Abstract
Aim
There is an urgent need for effective geriatric interventions to meet the health service demands of the growing older population. In this paper, we systematically review and update existing literature on interventions within emergency departments (ED) targeted towards reducing ED re‐visits, hospitalizations, nursing home admissions and deaths in older patients after initial ED discharge.
Methods
Databases Medline, CINAHL, Embase and Web of Science were searched to identify all articles published up to June 2012 that focused on older adults in the ED, included a comparison group, and reported quantitative results in four primary outcomes: ED re‐visits, hospitalizations, nursing home admissions and death after initial ED discharge.
Results
Of the 2826 titles screened, just nine studies met our inclusion criteria. The studies varied in their design and outcome measurements such that results could not be combined. Two trends surfaced: (i) more intensive interventions more frequently resulted in reduced adverse outcomes than did simple referral intervention types; and (ii) among the lowest intensity, referral‐based interventions, studies that used a validated prediction tool to identify high‐risk patients more frequently reported improved outcomes than those that did not use such a tool.
Conclusion
Of the few studies that met the inclusion criteria, there was a lack of consistency and clarity in study designs and evaluative outcomes. Despite this, more intensive interventions that followed patients beyond a referral and the use of a clinical risk prediction tool appeared to be associated with improved outcomes. The dearth of rigorous evaluations with standardized methodologies precludes further recommendations. Geriatr Gerontol Int 2015; 15: 1107–1117.
Keywords: death, emergency service, geriatric assessment, hospital, hospital readmission, nursing homes
Introduction
Older adults have a higher rate of emergency department (ED) use than any other age group and as they continue to age, this will result in approximately a 30% increase in their ED use.1, 2, 3 People aged 65 years and older account for 12–21% of all ED visits,4 while accounting for just 14% of the general population.5 Several studies suggest that even after being seen in the ED, the needs of older patients often remain unaddressed. Within 6 months of discharge from the index ED visit, 43.9% of older adults returned to the ED at least once, and 7.5% returned three or more times.6 Within 3 months of discharge, 12.4% of older patients died,7 18.3% were hospitalized and 2.6% subsequently entered a nursing home.8 Furthermore, approximately 80% of older adults discharged from the ED have at least one unaddressed health issue.9
Such high rates of re‐visits and other adverse outcomes after an initial ED admission reinforce concerns that traditional ED models do not meet the chronic and underlying needs of many older patients.10, 11, 12 The current ED model of rapid care, which was designed to predominantly deal with trauma and acute illness, results in treating only the patient's primary concern.4, 10, 13, 14 Without spending the necessary time required to determine the underlying, complex health problems and slowly evolving chronic conditions, the older patient is at risk for future health concerns.15 As a result, the serious health needs of older adults go unmet, and subsequently, ED re‐visits and other adverse outcomes can occur. Given their increasing complexity, the growing population of older adults will cause additional challenges to an already burdened ED system.1, 16, 17, 18
Importance
Many experts have recognized the need for change in ED organization and the current model of care used for older adults.4, 17, 19, 20, 21 A number of interventions have been implemented in an effort to reduce ED re‐visits and better accommodate the specific needs of older adults in the ED.22, 23, 24 These interventions often focus on incorporating a geriatric assessment into the ED to make care recommendations, particularly for those with complex issues including: compromised activities of daily living, cognitive impairment and multiple chronic conditions. To date, there has been little conclusive evidence regarding the effectiveness of these interventions, largely because of inconsistency in primary knowledge and study methods, which has made comparisons across studies difficult.17, 22, 25 Furthermore, many publications focus only on describing intervention types while the most promising models of care for older ED patients are yet to be evaluated.
Two early literature reviews, by Hastings and Heflin20 and McCusker and Verdon,21 examined interventions to improve outcomes after acute care for older adults. In neither review could a conclusion be drawn on the effectiveness of ED‐based interventions because of inconsistencies across interventions. In both reviews, authors recommended that future evaluations use standardized methods for evaluation and reporting to improve the quality of evidence. Since then, four reviews on similar topics have been published, but none of which took on the broad methodological approach that was used by either Hastings and Heflin20 or McCusker and Verdon.21 Nevertheless, their findings show that inconsistent results continue to persist. In two of these four reviews, interventions were not associated with improvements in ED re‐visits19, 26 or death.26 The review by Graf et al. suggested reductions in ED visits and possibly nursing home admissions; however, interventions evaluated had a “complete” comprehensive geriatric assessment design, eliminating the inclusion of interventions, such as fall risks assessments and other rapid‐based interventions.27 Sinha et al.17 used a substantially different methodology that aimed to analyze the core components of an intervention, and its impact on health, social and service outcomes.
To match a growing health service concern by older adults, it is evident that interventions are occurring in an attempt to improve older adult health status and healthcare burden;1, 16, 17, 18 however, it continues to be unclear which programs work and which are ineffective. Further research is required to impact decision‐making processes in the healthcare system.
Goal of this investigation
Our primary objective was to review the literature on ED‐based interventions and examine the evidence on reductions in ED re‐visits, hospitalizations, nursing home admissions and deaths among older adults. In an attempt to restrict our review to relatively more rigorous evaluations, we implemented inclusion criteria related to specific aspects of evaluation design. Although several interventions have been implemented in hopes to reduce ED re‐visits, the lack of consistency in evaluation of these interventions weakens the knowledge base, despite this being an area of such importance. This work will serve to update the aforementioned systematic reviews, determine if previously identified gaps in the research have since been addressed, and make recommendations to improve intervention design and evaluation methods based on a framework of intervention classifications developed in the present review.
Methods
Search strategy
Four electronic databases were searched to carry out the present systematic review in June 2012: Medline (PubMed), CINAHL, Embase and Web of Science. An expert librarian at the affiliated hospital aided in the formation of search statements used for each database. See Appendix I: Search strategy for a detailed summary of search statements used.
