Abstract
Objective
To identify factors associated with progression from pregnancy‐associated severe sepsis to death in the UK.
Design
A population‐based case‐control analysis using data from the UK Obstetric Surveillance System (UKOSS) and the UK Confidential Enquiry into Maternal Death (CEMD).
Setting
All pregnancy care and death settings in UK hospitals.
Population
All non‐influenza sepsis‐related maternal deaths (January 2009 to December 2012) were included as cases (n = 43), and all women who survived severe non‐influenza sepsis in pregnancy (June 2011 to May 2012) were included as controls (n = 358).
Methods
Cases and controls were identified using the CEMD and UKOSS. Multivariable logistic regression was used to estimate adjusted odds ratios (aOR) with 95% confidence intervals.
Main outcome measures
Odds ratios for socio‐demographic, medical, obstetric and management factors in women who died from sepsis, compared with those who survived.
Results
Four factors were included in the final regression model. Women who died were more likely to have never received antibiotics [aOR = 22.7, 95% confidence interval (CI) 3.64–141.6], to have medical comorbidities (aOR = 2.53, 95%CI 1.23–5.23) and to be multiparous (aOR = 3.57, 95%CI 1.62–7.89). Anaemia (aOR = 13.5, 95%CI 3.17–57.6) and immunosuppression (aOR = 15.0, 95%CI 1.93–116.9) were the two most important factors driving the association between medical comorbidities and progression to death.
Conclusions
There must be continued vigilance for the risks of infection in pregnant women with medical comorbidities. Improved adherence to national guidelines, alongside prompt recognition and treatment with antibiotics, may reduce the burden from sepsis‐related maternal deaths.
Tweetable abstract
Medical comorbidities, multiparity and antibiotic delays increase the risk of death from maternal sepsis.
Keywords: Maternal deaths, maternal sepsis, pregnancy, septic shock, severe acute maternal morbidity, severe sepsis
Introduction
The most recent confidential enquiry into maternal deaths has shown that in the UK, the rate of maternal death from genital tract sepsis has decreased substantially from 1.13 to 0.50 deaths per 100 000 maternities (2006–2008 to 2010–2012).1, 2 However, this promising trend obscures two main issues. Firstly, when infective causes beyond the genital tract are included this figure rises to 2.04 per 100 000 maternities (2009–2012).1 This is because infections such as influenza, pneumonia and urinary tract infections, which are not ‘caused’ by pregnancy, but may be exacerbated by the immunomodulated pregnant state,3 are not included in the classification of direct maternal deaths. Secondly, for each woman who dies, several more experience severe maternal morbidity or a ‘near‐miss’,4, 5 posing a substantial burden that is not represented sufficiently by mortality rates. This is evidenced by a recent UK population‐based study that documented all causes of sepsis and found 47 cases of severe sepsis morbidity per 100 000 maternities,6 compared with the 0.50 genital tract sepsis deaths per 100 000 maternities.
These figures, deduced from two active, prospective, population‐based surveillance systems for maternal deaths and maternal morbidities in the UK, merit further exploration. In particular, comparisons of women along the morbidity spectrum have potential to identify risk factors for progression of disease, and the availability of population‐based data reduces the effects of selection bias that are common in facility‐based studies.7, 8
Several studies have compared women with severe sepsis to those with uncomplicated sepsis or normal pregnancies,6, 9, 10, 11 which will reflect factors that affect the incidence of sepsis, as well as progression of disease. However, no population‐based studies have compared sepsis‐related maternal deaths with morbidities, although this approach can highlight factors more pertinent to the most severe outcomes.12, 13, 14
Furthermore, the role of factors specific to the management of sepsis, such as the timing and indication for antibiotic usage, have been understudied, despite clear guidelines from the Surviving Sepsis Campaign15, 16, 17 and Royal College of Obstetricians and Gynaecologists (RCOG)18, 19 on the importance of prompt diagnosis and resuscitation. This is most likely due to the difficulty of studying management factors through the use of administrative data sources, as access to medical records is often needed to delineate the time delay from diagnosis to antibiotics.20, 21
Thus, the aim of this study was to identify the demographic, socio‐economic, medical, obstetric and management factors associated with progression from pregnancy‐related severe sepsis to death.
Methods
A UK‐wide population‐based case case‐control analysis was carried out comparing ‘cases’, women who died from non‐influenza sepsis, with ‘controls’, women who survived severe non‐influenza sepsis.
Identification of cases
The Maternal, Newborn and Infant Clinical Outcome Review programme run by the MBRRACE‐UK collaboration is the current system used for surveillance and confidential enquiries of all maternal deaths in the United Kingdom (UK), occurring during pregnancy and up to 1 year after the end of pregnancy, regardless of the pregnancy outcome.22
Once a maternal death is notified to MBRRACE‐UK, the clinical notes, postmortem information and surveillance data are obtained from the reporting hospital. Initially, each case is classified according to a pathologist's assessment of probable cause of death. Further classification to the ‘sepsis’ category may occur after assessment by obstetricians, midwives, anaesthetists and infectious disease specialists.22 This process of case ascertainment from direct notification is supplemented with death registration information from the Office of National Statistics (England and Wales), and National Records of Scotland, pathologists, coroners and procurators fiscal, to maximise case identification.2
Women who died from non‐influenza sepsis between January 2009 and December 2012, in whom the onset of infection occurred during or up to 6 weeks after the end of pregnancy, were included as cases in this study.
