Abstract
Problem
Preeclampsia affects 3–17% of pregnancies worldwide and has serious consequences for both the mother and the fetus. As maternal–fetal immune tolerance is bidirectional, fetal immunopathology may play a significant role in the pathogenesis of pregnancy disorders. Nevertheless, the impact of preeclampsia on the fetal immune system is unclear.
Method of study
In this case–control study, we examined the phenotype of innate and adaptive immune cells from the cord blood of 3rd trimester babies born to healthy mothers and compared them to cord blood from 3rd trimester babies born to mothers with symptomatic preeclampsia.
Results
The ratio of CD56hi CD16− non‐activated/regulatory NK cells to CD56lo CD16+ activated/effector NK cells as well as the proportion of CD4+ T cells was significantly decreased in the cord blood of babies born to preeclamptic mothers. The percentage of FoxP3+ Treg, especially the FoxP3lo populations (resting Treg and cytokine Treg), were significantly reduced. Importantly, this reduction in FoxP3+ Treg affected the ratio of CD8+ effector T cells per FoxP3+ Treg in the cord blood of babies born to preeclamptic mothers.
Conclusion
These observations indicate that there are significant fetal immune system derangements during preeclampsia.
Keywords: fetus, preeclampsia, pregnancy, regulatory T cells
Introduction
The fetus and the mother are immunologically incompatible in nearly every pregnancy due to differences in transplantation antigens inherited from the father as well as non‐inherited maternal antigens.1, 2, 3 To cope with these significant antigenic differences, mechanisms of immune tolerance are invoked during pregnancy.2, 4, 5 Among these are the actions of the powerful T regulatory (Treg) cell that has the ability to negatively regulate major portions of the cellular immune system.6 During pregnancy, the mother develops Treg specificity for fetal antigens and, similarly, the fetus develops Tregs with specificity for non‐inherited maternal antigens.1, 2, 3 During preeclampsia, there is significant evidence demonstrating markers of maternal immune activation as part of the disease.7 In contrast, investigations of the fetal immune response during preeclampsia are limited with no published studies regarding fetal Treg changes in preeclampsia.
Despite the high incidence of preeclampsia and the significant morbidity and mortality associated with it that includes hypertension, organ failure, progression to seizures (eclampsia), prematurity, and death, the underlying cause is still a mystery.8 To date, there is limited information about the impact of maternal preeclampsia on the fetal immune system. To address the hypothesis that preeclampsia impacts the fetal immune system, we perform a case–control study analyzing cord blood lymphocytes from healthy babies born to healthy women in comparison with cord blood lymphocytes from healthy babies born to women with symptomatic preeclampsia. We enumerate and phenotypically characterize monocytes, natural killer (NK) cells, T cells, and regulatory T cells (Treg). In this process, we confirm prior observations regarding NK activation and add depth regarding important changes to Tregs during preeclampsia, highlighting that the fetal immune response also shows signs of deregulation.
Materials and methods
Ethics Statement
Human subjects were recruited for participation under an IRB approved protocol (University of California, Los Angeles, Office of Human Research Protection Program, Medical IRB Committee‐1 #11‐003962) providing written informed consent prior to enrollment.
Human Subjects
Healthy and preeclamptic women with otherwise uncomplicated, 3rd trimester, singleton pregnancies were recruited for participation between April 2013 and December 2014. Demographic and obstetrical characteristics from the recruited population are provided in Tables 1 and 2.
Table 1.
Subject Demographics
| Age | Healthy (n = 16) | Preeclampsia (n = 9) | P |
|---|---|---|---|
| Range | 19–39 | 20–40 | 0.5789 |
| Average | 31.1 ± 1.7 | 29.4 ± 2.6 | |
| Median | 32 | 29 | |
| Ethnicity | |||
| Caucasian | 62% (8) | 12.5% (1) | 0.1566 |
| Hispanic | 23% (3) | 37.5% (3) | |
| Black | 8% (1) | 25% (2) | |
| Asian | 8% (1) | 25% (2) | |
| Gravidity | 2.3 ± 0.4 | 1.4 ± 0.3 | 0.1186 |
| Parity | 0.6 ± 0.1 | 0.1 ± 0.1 | 0.0272 |
| Smoker | 8% (1) | 0% | |
| Comorbidities | |||
| Advanced maternal age | 41% (12) | 25% (2) | 0.6065 |
| IVF | 0% | 12.5% (1) | 0.3810 |
| Hypothyroidism | 23% (3) | 0% | 0.2571 |
Age, gravidity, and parity differences analyzed with an unpaired, parametric t‐test. Ethnicity analyzed using a chi‐squared test. Comorbidities (each individual) were analyzed using a Fisher's exact test.
Table 2.