Data collection and processing
A total of 2826 articles were identified: 694 through Medline, 450 through CINAHL, 707 through Embase, 949 through Web of Science and 26 through subsequent manual searches of references in relevant papers, and a search for studies that had cited these papers. Authors AG and LB screened Medline and CINAHL for relevance up until May 2008; GK and ZR screened Medline and CINAHL from May 2008 to June 2012, and Embase and Web of Science for all years. Authors independently reviewed each paper at each screening stage (title, abstract and full‐text) and discussed any discrepancies in judgment until consensus was reached; if consensus could not be reached, a third author was involved. The screening process is described below.
To determine which articles met the inclusion criteria, reviewers screened search results in four sequential steps. First, 1035 duplicates across databases were removed. Second, titles were scanned for relevance; 1534 articles were excluded, leaving 257 articles for further screening. Third, complete abstracts of the remaining articles were reviewed. A further 126 articles were eliminated. Titles and abstracts were screened based on the following criteria: (i) a focus on older adults or a subanalysis of older adults of which age cut‐offs correspond with the general understanding of older age groups; (ii) patients were discharged from ED (and not admitted directly to hospital or nursing home); and (iii) studies described an intervention aimed to reduce adverse events after an index ED visit. Literature reviews, letters to the editor, commentaries and editorials were excluded. Fourth, we completed a full‐text review of the remaining 131 articles using a standardized abstraction form. Information was extracted from each paper based on the inclusion criteria used in the titles and abstracts screening, as well as three additional requirements implemented at this stage: (i) the manuscript consisted of an evaluation of the intervention (rather than a mere description of a program); (ii) a concurrent or historical comparison group was used in order to make comparisons against the intervention group; and (iii) at least one of the specified health services events (ED re‐visit, subsequent hospitalization, nursing home admission or death) was included as a study outcome. Nine articles met our inclusion criteria (see Fig. 1 for a complete summary of the study exclusion criteria).
Figure 1.
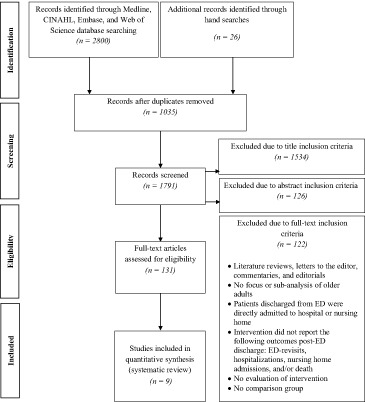
Flow diagram of selection process of included papers.
Outcome measures
The type of intervention design, sample size, follow‐up time, participant identification (for example, the use of a high‐risk prediction tool), intervention details and study results were abstracted from each study. All relevant outcome measures, including raw numbers and test statistics (i.e. estimates of risk difference, risk ratio, odds ratios and P‐values) were recorded where available. Outcome measures of interest were: ED re‐visits, hospitalizations, nursing home admissions and death.
Based on the initial readings of the included manuscripts, two trends emerged, which we then used to organize the results. These were: (i) the intensity of the intervention studied; and (ii) the use of a screening tool to identify high‐risk patients for inclusion in the study (this is further described in the Results section).
Results
Of the nine articles that met our inclusion criteria, the publication dates ranged from 199628 to 2008.29 Four were interventions tested in Australia, two in Canada, two in the USA and one in Italy. There was substantial variation in the study methods, and for this reason, more formal analyses or strategies to combine the data were not used. Included studies are summarized in Table 1.
Table 1.
Characteristics of included papers
| Manuscript number | Author, year | Country | Intervention type | Randomization: (yes/no) | Comparison group | Sample size | Major inclusion criteria | Follow‐up time | Outcome for intervention group |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ballabio et al., 200829 | Italy | CGE | No | 3 months before CGE |
|
|
3 months |
|
| 2 | Bird et al., 200730 | Australia | Care Facilitator Assessment a | No |
|
|
|
First 90 days post‐recruitment |
|
| 3 | Caplan et al., 200431 | Australia | CGA | Yes | Randomly selected control group that received usual care |
|
|
3, 6, 12 and 18 months | |
| 4 | Guttman et al., 200432 | Canada | NDPC | No | Pre‐phase control group that received usual care from May 1999 to December 1999; Post‐phase intervention group received NDPC from January 2000 to July 2000. |
|
|
1, 8 and 14 days | |
| 5 | Hegney et al., 200633 | Australia | Community Nurse Assessment | No | Compared with baseline 9‐months prior intervention | Total: n = 1102 |
|
Unclear |
|
| 6 | Lee et al., 200734 | Canada | PERS | Yes | Randomly selected control group that received usual care |
|
|
Between 60 and 67 days |
|
| 7 | Miller et al., 199628 | USA | CGA | No | Control group matched by same day of visit, gender, and age within 5 years |
|
3 months |
|
|
| 8 | Mion et al. 200335 | USA | CGA | Yes | Randomly selected control group that received usual care |
Total: n = 650 Intervention group: n = 326 Control group: n = 324 |
|
30 and 120 days |
|
| 9 | Moss et al., 200236 | Australia | CCT assessment | No | Compared to baseline 12 months before CCT |
|
|
12 months |
|
aCare Facilitator Assessment consisted of assessing patients' needs, coordinating care with a geriatrician, creating an individual care plan for each patient, and providing information, advice and education for self‐management and referrals. bInitially, recruitment required ≥3 emergency department (ED) visits in the 12 months prior; 6 months after project commenced, criteria changed to ≥2 ED visits in prior 12 months or were perceived to be at risk of ED admission. cHospital Admission is defined as a subsequent hospital visit after ED discharge, not a direct transfer to hospital from ED. dResults for ED re‐visits are shown for 1‐month follow up. eResults for Hospital Admissions are shown for 1‐ and 18‐month follow up. fBased on text, assumed the results correspond to 18‐month follow up. gResults for ED re‐visits are shown for 8‐ and 14‐day follow up. hResults for Hospital admissions are shown at 14‐day follow up. iSample sizes reflect both discharged and non‐discharged patients. Sample sizes that pertain to our interest in discharged patients only were not available. jTool created for the Department of Human Services by Thomas and Associates in 1998. A positive score resulted from yes to any one of the following: living alone and aged >65 years (although the initial tool stated >70 years), has caring responsibilities for others, is receiving community services and likely to have self‐care problems.37 CCT, Care Coordination Team; CGA, Comprehensive Geriatric Assessment; CGE, Comprehensive Geriatric Evaluation; ISAR, Identification of Seniors at Risk; NDPC, Nurse Discharge Plan Coordinator; PERS, Personal Emergency Response Systems; STEP, Tool for Elderly Patients.
