 0 | 0 | 0 | 2 | 2 | 4 |
| M25-P1 | 32 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 2 |
| M23-P0-FK506 | 54 | 1 | 3 | 3 | 7 | 2 | 0 | 0 | 0 | 2 | 3 | 6 |
| M24-P1-FK506 | 50 | 0 | 4 | 1 | 5 | 1 | 0 | 0 | 0 | 2 | 3 | 6 |
The histological status was determined by the modified HAI system (Kondell score), which grades necrosis and inflammation on a scale of 0 to 18 (periportal inflammation and necrosis, 0 to 10; lobular inflammation and necrosis, 0 to 4; portal inflammation, 0 to 4).
Fibrosis was scored as 0 to 6 (0, no fibrosis; 1 or 2, portal fibrosis; 3 or 4, bridging fibrosis; 5 or 6, cirrhosis).
The score was given according to the intensity of nucleic or cytoplasmic staining (no staining, 0; weak staining, 1; moderate staining, 2; strong staining, 3) and the extent of stained cells (0%, 0; 1 to 10%, 1;11 to 50%, 2; 51 to 80%, 3; 81 to 100%, 4). The final immunoreactivity score was determined by multiplying the intensity scores by the extent of positivity scores of stained cells, with a minimum score of 0 and a maximum score of 12.
Pathology of viral hepatitis changes in infected marmosets.
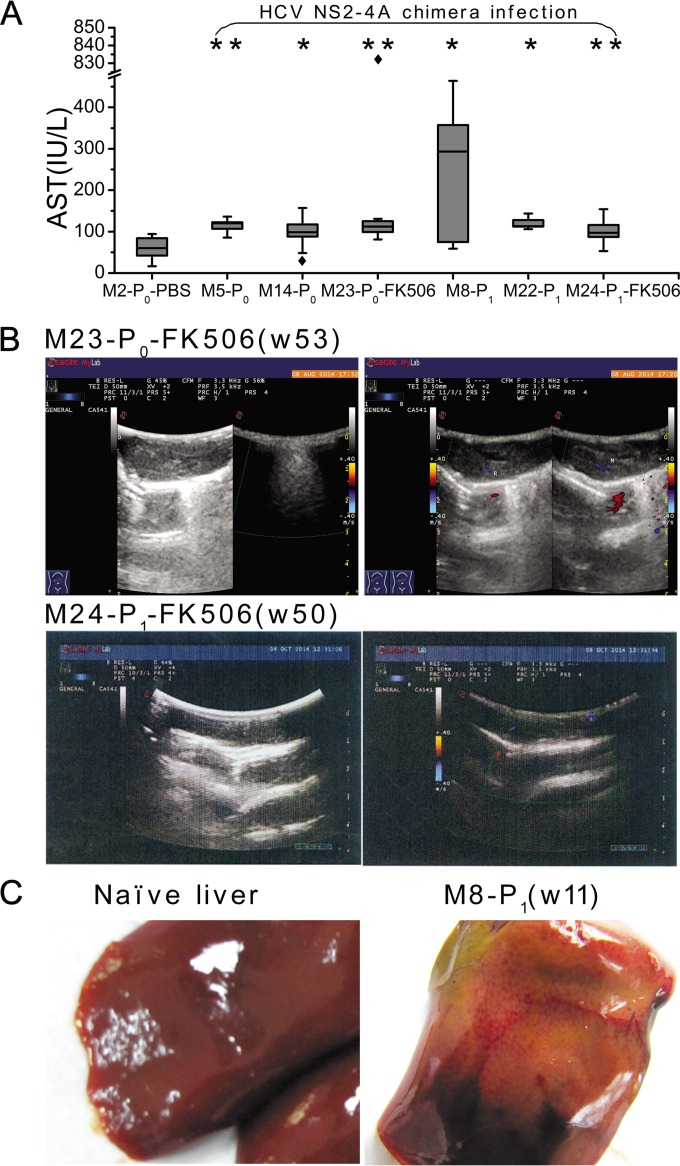
Serum ALT and AST levels were tested in the course of HCV NS2 to -4A chimera-infected or control marmosets. ALT levels varied insignificantly in either negative-control and chimera-infected marmosets or in chimera pre- and postinfection, but AST levels in HCV NS2 to -4A chimera-infected marmosets were significantly higher than that in the negative-control marmoset M2-P0-PBS (P = 0.049 to 0.001) (Fig. 5A).
FIG 5.
Blood and liver markers in infected marmosets. (A) AST levels were measured in sera of infected marmosets and a PBS control animal. Boxes indicate the upper and lower quartiles, whiskers indicate the maximum and minimum values, horizontal lines indicate median values, and diamond symbols indicate the value of outliers. P values were analyzed between chimera-infected and PBS control marmosets. **, P < 0.001; *, P < 0.01. (B) B-mode ultrasonogram examination of marmosets M23-P0 and M24-P1. The hepatic surface appeared dotted, the liver parenchyma was heterogeneous, and the vessel definition was unclear. (C) Morphological examination of livers from M8-P0 and control animals.