Subject Obstetrical Characteristics
| Healthy | Preeclampsia | P | |
|---|---|---|---|
| Maximum systolic blood pressure | 124.0 ± 12.6 | 163.8 ± 12.6 | <0.0001 |
| Mode of delivery | |||
| Vaginal | 19% (3) | 37.5% (3) | 0.3618 |
| Cesarean | 81% (13) | 62.5% (5) | |
| Fetal | |||
| Gestational age (weeks) | 39.2 ± 0.8 | 39.1 ± 2.4 | 0.2070 |
| Average birthweight (g) | 3352 ± 138.4 | 3379 ± 207.8 | 0.9112 |
| APGAR‐1 | 8.6 ± 0.2 | 8.3 ± 0.3 | 0.2894 |
| APGAR‐5 | 8.8 ± 0.1 | 9.0 ± 0.0 | 0.2655 |
Normality was assessed using Shapiro–Wilk test. Maximum systolic blood pressure differences were analyzed using a two‐tailed unpaired student's t‐test. Mode of delivery differences were analyzed using a two‐tailed Fisher's exact test. Gestational age differences were analyzed using Mann–Whitney U‐test. Fetal birthweight and APGAR score differences analyzed using an unpaired, parametric t‐test.
Tissue Collection
Cord blood was collected after delivery of the fetus, but prior to placental separation, into a sterile cord blood collection kit containing citrate phosphate dextrose solution (Medsep Corporation, Covina, CA, USA).
Lymphocyte Purification
Granulocytes were depleted utilizing the RosetteSep Granulocyte Depletion Cocktail (Stemcell Technologies, Vancouver, BC, Canada) following the manufacturer's recommendations; 1 mL of cocktail was used per 30 mL cord blood/anticoagulant citrate phosphate dextrose solution from the sterile cord blood collection unit. Granulocyte‐depleted mononuclear cells were isolated by gradient centrifugation over Ficoll‐Paque PLUS from GE Healthcare (Uppsala, Sweden) following the manufacturer's recommendations. Cells were washed twice with sterile PBS and enumerated utilizing an Accuri flow cytometer with propidium iodide exclusion of dead cells.
Phenotypic Analysis via Multicolor Flow Cytometry
Immediately after cell purification, 1 × 106 cells were stained with Fixable Viability Dye eF780 (eBioscience, San Diego, CA, USA) in 200 μL PBS in a 96‐well U‐bottom plate. The cells were pelleted and supernatant removed. Antibodies against surface antigens were added in 100 μL PBS and 1% FBS at the optimal concentrations determined by previous titration (Table 3) and incubated for 15 min at room temperature in the dark. Following two washes with PBS and 1% FBS, intranuclear FoxP3 staining was performed utilizing the FoxP3/Transcription Factor Staining Kit (eBioscience) per manufacturer's instructions including the recommended 15‐min incubation with mouse serum prior to intranuclear staining. Cells were resuspended in PBS and 1% FBS, transferred to FACS tubes, and analyzed within 6 hrs. Either isotype staining or Fluorescence Minus One (FMO) stains were performed. For isotype control staining, the appropriate antibody isotype or, if unavailable, a similar isotype coupled to the same fluorophore was used at the same concentration as the antibody. Histogram gating was performed using the isotype staining as a guide to set the gates. For FMO controls, all antibodies minus one (FoxP3) were stained.9 Analysis was performed on a BD SORP LSR II analytic flow cytometer with post‐acquisition analysis performed with flowjo (Treestar, Palo Alto, CA, USA).
Table 3.
Antibodies Used for Antigen Detection
| Antigen | Source | Clone | ng/test | Fluorochrome | Excitation | Long‐pass Dichroic Mirror | Bandpass filter |
|---|---|---|---|---|---|---|---|
| CD45RO | Biolegend | UCHL1 | 2500 | Brilliant violet 421 or Pacific Blue | 405 nm | Blank | 450/50 |
| CD4 | Biolegend | OKT4 | 150 | Brilliant violet 510 | 405 nm | 505LP | 525/50 |
| CD56 | Biolegend | HCD56 | 500 | Brilliant violet 510 | 405 nm | 505LP | 525/50 |
| CD16 | Biolegend | 3G8 | 500 | Brilliant violet 605 | 405 nm | 595LP | 605/40 |
| HLA‐DR | Biolegend | L243 | 180 | Brilliant violet 711 | 405 nm | 685LP | 710/50 |
| CD16 | Biolegend | 3G8 | 600 | Brilliant violet 785 | 405 nm | 750LP | 780/60 |
| CD45RA | Biolegend | HI100 | 400 | AF488 | 488 nm | 505LP | 530/30 |
| CD3 | eBioscience | UCHT1 | 500 | PerCP‐Cy5.5 | 488 nm | 685LP | 695/40 |
| CD8 | Biolegend | HIT8a | 2500 | AF700 | 640 nm | 685LP | 710/50 |
| Viability marker | eBioscience | Cat# 65‐0865 | 1:1000 | eF780 | 640 nm | 755LP | 780/60 |
| FoxP3 | BD | 259D/C7 | 500 | PE | 561 nm | Blank | 582/15 |
Intranuclear antigens are noted in italics.