Two themes emerged and were used to create a framework for presenting the results. These were: (i) the intensity of the intervention design; and (ii) the type of strategy used to identify eligible study participants. Each intervention was assigned to one of three mutually exclusive categories based on the intensity of the intervention; from the least to most intense, these categories were: (i) referral; (ii) program/follow up; and (iii) integrated model of care. To identify eligible participants, three of the nine papers used a risk prediction tool to identify ED patients potentially at risk for poor outcomes after the visit;28, 32, 33, 35, 36 the other six studies did not use such a strategy and relied only on general study inclusion criteria that commonly included items such as: age, daily intake of more than three drugs, living alone or lacking adequate support, speaks English, a certain number of presentations to the ED and cognitive impairment.28, 29, 30, 31, 32, 34 Each article was categorized according to both themes. The results are presented by intervention type with subcategories based on participant selection strategy. A summary of results is shown in Table 2.
Table 2.
Outcomes of included interventions categorized by intervention type and participant selection criteria
| Manuscript number | Follow‐up times | Outcomes | |||
|---|---|---|---|---|---|
| ED re‐visits | Hospital Admissions | Nursing Home Admissions | Deaths | ||
| Intervention type: Referral | |||||
| 5 a | Unclear |
Intervention: — Control: — OR: — χ2 = 15.59 P < 0.001 |
— b | — b | — b |
| 8 | 30 days |
Low Risk Group
c
Intervention: 31/180 (17%) Control: 18/179 (10%) OR: 1.9 (95% CI 1.0–3.5) P: — High Risk Group d Intervention: 35/146 (24%) Control: 31/145 (21%) OR: 1.2 (95% CI 0.7–2.0) P: — |
Low Risk Group
c
Intervention: 16/180 (9%) Control: 9/179 (5%) OR: 1.8 ([95% CI] 0.8–4.3) P: — High Risk Group d Intervention: 30/146 (21%) Control: 37/145 (26%) OR: 0.8 (95% CI 0.4–1.3) P: — |
Low Risk Group
c
Intervention: 0/180 (0%) Control: 0/179 (0%) OR: — P: — High Risk Group d Intervention: 2/146 (2%) Control: 9/145 (7%) OR: 0.2 (95% CI 0.04–0.96) P: — |
Combined Groups
e
Intervention: 4/326 (0.6%) Control: 2/324 (0.3%) OR: 2.00 (95% CI 0.36–11.00) P: — |
| 120 days |
Low Risk Group
c
Intervention: 55/180 (31%) Control: 58/179 (32%) OR: 0.9 (95% CI 0.6–1.4) P: — High Risk Group d Intervention: 66/146 (45%) Control: 70/145 (48%) OR: 0.9 (95% CI 0.6–1.4) P: — |
Low Risk Group
c
Intervention: 37/180 (21%) Control: 29/179 (16%) OR: 1.3 (95% CI 0.8–2.3) P: — High Risk Group d Intervention: 54/146 (37%) Control: 58/145 (40%) OR: 0.9 (95% CI 0.5–1.4) P: — |
Low Risk Group
c
Intervention: 2/180 (1.3%) Control: 1/179 (0.7%) OR: 2.0 (95% CI 0.3–22.4) P: — High Risk Group d Intervention: 3/146 (3%) Control: 11/145 (10%) OR: 0.3 (95% CI 0.07–0.94) P: — |
Combined Groups
e
Intervention: 9/326 (1%) Control: 10/324 (2%) OR: 0.89 (95% CI 0.36–2.72) P: — |
|
| 9 f | 12 months |
Intervention: 3744 (8.6%) (95% CI 8.4%–8.9%) Control: 3856 (8.8%) (95% CI 8.6%–9.1%) OR: — χ2 = 1.19 P = 0.28 |
— b | — b | — b |
| 4 | 8 days |
Intervention 8.5% incidence Control: 11.6% incidence Adjusting for all covariates g RR: 0.70 (95% CI 0.44–1.10) OR: — P: — |
— b | — b | — b |
| 14 days |
Intervention: 12.9% incidence Control: 16.1% incidence Adjusting for all covariates g RR: 0.80 (95% CI 0.55–1.15) OR: — P: — |
Intervention: 39 Control: 47 Change in admissions: −17% RR: — OR: 0.92 (95% CI 0.59–1.42) P: — |
— b | — b | |
| 7 | 3 months |
Intervention: 0.29 Control: 0.34 OR: — CI: — P = — |
— b |
Intervention: 3.1% Control: 2.4% OR: — CI: — P = — |
Intervention: 7.5% Control: 6.5% OR: — CI: — P = — |
| Intervention type: Program/follow up; no high‐risk selection tool used to identify eligible participants | |||||
| 3 | 30 days |
Intervention: 58/370 (15.7%) Control: 49/369 (13.3%) DP: 2.4 (95% CI −2.7 to 7.5) P = 0.349 |
Intervention: 42/370 (11.9%) Control: 51/369 (14.4%) DP: −2.5 (95% CI −7.4 to 2.4) P = 0.312 h |
— b | — b |
| 18 months | — b |
Intervention: 164/370 (44.4%) Control: 201/369 (54.3%) DP: −9.9 (95% CI −17.1 to −2.7) P = 0.007 h |
— b |
Intervention: 55/370 (14.9%) Control: 53/369 (14.4%) DP: 0.5 (95% CI −4.6 to 5.5) P = 0.765 i |
|
| 1 | 3 months |
Intervention: 21/196 (11%) Control: 44/222 (20%) OR: — P = 0.014 (95% CI −0.160 to −0.020) |
— b | — b | — b |
| 6 | Between 60 and 67 days |
Intervention: 8/43 (19%) Control: 8/43 (19%) RD: 0.0% (95% CI −16% to 16%) P = 1.0 |