The liver condition of HCV NS2 to -4A chimera-infected marmosets was examined by B-mode ultrasonography. Dots on the liver surface and unclear liver vessels were observed in marmosets M23-P0-FK506 and M24-P1-FK506 (Fig. 5B), suggesting liver injury in the two animals. Marmoset livers were examined for pathological and histopathological changes mostly at the time when the animals were sacrificed or dead. Severe liver abnormalities with brownish yellow particles were observed in M8-P1 (Fig. 5C), indicating heterogeneous alteration of the marmoset liver parenchyma, but the livers of other infected animals did not show such morphological changes compared with the livers of negative-control animals.
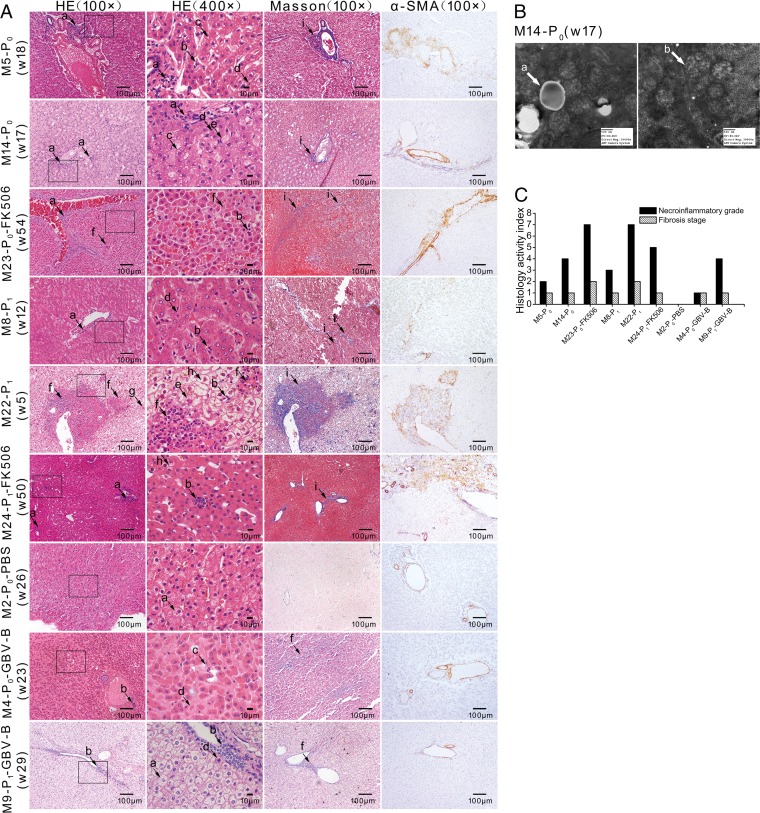
Histopathological changes were examined by H&E staining and Masson's trichrome staining in sections of liver tissue from infected marmosets at the indicated time points and were compared with control animals M2-P0-PBS, M4-P0-GBV-B, and M9-P1-GBV-B. The liver tissue from six primary (P0) and passaged (P1) HCV NS2 to -4A chimera intrahepatically or intravenously infected marmosets presented lymphocytic infiltrates, ballooning degeneration, ground glass liver cells, eosinophilic cells, Kupffer cell enlargement, and necrosis (Fig. 6A), indicating typical viral hepatitis. Steatosis was seen in the livers of M5-P0 and M14-P0 (Fig. 6A). In addition, an increase of lipid droplets and abnormal mitochondria was observed in M14-P0 examined by transmission electron microscopy (Fig. 6B).
FIG 6.
Histopathological observation of liver tissues from infected marmosets. (A) Liver tissues of marmosets were stained with H&E, Masson's trichrome, and α-SMA. The lowercase letters with arrows indicate histopathological features: lymphocytic infiltrates (a), focal necrosis (b), steatosis (c), eosinophilic cells (d), ground glass liver cells (e), piecemeal necrosis (f), Kupffer cell enlargement (g), ballooning degeneration (h), and fibrous expansion (i). (B) Electron microscope image of marmoset M14-P0. The lowercase letters with arrows indicate histopathological changes: lipid droplets (a) and mitochondria (b). (C) Necroinflammatory grade and fibrosis stage were scored by the modified HAI system, in which necrosis and inflammation grades were on a scale of 0 to 18 and fibrosis was scored as 0 to 6.
Fibrous expansion in some portal areas or liver parenchyma was revealed in Masson's trichrome-stained liver tissues from all chimera-infected marmosets (Fig. 6A). The most severe fibrosis was accompanied by piecemeal necrosis in the liver parenchyma of marmoset M22-P1. An increase of α-SMA expression was found in three of six animals, particularly in M22-P1 (Fig. 6A), suggesting that the hepatic stellate cells were activated and correlated with the progression of liver injury and fibrosis. The necroinflammation and fibrosis stages in the livers of infected marmosets were graded according to the HAI index score (Table 2 and Fig. 6C). Marmoset M22-P1 presented the highest grade.
Specific antibody response to the HCV NS2 to -4A chimera in infected marmosets.