Identification of controls
The United Kingdom Obstetric Surveillance System (UKOSS) is a national research platform used to study women who have experienced specific pregnancy‐related morbidities.23 Each consultant‐led maternity unit in the UK nominates up to four clinicians to report to the system, which may include obstetricians, midwives, anaesthetists and risk managers. Nominated clinicians are sent a monthly notification card containing a list of specific conditions under surveillance and are asked to report any women within their unit with any of these conditions.
If no women have had these conditions, a ‘nil to report’ return is submitted to distinguish this from a lack of response. Once a woman with a particular condition has been notified, a condition‐specific data collection form is dispatched to the unit to collect detailed, anonymised information.
A UKOSS study of severe sepsis was conducted in 2011–2012.6 Due to the lack of a standardised definition, pregnancy‐related severe sepsis was defined based on previous literature24 and by consensus discussion of the UKOSS steering committee (see Box 1). Initial results of the main study were reported by Acosta et al.,6 in which severe sepsis morbidity cases were compared with uncomplicated pregnancies as controls. There were no cases of sepsis due to influenza reported to the UKOSS study during this period. For the analysis reported here, women who survived severe sepsis morbidity occurring between June 2011 and May 2012 were included as controls.
Box 1. UKOSS definition of severe sepsis. Source: Acosta et al.6 .
Any pregnant or recently pregnant woman (up to 42 days postpartum) diagnosed with severe sepsis, irrespective of the source of infection. This would be expected to include women in any of the following groups:
Death related to infection or suspected infection
Any women requiring level 2 (high dependency unit) or level 3 critical care (intensive care unit) with severe sepsis or suspected severe sepsis
- A clinical diagnosis of severe sepsis, which would usually be associated with two or more of the following:
- Temperature >38°C or <36°C measured on two occasions, at least 4 hours apart
- Heart rate >100 beats/minute measured on two occasions, at least 4 hours apart
- Respiratory rate >20/minute measured on two occasions, at least 4 hours apart
- White cell count >17 × 109/l or <4 × 109/l or with >10% immature band forms, measured on two occasions
Exclusion criteria
Due to the time periods used to identify cases and controls, pandemic influenza (H1N1) contributed to sepsis in 46% (34 of 77) of cases, but in none of the controls. These cases were excluded from the analysis, due to the lack of comparability with controls, who were identified outside the pandemic period, leaving 43 cases and 358 controls.
Measurement of risk factors
The data collection forms from the MBRRACE‐UK and UKOSS surveillance systems contain questions about demographic characteristics, medical and obstetric history, pregnancy and delivery, which were used to construct the variables included in the study.
Demographic characteristics, medical and obstetric factors would have been documented in the medical records at booking, whereas other factors relating to pregnancy and delivery may be recorded at any point in the pregnancy. All data were extracted from the medical records onto the data collection forms.
However, while the UKOSS data collection form contained specific information on the diagnosis and management of sepsis, the MBRRACE‐UK surveillance form was developed to cover all causes of death and did not include this specific information. Thus, the medical records of each woman who died were reviewed by one author (OMA) to determine the date and time of diagnosis and administration of antibiotics using clinical entries and drug charts, a method that has been used in other studies of sepsis.20, 21
It was not possible to review the medical records of each woman who survived, as anonymised data collection forms (without medical records) are submitted by clinicians to the UKOSS study. This meant that only date, not time, of antibiotic administration was available for controls.
The date and time of diagnosis were used to determine two new variables. First, the date and time of diagnosis and the date and time of delivery, termination of pregnancy or miscarriage, was used to classify cases and controls into antenatal or postpartum sepsis. Secondly, the date (and time, where available) antibiotics were first administered was used to determine the timing of administration in relation to the timing of the diagnosis of sepsis.
Study sample, size and power
Forty‐three cases and 358 controls were included in the analysis. At the lowest risk factor prevalence of 6% in controls and highest risk factor prevalence of 46%, the analysis had 80% power at the 5% level of statistical significance to detect an odds ratio of 3.4 or greater and 2.3 or greater, respectively.25
Statistical analysis
All statistical analyses were carried out using STATA 13 SE statistical software (StataCorp, College Station, TX, USA). Continuous variables were summarised as means (standard deviations) or medians (interquartile range) for non‐normally distributed data. Categorical variables were summarised as frequencies. Chi‐square tests were used to test differences in proportions between groups. Results with P < 0.05 were considered statistically significant.
A priori risk factors were identified based on pre‐existing literature and plausible relationships and were included if data were available. Most variables were included as binary variables, including ethnicity, age and parity. Pre‐existing medical conditions such as anaemia, asthma, autoimmune conditions, diabetes, immunosuppression and mental health problems, which are common and have a plausible association with maternal sepsis, were grouped to form one binary variable. Unconditional logistic regression was used to estimate unadjusted odds ratios (uOR), 95% confidence intervals (CI) and P‐values, for each of the 12 variables.