Intervention: 3/43 (7%) Control: 6/43 (14%) RD: −7.0% (95% CI −19.8% to 5.9%) P = 0.29 |
— b | — b |
| Intervention type: Integrated Model of Care; no high risk selection tool used to identify eligible participants | |||||
| 2 j | First 90‐days post‐recruitment |
Post‐intervention rate: 0.0099 Pre‐intervention rate: 0.0125 Percentage change: −20.8% P < 0.001 |
Post‐intervention rate: 0.0049 Pre‐intervention rate: 0.0068 Percentage change: −27.9% P < 0.001 |
— b | — b |
aOnly analyzed the High Risk Group as determined by the Screening Tool for Elderly Patients: Score >2. b—: Not reported. cTriage Risk Screen Tool: Score <2 and no cognitive impairment. dTriage Risk Screening Tool: Score of ≥2 or cognitive impairment. eDeath outcomes were not stratified into low‐ and high‐risk groups. fOnly analyzed the High Risk Group as determined by a validated risk tool created for the Department of Human Services by Thomas and Associates in 1998. A positive score resulted from yes to any one of the following: living alone and aged >65 years (although the tool initially created using >70 years), has caring responsibilities for others, receiving community services and likely to have self‐care problems.37 g(a) Unadjusted multivariate Cox proportional hazards regression: day 8: RR = 0.70 ([95% CI] 0.51–0.98); day 14: RR = 0.79 (95% CI 0.62–1.02). (b) Unadjusted RR for unscheduled revisits: day 8: Reduced by 27% (95% CI 0–44); day 14: Reduced by 19% (95% CI −2 to 36). (c) Adjusting for patients' perceived severity of illness and functional autonomy: day 8: RR = 0.70 (95% CI 0.51–0.96; day 14: RR = 0.74 (95% CI 0.57–0.96). hHospital Admission defined as “Emergency admissions to hospital”. iBased on text, assumed results correspond to 18‐month follow up. jFor simplicity, we focused on comparing only the intervention group 12 months pre‐recruitment versus 12 months post‐recruitment; however, the authors also reported findings on a comparison group who declined participation 12 months pre‐ and post‐recruitment. See text for descriptions of both comparisons. CI, confidence interval; DP, difference in percentage; OR, odds ratio; RD, risk difference treatment – control; RR, relative risk.
Intervention type: Referral
A “referral” was defined as an assessment of the patient by a care provider (usually a nurse or social worker) in the ED, followed by recommendations to community‐based agencies or referral for follow up with the regular physician.28, 32, 33, 35, 36 Five articles described interventions that were classified as a referral.
Referral interventions with a risk prediction tool for patient screening
Three studies reported using a risk prediction tool to identify participants. Hegney et al.33 used the Screening Tool for Elderly Patients, which was adapted from the previously validated Identification of Seniors at Risk tool.38, 39 The Screening Tool for Elderly Patients was completed for each patient by a community nurse in the ED, and eligible patients were then referred to community services. Compared with baseline results, patients experienced fewer return visits to the ED (χ2 = 15.59, P < 0.001). In the second study, Mion et al.35 used the Triage Risk Screening Tool to stratify patients into risk groups.37 The Triage Risk Screening Tool was completed by an advanced practice nurse in the ED, and followed by referrals to various community resources before discharge. Among the high‐risk group, the intervention group had fewer admissions to nursing homes when compared with the randomly selected control group at 30‐ and 120‐day follow up (30 days: 2% intervention vs 7% control, odds ratio [OR] 0.2, 95% confidence interval [CI] 0.04–0.96; 120 days: 3% intervention vs 10% control, OR 0.3, 95% CI 0.07–0.94). Within the low‐risk cohort, Mion et al. also found an overall reduction in nursing home admissions (30 days: 0.7% intervention vs 3.0% control, OR 0.21, 95% CI 0.05–0.99).35 No differences were observed in other outcomes. Finally, Moss et al. screened patients with a validated risk prediction tool administered by triage staff and subsequently referred patients to community services if deemed at risk.36 Data on ED re‐visits in the 12 months after intervention implementation were compared against data on re‐visits among older patients who had been seen in the ED in the year before implementation. Results at 12‐month follow up did not suggest any change in ED re‐visits (intervention: 8.6%, 95% CI 8.4–8.9% vs control: 8.8%, 95% CI 8.6–9.1%; χ2 = 1.19, P = 0.28).