The reactivity of antibody to HCV NS3 in sera of chimera-infected marmosets was assessed as the S/CO ratio by ELISA (Fig. 7). Overall anti-HCV reactivity levels in primary and passaged HCV NS2 to -4A chimera-infected immunocompetent or FK506-treated immunosuppressed marmosets were significantly higher than in PBS- and GBV-B-injected control animals (P < 0.001). The reactivity of antibodies to HCV NS3 had S/CO ratios of <1 in sera of control marmosets (Fig. 7A), while high antibody reactivity was observed in both primary and passaged chimera-infected marmosets (Fig. 7B and C), likely reflecting the fluctuations of the viral load (Fig. 2A and B). Antibody response to HCV NS3 in marmoset M22-P1 was considerably stronger than that in marmoset M8-P1 (Fig. 7C), a result consistent with the fluctuations of the viral loads in the two animals (Fig. 2B). In contrast, low antibody reactivity was observed in immunosuppressed animals (M23-P0-FK506 and M24-P1-FK506), which strengthened progressively when FK506 was discontinued at week 26 or at week 14 (P = 0.042 or P < 0.001), respectively (Fig. 7D).
FIG 7.
Reactivity of antibody to HCV NS3 in ELISA. (A) Control marmosets. (B) Primary-infected marmosets. (C) Passage-infected marmosets. (D) FK506 immunosuppression-infected marmosets. An S/CO ratio of >1 was considered reactive. Antibody reactivity between control and HCV NS2 to -4A chimera-infected marmosets was significantly different by the nonparametric Mann-Whitney U test (P < 0.001).
Viremic clearance but hepatic chimera persistence in an HCV NS2 to -4A chimera-infected marmoset.
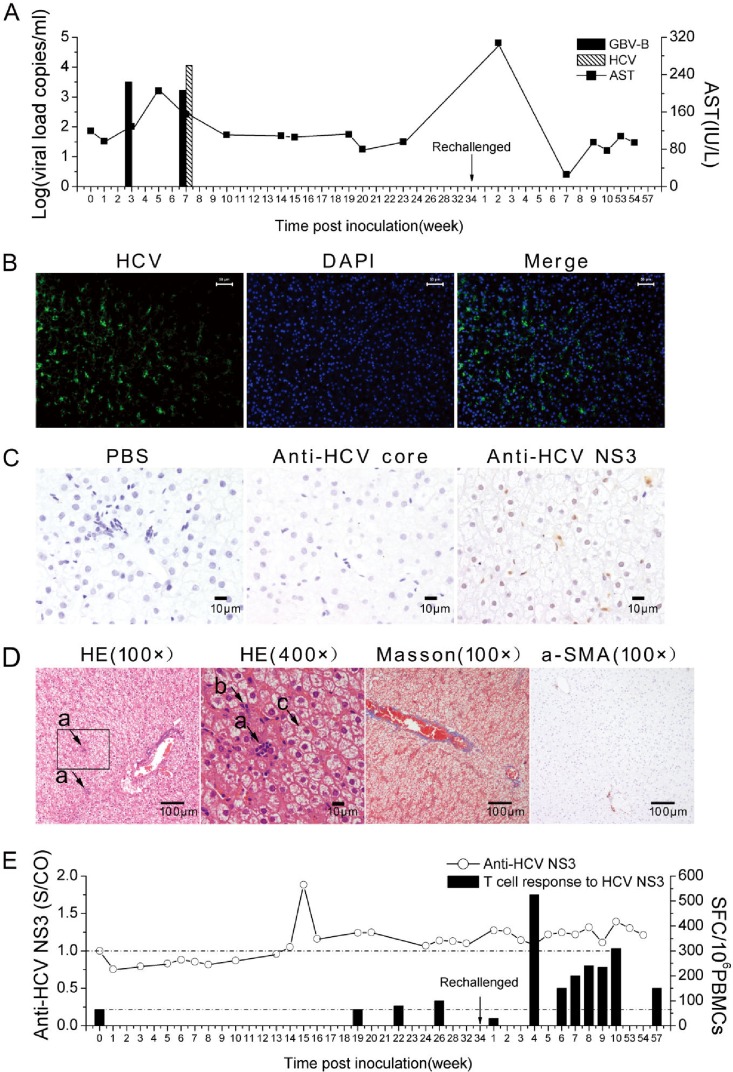
Among four passage chimera-infected marmosets (Table 1), M25-P1 was intravenously inoculated with 2 × 104 viral-RNA copies in P0 sera collected from M23-P0-FK506 after discontinuing administration of FK506. Within the 34 weeks of follow-up of M25-P1, viremia and elevated ALT were detected only at weeks 3 and 7 (Fig. 8A). Liver tissue collected by biopsy at week 32 contained 22% and 4% of hepatocytes positive for HCV NS3 RNA and protein by FISH and IHC staining, respectively (Fig. 8B and C). Mild focal necrosis, eosinophilic cells, and ballooning degeneration were observed in liver tissues by H&E staining, but no fibrosis was found by Masson's trichrome staining and a-SMA (Fig. 8D). Antibody to HCV NS3 was detected at relatively high levels at weeks 14 to 17 and then declined, while the specific T-cell response was weak or undetectable until week 34 (Fig. 8E). In order to detect the memory immune response to the HCV NS2 to -4A chimera, M25-P1 was challenged at week 34 with P1 virus-containing serum (2 × 104 viral-RNA copies) collected from M24-P1 after discontinuation of FK506 administration. The reactivity of antibody to HCV NS3 significantly increased postchallenge (P = 0.001) (Fig. 8E). The T-cell response to HCV NS3 was equally triggered (Fig. 8E). The level of specific IFN-γ production was significantly higher than that found before challenge (P = 0.019), but no viremia was detected during the 57 weeks of follow-up. Taken together, these results suggest that M25-P1 cleared HCV NS2 to -4A chimera infection in serum but not in liver tissue.