All variables were tested for collinearity using Pearson's correlation coefficient. Mode of delivery was found to be correlated with antenatal and postpartum sepsis (co‐efficient 0.6629), which was consistent with the literature.6, 11 This is most likely to be a result of antenatal sepsis leading to expedited delivery, particularly by caesarean section. No other variables were correlated.
Due to the small number of cases, a statistical approach was used to derive a parsimonious multivariate logistic regression model. Each variable was added into the model in order of the strength of the univariate association, with subsequent likelihood ratio testing. Variables were retained in the model if they significantly affected the fit of the data using a 10% significance level. This resulted in a regression model with four variables (employment status, pre‐existing medical conditions, parity and antibiotic delay). An exploratory analysis considered which of the six medical conditions in the ‘pre‐existing medical conditions’ category were driving the association and how each contributed.
Cases and controls were compared in terms of the severity of infection, by looking at admission to intensive care units and lactate levels. These factors are measures of severity progression and were therefore not included in the adjusted analysis due to the risk of over‐adjustment.26
Sensitivity analyses
Two sensitivity analyses were conducted to assess the role of missing data and confounding variables on the regression model.
First, missing data were ≤2% for all variables except for employment status (28%), body mass index (BMI, 4%) and lactate levels (54%). Previous studies utilising UKOSS datasets have shown this information is unlikely to be missing at random27 and therefore unsuitable for multiple imputation.28 As a result, where missing data represented 10% or more of the data, we included a category in the variable to represent the missing data. As employment status was included in the final regression model, we assessed the effect of redistributing the missing observations on the results.
The second sensitivity analysis assessed the effect of including well‐established confounders such as age, ethnicity, smoking and BMI on the findings.
Results
Forty‐three women who died from (non‐influenza) sepsis and 358 women who survived severe pregnancy‐associated sepsis were identified, using the MBRRACE‐UK and UKOSS surveillance systems, and included in the analysis.
For cases and controls, the genital tract represented the largest single source of infections (44% in cases and 31% in controls), with the remainder due to other or unclear sources of infection (Table 1). For cases, this category primarily consisted of women with meningitis (30% of other, n = 7). For controls, non‐genital tract sources of infection tended to be due to an ‘unclear’ source of infection (38% of other, n = 93), urinary tract infections (29% of other, n = 71) and wound infections (13% of other, n = 33).
Table 1.
Source and severity of infection in cases and controls
| Variables | Number (%) of cases (n = 43) | Number (%) of controls (n = 358) | P‐valuea |
|---|---|---|---|
| Primary source of infection | |||
| Genital tract | 19 (44) | 110 (31) | 0.074 |
| Other or unclear | 24 (56) | 248 (69) | |
| Admission to intensive care unit (Level 3) b | |||
| No | 10 (23) | 249 (70) | <0.001 |
| Yes | 32 (74) | 108 (30) | |
| Missing | 1 (2) | 1 (0) | |
| Highest lactate >2 mmol/l | |||
| No | 4 (9) | 86 (24) | <0.001 |
| Yes | 31 (72) | 62 (17) | |
| Missing or not measured | 8 (19) | 210 (59) | |
| Highest lactate >4 mmol/l | |||
| No | 13 (30) | 126 (35) | <0.001 |
| Yes | 22 (51) | 22 (6) | |
| Missing | 8 (19) | 210 (59) | |
Calculated using chi‐square test for difference in proportions.
Level 3 care refers to patients requiring advanced respiratory support or basic respiratory support and support for at least two organ systems, as classified by the Department of Health (2000).
Cases and controls differed in the severity of infection, with more cases than controls admitted to an intensive care unit (74 versus 30%, respectively, P < 0.001). Similarly, 72% of cases (n = 31) had a highest lactate above 2 mmol/l, compared with only 17% of controls (n = 62, P < 0.001), while 51% of cases (n = 22) and only 6% of controls (n = 22) had a highest lactate above 4 mmol/l (P < 0.001). Importantly, in a large proportion (54%), lactate levels were not measured (cases 19%, controls 59%).
Descriptive statistics and univariable analysis for the 12 variables included in the study are displayed in Table 2. In general, the distribution of socio‐demographic characteristics was similar between the groups. However, women who died from sepsis, when compared with women who survived, were more likely to be over the age of 35 (uOR = 2.11, 95%CI 1.04–4.28).
Table 2.