Referral interventions without a risk prediction tool for patient screening
Two of the interventions identified as referrals did not use a risk prediction tool to select participants. In both cases, patients were identified through other eligibility criteria, and were assessed by a nurse in the ED who then made recommendations to the patient and caregivers about community‐based services. Guttman et al. found a reduction in ED re‐visits 8 and 14 days after discharge in the unadjusted multivariable Cox proportional hazards regression and the unadjusted relative risk for unscheduled re‐visits, but differences did not persist after controlling for all other patient characteristics including perceived severity of illness and functional autonomy (day 8: adjusted relative risk 0.70, 95% CI 0.44–1.10; day 14: adjusted RR 0.80, 95% CI 0.55–1.15).32 Similarly, no differences in subsequent hospitalizations were observed between groups. Miller et al. reported on ED re‐visits, nursing home admissions and deaths.28 They found slight changes between the intervention and control groups at 3‐month follow up (ED re‐visits: 0.29 intervention vs 0.34 control; nursing home admissions: 3.1% intervention vs 2.4% control; and death: 7.5% intervention vs 6.5% control).
Referral interventions showed only small to moderate success in reducing the occurrence of adverse outcomes after an ED visit; however, there appeared to be slightly better results among those that used a risk prediction tool to target the intervention population.
Intervention type: Program/follow up
An intervention categorized as “program/follow up” consisted of on‐going support or care for the patient after discharge from the index ED visit. Three interventions met this criterion; two consisted of a comprehensive assessment, care plan development and care plan implementation by a coordinated team,29, 31 whereas the third consisted of an at‐home monitoring device.34 None of these interventions used a risk prediction tool to identify eligible participants, but instead used a general inclusion criterion.
Caplan et al. introduced a comprehensive geriatric assessment into the ED, and followed up with a nurse‐created individualized care plan, a home visit after ED discharge and referrals by an interdisciplinary team.31 Participants were followed up to 18 months to determine ED re‐visits, hospitalizations and death. Only reductions in hospitalizations were found at follow up (30 days: 11.9% intervention vs 14.4% control, difference in percentage [DP] −2.5, 95% CI −7.4 to 2.4, P = 0.312; 18 months: 44.4% intervention vs 54.3% control, DP −9.9, 95% CI −17.1 to −2.7, P = 0.007). Ballabio et al. used a comprehensive geriatric evaluation that involved a geriatrician, a nurse, and a social worker to provide counseling, education, treatment changes, referrals to community services and an individualized care plan.29 A subsequent assessment occurred 3 months after baseline. Rates of ED re‐visits were reduced (3 months: 11% intervention vs 20% control, 95% CI −0.160 to −0.020, P = 0.014).
The final program, reported by Lee et al., was the only intervention that did not focus on connecting patients with community‐based services.34 Instead, each patient was trained in the use of a Personal Emergency Response System device that they received on ED discharge. A follow‐up telephone call was made 60–67 days post‐discharge to assess ED outcomes. There was no difference between subsequent ED re‐visits (day 60–67: 19% intervention vs 19% control, risk difference 0.0%, 95% CI −16 to 16%, P = 1.0) and only a slight change in hospitalizations (day 60–67: 7% intervention vs 14% control, risk difference −7.0%, 95% CI −19.8% to 5.9%, P = 0.29).
The interventions that incorporated a geriatric assessment within the ED and follow up by a geriatric care team showed some success, but were not consistent across outcomes. The intervention that focused on electronic monitoring did not have any substantial effect on ED re‐visits or subsequent hospitalizations.
Intervention type: Integrated model of care
“Integrated models of care,” the highest intensity of the interventions identified, were defined as those in which a care facilitator was embedded into the patient's individual care plans. The study by Bird et al.30 was the only intervention that met the criteria for this category; no risk prediction tool was used. This intervention aimed to improve the communication and coordination between all care partners on an on‐going basis. The study incorporated an assessment, care coordination team to identify the patient needs, patient education and advice, individualized care plan, and referrals to community‐based agencies.30 Patients' use of acute hospital services 12 months prerecruitment and 12 months post‐recruitment were studied in both the intervention and comparison groups (those who declined participation). Thus, two sets of comparisons were reported. Within the intervention group, there was a reduction in ED re‐visits (90 days post‐recruitment: 0.0125 pre‐intervention rate vs 0.0099 post‐intervention rate, percentage change [PC] −20.8%, P < 0.001). Fewer hospitalizations also occurred within the intervention cohort (90 days post‐recruitment: 0.0068 pre‐intervention rate vs 0.0049 post‐intervention rate, PC −27.9%, P < 0.001). In the second comparison group, authors also reported the pre‐ and post‐intervention rates for ED re‐visits among patients who declined participation (90 days post‐recruitment: 0.0115 pre‐intervention rate vs 0.0121 post‐intervention rate, PC +5.2, P = 0.246) and for hospitalizations (90‐day post‐recruitment: 0.0068 pre‐intervention rate vs 0.0065 post‐intervention rate, PC −4.4%, P = 0.390).
Inconsistencies across interventions and their reported outcomes make it difficult to reach a conclusion about their efficacy in improving outcomes in older adults after ED discharge; however, there is some suggestion that more involved intervention, or the use of a risk prediction tool, can lead to better outcomes.
Discussion
The present findings suggest that interventions were more successful if they extended beyond referral and if they used a validated risk prediction tool to identify potential candidates. However, the variability in evaluation methodologies and other limitations make summaries across studies difficult. There continues to be a need for rigorous evaluation of interventions to better understand their impact.
Our results suggest two important points despite the heterogeneity of methods. First, the implementation of a clinical risk prediction tool in the ED setting might lead to better targeting of interventions to those older patients most likely to benefit. Clinical risk prediction tools were only reported as a means of identifying eligible participants in studies of low intensity interventions; however, those that used such a tool appeared to have somewhat better results than those that did not.33, 35, 36 In one study, the authors suggested that the use of a risk prediction tool allowed for earlier discharge planning and improved ED efficiency.33 The present review found three different tools used to identify a high risk cohort: (i) Screening Tool for Elderly Patients (adapted from the Identification of Seniors at Risk);33 (ii) an ED nursing tool based on the Triage Risk Screening Tool;35, 37 and (iii) a tool developed in 1998 for the Department of Human Resources Services in Melbourne, Australia.36, 40 Although these tools were independently developed, consisted of different psychometric properties, and were used in different locations, they tended to be comprised of similar items, such as multiple medications, previous ED use, hospital or community services, issues of self‐care, history of falls, caregiving responsibilities and living alone. These common elements focus on the underlying issues relevant to geriatric self‐care and frailty, and reinforce the need for supportive care that extends beyond the acute care sector. The commonality among the tools suggests that the specific tool used might not be as important as the actual implementation of one to screen patients and target interventions to those older adults mostly likely to benefit. By focusing on patients at high risk for poor health outcomes, there is a greater opportunity to reduce the adverse event rate for patients.