Statistical Analysis
Differences in normally distributed populations were statistically analyzed using unpaired Student's t‐test, chi‐square test, or Fisher's exact test as indicated. Analysis was accomplished with prism (Graphpad, La Jolla, CA, USA).
Results
Effector NK (CD56lo CD16+) to Regulatory NK (CD56hi CD16+) Ratio is Significantly Higher in the Cord Blood of Babies Born to Preeclamptic Mothers
Innate immune cells are sensitive to changes in the inflammatory milieu.10, 11 During preeclampsia, the mother experiences substantial physical alterations such as high blood pressure, serum inflammatory cytokine elevations, and endothelial dysfunction. To inquire whether these changes in the mother are accompanied by alterations in the fetal immune system, we performed a case–control study that analyzed cord blood samples from healthy babies born to preeclamptic mothers for different immune parameters and compared them to cord blood samples from healthy babies born to healthy mothers. For analysis of monocytes, granular (SSC‐H hi) HLA‐DR+ cells were identified (Fig. 1a) and live singlets were isolated in three more gating steps (Fig. 1b). The proportion of the CD3− negative portion of the resulting population on the total live lymphocyte population (as gated in Fig. 1e) was then calculated. Assessment of monocytes in the cord blood from healthy and preeclamptic mothers did not reveal any quantitative differences (Fig. 1d). NK cells have disparate phenotypes according to their activation status and functionality; one model suggests that CD56hi CD16− NK cells are non‐activated NK cells that can even exert regulatory functions (IL‐10 production), CD56hi CD16± cells are recently activated cells in transition toward the fully activated effector phenotype (CD56lo CD16+).12, 13, 14 In adherence to this model, we quantified the three different NK cell subtypes CD56hi CD16− NK cells, transitioning NK cells (CD56int CD16int), and effector NK (CD56lo CD16+) also did not reveal any statistically significant differences between the two patient groups (Fig. 1e–h). A skewed numeric relationship between effector NK cells and CD56hi CD16− NK cells has been observed in several human pathological conditions.12, 15 Calculating the relationship between the two populations reveals that babies born to preeclamptic mothers have significantly more effector NK cells per CD56hi CD16− NK cell in their cord blood than babies born to healthy mothers (Fig. 1i).
Figure 1.
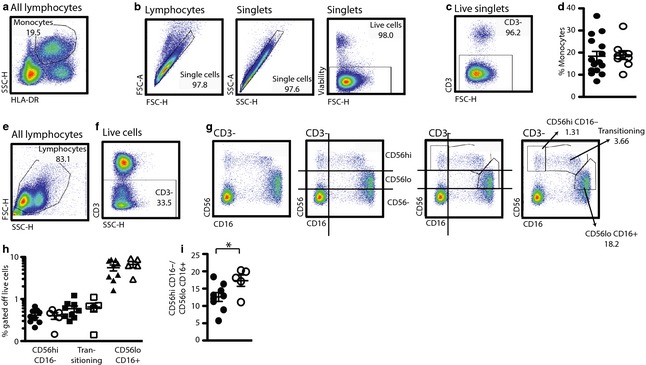
The NK cell profile of babies born to preeclamptic women is shifted toward CD56loCD16+ effector NK cells. Fresh granulocyte reduced cord blood samples from babies born to healthy 3rd trimester pregnant women and preeclamptic women. (a–d) Gating strategy for the identification of monocytes. (a) Starting with a side scatter versus HLA‐DR blot, granular HLA‐DR+ cells are identified and (b) subjected to live singlet identification gating steps. (c) CD3− lymphocytes are identified. (d) The monocyte population percentage off total live cells is calculated. (e–g) Gating strategy for the identification of NK cells. (e) Starting with all lymphocytes, a lymphocyte subgate is placed and live singlets are identified as in (b). (f) Then, CD3− cells are identified. (g) Depiction of the same CD3− population in four different blots. Guidance for the gate placement is provided by the CD56‐CD16− population that allows for distinguishing CD16‐negative and CD16‐positive populations as well as the three expression levels of CD56: negative, lo and hi. Based on these guidelines, CD56hiCD16−, transitioning and CD56loCD16+ NK cells are identified (right hand side dot blot). (h+i) Closed symbols: babies born to healthy mothers, open symbols: babies born to preeclamptic mothers. (h) Percentages of the different NK cell populations calculated off the total live lymphocytes. (i) CD56loCD16+ NK cells per CD56hiCD16− NK cells. (d, h, i) Student's t‐test, unpaired. *P = 0.0347.
