Abstract
Titanium dioxide nanoparticles are photoactive and produce reactive oxygen species under natural sunlight. Reactive oxygen species can be detrimental to many organisms, causing oxidative damage, cell injury, and death. Most studies investigating TiO2 nanoparticle toxicity did not consider photoactivation and performed tests either in dark conditions or under artificial lighting that did not simulate natural irradiation. The present study summarizes the literature and derives a phototoxicity ratio between the results of nano‐titanium dioxide (nano‐TiO2) experiments conducted in the absence of sunlight and those conducted under solar or simulated solar radiation (SSR) for aquatic species. Therefore, the phototoxicity ratio can be used to correct endpoints of the toxicity tests with nano‐TiO2 that were performed in absence of sunlight. Such corrections also may be important for regulators and risk assessors when reviewing previously published data. A significant difference was observed between the phototoxicity ratios of 2 distinct groups: aquatic species belonging to order Cladocera, and all other aquatic species. Order Cladocera appeared very sensitive and prone to nano‐TiO2 phototoxicity. On average nano‐TiO2 was 20 times more toxic to non‐Cladocera and 1867 times more toxic to Cladocera (median values 3.3 and 24.7, respectively) after illumination. Both median value and 75% quartile of the phototoxicity ratio are chosen as the most practical values for the correction of endpoints of nano‐TiO2 toxicity tests that were performed in dark conditions, or in the absence of sunlight. Environ Toxicol Chem 2015;34:1070–1077. © 2015 The Author. Published by SETAC.
Keywords: Titanium dioxide, Nanoparticles, Photoxicity, Simulated solar radiation, Photoactivation
INTRODUCTION
Titanium dioxide (TiO2) is a component of many sunscreens, soaps, shampoos, toothpastes, cosmetics, paper products, plastics, ink, paint, and building materials 1 in both its bulk form and its nanoform. It is also used in human food as a colorant and inactive ingredient, where it can also be present in both forms 1, 2. From 1916 to 2011, an estimated total of 165 050 000 metric tonnes of TiO2 pigment were produced worldwide (bulk form and nanoform combined), with a current annual estimated production of more than 6 million tonnes/yr 2. Reviews of nano‐TiO2 toxicology are available across various evolutionary groups of species 3, 4, 5, 6, 7, 8, often summarizing half maximal effective concentration (EC50), half maximal inhibitory concentration (IC50), and median lethal concentration (LC50) values. Nano‐TiO2 is also photoactive and produces reactive oxygen species (ROS) on illumination 9. Reactive oxygen species can be detrimental to many organisms, causing oxidative damage, cell injury, and ultimately death 10. Recently, it has been argued that photoactivation of nano‐TiO2 under natural levels of sunlight is sufficient to affect the output of LC50 and EC50 values in standard toxicology tests 11, 12. The majority of studies investigating nano‐TiO2 toxicity did not take photoactivation into account and performed tests either in dark conditions or under indoor commercial artificial lighting that did not simulate natural solar irradiation.
The aim of present study was to derive a phototoxicity ratio between the results of the nano‐TiO2 experiments conducted in the absence of sunlight and conducted in the presence of solar or simulated solar radiation (SSR). To achieve this aim, we searched the literature for studies that included nano‐TiO2 experiments both with and without irradiance under the same experimental setup and otherwise identical conditions. Therefore, the phototoxicity ratio can be used to correct endpoints of the toxicity tests with nano‐TiO2 that were performed in absence of natural sunlight or SSR. Such corrections also may be important for regulators and risk assessors when reviewing previously published data. Another aim is to provide information for improvement of risk assessment of nano‐TiO2. For example, one of the current challenges for conducting risk assessment of nanoparticles such as TiO2 is lack of consistent toxicity data because of the varieties of materials and test conditions. A phototoxicity ratio derived from existing literature will help to harmonize the toxicity data. Regulatory thresholds for nano‐TiO2 do not exist currently; however, regulation of nanoparticles discharge and monitoring in aquatic environment is anticipated in the future. It is expected that regulators will use the phototoxicity ratio when deriving thresholds for nano‐TiO2 because the majority of the published literature reported toxicity endpoints for nano‐TiO2 in the absence of natural sunlight or SSR.
Of course, the phototoxicity ratio value calculated in present study is not absolutely precise and correct for all environmental conditions and species; it does, however, considerably reduce the possible error of data endpoints obtained in the absence of natural sunlight or SSR. It also mitigates uncertainties in the risk assessment process by taking into account the photoactivation and phototoxicity of nano‐TiO2.