Second, more intensive interventions, particularly those that involved more than a simple referral, more frequently found positive results than did studies of less intensive interventions. Studies that utilized a referral type of intervention appeared to be less successful in terms of reducing ED re‐visits, subsequent hospital admissions and other outcomes after an ED visit. One reason could be that the simple act of a referral was insufficient to actually put patients and caregivers in contact with appropriate services for follow up. For instance, it is possible that older patients did not understand the information given at discharge and had low‐compliance with instructions.41, 42 In contrast, the most intense intervention style (the integrated model of care) seemed to result in the greatest benefits for patients. Such programs generally included an interdisciplinary care team consisting of a nurse in addition to at least one other health professional. This diverse team could address a range of patient needs and better manage transitions between healthcare settings and providers.30 Other integrated models (implemented through mechanisms other than through the ED) have shown improved outcomes for frail older adults, likely because they provide comprehensive services that span the full spectrum of older adults' needs. The System of Integrated Services for Older Persons intervention model was a randomized control trial developed by Beland et al.43 that successfully integrated older adult services, increased access to community‐based services, and reduced both ED visits and nursing home admissions by 10%.43, 44 Such integrated models of care appear to be better able to meet the needs of older adults than the current more fragmented health system, but they have been difficult to implement more broadly because of their complex nature.44, 45
The present literature review highlights on‐going gaps in this field of research. Two of the earliest reviews by Hastings and Heflin20 and McCusker and Verdon21 evaluated studies on interventions published between 1966–2005 and 1965–2004, respectively. We identified five studies in the present review that were included in either of these older reviews,28, 31, 32, 35, 36 as well as an additional four studies that have been published since the reviews.29, 30, 33, 34 These more recent studies continued to have inconsistencies in evaluation methods and reporting measures suggesting that recommendations by Hastings and Heflin20 and McCusker and Verdon21 have yet to be adopted.
The other reviews published since Hastings and Heflin20 and McCusker and Verdon21 took a different approach to identifying interventions for review. Fealy et al. exclusively examined nurse‐led interventions,19 whereas Graf et al. included only those that incorporated comprehensive geriatric assessment or a risk tool.27 Conroy et al. focused on any short‐term discharge from hospital (<72 h), which meant that interventions for ED discharge were mixed with those for inpatient hospital discharge.26 Sinha et al. used an adherence analysis methodology to examine associations between core operational components of interventions and utilization outcome.17 In contrast to these reviews,17, 19, 26, 27 we focused solely on interventions that took place in the ED, included a comparison group and studied four specific outcomes: ED re‐visits, nursing home admissions, hospitalizations and mortality. With these criteria, we identified three evaluations that were not included in these prior reviews.29, 30, 34 Regardless of review inclusion criteria, it is clear that inconsistencies in evaluation design and reporting preclude the ability to come to a consensus on effective interventions using the published literature.
Our requirement to only include studies with a comparison group resulted in the exclusion of many manuscripts. Even among those included in the present review, in which we attempted to limit inclusion to studies with specific criteria, there were difficulties in extracting data because of inconsistencies in reporting methods and in reporting results. For example, details regarding follow‐up time were inconsistently reported across studies. In the study by Caplan et al., we found it unclear if the authors collected all outcome measures at several follow‐up periods, but then selectively chose to report outcomes at only 1‐ and/or 18‐month follow up.31 Ballabio et al. stated in their abstract that ED re‐visits and hospitalizations were lower in the 3 months before the intervention; however, we could not find statistics regarding hospitalizations reported later within the paper, and details pertaining to ED re‐visits were vague.29 We also found it difficult to determine an accurate follow‐up time in the study by Hegney et al., and the sample sizes in the comparison and intervention groups after subsequent ED re‐visit were not shown alongside the χ2 and P‐value.33 The lack of clarity in reporting prevents identification of interventions that are worth spending the time and resources required to implement them. Standardizing outcome definitions and requirements (such as clarifying if a hospital admission is directly from the ED), reporting statistics for all follow‐up times studied and thoroughly displaying all outcome measurements would allow for a more accurate analysis of ED‐based interventions for older adults.
The most recent paper included in the present review was published in 2008.29 Given ongoing and increasing concerns about overburdened ED and the aging population, it is surprising that more recent evaluations could not be identified.2, 4, 12, 17 It is not yet clear how to best meet the needs of older adults who visit the ED, but it is evident that research on this issue is urgently required. Our findings, and those of others, show that innovative interventions need to be rigorously evaluated and the results disseminated so that successful programs can be implemented elsewhere.
From our systematic review, we suggest that the development of targeted interventions (potentially with the use of a high‐risk prediction tool), and the implementation of rigorous evaluations (use of a comparison group, and the standardization of evaluation design, outcome definitions, follow‐up times and statistical analyses) needs to continually occur to keep up with the growing health service needs of older adults. Without better evidence on which programs work and why, it is difficult to determine how, where and when such programs should be implemented.
We found just nine studies that were relevant for the purpose of this systematic review. Future evaluations should consider standardizations of methods (especially with the use of a comparison group), outcome measures and statistical analyses that would allow for better understanding as to which types of interventions would be most effective in reducing adverse outcomes in older adults after ED discharge. The present review suggests the use of a validated risk prediction tool to stratify patients into high‐ and low‐risk groups could lead to improved patient outcomes. Furthermore, interventions that extend beyond a simple referral might reduce rates of adverse outcomes after ED discharge and should be considered in future intervention design.