CD4/CD8 T‐cell Ratio is Significantly Lower in Babies Born to Preeclamptic Mothers
To assess potential consequences of maternal preeclampsia on the fetal adaptive immune system, we enumerated total CD4+ and CD8+ T‐cell populations. This revealed a significant decrease of the CD4+ (healthy: 73.47 ± 4.34%, preeclamptic 67.95 ± 5.82%, P = 0.0302) and a non‐significant increase of the CD8+ populations (healthy: 21.62 ± 4.03, preeclamptic 24.28 ± 8.1, P = 0.2032) in cord blood lymphocytes from babies born to preeclamptic mothers (Fig. 2a,b). Naïve and memory T cells were identified by visualizing CD45RA (naïve) and CD45RO (memory)‐ protein expression within the different T‐cell populations. To exclusively study non‐Treg populations, we excluded the FoxP3+ T cells from this particular analysis of the CD4 T cells and instead started with the FoxP3− CD4+ population (Fig. 2c,d). This gating strategy revealed an incomplete discriminatory but exclusively positive (Fig. 2d) expression pattern of these two isoforms. To account for this fact, we employed a two‐step histogram gating strategy that identifies CD45RA+ T cells (Fig. 2e) but excludes CD45RO+ T cells from this population (Fig. 2e, right hand side histogram, CD45RA+CD45RO−, henceforth CD45RA+). Similarly, to identify cells that exclusively express CD45RO, CD45RA+ cells were excluded (Fig. 2f, right hand side histogram, CD45RO+ CD45RA−, henceforth CD45RO+). Cells expressing both CD45RA and CD45RO were identified in the same histogram (Fig. 2f, right hand side histogram, CD45RA+/RO+) as we have previously outlined for maternal samples.16 Comparison of the proportion of naïve, memory, and CD45RO+RA+ cells between children born to healthy or preeclamptic women did not reveal a statistically significant difference (Fig. 2g). Further, we quantified the expression of HLA‐DR as a marker of activation by cells of the different T‐cell subtypes and did not detect any statistically significant differences between the two patient groups (Fig. 2h).17
Figure 2.
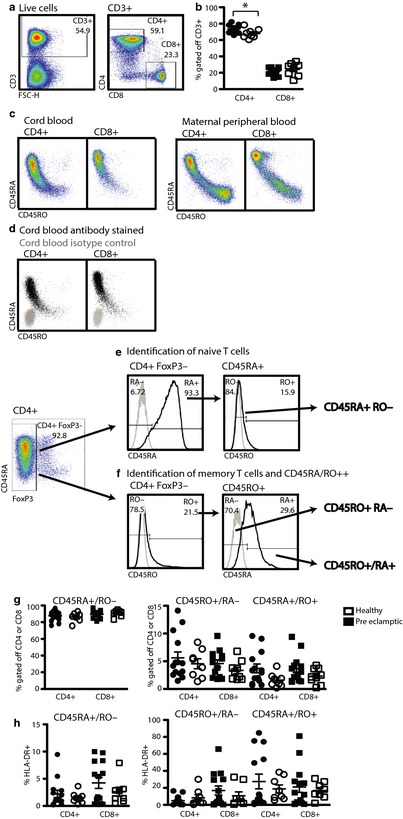
CD4+ T cells in the cord blood of babies born to preeclamptic women are significantly reduced. Fresh cord blood samples from babies born to healthy 3rd trimester pregnant women and preeclamptic women were processed and analyzed as described in Materials and methods. (a) Gating strategy for the identification of CD4+ and CD8+ T cells. Starting with live lymphocytes as in Fig. 1e, live singlets are identified as in Fig. 1b. Gating for CD4+ and CD8+ T cells is depicted in the right hand side dot blot. (b) The percentage of CD4+ or CD8+ T cells within the total CD3+ population. Closed symbols: babies born to healthy mothers, open symbols: babies born to preeclamptic mothers. (c) Dot blot depiction of CD45RA and RO in CD4+ and CD8+ T cells of cord blood (left hand side) and maternal peripheral blood right hand side) (d) Dot blot overlay of antibody stained (black) or isotype control stained (gray) T‐cell populations (same sample as in 2c). (e+f) Gating strategy for the identification of three T‐cell subpopulations with distinct history. For the CD4+ population, only FoxP3‐negative T cells are analyzed. (e) CD45RA+ T cells are identified using a CD4+ T‐cell population stained with isotype control antibody (gray line, left hand side) to set a histogram gate including all CD45RA‐ antibody stained cells (black line, left hand side). Within this CD45RA+ population, CD45RO− T cells are identified using isotype control antibody stained cells (gray line, right hand side) to identify and exclude CD45RO− antibody stained cells (black line, right hand side). (f) Starting with the same population and similarly as in (e), CD45RO+ cells are defined as CD45RO+ in a histogram overlay of the isotype control stained cells (gray line, left hand side) with cells stained with CD45RO (black line, left hand side). Cells expressing CD45RO are analyzed for the absence or presence of CD45RA dividing them into two populations (right hand side). Based on the isotype control stained cells (gray line, right hand side), absence of CD45RA identifies CD45RO+ cells, while presence of CD45RA indicates CD45RO+/RA+ cells. (g) Percentage of the live cells identified as CD45RA+, CD45RO+, and CD45RA+/RO+ gated off the total CD4+FoxP3− or CD8+ population. (h) Percentage of HLA‐DR+ cells within the indicated populations. Student's t‐test, unpaired *P < 0.0174.