Characteristics of cases and controls
| Independent variables | Number (%) of cases (n = 43) | Number (%) of controls (n = 358) | uOR | 95%CI | P‐value |
|---|---|---|---|---|---|
| Maternal age (years) | |||||
| <35 | 30 (70) | 297 (83) | 1.00 | ||
| ≥35 | 13 (30) | 61 (17) | 2.11 | 1.04–4.28 | 0.038 |
| Employment status | |||||
| Employed | 28 (65) | 223 (62) | 1.00 | ||
| Unemployeda | 8 (19) | 28 (8) | 2.28 | 0.95–5.48 | 0.067 |
| Missing | 7 (16) | 107 (30) | 0.52 | 0.22–1.23 | 0.137 |
| Ethnicity | |||||
| White | 28 (65) | 255 (71) | 1.00 | ||
| Black, Asian and minority ethnic | 15 (35) | 102 (28) | 1.34 | 0.69–2.61 | 0.391 |
| Missing | 0 (0) | 1 (0) | Omitted | ||
| Smoking status | |||||
| Did not smoke during pregnancy | 30 (70) | 258 (72) | 1.00 | ||
| Smoked during pregnancy | 9 (21) | 96 (27) | 0.81 | 0.37–1.76 | 0.589 |
| Missing | 4 (9) | 4 (1) | Omitted | ||
| Body mass index | |||||
| Underweight (<18.5) | 3 (7) | 14 (4) | 1.77 | 0.47–6.73 | 0.402 |
| Normal (18.5–24.9) | 19 (44) | 157 (44) | 1.00 | ||
| Overweight (25–29.9) | 9 (21) | 93 (26) | 0.80 | 0.35–1.84 | 0.599 |
| Obese (≥30) | 8 (19) | 79 (22) | 0.84 | 0.35–2.00 | 0.688 |
| Missing | 4 (9) | 15 (4) | 2.20 | 0.66–7.33 | 0.197 |
| Pre‐existing medical conditions b | |||||
| No | 24 (56) | 290 (81) | 1.00 | ||
| Yes | 19 (44) | 68 (19) | 3.38 | 1.75–6.51 | <0.001 |
| Parity | |||||
| Nulliparous | 9 (21) | 194 (54) | 1.00 | ||
| Multiparous | 34 (79) | 163 (46) | 4.50 | 2.10–9.65 | <0.001 |
| Missing | 0 (0) | 1 (0) | Omitted | ||
| Previous caesarean section | |||||
| No | 33 (77) | 311 (87) | 1.00 | ||
| Yes | 10 (23) | 47 (13) | 2.01 | 0.93–4.34 | 0.077 |
| Missing | 0 (0) | 0 (0) | Omitted | ||
| Mode of delivery c | |||||
| Vaginal delivery | 12 (28) | 84 (23) | Excluded | ||
| Operative vaginal delivery | 5 (12) | 47 (13) | |||
| Caesarean section | 17 (40) | 198 (55) | |||
| Early pregnancy loss or undelivered at time of death | 8 (19) | 27 (8) | |||
| Missing | 1 (2) | 2 (1) | |||
| Induction of labour | |||||
| No | 32 (74) | 259 (72) | 1.00 | ||
| Yes | 10 (23) | 98 (27) | 0.83 | 0.39–1.74 | 0.616 |
| Missing | 1 (2) | 1 (0) | Omitted | ||
| Timing of sepsis | |||||
| Postnatal sepsis | 21 (49) | 211 (59) | 1.00 | ||
| Antenatal sepsis | 22 (51) | 144 (40) | 1.54 | 0.81–2.89 | 0.186 |
| Missing | 0 (0) | 3 (1) | Omitted | ||
| Number of days from diagnosis to administration of antibiotics | |||||
| Same day or before diagnosis | 29 (67) | 305 (85) | 1.00 | ||
| 1 or more days | 8 (19) | 51 (14) | 1.65 | 0.71–3.81 | 0.241 |
| Never started on antibiotic | 6 (14) | 2 (1) | 31.6 | 6.09–163.5 | <0.001 |
| Group A Streptococcal | |||||
| No | 34 (79) | 337 (94) | 1.00 | ||
| Yes | 9 (21) | 21 (6) | 4.25 | 1.80–10.0 | 0.001 |
‘Unemployed’ refers to women from households where either she (for single mothers) or both she and her partner (for women who were married or cohabiting) were unemployed.
‘Pre‐existing medical conditions’ refers to any woman with anaemia, asthma, autoimmune conditions, diabetes, immunosuppression or mental health problems.
Mode of delivery was highly correlated with the timing of sepsis, as it can be a potential outcome of antenatal sepsis, and a potential risk factor for postpartum sepsis. Therefore, it was excluded from the main analysis of all variables.
uOR unadjusted odds ratio; 95%CI, 95% confidence interval.
The majority of women who died were multiparous (79%, n = 34), whereas the majority of controls were nulliparous (54%, n = 194). A minority of cases and controls had a caesarean section prior to their current pregnancy (cases 23%, n = 10, and controls 13%, n = 47). Conversely, for 49% of cases (n = 36) and 55% of controls (n = 198), their current pregnancy was delivered via caesarean section. Only 28% of cases (n = 21) and 23% of controls (n = 84) had an unassisted vaginal delivery.
For nine of the women who died (21%) and 21 of the women who survived (6%), Group A streptococcus was identified as the causative organism. Despite a significant association on univariable analysis (uOR = 4.25, 95%CI 1.80–10.0), organism type did not contribute to the fit of the final model.
For 51% of cases (n = 22) and 40% of controls (n = 144), sepsis was diagnosed during pregnancy (antepartum sepsis), with the remainder diagnosed after pregnancy (postpartum sepsis). In both cases and controls, the majority were started on antibiotics on the same day, or before, diagnosis of severe sepsis (67% of cases, 85% of controls). However, more cases than controls (19% versus 14%) were started on antibiotics at least 1 day after diagnosis, though this difference was not statistically significant (uOR 1.65, 95%CI 0.71–3.81). Six women who died (14%), and two women who survived (1%), were never started on antibiotics (uOR = 31.6, 95%CI 6.09–163.5).