FIG 8.
Characteristics of marmoset M25-P1 with chimera viremic clearance but hepatic viral-RNA persistence. M25-P1 was inoculated intravenously with the P0 serum containing 2 × 104 chimera RNA copies and rechallenged with the same dose of chimera P1 in week 34 following the first inoculation. (A) Chimeric viremia and serum AST detection in the course of infection of M25-P1. (B) FISH detection of chimeric RNA specific to HCV NS3 probes. (C) Immunohistochemical staining of liver tissue. (D) Histopathological observation of liver biopsy specimens collected at week 32. The lowercase letters with arrows indicate histopathological changes: focal necrosis (a), eosinophilic cells (b), and ballooning degeneration (c). (E) Antibody and T-cell immune responses specific to HCV NS3 in ELISA or ELISpot. The difference in specific antibody or IFN-γ production levels between the first challenge and rechallenge courses was calculated by the nonparametric Mann-Whitney U test (P = 0.001 or 0.019).
DISCUSSION
HCV is a single-stranded RNA virus containing an open reading frame (ORF) encoding a single polypeptide chain, which is cleaved into structural (core, E1, E2, and p7) and nonstructural (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins (28). NS2-NS3 protease has been known as the autoprotease, while NS2 plays a role in mediating the processing at the NS2-NS3 site (29). The NS3 protein encompasses both serine protease (the N-terminal one-third) and helicase (the C terminus) domains and plays a critical role in the life cycle of HCV. The NS4A polypeptide acts as a cofactor of NS3 serine protease for efficient polyprotein processing (30). The HCV NS3-NS4A complex is a heterodimeric serine protease that has been considered a prime target for developing direct-acting antivirals (31, 32), such as novel protease inhibitors of noncovalent macrocyclic simeprevir/TMC435 (33, 34), covalent linear boceprevir (BOC) (35–37), and telaprevir analog (TVR) (38, 39). From an immunologic point of view, the HCV NS2 to -4A proteins are major antigens in HCV vaccines, a few of which are currently in clinical trials for induction of a protective T-cell response against HCV infection (40–42).
There is approximately 34% amino acid sequence homology between HCV and GBV-B NS2 to -4A proteins (43). However, HCV and GBV-B share common NS2 to -4A protease activity and substrate specificity in vitro (44–46), which may allow HCV NS2 to -4A/GBV-B chimeric virus to remain replicating and infectious in marmosets. In the present study, an HCV NS2 to -4A chimera was generated and revealed to be infectious and able to transmit to naive marmosets, supporting the above-mentioned hypothesis. Six of seven HCV NS2 to -4A chimera-infected animals presented viremic persistence, and one animal (M25-P1) appeared to present viremic clearance (Fig. 2 and 8). Significant levels of chimera viremia and evidence of liver disease in all immunocompetent, as well as immunosuppressed, infected animals detected intermittently or consistently for up to at least 18 weeks strongly suggest that HCV/GBV-B chimeras are infectious and replicative in marmosets. HCV NS2 to -4A chimera-infected marmosets exhibited viral hepatitis similar to human hepatitis C, as evidenced by persistent viremia, viral-genome and protein detection in liver tissue, histopathologic changes indicating hepatic inflammation and fibrosis, and adaptive antibody response specific to HCV NS3. Whether the presence of HCV structural or nonstructural protein genes converted generally resolved GBV-B infection into persistent infection needs to be further investigated.
The replicative fitness of the HCV NS2 to -4A chimera was preliminarily analyzed in this study. The frequency of random substitution was detected at 1/281 at a single site of nucleotide sequence from the immunocompetent marmoset (M22-P1) or 1/175 from the immunosuppressed animal (M24-P1-FK506), which suggested that quasispecies of the virus existed. Amino acid mutations W1002R, S1497A, and P1525L were detected twice in the 7 to 9 reads of NS3 region sequence of the HCV NS2 to -4A chimera from M22-P1, but no common mutation was found in the consensus sequence. However, the significance of these mutations in the NS3 region of the virus in vivo should be further investigated.
In six of seven marmosets infected with the HCV NS2 to -4A chimera, viremia was consistently detected during the experimental course of each animal infection (Fig. 2), with viral replication patterns similar to those seen in HCV-infected patients and in previously reported HCV C-E1-E2-p7 or E1-E2-p7 chimera-infected marmosets (22, 47). The viral loads in two FK506-treated immunosuppressed marmosets (M23-P0 and M24-P1) were significantly higher than those in immunocompetent animals (P = 0.02). In animals immunosuppressed with FK506, the viral loads observed during therapy or after discontinuation of therapy were not significantly different, though they were higher than in immunocompetent infected animals, suggesting that once HCV NS2 to -4A chimera infection is established, persistent infection is sustained. However, this persistent replication pattern of the HCV chimera in marmosets after discontinuing FK506 treatment was different from spontaneous clearance of GBV-B in tamarins 18 weeks after stopping FK506 therapy (15). Marmoset M22-P1 died 5 weeks after the passage chimera infection with intravenous inoculation of M23-P0 serum (Fig. 2C). At the time of death, the animal presented with a high viremia load and HAI scores of necroinflammation (grade 7) and fibrosis (stage 2) in the liver tissue (Fig. 6A and C), strongly suggesting severe viral hepatitis related to HCV NS2 to -4A chimera infection and the potential cause of death. Simultaneously, the level of anti-HCV NS3 antibody rose more rapidly than in five other persistently infected animals with milder viral hepatitis (Fig. 7).