The Proportion of Tregs is Significantly Lower in the Cord Blood of Babies Born to Preeclamptic Mothers
To identify regulatory FoxP3+ T cells, Fluorescence Minus One (FMO)‐isotype staining was used for gate placement (Fig. 3a). First, the percentage of the total population of CD4+FoxP3+ T cells was assessed revealing a significant reduction of Tregs in the cord blood of babies born to preeclamptic mothers (Fig. 3c). Adult Tregs are now appreciated to have differential functionality that is characterized by the degree of FoxP3− and CD45RA expression.18 Subquantification of the FoxP3low and FoxP3hi fractions revealed a significant reduction in the FoxP3low, but not in the FoxP3hi fraction in cord blood from babies born to preeclamptic mothers (Fig. 3b,c).
Figure 3.
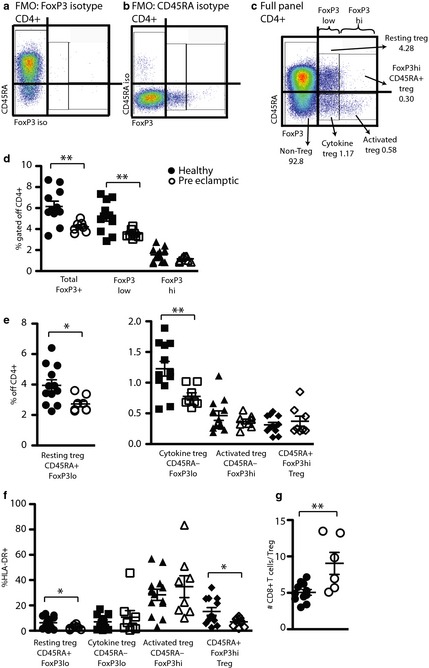
The proportion of Regulatory T cells is significantly reduced in babies born to preeclamptic women. (a–c) Gating strategy for Treg identification. Starting with live lymphocyte singlets as in Fig. 1b and e, Fluorescence Minus One (FMO)‐isotype control stainings are performed to aid gate placement. (a) Full antibody panel minus FoxP3 antibody staining. (b) Full antibody and FoxP3 staining minus CD45RA‐ staining. (c) Full panel staining. (d) Proportion of total FoxP3+ Treg gated off the total CD4+ populations. (e) % of four Treg subtypes gated off the total CD4+ populations in cord blood. (f) % of HLA‐DR+ Treg within the indicated subpopulations. (g) Number of CD8+ T cells divided by total Treg as determined in (d). Statistics in (d–g) Student's t‐test, unpaired. *P < 0.0487, **P < 0.0091.
Resting and Cytokine Tregs are Significantly Reduced in Babies Born to Preeclamptic Mothers
Sakaguchi et al.18 developed a simple staining and gating strategy that allows for the identification of three Treg subtypes with distinct biological features distinguished by the surface expression of CD45RA in combination with the intranuclear expression of FoxP3 (Table 4). These three subtypes, resting Treg (CD45RAhi FoxP3lo), cytokine Treg (CD45RA− FoxP3lo), and activated Treg (CD45RA− FoxP3hi) differ in their proliferation potential (resting Treg > cytokine Treg > activated Treg) and in their suppression potential (activated Treg > cytokine Treg > resting Treg). Application of this gating strategy (Fig. 3a–c) to lymphocyte specimens from cord blood of babies born to healthy or preeclamptic women illustrated significant reduction of the percentages of resting Tregs and cytokine Tregs (Fig. 3d). Additionally, we observed a population of Tregs that does express CD45RA but also expresses high levels of FoxP3 (FoxP3hi CD45RA+ Treg, Fig. 3c). This unique fetal population (CD45RA+FoxP3hi healthy: 0.3 ± 0.14%, preeclamptic 0.37 ± 0.23%, P = 0.4778) was present with a similar frequency as the activated Treg population (CD45RA− FoxP3+ healthy: 0.46 ± 0.27%, preeclamptic: 0.37 ± 0.11, P = 0.3519). HLA‐DR expression identifies highly suppressive Tregs in adults, and for that reason, we analyzed the expression of this molecule on the surface of the Treg subtypes in both subject groups (Fig. 3d).19 As expected, we found the highest proportion of HLA‐DR+ FoxP3+ cells in the population of activated Treg (HLA‐DR+ FoxP3hi CD45RA− healthy: 28.42 ± 16.06%, preeclamptic: 34.9 ± 24.39%, P = 0.4696, Fig. 3d). The expression of HLA‐DR was significantly reduced in the uniquely fetal CD45RA+ FoxP3hi population of in cord blood of babies born to preeclamptic mothers compared to babies born to healthy mothers (HLA‐DR+ CD45RA+ FoxP3hi healthy: 15.29 ± 10.52%, preeclamptic: 7.05 ± 3.68%, P = 0.0487).
Table 4.