The present study had limitations. First, only English‐language articles were evaluated. As well, it is possible that our specific search criteria did not identify all relevant studies; however, even with our intention of developing a highly focused review, we did use a very broad set of search criteria and identified titles outside of our scope of interest. As with all systematic reviews, publication bias (or the likelihood to publish interventions with positive outcomes) might have occurred.
Disclosure statement
No potential conflicts of interest were disclosed.
Acknowledgments
This research was funded by two operating grants (Transitions Across the Health Care System and Risk of Rehospitalization in Older Women and Men, MOP 119330 and Emergency Department Visits by Nursing Home Residents in Ontario: Descriptive Patterns of Resident Visits by Timing, Location, and Physician Availability, MOP 89943) from the CIHR Institute of Health Services and Policy Research. Dr Gruneir is partially funded by a Team Grant from the CIHR Institute of Health Research (Pharmacologic Management of Chronic Disease in Older Adults – II, FRN OTG‐88591). The funder played no role in the design, analysis or writing of this manuscript.
References
- 1. Chan BS, Schull MJ, Schultz SE. Atlas Report – Emergency Department Services in Ontario 1993–2000. Toronto, ON: ICES, 2001; 54. [Google Scholar]
- 2. Kaskie B, Obrizan M, Jones MP et al Older adults who persistently present to the emergency department with severe, non‐severe, and indeterminate episode patterns. BMC Geriatr 2011; 11: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCaig LF, Burt CW. National Hospital Ambulatory Medical Care Survey: 2002 emergency department summary. Adv Data 2004; 340: 1–34. [PubMed] [Google Scholar]
- 4. Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002; 39: 238–247. [DOI] [PubMed] [Google Scholar]
- 5. Canadian Institute for Health Information . Health Care in Canada, 2011: a Focus on Seniors and Aging. Ottawa, ON: CIHI, 2001; 162. [Google Scholar]
- 6. McCusker J, Cardin S, Bellavance F, Belzile E. Return to the emergency department among elders: patterns and predictors. Acad Emerg Med 2000; 7: 249–259. [DOI] [PubMed] [Google Scholar]
- 7. Richardson DB. Elderly patients in the emergency department: a prospective study of characteristics and outcome. Med J Aust 1992; 157: 234–239. [DOI] [PubMed] [Google Scholar]
- 8. Hastings SN, Oddone EZ, Fillenbaum G, Sloane RJ, Schmader KE. Frequency and predictors of adverse health outcomes in older Medicare beneficiaries discharged from the emergency department. Med Care 2008; 46: 771–777. [DOI] [PubMed] [Google Scholar]
- 9. Rosted E, Wagner L, Hendriksen C, Poulsen I. Geriatric nursing assessment and intervention in an emergency department: a pilot study. Int J Older People Nurs 2012; 7: 141–151. [DOI] [PubMed] [Google Scholar]
- 10. Adams JG, Gerson LW. A new model for emergency care of geriatric patients. Acad Emerg Med 2003; 10: 271–274. [DOI] [PubMed] [Google Scholar]
- 11. Gruneir A, Silver MJ, Rochon PA. Emergency department use by older adults: a literature review on trends, appropriateness, and consequences of unmet health care needs. Med Care Res Rev 2011; 68: 131–155. [DOI] [PubMed] [Google Scholar]
- 12. Schumacher JG. Emergency medicine and older adults: continuing challenges and opportunities. Am J Emerg Med 2005; 23: 556–560. [DOI] [PubMed] [Google Scholar]
- 13. Hwang U, Morrison RS. The geriatric emergency department. J Am Geriatr Soc 2007; 55: 1873–1876. [DOI] [PubMed] [Google Scholar]
- 14. Lowenstein SR, Crescenzi CA, Kern DC, Steel K. Care of the elderly in the emergency department. Ann Emerg Med 1986; 15: 528–535. [DOI] [PubMed] [Google Scholar]
- 15. Foo CL, Siu VW, Tan TL, Ding YY, Seow E. Geriatric assessment and intervention in an emergency department observation unit reduced re‐attendance and hospitalisation rates. Australas J Ageing 2012; 31: 40–46. [DOI] [PubMed] [Google Scholar]
- 16. Hampton T. Experts predict visits by baby boomers will soon strain emergency departments. JAMA 2008; 299: 2613–2614. [DOI] [PubMed] [Google Scholar]
- 17. Sinha SK, Bessman ES, Flomenbaum N, Leff B. A systematic review and qualitative analysis to inform the development of a new emergency department‐based geriatric case management model. Ann Emerg Med 2011; 57: 672–682. [DOI] [PubMed] [Google Scholar]
- 18. Turcotte M, Schellenberg G. A Portrait of Seniors in Canada. Ottawa, ON: Statistics Canada, 2006; 301. [Google Scholar]
- 19. Fealy G, McCarron M, O'Neill D et al Effectiveness of gerontologically informed nursing assessment and referral interventions for older persons attending the emergency department: systematic review. J Adv Nurs 2009; 65: 934–935. [DOI] [PubMed] [Google Scholar]
- 20. Hastings SN, Heflin MT. A systematic review of interventions to improve outcomes for elders discharged from the emergency department. Acad Emerg Med 2005; 12: 978–986. [DOI] [PubMed] [Google Scholar]
- 21. McCusker J, Verdon J. Do geriatric interventions reduce emergency department visits? A systematic review. J Gerontol A Biol Sci Med Sci 2006; 61: 53–62. [DOI] [PubMed] [Google Scholar]