METHODS
A comprehensive literature review was conducted to collect available toxicity endpoints for nano‐TiO2. The literature search (September 2014) was performed within 4 databases—Web of Science, Scopus, Google Scholar, and the University of British Columbia library database—using the following keywords in various combinations: titanium dioxide, TiO2, nanoparticles, phototoxicity, photoactivation, EC50, LC50, IC50, and lowest‐observed‐effect concentration (LOEC). Abstracts of numerous hits were read, and downloaded papers were checked for useful information. Only papers that reported results under the same environmental conditions for 2 different nano‐TiO2 exposure groups (with and without SSR) in the form of EC50, LC50, IC50, LOEC, or a ratio were selected. Thus, all included data were based on dose–response curves, ensuring the highest possible quality. Such methodology was selected based on the previous study of nano toxicity ratios 13. The phototoxicity ratio (PR) was calculated in the form of a ratio:
A phototoxicity ratio greater than 1 means nano‐TiO2 is phototoxic.
In a few isolated cases, results were not given in the form of a number; but it was possible to derive a number based on figures provided. Because the papers reported only irradiance (power of electromagnetic radiation per unit area) intensity and not actual insolation (total amount of solar radiation or SSR energy received on a given surface area during a given time), insolation value was calculated when possible. Insolation was calculated based on the irradiance (W/m2), actual duration of irradiance (h), and total duration of the toxicity test. In cases in which irradiance intensity was reported in units other than W/m2, the data were converted for consistency. For the studies in which data existed only in the form of full spectrum insolation, it was important to at least approximate the levels of ultraviolet A (UVA) and ultraviolet B (UVB) used in the studies. At sea level, UVA spectra is accountable for 5.7% of the total sunlight, whereas UVB is accountable for 0.3% of the total sunlight 14. Thus, UVA and UVB approximations were performed on the studies reporting full spectrum with factors of 0.057 and 0.003 for UVA and UVB, respectively. Such conversions allowed us to determine whether UVA and UVB levels used in the studies were of environmental relevance by comparing the data with published averaged UVA and UVB levels over Europe. The original data of insolation of the full spectrum also are presented.
Because the focus of the present study is environmental relevance, in all cases, data points were excluded from evaluation if testing conditions did not represent environmentally relevant exposure conditions, such as in vitro toxicity tests with cells. Only data from in vivo studies were used. In several studies, nano‐TiO2 toxicity was reported as greater than the highest exposure concentration with no negative toxic effects. In those cases, the highest tested concentration value was used to derive phototoxicity ratio. This procedure was only applied if the reported “greater than” value was from the control TiO2 group that was not exposed to SSR or sunlight. This might have led to a slight underestimation of the phototoxicity ratio value, which can be seen as a conservative approach. In all cases, the crystal structure of the nano‐TiO2 particles, their primary particle size, and their hydrodynamic diameter were reported, and the data are presented in Table 1. Therefore, collected data are a mixture of both anatase and rutile crystal forms as well as various particle sizes. Ecosystems generally contain a mixture of all sizes and types of crystal structures of anthropogenically introduced nanoparticles with which decision makers have to cope simultaneously; thus, the aim of the phototoxicity ratio is to provide a distinct value within a muddle. The coating of nano‐TiO2 was not taken into account when evaluating phototoxicity of nano‐TiO2, since all of the collected studies have investigated exclusively bare nano‐TiO2.
Table 1.