Treg Subset Identification Strategy18
| FoxP3 | CD45RA | |
|---|---|---|
| Resting Treg | Low | Positive |
| Cytokine Treg | Low | Negative |
| Activated | High | Negative |
CD8+ Responder T‐cell to Treg Ratio is Significantly Increased in Cord Blood of Babies Born to Preeclamptic Mothers
To date, the precise mechanism by which Tregs limit effector immune responses is incompletely understood, but the importance of a numeric relationship between responder cells and suppressor cells is apparent.6 To assess this numeric relationship, we calculated the number of CD8+ responder cells per Treg in both patient groups (Fig. 3e). We found a significantly higher number of CD8+ responder T cells per Treg in children born to preeclamptic mothers (healthy 5.06 ± 1.29 CD8+ T cells/Treg, preeclamptic 9.0 ± 3.7 CD8+ T cells/Treg, P = 0.0050).
Discussion
Our analysis of the fetal immune response during preeclampsia confirms previous reports of NK cell activation.20 Furthermore, in this comprehensive analysis of lymphocyte changes, we find that there is a shift toward a greater percentage of CD8 cells compared with CD4 cells, an observation that has been previously reported for small for gestational age (SGA) fetuses.21 Lastly, we find that there is evidence of a deficient fetal Treg response during preeclampsia resulting in a skewed relationship between regulatory and effector elements of the adaptive immune system.
To date, there are few studies examining the consequences of maternal preeclampsia on the fetal immune system. Increased inflammation and activation of the Alternative Complement Pathway in cord blood of babies born to preeclamptic mothers have been observed suggesting that maternal preeclampsia does indeed have consequences for the fetal immune system.22, 23 Bujold et al.20 reported a higher percentage of NK cells in cord blood of babies born to preeclamptic mothers compared to those born to healthy mothers. While we observe a similar trend, our results do not reach statistical significance likely due to the small sample size in our study. Identification of individual NK cell populations, according to the respective model CD56hi CD16−, transitioning, and effector NK cells (CD56lo CD16+), reveals that the effector NK population is expanded resulting in a significant skewing of the ratio of CD56hi CD16− to‐ effector NK cells. This observation is in line with previous reports of increased expression of NK activation receptors during preeclampsia typically present on effector NK cells.24 These data are especially important in light of the observations in human autoimmune disorders (uveitis and multiple sclerosis) treated with either combinations of IFN‐β and declizumab (monoclonal antibody against the human IL‐2 receptor α chain) or declizumab alone.25, 26 The authors found the CD56hi CD16− NK cell population expanding and a reduction of disease symptoms. Uveitis patient NK cells produced IL‐10, while multiple sclerosis patients’ NK cells inhibited T‐cell proliferation in vitro suggesting that this population exerted an anti‐inflammatory effect.
The CD4/CD8 T‐cell ratio is an important marker of many human diseases most prominently human immunodeficiency virus infection, but also idiopathic aplastic anemia, leprosy, sarcoidosis, and others with an inverted ratio correlating to increased inflammation.27, 28, 29, 30 In normal fetuses, the CD4/CD4 ratio is comparable to adults, while in the cord blood of babies affected by fetal growth restriction, higher CD4/CD8 ratios have been observed.21, 31 In line with this observation, we found increased CD4/CD8 T‐cell ratios in the cord blood of fetuses born to preeclamptic mothers indicating increased inflammation.
Fetal T cells have the capability to be activated in utero. Fetal adaptive immune responses are dominated by the generation of a Treg response, for example, against non‐inherited maternal antigens present on immigrating maternal cells.1, 32 Cord blood T cells from babies born under the circumstances of preterm labor or chorioamnionitis are characterized by increased activation (CD25, HLA‐DR, and CD69‐expression).33 In contrast, our observations with cord blood T cells from babies born to preeclamptic mothers show no additional activation over those born to healthy mothers. Additionally, Luciano et al. describe an unexpected increased frequency of memory (CD45RO+) T cells in preterm neonates with near‐adult phenotype reflecting long‐standing immune activation thought not to occur during fetal life. Whether these expanded CD45RO+ T cells were Treg was not explored by Luciano et al.33, 34 We do not observe an increase in fetal CD4 memory effector (FoxP3−) or regulatory (FoxP3+) T cells during preeclampsia. In this regard, preeclampsia and preterm labor are phenotypically diverged. In the maternal compartment, innate and adaptive immune activation is a feature of both preterm labor and preeclampsia. In contrast, in the cord blood of babies born to preeclamptic mothers, we find a depressed immunoregulatory response without accompanying activation or progression to memory phenotype while those born under preterm labor circumstances show immune activation in that compartment.
Maternal Tregs have been studied in the context of healthy and pathologic pregnancies.2, 4, 5, 35 In contrast, very little is known about a potential role of fetal Tregs in pregnancy disorders. Fetuses who are small for gestational age display alterations in their Treg compartments with lower percentages of Tregs and FoxP3−protein levels than appropriate for gestational age (AGA) fetuses suggesting a reciprocal relationship between fetal pathology and fetal Treg compartments.36 Expanding on this observation, Xiong et al.21 reported increased kill activity in cord blood mononuclear cells from SGA fetuses compared with AGA fetuses. In the pathological condition of preeclampsia, we find a significant reduction in the total fetal FoxP3+ population that is mostly restricted to the FoxP3 low population as found in SGA.36 Our analysis includes Treg subtype determination using CD45RA versus FoxP3 in which we find that the reduction in FoxP3 low includes both the resting Treg and cytokine Treg, an analysis that cannot be compared to the results by Steinborn et al.