A breakdown of the time delay in administration of antibiotics is displayed in Figure 1. As the time of administration was not available for controls, this is presented only for cases (n = 43). Fourteen of the cases (33%) received antibiotics within 1 hour of diagnosis and 25 (58%) within 3 hours. Of the six women (14%) who were never started on antibiotics, three died at home or on arrival at the hospital (n = 3, 7%).
Figure 1.
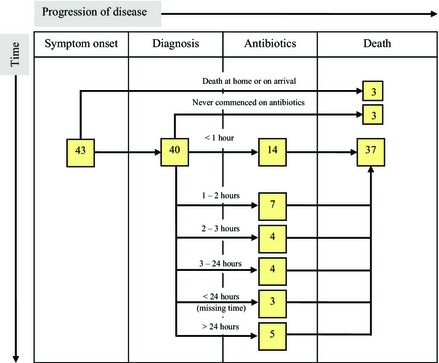
Time delay from diagnosis to administration of antibiotics, in cases only.
The result of the multivariable analysis is shown in Table 3. Antibiotic delay, multiparity, employment status, and the presence of medical comorbidities significantly affected the fit of the data and were retained in the final model (Model 1, Table 3). After adjustment for these factors, women who died from sepsis were significantly more likely to have never been started on antibiotics, than were those who survived sepsis [adjusted odds ratio (aOR) 22.7, 95%CI 3.64–141.6].
Table 3.
Factors associated with death from sepsis (main multivariable analysis and sensitivity analyses)
| Employment status | ||||
| Employed | Baseline | |||
| Unemployed | 2.25 (0.86–5.91) | 2.70 (1.05–6.93) | 0.86 (0.42–1.76) | 2.55 (0.86–7.52) |
| Pre‐existing medical conditions a | ||||
| No | Baseline | |||
| Yes | 2.53 (1.23–5.23) | 2.51 (1.21–5.18) | 2.58 (1.26–5.30) | 2.43 (1.12–5.25) |
| Parity | ||||
| Nulliparous | Baseline | |||
| Multiparous | 3.57 (1.62–7.89) | 3.65 (1.65–8.05) | 3.57 (1.62–7.85) | 3.29 (1.39–7.78) |
| Number of days from diagnosis to administration of antibiotics | ||||
| Same day or before diagnosis | Baseline | |||
| 1 or more days | 1.44 (0.59–3.52) | 1.36 (0.56–3.34) | 1.38 (0.57–3.34) | 1.65 (0.65–4.22) |
| Never commenced on antibiotics | 22.69 (3.64–141.6) | 21.68 (3.69–127.4) | 20.43 (3.53–118.1) | 37.73 (5.37–265.0) |
aOR, adjusted odds ratio; 95%CI, 95% confidence interval.
Model 1: adjusted for antibiotic delay, multiparity, employment status and medical history.
Model 1a: adjusted for all factors in Model 1, but assumes that all ‘missing’ employment status were employed.
Model 1b: adjusted for all factors in Model 1, but assumes that all ‘missing’ employment status were unemployed.
Model 2: adjusted for all factors in Model 1 plus the potential confounders: age, ethnicity, smoking, BMI.
‘Pre‐existing medical conditions’ includes anyone with anaemia, asthma, autoimmune conditions, diabetes, immunosuppression or mental health problems.
The odds of being multiparous and having pre‐existing medical conditions were 3.57 (95%CI 1.62–7.89) and 2.53 (95%CI 1.23–5.23) times higher in women who died than those who survived, and both odds were statistically significant.
After adjustment, women who died were 2.25 times more likely to be from a household in which either she (single mothers) or both she and her partner (women who were married or cohabiting) were unemployed compared with those who survived, though this was not statistically significant (95%CI 0.86–5.91). Redistribution of missing data, which represented 16% for cases (n = 7) and 30% for controls (n = 107), did not materially alter the results, apart from for unemployment (Models 1a and 1b, Table 3). Assuming that all women with missing employment information were actually employed resulted in a small change in the estimated odds ratio (aOR = 2.70, 95%CI 1.05–6.93). Conversely, classifying all ‘missing’ as unemployed reduced the estimated odds ratio (aOR = 0.86, 95%CI 0.42–1.76).
As unemployment was the only socio‐demographic factor which remained in the regression model, a second sensitivity analysis considered the effect of adding age, ethnicity, BMI and smoking into the model (Model 2, Table 3), as these are established confounders in previous studies. This had no material effect on the findings.
An exploratory analysis separating different pre‐existing medical conditions showed that the association with progression to death appeared to be particularly driven by the presence of anaemia and immunosuppression. After adjustment, women who died were 13.5 times more likely to be anaemic (95%CI 3.17–57.6) and 15.0 times more likely to be immunosuppressed (95%CI 1.93–116.9) than those who survived, although with wide confidence intervals due to the limited statistical power of this analysis (Supporting Information Table S1).