Review of nano‐titanium dioxide (TiO2) phototoxicity to various species
| Organism | TiO2 primary particle size (nm) a | Hydrodynamic diameter of TiO2 (nm) | Endpoint | Control group | Experimental group | Irradiance (W/m2) | Test duration (h) | Irradiance duration (h:min) | Full spectrum insolation (Wh/m2) | UVA insolation (Wh/m2) | UVB insolation (Wh/m2) | PR | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50; IC50; LC50; LOEC (mg/L) | EC50; IC50; LC50; LOEC (mg/L) | |||||||||||||||
| Dark | Indoor light | UVA | UVB | Full spectrum | ||||||||||||
| Aeromonas hydrophilla | 81 A | NA | EC50 | 25 b | 2.5 b | 200 | 2 | 2:00 | 200.00 | 11.4 | 0.6 | 10.0 | 26 | |||
| Aeromonas hydrophilla | 50–120 A | 299–666 | IC50 | 100 | 40 | 1/3 | 2.5 | 27 | ||||||||
| Aeromonas hydrophilla | 20–50 A | 253–608 | IC50 | 100 | 50 | 1/3 | 2.0 | 27 | ||||||||
| Aeromonas hydrophilla | 50–130 A | 236–618 | IC50 | 100 | 60 | 1/3 | 1.7 | 27 | ||||||||
| Aeromonas hydrophilla | 70–200 A | 279–427 | IC50 | 100 | 100 | 1/3 | 1.0 | 27 | ||||||||
| Aeromonas hydrophilla | 15–25 A/R | 401–872 | IC50 | 100 | 100 | 1/3 | 1.0 | 27 | ||||||||
| Artemia salina | 25 A | 1600–2400 | EC50 | 480.7 | 4.05 | 6 | 48 | 48:00 | 6 | 0.342 | 0.018 | 118.7 | 28 | |||
| Artemia salina | 25 A/R | 1400–3700 | EC50 | 284.8 | 4.03 | 6 | 48 | 48:00 | 6 | 0.342 | 0.018 | 70.7 | 28 | |||
| Bacillus licheniformis | 25 A | 20–2000 | EC50 | 19.57 | 5.23 | 3.7 | 29 | |||||||||
| Bacillus licheniformis | 25 A | 20–2000 | EC50 | 17.66 | 4.98 | 2 | 2:00 | 3.5 | 29 | |||||||
| Bacillus subtilis | 66 A/R | 320 | Ratio | X | X | 20 | 6:00 | 2.5 | 30 | |||||||
| Bacillus subtilis | 81 A | NA | EC50 | 25 b | 0.5 b | 200 | 1 | 1:00 | 200.00 | 11.4 | 0.6 | 50.0 | 26 | |||
| Bacillus subtilis | <50 | 828 | EC50 | 300 | 230 | 8.2 | 1/3 | 0:20 | 2.73 | 0 | 1.3 | 31 | ||||
| Bacillus subtilis | <50 | 828 | EC50 | 300 | 300 | 5.68 | 1/3 | 0:20 | 1.89 | 0 | 1.89 | 1 | 31 | |||
| Caenorhabditis elegans | 21 A/R | 300–1500 | EC50 | 100 | 53 | 231 | 96 | 0:30 | 1.20 | 0.0686 | 0.0036 | 1.9 | 32 | |||
| Ceriodaphnia dubia | 25 A | 200–1000 | EC50 | 27.45 | 8.26 | 48 | 16:00 | 3.3 | 33 | |||||||
| Danio rerio | 23 A/R | 50–1000 | LC50 | 1 | 0.3 | 100 c | 96 | 32:00 | 33.33 | 0 | 3.3 | 34 | ||||
| Danio rerio | 21 A/R | 1243 | Ratio | X | 50 | 168 | 24:00 | 7.14 | 0.4071 | 0.0214 | 1.5 | 35 | ||||
| Danio rerio | 21 A/R | 200–2000 | LC50 | 500 | 500 | 17 | 96 | 16 | 2.83 | 2.83 | 0 | 1 | 19 | |||
| Danio rerio | 21 A/R | 200–2000 | LC50 | 500 | 34 | 17 | 96 | 16 | 2.83 | 2.83 | 0 | 14.7 | 19 | |||
| Danio rerio | 21 A/R | 200–2000 | LC50 | 500 | 135 | 17 | 96 | 16 | 2.83 | 2.83 | 0 | 3.7 | 19 | |||
| Danio rerio | 21 A/R | 200–2000 | LC50 | 500 | 20.3 | 17 | 96 | 16 | 2.83 | 2.83 | 0 | 24.6 | 19 | |||
| Daphnia magna | 21 A/R | 345 | EC50 | 29.7 | 1.2 | 5.6 | 48 | 32:00 | 3.73 | 3.73 | 0 | 24.8 | 36 | |||
| Daphnia magna | 21 A/R | 358 | EC50 | 33.6 | 3.4 | 5.6 | 48 | 32:00 | 3.73 | 3.73 | 0 | 9.9 | 36 | |||
| Daphnia magna | 21 A/R | 1600–3400 | LC50 | 118 | 0.06 | 17 | 48 | 8:00 | 2.83 | 0.1615 | 0.0085 | 1967 | 37 | |||
| Daphnia magna | 25 A/R | 150–190 | LC50 | 500 | 0.0298 | 17 | 48 | 8:00 | 2.83 | 0.1613 | 0.0085 | 16778 | 12 | |||