In adults, subtyping of Tregs with a CD45‐RA versus FoxP3 blot identifies three Treg populations (resting, cytokine, and activated Treg) but does not include a CD45RA+ FoxP3hi population as that gate only includes negligible numbers of cells (personal observation).18 In contrast to adults, this gate identifies a significant population in cord blood that is therefore unique to fetuses (Fig. 3). Interestingly, we find that the CD45RA+ FoxP3hi Treg population in the cord blood of babies born to healthy mothers includes a population that expresses the activation marker HLA‐DR to a comparable proportion as in activated Treg in the same sample. Possibly, these cells are freshly stimulated Tregs that in time will lose the naïve marker CD45RA as they become activated Tregs. The Treg population unique to fetuses (CD45RA+ FoxP3hi) displays a significantly lower proportion of activated (HLA‐DR+) cells in the cord blood of babies born to preeclamptic mothers; possibly the phenotypic signature of an activation/developmental defect that may contribute to the overall reduction of Tregs observed. Suppressive capacity and biological significance of these unique fetal Tregs requires further study.
Conclusion
The cord blood of babies born to preeclamptic mothers displays a significant shift toward an effector NK innate immune phenotype as well as a reduction of the effector T‐cell to Treg ratio. These previously undescribed immune derangements may represent an aspect of the pathogenesis of preeclampsia that merits further exploration. In how far this phenotype represents a cause or effect of preeclampsia will require additional study.
Acknowledgments
Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards CA‐16042 and AI‐28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA. We acknowledge the support of Harvey L. Karp Discovery Award, UCLA‐Caltech Joint Center for Translational Medicine, a gift from the UCLA Scholars in Translational Medicine, and Reproductive Scientist Development Program (D.A.K.) NIH/NICHD 2K12HD000849‐26 (Moley).
Loewendorf AI, Nguyen TA, Yesayan MN, Kahn DA. Preeclampsia is characterized by fetal NK cell activation and a reduction in regulatory T cells. Am J Reprod Immunol 2015; 74: 258–267
References
- 1. Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM: Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008; 322:1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rowe JH, Ertelt JM, Xin L, Way SS: Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012; 490:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zenclussen ML, Thuere C, Ahmad N, Wafula PO, Fest S, Teles A, Leber A, Casalis PA, Bechmann I, Priller J, Volk HD, Zenclussen AC: The persistence of paternal antigens in the maternal body is involved in regulatory T‐cell expansion and fetal‐maternal tolerance in murine pregnancy. Am J Reprod Immunol 2010; 63:200–208. [DOI] [PubMed] [Google Scholar]
- 4. Kahn DA, Baltimore D: Pregnancy induces a fetal antigen‐specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA 2010; 107:9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY: Extrathymic generation of regulatory T cells in placental mammals mitigates maternal‐fetal conflict. Cell 2012; 150:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY: Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005; 22:329–341. [DOI] [PubMed] [Google Scholar]
- 7. Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW: Tumor necrosis factor‐alpha, interleukin‐6, and interleukin‐10 levels are altered in preeclampsia: a systematic review and meta‐analysis. Am J Reprod Immunol 2013; 70:412–427. [DOI] [PubMed] [Google Scholar]
- 8. Cudihy D, Lee RV: The pathophysiology of pre‐eclampsia: current clinical concepts. J Obstet Gynaecol 2009; 29:576–582. [DOI] [PubMed] [Google Scholar]
- 9. Roederer M, Hardy RR: Frequency difference gating: a multivariate method for identifying subsets that differ between samples. Cytometry 2001; 45:56–64. [DOI] [PubMed] [Google Scholar]
- 10. Marcenaro E, Dondero A, Moretta A: Multi‐directional cross‐regulation of NK cell function during innate immune responses. Transpl Immunol 2006; 17:16–19. [DOI] [PubMed] [Google Scholar]
- 11. McKenzie AN, Spits H, Eberl G: Innate lymphoid cells in inflammation and immunity. Immunity 2014; 41:366–374. [DOI] [PubMed] [Google Scholar]
- 12. Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J: CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009; 126:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saito S, Nakashima A, Myojo‐Higuma S, Shiozaki A: The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol 2008; 77:14–22. [DOI] [PubMed] [Google Scholar]
- 14. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S: Functions of natural killer cells. Nat Immunol 2008; 9:503–510. [DOI] [PubMed] [Google Scholar]