Discussion
Main findings
This study highlights that women who died from sepsis were more likely to have pre‐existing medical conditions (particularly anaemia and immunosuppression), be multiparous and to never have been started on antibiotics compared with women who survived. Socio‐economic circumstances as represented by unemployment played an important role in these associations. No other socio‐demographic factors or obstetric factors were found to be associated with death, although the limited power of this study based on relatively small numbers means that we cannot exclude the possibility of other associated factors which we were not able to detect.
As would be anticipated, women who died experienced more severe infections, as suggested by higher admission rates to intensive care units and higher serum lactate levels. For both cases and controls, genital tract sepsis contributed to less than half of all infections. Importantly, half of the women did not have lactate measured and only a third of women who died from sepsis received antibiotics within 1 hour of diagnosis. Unfortunately we were not able to assess the speed of administration of antibiotics for the survivors, but a recent confidential enquiry which included some of the severe sepsis morbidity cases from this same dataset suggested that the survivors received antibiotics and other appropriate management more rapidly than did the women who died.1
Strength and limitations
The major strengths of this study relate to the study design and study populations. Both data sources used population‐based, prospective surveillance systems with significant efforts to ensure good ascertainment, thus reducing the risk of selection bias, which is often a problem in case‐control and facility‐based studies.29 In addition, the extensive information available from both systems enabled detailed investigation of the management of sepsis and exploration of a range of putative risk factors for progression.
However, several limitations exist. Despite the use of 4 years' worth of national data, the fortunately low maternal death rate meant there were few cases, and thus low statistical power. Our parsimonious approach to adjustment excluded many potential confounders which may have been associated with smaller and thus undetectable odds ratios given the power profile of the study and there remains a risk of residual confounding. Data collected were from clinical records and therefore may have been recorded at any point during pregnancy. However, three of the four risk factors that were significant in the multivariable analysis (parity, antibiotic delay and medical comorbidities) would not have been affected by this. In addition, this study used observational data, which cannot prove causality, although the results are consistent with current research. Finally, it is worth noting that, as this study was conducted in the UK, where termination of pregnancy is legally available, the findings are unlikely to be generalisable to settings where sepsis is frequently associated with illegal and unsafe terminations of pregnancy.
Interpretation
Several population‐based studies have considered risk factors for poor outcomes that are relevant to this study. These include studies of sepsis in obstetric populations,6, 9, 10, 11, 30 and other morbidities in obstetric populations.12, 13, 14, 27, 31, 32 Interestingly, all those studies found an effect of at least one socio‐demographic factor, most commonly age, body mass index (BMI) or ethnicity. In contrast, our study found no consistent effect any of these factors, except for unemployment, which contributed to the fit of the data but did not have a statistically significant association. Unemployment has been identified as a risk factor in other studies12, 27 and is likely to be a marker of social exclusion, affecting how women access maternity care, and contributing to late presentation.33 One possible explanation for these differences is that factors such as age, BMI and ethnicity may predispose to infection and morbidity, but are not necessarily associated with death. A second explanation is that these factors are associated with progression to death but at odds ratios levels that were not detectable as statistically significant in this study of limited sample size and thus power.
This study showed that medical comorbidities appear to have a strong association with adverse outcomes, which is consistent with the literature6, 11, 13, 14, 27, 31 and current management strategies. However, the particular medical conditions vary between studies. In our study, anaemia and immunosuppression appeared to drive the association, although asthma and mental health problems were associated with non‐significantly raised odds.
Immunosuppression is known to make women more susceptible to severe infection1, 2, 34, 35 and anaemia has been increasingly highlighted as a potential risk factor for severe obstetric complications.9, 13, 31 However, anaemia can be a risk factor, outcome or confounding variable for sepsis. For example, the inflammatory processes associated with sepsis may cause destruction of the red blood cells, leading to anaemia.36 Although in our study we could not differentiate the onset of anaemia, a study that used ‘taking iron tablets at booking’, as a marker of anaemia, found a strong association with sepsis (aOR = 29.5).24
The association between sepsis and parity, which was not explained by age, ethnicity, employment status, smoking or BMI in our study, appears to be inconsistent in the literature, with studies finding increased risk for multiparous9 or nulliparous women.6, 10 This may be due to differences in the study populations, methods or adjustment for confounding. In particular, multiparity may affect exposure to community‐acquired infections or ease of accessing health services. Interestingly, studies comparing deaths with severe morbidities in obstetric populations have not found that parity has an effect.12, 13
Several findings in this study highlight the importance of management factors and adherence to clinical guidelines. Our access to clinical records, for women who died, showed that timely administration of antibiotics and measurement of lactate levels were inadequate. This is despite the emphasis of both factors in the Surviving Sepsis Campaign bundles and guidelines from the Royal College of Obstetricians and Gynaecologists.16, 18, 19 Given the evidence supporting early interventions, such findings emphasise the role of clinical audit as a means of improving adherence to guidelines. At the local level, it is possible for each maternity unit to review performance towards meetings these goals, and quality improvement programmes have been successful in reducing time to first antibiotic21 and mortality from sepsis.37
Conclusions
Several risk factors, including those relating to medical comorbidities, unemployment, parity and management factors, such as timing of antibiotic administration, have been found to play an important role in progression from severe pregnancy‐associated sepsis to death.