In marmosets infected with the HCV NS2 to -4A chimera, all seven animals developed necroinflammation (grades 1 to 7), 2 of 7 developed steatosis, and 6 of 7 developed fibrosis (stages 1 and 2) in the 5- to 54-week course of infection (Fig. 2 and Table 2), but no evidence of cirrhosis or hepatocellular carcinoma (HCC) was found. The absence of cirrhosis and HCC might be explained by the relatively short period of follow-up. However, to classify the outcomes of HCV chimera-infected marmosets should require 16 weeks or even longer follow-up detection, which was observed for GBV-B clearance or persistence in tamarins (48–50).
Approximately 30% spontaneous viral clearance is found in HCV-infected patients (47). However, a study examining diagnostic liver biopsy specimens in individuals with resolved infection (HCV antibody positive but RNA negative) found that 82% of nonviremic patients had fibrosis and might have HCV hepatic persistence (51). In our study, a single, admittedly anecdotal, case of a chimera-infected marmoset (M25-P1) was classified as spontaneous clearance of viremia but persistence of detectable viral RNA in liver tissue (Fig. 8). M25-P1 presented mild histopathological changes with mild focal necrosis, eosinophilic cells, and ballooning degeneration. FISH and IHC staining revealed relatively weak positivity of HCV NS2 to -4A chimeric RNA and HCV NS3 protein in liver tissue collected at week 32 but remained consistently negative for viral RNA in serum beyond week 7 of follow-up. M25-P1 was rechallenged with HCV NS2 to -4A chimera (M24-P1) at week 34 after the initial inoculation. Both antibody and T-cell responses to HCV NS3 were significantly stronger than that observed before rechallenge, suggesting the triggering of an anamnestic response. No emergence or reoccurrence of chimera viremia in M25-P1 was detected following rechallenge, suggesting that sufficient immune protection had been developed. A previous study found that in four GBV-B-infected marmosets (including two reinfected animals), the T-cell response correlated with clearance of viremia. This T-cell response was mostly specific for NS3 and NS4A epitopes of GBV-B (52). In our study, however, with HCV NS2 to -4A replacing the corresponding GBV-B elements, whether the T-cell response to HCV NS3 and 4A played an important role in controlling HCV NS2 to -4A chimera infection in marmoset M25-P1 remains to be further investigated.
Recently, animal models for the study of hepatitis C virus infection and related liver disease were extensively described (2, 21), including chimpanzees for study of the immune response and humanized mice for study of the virus life cycle. The HCV/GBV-B chimera-infected marmoset model, with relatively low cost and ready availability, can be considered a potential tool for future development of novel antiviral drugs, immunotherapies, and vaccines against HCV infection (21). Besides the differences between GBV-B and HCV, GBV-B infection in marmosets mostly resulted in an acute and self-limited infection with rare cases of persistence (13, 14, 21, 52). HCV/GBV-B chimeras carrying a short section of the HCV genome hardly mimicked the functions of HCV structural or nonstructural proteins (17, 19, 20), which limited the utility of these small-primate models for understanding the mechanisms of persistent HCV infection. In our previously reported study, we established a marmoset model infected with HCV/GBV-B chimeras carrying either all the HCV structural proteins (C, E1, E2, and p7) or full-length envelope proteins (E1, E2, and p7) (22). In this study, we successfully established a model of marmosets infected with a HCV NS2 to -4A/GBV-B chimera containing the major HCV nonstructural proteins NS2 to -4A. The data presented suggest the suitability of this small-primate animal model for studying novel antiviral drugs targeting HCV NS3-NS4A protease and a new concept of T-cell-based HCV vaccines.
ACKNOWLEDGMENTS
We thank Jens Bukh (NIH, Bethesda, MD, USA) for kindly providing the plasmid pGBB; Yuanping Zhou (Department of Infectious Disease, Nanfang Hospital, Guangzhou, China) for kindly providing the liver biopsy sample from an HCV patient; Mourong Liu, Xianghui Wu, and Junling Zeng (Experimental Animal Center, Nanfang Hospital, Guangzhou, China) for assistance in animal surgery and blood sample collection; and Ling Dai (Hepatology Center, Nanfang Hospital, Guangzhou, China) for examination of animals by B-mode ultrasonography.
Chengyao Li and Tingting Li designed research; Shaomei Zhu, Tingting Li, Bochao Liu, Yuxia Xu, Yachun Sun, Yilin Wang, Zixuan Chen, Yuanzhan Wang, and Lifang Shuai performed research; Chengyao Li, Shaomei Zhu, Tingting Li, and Jean-Pierre Allain analyzed data; and Chengyao Li, Tingting Li, Shaomei Zhu, and Jean-Pierre Allain wrote the paper.
We declare that we have no competing interests.