- 15. Beziat V, Descours B, Parizot C, Debre P, Vieillard V: NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS ONE 2010; 5:e11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loewendorf AI, Nguyen TA, Yesayan MN, Kahn DA: Normal human pregnancy results in maternal immune activation in the periphery and at the uteroplacental interface. PLoS ONE 2014; 9:e96723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gourley TS, Wherry EJ, Masopust D, Ahmed R: Generation and maintenance of immunological memory. Semin Immunol 2004; 16:323–333. [DOI] [PubMed] [Google Scholar]
- 18. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S: Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009; 30:899–911. [DOI] [PubMed] [Google Scholar]
- 19. Baecher‐Allan C, Wolf E, Hafler DA: MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol 2006; 176:4622–4631. [DOI] [PubMed] [Google Scholar]
- 20. Bujold E, Chaiworapongsa T, Romero R, Gervasi MT, Espinoza J, Goncalves LF, Berman S, Yoon BH, Kim YM: Neonates born to pre‐eclamptic mothers have a higher percentage of natural killer cells (CD3−/CD56+16+) in umbilical cord blood than those without pre‐eclampsia. J Matern Fetal Neonatal Med 2003; 14:305–312. [DOI] [PubMed] [Google Scholar]
- 21. Xiong F, Tong Y, You Y, Li P, Huo T, Tu W, Mao M: Prospective cohort study about the lymphocyte subpopulations’ change and impact on the pregnancy outcome in fetal growth restriction. J Matern Fetal Neonatal Med 2012; 25:2773–2777. [DOI] [PubMed] [Google Scholar]
- 22. Catarino C, Santos‐Silva A, Belo L, Rocha‐Pereira P, Rocha S, Patricio B, Quintanilha A, Rebelo I: Inflammatory disturbances in preeclampsia: relationship between maternal and umbilical cord blood. J Pregnancy 2012; 2012:684384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffman MC, Rumer KK, Kramer A, Lynch AM, Winn VD: Maternal and fetal alternative complement pathway activation in early severe preeclampsia. Am J Reprod Immunol 2014; 71:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sohlberg E, Saghafian‐Hedengren S, Bachmayer N, Hamad RR, Bremme K, Holmlund U: Pre‐eclampsia affects cord blood NK cell expression of activation receptors and serum cytokine levels but not CB monocyte characteristics. Am J Reprod Immunol 2014; 71:178–188. [DOI] [PubMed] [Google Scholar]
- 25. Bielekova B, Catalfamo M, Reichert‐Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R: Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL‐2Ralpha‐targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA 2006; 103:5941–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB: Cutting edge: in vivo blockade of human IL‐2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol 2005; 174:5187–5191. [DOI] [PubMed] [Google Scholar]
- 27. Hussain T, Kulshreshtha KK, Yadav VS, Katoch K: CD4+, CD8+, CD3+ cell counts and CD4+/CD8+ ratio among patients with mycobacterial diseases (leprosy, tuberculosis), HIV infections, and normal healthy adults: a comparative analysis of studies in different regions of India. J Immunoassay Immunochem 2015; 36:420–443. [DOI] [PubMed] [Google Scholar]
- 28. Oda K, Ishimoto H, Yatera K, Yamada S, Nakao H, Ogoshi T, Noguchi S, Yamasaki K, Kawanami T, Mukae H: Relationship between the ratios of CD4/CD8 T‐lymphocytes in the bronchoalveolar lavage fluid and lymph nodes in patients with sarcoidosis. Respir Investig 2014; 52:179–183. [DOI] [PubMed] [Google Scholar]
- 29. Thornhill J, Inshaw J, Oomeer S, Kaleebu P, Cooper D, Ramjee G, Schechter M, Tambussi G, Fox J, Miro JM, Weber J, Babiker A, Porter K, Fidler S: Enhanced normalisation of CD4/CD8 ratio with early antiretroviral therapy in primary HIV infection. J Int AIDS Soc 2014; 17(4 Suppl 3):19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Q, Zhang J, Shi J, Ge M, Li X, Shao Y, Yao J, Zheng Y: Increased bone marrow (BM) plasma level of soluble CD30 and correlations with BM plasma level of interferon (IFN)‐gamma, CD4/CD8 T‐cell ratio and disease severity in aplastic anemia. PLoS ONE 2014; 9:e110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. D'Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, Carotenuto M: Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica 1998; 83:197–203. [PubMed] [Google Scholar]
- 32. Burt TD: Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. Am J Reprod Immunol 2013; 69:346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luciano AA, Yu H, Jackson LW, Wolfe LA, Bernstein HB: Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS ONE 2011; 6:e16698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Byrne JA, Stankovic AK, Cooper MD: A novel subpopulation of primed T cells in the human fetus. J Immunol 1994; 152:3098–3106. [PubMed] [Google Scholar]
- 35. Rowe JH, Ertelt JM, Xin L, Way SS: Regulatory T cells and the immune pathogenesis of prenatal infection. Reproduction 2013; 146:R191–R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steinborn A, Engst M, Haensch GM, Mahnke K, Schmitt E, Meuer S, Sohn C: Small for gestational age (SGA) neonates show reduced suppressive activity of their regulatory T cells. Clin Immunol 2010; 134:188–197. [DOI] [PubMed] [Google Scholar]