As such, there are sever al clinical implications which emphasise the need for improved adherence to current national and international guidelines.16, 18, 19, 38, 39 First, there must be continued vigilance for the risks of infection in women with medical comorbidities, including anaemia, the significance of which can be under‐appreciated. Secondly, unemployment, which is likely to relate to social exclusion, will require intersectoral and interagency work between health and social services to ensure that women's social, psychological and health needs are addressed. Finally, this study supports the international consensus guidelines for the early recognition and treatment of sepsis,16 recognising the role of local audits and quality improvement programmes in improving adherence to the most important interventions.15, 21
Disclosure of interests
Full disclosure of interests available to view online as supporting information.
Contribution to authorship
OMA assisted with design of the study, carried out the analysis and wrote the first draft of the manuscript, which formed part of a thesis submitted in partial fulfilment of the Masters in Global Health Sciences, University of Oxford. MK conceived and designed the study, obtained funding and contributed to analysis of the data and writing of the manuscript. MN contributed to analysis of the data and writing of the manuscript. CA conceived and designed the study, and contributed to writing of the manuscript. JJK conceived and designed the study, and contributed to analysis of the data and writing of the manuscript.
Details of ethics approval
As a mandatory national audit, research ethics committee approval for data collection is not required for the MBRRACE surveillance system. It has been approved by the Secretary of State for Health through the National Information Governance Board (NIGB) processes (and its successor organisation the Confidentiality Advisory Group at the Health Research Authority) to collect fully identifiable data [ECC 5‐05 (f)/2012]. Permission was obtained to use the data for the purposes of this project.
The UKOSS general methodology (04/MRE02/45) and severe sepsis study (10/H0717/20) were approved by the London Research Ethics Committee. No additional ethical review was required for this secondary data analysis.
Funding
The Maternal, Newborn and Infant Clinical Outcome Review programme, delivered by MBRRACE‐UK, is commissioned by the Healthcare Quality Improvement Partnership (HQIP) on behalf of NHS England, NHS Wales, the Health and Social Care division of the Scottish government, the Northern Ireland Department of Health, Social Services and Public Safety (DHSSPS), the States of Jersey, Guernsey, and the Isle of Man.
This study was part‐funded by the National Institute for Health Research (NIHR, programme grant RP‐PG‐0608‐10038) and part‐funded by the Policy Research Programme in the Department of Health. MK is funded by an NIHR Research Professorship. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Supporting information
Table S1. Role of specific medical conditions as risk factors for death from sepsis.
Acknowledgements
This study would not have been possible without the contribution and enthusiasm of the MBRRACE‐UK and UKOSS reporting clinicians who notified cases and completed the data collection and surveillance forms.
Mohamed‐Ahmed O, Nair M, Acosta C, Kurinczuk JJ, Knight M. Progression from severe sepsis in pregnancy to death: a UK population‐based case‐control analysis. BJOG 2015;122:1506–1515.
Linked article This article is commented on by D Eschenbach, p. 1516 in this issue. To view this mini commentary visit http://dx.doi.org/10.1111/1471-0528.13558.
References
- 1. Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk J, et al. Saving Lives, Improving Mothers' Care—Lessons Learned to Inform Future Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–12. Oxford: National Perinatal Epidemiology Unit, University of Oxford, 2014. [Google Scholar]
- 2. Cantwell R, Clutton‐Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118(Suppl 1):1–203. [DOI] [PubMed] [Google Scholar]
- 3. Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010;63:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Filippi V, Alihonou E, Mukantaganda S, Graham WJ, Ronsmans C. Near misses: maternal morbidity and mortality. Lancet 1998;351:145–6. [DOI] [PubMed] [Google Scholar]
- 5. Say L, Souza JP, Pattinson RC, Mortality WHO, Working Group on Maternity and Morbidity classification . Maternal near miss—towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol 2009;23:287–96. [DOI] [PubMed] [Google Scholar]
- 6. Acosta CD, Kurinczuk JJ, Lucas DN, Tuffnell DJ, Sellers S, Knight M, et al. Severe maternal sepsis in the UK, 2011–2012: a national case‐control study. PLoS Med 2014;11:e1001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell O, Graham W. Measuring the Determinants of Maternal Mortality and Morbidity. London: Maternal and Child Epidemiology Unit, London School of Hygiene and Tropical Medicine, 1990. [Google Scholar]
- 8. Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74. [DOI] [PubMed] [Google Scholar]