REFERENCES
- 1.Rehermann B. 2013. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med 19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bukh J. 2012. Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology 142:1279–1287. doi: 10.1053/j.gastro.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Alter HJ, Purcell RH, Holland PV, Popper H. 1978. Transmissible agent in non-A, non-B hepatitis. Lancet i:459–463. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu YK, Weiner AJ, Rosenblatt J, Wong DC, Shapiro M, Popkin T, Houghton M, Alter HJ, Purcell RH. 1990. Early events in hepatitis C virus infection of chimpanzees. Proc Natl Acad Sci U S A 87:6441–6444. doi: 10.1073/pnas.87.16.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Major ME, Dahari H, Mihalik K, Puig M, Rice CM, Neumann AU, Feinstone SM. 2004. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology 39:1709–1720. doi: 10.1002/hep.20239. [DOI] [PubMed] [Google Scholar]
- 6.Barth H, Rybczynska J, Patient R, Choi Y, Sapp RK, Baumert TF, Krawczynski K, Liang TJ. 2011. Both innate and adaptive immunity mediate protective immunity against hepatitis C virus infection in chimpanzees. Hepatology 54:1135–1148. doi: 10.1002/hep.24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drexler JF, Corman VM, Müller MA, Lukashev AN, Gmyl A, Coutard B, Adam A, Ritz D, Leijten LM, van Riel D, Kallies R, Klose SM, Gloza-Rausch F, Binger T, Annan A, Adu-Sarkodie Y, Oppong S, Bourgarel M, Rupp D, Hoffmann B, Schlegel M, Kümmerer BM, Krüger DH, Schmidt-Chanasit J, Setién AA, Cottontail VM, Hemachudha T, Wacharapluesadee S, Osterrieder K, Bartenschlager R, Matthee S, Beer M, Kuiken T, Reusken C, Leroy EM, Ulrich RG, Drosten C. 2013. Evidence for novel hepaciviruses in rodents. PLoS Pathog 9:e1003438. doi: 10.1371/journal.ppat.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firth C, Bhat M, Firth MA, Williams SH, Frye MJ, Simmonds P, Conte JM, Ng J, Garcia J, Bhuva NP, Lee B, Che X, Quan PL, Lipkin WI. 2014. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 5:e01933–1914. doi: 10.1128/mBio.01933-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor A, Simmonds P, Gerold G, Qaisar N, Jain K, Henriquez JA, Firth C, Hirschberg DL, Rice CM, Shields S, Lipkin WI. 2011. Characterization of a canine homolog of hepatitis C virus. Proc Natl Acad Sci U S A 108:11608–11613. doi: 10.1073/pnas.1101794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsay JD, Evanoff R, Wilkinson TE Jr, Divers TJ, Knowles DP, Mealey RH. 2015. Experimental transmission of equine hepacivirus in horses as a model for hepatitis C virus. Hepatology 61:1533–1546. doi: 10.1002/hep.27689. [DOI] [PubMed] [Google Scholar]
- 11.Dorner M, Horwitz JA, Donovan BM, Labitt RN, Budell WC, Friling T, Vogt A, Catanese MT, Satoh T, Kawai T, Akira S, Law M, Rice CM, Ploss A. 2013. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature 501:237–241. doi: 10.1038/nature12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Zhao Y, Zhang C, Chen H, Feng J, Chi X, Pan Y, Du J, Guo M, Cao H, Chen H, Wang Z, Pei R, Wang Q, Pan L, Niu J, Chen X, Tang H. 2014. Persistent hepatitis C virus infections and hepatopathological manifestations in immune-competent humanized mice. Cell Res 24:1050–1066. doi: 10.1038/cr.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukh J, Apgar CL, Yanagi M. 1999. Toward a surrogate model for hepatitis C virus: an infectious molecular clone of the GB virus-B hepatitis agent. Virology 262:470–478. doi: 10.1006/viro.1999.9941. [DOI] [PubMed] [Google Scholar]
- 14.Jacob JR, Lin KC, Tennant BC, Mansfield KG. 2004. GB virus B infection of the common marmoset (Callithrix jacchus) and associated liver pathology. J Gen Virol 85:2525–2533. doi: 10.1099/vir.0.80036-0. [DOI] [PubMed] [Google Scholar]
- 15.Lanford RE, Chavez D, Notvall L, Brasky KM. 2003. Comparison of tamarins and marmosets as hosts for GBV-B infections and the effect of immunosuppression on duration of viremia. Virology 311:72–80. doi: 10.1016/S0042-6822(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 16.Bright H, Carroll AR, Watts PA, Fenton RJ. 2004. Development of a GB virus B marmoset model and its validation with a novel series of Hepatitis C virus NS3 protease inhibitors. J Virol 78:2062–2071. doi: 10.1128/JVI.78.4.2062-2071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rijnbrand R, Yang Y, Beales L, Bodola F, Goettge K, Cohen L, Lanford RE, Lemon SM, Martin A. 2005. A chimeric GB virus B with 5′ nontranslated RNA sequence from hepatitis C virus causes hepatitis in tamarins. Hepatology 41:986–994. doi: 10.1002/hep.20656. [DOI] [PubMed] [Google Scholar]
- 18.Takikawa S, Engle RE, Emerson SU, Purcell RH, St Claire M, Bukh J. 2006. Functional analyses of GB virus B p13 protein: development of a recombinant GB virus B hepatitis virus with a p7 protein. Proc Natl Acad Sci U S A 103:3345–3350. doi: 10.1073/pnas.0511297103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haqshenas G, Dong X, Netter H, Torresi J, Gowans EJ. 2007. A chimeric GB virus B encoding the hepatitis C virus hypervariable region 1 is infectious in vivo. J Gen Virol 88:895–902. doi: 10.1099/vir.0.82467-0. [DOI] [PubMed] [Google Scholar]