| Daphnia magna | <40 A | <112 | LC50 | 500 d | 0.14 | 47 | 8 | 8:00 | 47 | 2.6790 | 0.0141 | 3597 | 38 | |||
| Daphnia similis | 25 A | 580–1020 | EC50 | 1000 | 750.55 | 0.46 | 48 | 48:00 | 0.46 | 0.0262 | 0.0014 | 1.3 | 28 | |||
| Daphnia similis | 25 A/R | 780–1400 | EC50 | 1000 | 60.16 | 0.46 | 48 | 48:00 | 0.46 | 0.0262 | 0.0014 | 16.6 | 28 | |||
| Daphnia similis | 35 R | 350 | EC50 | 100 | 100 | 48 | 1 | 39 | ||||||||
| Daphnia similis | 25 A/R | 400 | EC50 | 100 | 7.8 | 48 | 12.8 | 39 | ||||||||
| Dunaliella tertiolecta | <30 A/R | NA | NOEC | 7 | 3 | 8.6 | 168 | 98:00 | 5.02 | 0.2860 | 0.0151 | 2.3 | 40 | |||
| Escheria coli | 66 A/R | 320 | Ratio | X | X | 20 | 6:00 | 1.8 | 30 | |||||||
| Escheria coli | 42 | NA | LC50 | 583 | 1.68 | 1/2 | 0:30 | 347 | 41 | |||||||
| Escheria coli | <50 | 828 | EC50 | 300 | 300 | 8.2 | 1/3 | 0:20 | 2.73 | 2.73 | 0 | 1 | 31 | |||
| Escheria coli | <50 | 828 | EC50 | 300 | 168 | 5.68 | 1/3 | 0:20 | 1.89 | 0 | 1.89 | 1.8 | 31 | |||
| Escheria coli | 23 A/R | 970 | IC50 | 25 | 2.7 | 2 | 2:00 | 9.3 | 42 | |||||||
| Escheria coli | 79 A | 461 | IC50 | 25 | 4.2 | 6.0 | 42 | |||||||||
| Escheria coli | 15 A | 639 | IC50 | 25 | 9.1 | 2.7 | 42 | |||||||||
| Escheria coli | 81 A | 436 | IC50 | 25 | 5 | 5.0 | 42 | |||||||||
| Escheria coli | 15–25 A/R | 401–872 | IC50 | 100 | 5.9 | 1 | 16.9 | 27 | ||||||||
| Escheria coli | 70–200 A | 279–427 | IC50 | 100 | 5.3 | 1 | 18.9 | 27 | ||||||||
| Escheria coli | 20–50 A | 253–608 | IC50 | 100 | 11.5 | 1 | 8.7 | 27 | ||||||||
| Escheria coli | 50–130 A | 236–618 | IC50 | 100 | 29.3 | 1 | 3.4 | 27 | ||||||||
| Escheria coli | 50–120 A | 299–666 | IC50 | 100 | 66.8 | 1 | 1.5 | 27 | ||||||||
| Gammarus fossarum | 21 A/R | 97 | X | X | 28.9 | 168 | 84:00 | 14.45 | 0.8237 | 0.0434 | 2 b | 43 | ||||
| Hyalella azteca | 25 A/R | 616–972 | LC50 | 631 | 29.9 | 2.2 | 96 | 16:00 | 0.37 | 0.0209 | 0.0011 | 21.1 | 44 | |||
| Isochrysis galbana | <30 A/R | NA | NOEC | 7 | 1 | 8.6 | 168 | 98:00 | 5.02 | 0.2860 | 0.0151 | 7.0 | 40 | |||
| Moina macropoa | 21 A/R | 298 | EC50 | 3.6 | 0.0071 | 1.7 | 48 | 48:00 | 1.7 | 1.7 | 0 | 507 | 45 | |||
| Moina macropoa | 21 A/R | 132 | EC50 | 2.8 | 0.0033 | 1.7 | 48 | 48:00 | 1.7 | 1.7 | 0 | 848.5 | 45 | |||
| Moina macropoa | 21 A/R | 72 | EC50 | 19 | 0.0372 | 1.7 | 48 | 48:00 | 1.7 | 1.7 | 0 | 510.8 | 45 | |||
| Oryzias latipes | 21 A/R | 1600–3400 | LC50 | 500 | 8.5 | 17 | 48 | 8:00 | 2.83 | 0.1615 | 0.0085 | 58.8 | 37 | |||
| Oryzias latipes | 25 A/R | 200–2400 | LC50 | 294 | 2.46 | 17 | 96 | 16 | 2.83 | 0.1615 | 0.0085 | 119.5 | 12 | |||
| Pseudokirchneriella subcapitata | 21 A/R | 486 | EC50 | 2.53 | 3 | 82 | 72 | 0:01 | 0.02 | 0.02 | 0 | 0.8 | 46 | |||
| Pseudokirchneriella subcapitata | 21 A/R | 486 | EC50 | 2.53 | 2.95 | 56.8 | 72 | 0:01 | 0.01 | 0 | 0.01 | 0.9 | 46 | |||
| Skeletonema costatum | <30 A/R | NA | NOEC | 7 | 7 | 8.6 | 168 | 98:00 | 5.02 | 0.2860 | 0.0151 | 1 | 40 | |||
| Thalassiosira pseudonana | <30 A/R | NA | NOEC | 7 | 3 | 8.6 | 168 | 98:00 | 5.02 | 0.2860 | 0.0151 | 2.3 | 40 | |||
| Xenopus laevis | 32 A | 103–1354 | NOEC | 77.7 | 77.7 | 40 | 336 | 196:00 | 23.33 | 23.33 | 0 | 1.0 | 47 | |||
| Xenopus laevis | 10 A | 177–666 | NOEC | 281.2 | 30.9 | 40 | 336 | 196:00 | 23.33 | 23.33 | 0 | 9.1 | 47 | |||