- 9. Acosta CD, Bhattacharya S, Tuffnell D, Kurinczuk JJ, Knight M. Maternal sepsis: a Scottish population‐based case‐control study. BJOG 2012;119:474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Acosta CD, Knight M, Lee HC, Kurinczuk JJ, Gould JB, Lyndon A. The continuum of maternal sepsis severity: incidence and risk factors in a population‐based cohort study. PLoS ONE 2013;8:e67175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bauer ME, Bateman BT, Bauer ST, Shanks AM, Mhyre JM. Maternal sepsis mortality and morbidity during hospitalization for delivery: temporal trends and independent associations for severe sepsis. Anesth Analg 2013;117:944–50. [DOI] [PubMed] [Google Scholar]
- 12. Kayem G, Kurinczuk J, Lewis G, Golightly S, Brocklehurst P, Knight M. Risk factors for progression from severe maternal morbidity to death: a national cohort study. PLoS ONE 2011;6:e29077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nair M, Kurinczuk J, Brocklehurst P, Sellers S, Lewis G, Knight M. Factors associated with maternal death from direct pregnancy complications: a UK national case‐control study. BJOG 2015;22:653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mhyre JM, Bateman BT, Leffert LR. Influence of patient comorbidities on the risk of near‐miss maternal morbidity or mortality. Anesthesiology 2011;115:963–72. [DOI] [PubMed] [Google Scholar]
- 15. Daniels R, Nutbeam T, McNamara G, Galvin C. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J 2011;28:507–12. [DOI] [PubMed] [Google Scholar]
- 16. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- 17. Townsend SR, Schorr C, Levy MM, Dellinger RP. Reducing mortality in severe sepsis: the Surviving Sepsis Campaign. Clin Chest Med 2008;29:721–33, x. [DOI] [PubMed] [Google Scholar]
- 18. Royal College of Obstetricians and Gynaecologists . Bacterial Sepsis in Pregnancy: Green‐top Guideline no. 64a. London: Royal College of Obstetricians and Gynaecologists (RCOG), 2012. [Google Scholar]
- 19. Royal College of Obstetricians and Gynaecologists . Bacterial Sepsis following Pregnancy: Green‐top Guideline no. 64b. London: Royal College of Obstetricians and Gynaecologists (RCOG), 2012. [Google Scholar]
- 20. Appelboam R, Tilley R, Blackburn J. Time to antibiotics in sepsis. Crit Care 2010;14(Suppl 1):50. [Google Scholar]
- 21. McGregor C. Improving time to antibiotics and implementing the ‘Sepsis 6’. BMJ Qual Improv Rep 2014;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurinczuk J, Draper ES, Field DJ, Bevan C, Brocklehurst P, Gray R, et al. Experiences with maternal and perinatal death reviews in the UK: the MBRRACE‐UK programme. Br J Obstet Gynaecol 2014;121(Suppl 4):41–6. [DOI] [PubMed] [Google Scholar]
- 23. Knight M, Kurinczuk JJ, Tuffnell D, Brocklehurst P. The UK Obstetric Surveillance System for rare disorders of pregnancy. BJOG 2005;112:263–5. [DOI] [PubMed] [Google Scholar]
- 24. Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case‐control study. BMJ 2001;322:1089–93; discussion 93–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rothman KJ, Boice JD. Epidemiologic Analysis with a Programmable Calculator. Bethesda, MD: National Institutes of Health, 1979. [Google Scholar]
- 26. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindquist A, Knight M, Kurinczuk JJ. Variation in severe maternal morbidity according to socioeconomic position: a UK national case‐control study. BMJ Open 2013;3:e002742. doi: 10.1136/bmjopen‐2013‐002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case‐control studies. II. Types of controls. Am J Epidemiol 1992;135:1029–41. [DOI] [PubMed] [Google Scholar]
- 30. Kramer HM, Schutte JM, Zwart JJ, Schuitemaker NW, Steegers EA, van Roosmalen J. Maternal mortality and severe morbidity from sepsis in the Netherlands. Acta Obstet Gynecol Scand 2009;88:647–53. [DOI] [PubMed] [Google Scholar]
- 31. Nair M, Kurinczuk JJ, Knight M. Ethnic variations in severe maternal morbidity in the UK—a case control study. PLoS ONE 2014;9:e95086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029–36. [DOI] [PubMed] [Google Scholar]
- 33. Clutton‐Brock T, Cooper G, Hall M, Harper A, Hepburn M, Nielson J, et al. Why Mothers Die 2000–20002. The Sixth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: Royal College of Obstetricians and Gynaecology (RCOG), 2004. [Google Scholar]
- 34. Lewis G. Saving Mothers' Lives: Reviewing Maternal Deaths to make Motherhood Safer—2003–2005. London: The Confidential Enquiry into Maternal and Child Health (CEMACH), 2007. [Google Scholar]
- 35. Poutsiaka DD, Davidson LE, Kahn KL, Bates DW, Snydman DR, Hibberd PL. Risk factors for death after sepsis in patients immunosuppressed before the onset of sepsis. Scand J Infect Dis 2009;41:469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piagnerelli M, Boudjeltia KZ, Gulbis B, Vanhaeverbeek M, Vincent J‐L. Anemia in sepsis: the importance of red blood cell membrane changes. Transfus Altern Transfus Med 2007;9:143–9. [Google Scholar]
- 37. Castellanos‐Ortega A, Suberviola B, Garcia‐Astudillo LA, Holanda MS, Ortiz F, Llorca J, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med 2010;38:1036–43. [DOI] [PubMed] [Google Scholar]
- 38. Health Protection Services . HPA Guidance on Use of Antiviral Agents for the Treatment and Prophylaxis of Influenza. Version 3. London: Health Protection Agency, 2012. [Google Scholar]
- 39. Pavord S, Myers B, Robinson S, Allard S, Strong J, Oppenheimer C, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 2012;156:588–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Role of specific medical conditions as risk factors for death from sepsis.