- 20.Griffin S, Trowbridge R, Thommes P, Parry N, Rowlands D, Harris M, Bright H. 2008. Chimeric GB virus B genomes containing hepatitis C virus p7 are infectious in vivo. J Hepatol 49:908–915. doi: 10.1016/j.jhep.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manickam C, Reeves RK. 2014. Modeling HCV disease in animals: virology, immunology and pathogenesis of HCV and GBV-B infections. Front Microbiol 5:690. doi: 10.3389/fmicb.2014.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T, Zhu S, Shuai L, Xu Y, Yin S, Bian Y, Wang Y, Zuo B, Wang W, Zhao S, Zhang L, Zhang J, Gao GF, Allain JP, Li C. 2014. Infection of common marmosets with hepatitis C virus/GB virus-B chimeras. Hepatology 59:789–802. doi: 10.1002/hep.26750. [DOI] [PubMed] [Google Scholar]
- 23.Sbardellati A, Scarselli E, Verschoor E, De Tomassi A, Lazzaro D, Traboni C. 2001. Generation of infectious and transmissible virions from a GB virus B full-length consensus clone in tamarins. J Gen Virol 82:2437–2448. doi: 10.1099/0022-1317-82-10-2437. [DOI] [PubMed] [Google Scholar]
- 24.Bian Y, Zhao S, Zhu S, Zeng J, Li T, Fu Y, Wang Y, Zheng X, Zhang L, Wang W, Yang B, Zhou Y, Allain JP, Li C. 2013. Significance of monoclonal antibodies against the conserved epitopes within non-structural protein 3 helicase of hepatitis C virus. PLoS One 8:e70214. doi: 10.1371/journal.pone.0070214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koo CL, Kok LF, Lee MY, Wu TS, Cheng YW, Hsu JD, Ruan A, Chao KC, Han CP. 2009. Scoring mechanisms of p16INK4a immunohistochemistry based on either independent nucleic stain or mixed cytoplasmic with nucleic expression can significantly signal to distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. J Transl Med 7:25. doi: 10.1186/1479-5876-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. 1981. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 27.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, Phillips KMJ, Portmann LBG, Poulsen MH, Scheuer PJ, Schmid NM, Thalero H. 1995. Histological grading and staging of chronic hepatitis. J Hepatol 22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 28.Moradpour D, Penin F, Rice CM. 2007. Replication of hepatitis C virus. Nat Rev Microbiol 5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 29.Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset FL, Bartenschlager R. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A 103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallinari P, Brennan D, Nardi C, Brunetti M, Tomei L, Steinkühler C, De Francesco R. 1998. Multiple enzymatic activities associated with recombinant NS3 protein of hepatitis C virus. J Virol 72:6758–6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiering N, D'Arcy A, Villard F, Simic O, Kamke M, Monnet G, Hassiepen U, Svergun DI, Pulfer R, Eder J, Raman P, Bodendorf U. 2011. A macrocyclic HCV NS3/4A protease inhibitor interacts with protease and helicase residues in the complex with its full-length target. Proc Natl Acad Sci U S A 108:21052–21056. doi: 10.1073/pnas.1110534108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghemo A, De Francesco R. 2013. New horizons in hepatitis C antiviral therapy with direct-acting antivirals. Hepatology 58:428–438. doi: 10.1002/hep.26371. [DOI] [PubMed] [Google Scholar]
- 33.Cummings MD, Lindberg J, Lin TI, de Kock H, Lenz O, Lilja E, Felländer S, Baraznenok V, Nyström S, Nilsson M, Vrang L, Edlund M, Rosenquist A, Samuelsson B, Raboisson P, Simmen K. 2010. Induced-fit binding of the macrocyclic noncovalent inhibitor TMC435 to its HCV NS3/NS4A protease target. Angew Chem Int Ed Engl 49:1652–1655. doi: 10.1002/anie.200906696. [DOI] [PubMed] [Google Scholar]
- 34.Lin TI, Lenz O, Fanning G, Verbinnen T, Delouvroy F, Scholliers A, Vermeiren K, Rosenquist A, Edlund M, Samuelsson B, Vrang L, de Kock H, Wigerinck P, Raboisson P, Simmen K. 2009. In vitro activity and preclinical profile of TMC435350, a potent hepatitis C virus protease inhibitor. Antimicrob Agents Chemother 53:1377–1385. doi: 10.1128/AAC.01058-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatraman S, Bogen SL, Arasappan A, Bennett F, Chen K, Jao E, Liu YT, Lovey R, Hendrata S, Huang Y, Pan W, Parekh T, Pinto P, Popov V, Pike R, Ruan S, Santhanam B, Vibulbhan B, Wu W, Yang W, Kong J, Liang X, Wong J, Liu R, Butkiewicz N, Chase R, Hart A, Agrawal S, Ingravallo P, Pichardo J, Kong R, Baroudy B, Malcolm B, Guo Z, Prongay A, Madison V, Broske L, Cui X, Cheng KC, Hsieh Y, Brisson JM, Prelusky D, Korfmacher W, White R, Bogdanowich-Knipp S, Pavlovsky A, Bradley P, Saksena AK, Ganguly A, Piwinski J, Girijavallabhan V, Njoroge FG. 2006. Discovery of (1R,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(1,1-dimethylethyl) amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl] -6,6-dimethyl-3-azabicyclo [3.1.0]hexan-2(S)-carboxamide (SCH 503034), a selective, potent, orally bioavailable hepatitis C virus NS3 protease inhibitor: a potential therapeutic agent for the treatment of hepatitis C infection. J Med Chem 49:6074–6086. doi: 10.1021/jm060325b. [DOI] [PubMed] [Google Scholar]