- 22. Basic D, Conforti DA. A prospective, randomised controlled trial of an aged care nurse intervention within the Emergency Department. Aust Health Rev 2005; 29: 51–59. [DOI] [PubMed] [Google Scholar]
- 23. McCoy HV, Kipp CW, Ahern M. Reducing older patients' reliance on the emergency department. Soc Work Health Care 1992; 17: 23–37. [DOI] [PubMed] [Google Scholar]
- 24. McCusker J, Dendukuri N, Tousignant P, Verdon J, Poulin de Courval L, Belzile E. Rapid two‐stage emergency department intervention for seniors: impact on continuity of care. Acad Emerg Med 2003; 10: 233–243. [DOI] [PubMed] [Google Scholar]
- 25. Hickman L, Newton P, Halcomb EJ, Chang E, Davidson P. Best practice interventions to improve the management of older people in acute care settings: a literature review. J Adv Nurs 2007; 60: 113–126. [DOI] [PubMed] [Google Scholar]
- 26. Conroy SP, Stevens T, Parker SG, Gladman JR. A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: ‘interface geriatrics’. Age Ageing 2011; 40: 436–443. [DOI] [PubMed] [Google Scholar]
- 27. Graf CE, Zekry D, Giannelli S, Michel JP, Chevalley T. Efficiency and applicability of comprehensive geriatric assessment in the emergency department: a systematic review. Aging Clin Exp Res 2011; 23: 244–254. [DOI] [PubMed] [Google Scholar]
- 28. Miller DK, Lewis LM, Nork MJ, Morley JE. Controlled trial of a geriatric case‐finding and liaison service in an emergency department. J Am Geriatr Soc 1996; 44: 513–520. [DOI] [PubMed] [Google Scholar]
- 29. Ballabio C, Bergamaschini L, Mauri S et al A comprehensive evaluation of elderly people discharged from an Emergency Department. Intern Emerg Med 2008; 3: 245–249. [DOI] [PubMed] [Google Scholar]
- 30. Bird SR, Kurowski W, Dickman GK, Kronborg I. Integrated care facilitation for older patients with complex health care needs reduces hospital demand. Aust Health Rev 2007; 31: 451–461, discussion 449–450. [DOI] [PubMed] [Google Scholar]
- 31. Caplan GA, Williams AJ, Daly B, Abraham K. A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department – the DEED II study. J Am Geriatr Soc 2004; 52: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 32. Guttman A, Afilalo M, Guttman R et al An emergency department‐based nurse discharge coordinator for elder patients: does it make a difference? Acad Emerg Med 2004; 11: 1318–1327. [DOI] [PubMed] [Google Scholar]
- 33. Hegney D, Buikstra E, Chamberlain C et al Nurse discharge planning in the emergency department: a Toowoomba, Australia, study. J Clin Nurs 2006; 15: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 34. Lee JS, Hurley MJ, Carew D, Fisher R, Kiss A, Drummond N. A randomized clinical trial to assess the impact on an emergency response system on anxiety and health care use among older emergency patients after a fall. Acad Emerg Med 2007; 14: 301–308. [DOI] [PubMed] [Google Scholar]
- 35. Mion LC, Palmer RM, Meldon SW et al Case finding and referral model for emergency department elders: a randomized clinical trial. Ann Emerg Med 2003; 41: 57–68. [DOI] [PubMed] [Google Scholar]
- 36. Moss JE, Flower CL, Houghton LM, Moss DL, Nielsen DA, Taylor DM. A multidisciplinary Care Coordination Team improves emergency department discharge planning practice. Med J Aust 2002; 177: 435–439. [DOI] [PubMed] [Google Scholar]
- 37. Mion LC, Palmer RM, Anetzberger GJ, Meldon SW. Establishing a case‐finding and referral system for at‐risk older individuals in the emergency department setting: the SIGNET model. J Am Geriatr Soc 2001; 49: 1379–1386. [DOI] [PubMed] [Google Scholar]
- 38. McCusker J, Verdon J, Tousignant P, de Courval LP, Dendukuri N, Belzile E. Rapid emergency department intervention for older people reduces risk of functional decline: results of a multicenter randomized trial. J Am Geriatr Soc 2001; 49: 1272–1281. [DOI] [PubMed] [Google Scholar]
- 39. Salvi F, Morichi V, Lorenzetti B et al Risk stratification of older patients in the emergency department: comparison between the Identification of Seniors at Risk and Triage Risk Screening Tool. Rejuvenation Res 2012; 15: 288–294. [DOI] [PubMed] [Google Scholar]
- 40. Thomas S and Associates . Report of the Development of a Risk Screening Tool for Services Needs Following Discharge From Acute Care. Melbourne, Vic: Department of Human Services, 1998; 75. [Google Scholar]
- 41. Hastings SN, Barrett A, Weinberger M et al Older patients' understanding of emergency department discharge information and its relationship with adverse outcomes. J Patient Saf 2011; 7: 19–25. [DOI] [PubMed] [Google Scholar]
- 42. Salvi F, Morichi V, Grilli A, Giorgi R, De Tommaso G, Dessi‐Fulgheri P. The elderly in the emergency department: a critical review of problems and solutions. Intern Emerg Med 2007; 2: 292–301. [DOI] [PubMed] [Google Scholar]
- 43. Beland F, Bergman H, Lebel P, Dallaire L. Integrated services for frail elders (SIPA): a trial of a model for Canada. Can J Aging 2006; 25: 5–42. [PubMed] [Google Scholar]
- 44. Kodner DL. Whole‐system approaches to health and social care partnerships for the frail elderly: an exploration of North American models and lessons. Health Soc Care Community 2006; 14: 384–390. [DOI] [PubMed] [Google Scholar]
- 45. Glendinning C. Breaking down barriers: integrating health and care services for older people in England. Health Policy (New York) 2003; 65: 139–151. [DOI] [PubMed] [Google Scholar]