- 36.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R, HCV RESPOND-2 Investigators . 2011. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang MH, Gordon LA, Fung HB. 2012. Boceprevir: a protease inhibitor for the treatment of hepatitis C. Clin Ther 34:2021–2038. doi: 10.1016/j.clinthera.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Perni RB, Chandorkar G, Cottrell KM, Gates CA, Lin C, Lin K, Luong YP, Maxwell JP, Murcko MA, Pitlik J, Rao G, Schairer WC, Van Drie J, Wei Y. 2007. Inhibitors of hepatitis C virus NS3.4A protease. Effect of P4 capping groups on inhibitory potency and pharmacokinetics. Bioorg Med Chem Lett 17:3406–3411. [DOI] [PubMed] [Google Scholar]
- 39.Forestier N, Zeuzem S. 2012. Telaprevir for the treatment of hepatitis C. Expert Opin Pharmacother 13:593–606. doi: 10.1517/14656566.2012.660524. [DOI] [PubMed] [Google Scholar]
- 40.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, Lahm A, Luzzago A, Vitelli A, Colloca S, Cortese R, Nicosia A. 2006. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med 12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 41.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O'Hara G, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. 2012. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, Kurioka A, Ammendola V, Del Sorbo M, Grazioli F, Esposito ML, Siani L, Traboni C, Hill A, Colloca S, Davis M, Nicosia A, Cortese R, Folgori A, Klenerman P, Barnes E. 2014. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med 6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muerhoff AS, Leary TP, Simons JN, Pilot-Matias TJ, Dawson GJ, Erker JC, Chalmers ML, Schlauder GG, Desai SM, Mushahwar IK. 1995. Genomic organization of GB viruses A and B: two new members of the Flaviviridae associated with GB agent hepatitis. J Virol 69:5621–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarselli E, Urbani A, Sbardellati A, Tomei L, De Francesco R, Traboni C. 1997. GB virus B and hepatitis C virus NS3 serine proteases share substrate specificity. J Virol 71:4985–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butkiewicz N, Yao N, Zhong W, Wright-Minogue J, Ingravallo P, Zhang R, Durkin J, Standring DN, Baroudy BM, Sangar DV, Lemon SM, Lau JY, Hong Z. 2000. Virus-specific cofactor requirement and chimeric hepatitis C virus/GB virus B nonstructural protein 3. J Virol 74:4291–4301. doi: 10.1128/JVI.74.9.4291-4301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boukadida C, Marnata C, Montserret R, Cohen L, Blumen B, Gouttenoire J, Moradpour D, Penin F, Martin A. 2014. NS2 proteins of GB virus B and hepatitis C virus share common protease activities and membrane topologies. J Virol 88:7426–7444. doi: 10.1128/JVI.00656-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farci P, Alter HJ, Wong D, Miller RH, Shih JW, Jett B, Purcell RH. 1991. A long-term study of hepatitis C virus replication in non-A, non-B hepatitis. N Engl J Med 325:98–104. doi: 10.1056/NEJM199107113250205. [DOI] [PubMed] [Google Scholar]
- 48.Beames B, Chavez D, Guerra B, Notvall L, Brasky KM, Lanford RE. 2000. Development of a primary tamarin hepatocyte culture system for GB virus-B: a surrogate model for hepatitis C virus. J Virol 74:11764–11772. doi: 10.1128/JVI.74.24.11764-11772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin A, Bodola F, Sangar DV, Goettge K, Popov V, Rijnbrand R, Lanford RE, Lemon SM. 2003. Chronic hepatitis associated with GB virus B persistence in a tamarin after intrahepatic inoculation of synthetic viral RNA. Proc Natl Acad Sci U S A 100:9962–9967. doi: 10.1073/pnas.1731505100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nam JH, Faulk K, Engle RE, Govindarajan S, St Claire M, Bukh J. 2004. In vivo analysis of the 3′ untranslated region of GB virus B after in vitro mutagenesis of an infectious cDNA clone: persistent infection in a transfected tamarin. J Virol 78:9389–9399. doi: 10.1128/JVI.78.17.9389-9399.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoare M, Gelson WT, Rushbrook SM, Curran MD, Woodall T, Coleman N, Davies SE, Alexander GJ. 2008. Histological changes in HCV antibody-positive, HCV RNA-negative subjects suggest persistent virus infection. Hepatology 48:1737–1745. doi: 10.1002/hep.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woollard DJ, Haqshenas G, Dong X, Pratt BF, Kent SJ, Gowans EJ. 2008. Virus-specific T-cell immunity correlates with control of GB virus B infection in marmosets. J Virol 82:3054–3060. doi: 10.1128/JVI.01153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]