| Xenopus laevis | 5 A | 39–398 | NOEC | 90.2 | 9.5 | 40 | 336 | 196:00 | 23.33 | 23.33 | 0 | 9.5 | 47 | |||
| Xenopus laevis | 32 A | 103–1354 | LC50 | 295.1 | 268 | 40 | 336 | 196:00 | 23.33 | 23.33 | 0 | 1.1 | 47 | |||
| Xenopus laevis | 5 A | 39–398 | LC50 | 210.2 | 57.9 | 40 | 336 | 196:00 | 23.33 | 23.33 | 0 | 3.6 | 47 | |||
Anatase (A) or rutile (R).
Derived data based on the presented data.
Calculated as 0.45 m distance; 30% shadow angle; and 250 W light source.
Not in total darkness. Control received 10% of light source.
EC50 = median effective concentration; IC50 = median inhibitory concentration; LC50 = median lethal concentration; LOEC = lowest‐observed‐effect concentration; UVA = ultraviolet A; UVB = ultraviolet B; PR = phototoxicity ratio; NOEC = no‐observed‐effect concentration; NA = data are not available.
Data were checked for normality with the Kolmogorov‐Smirnov test and were found not to be of normal distribution. Spearman rank correlation was performed between the phototoxicity ratio value and time duration of the reported toxicity test, time duration of irradiance, irradiance intensity, insolation, and the organism taxa to determine whether any of these variables drove the output value. In the case of organism taxa, for the purpose of analysis, a code of 5 different digits was assigned to bacteria, algae, invertebrates, fish, and amphibians. Kruskal‐Wallis analysis of variance with a post hoc multiple comparison and/or Mann‐Whitney U test were also performed where applicable.
Validation of phototoxicity ratios in correction of toxicity tests endpoint values was performed on data obtained in absence of sunlight or SSR. “True” phototoxicity data summarized in Table 1 (obtained in the presence of sunlight or SSR), served as a control group. Results were log 10 transformed and then statistically compared with either log 10 (data), log 10 (data/median phototoxicity ratio), or log 10 (data/75% phototoxicity ratio quartile). These 3 groups of results originated from the same set of analyzed studies but were obtained in the absence of sunlight or SSR.
RESULTS
The literature search resulted in 25 usable references, from which 62 pairs of data were generated for calculation of a phototoxicity ratio (Table 1). In total, experiments were performed on 20 different species, ranging from bacteria to amphibians. Applied total irradiance was between 0.46 W/m2 and 231 W/m2 (mean, 35.63 W/m2; median, 17 W/m2), and effective total insolation was between 0.013 Wh/m2 and 200 Wh/m2 (mean, 17.44 Wh/m2; median, 2.83 Wh/m2). The recalculated and approximated insolation mean and median data are, respectively, 5.64 W/m2 and 1.7 W/m2 for UVA, and 0.243 W/m2 and 0.015 W/m2 for UVB. The majority of the studies have used the same nano‐TiO2 products (P25 Degussa), resulting in a fairly similar size span of primary particle diameter.
Phototoxicity ratio minimum and maximum values were 0.84 and 16 778, respectively, and mean and median values were 407.5 and 3.7. The discrepancy between the mean and median was caused primarily by the data associated with the Cladocera taxon. When the data were analyzed for susceptibility of bacteria, algae, invertebrates, fish, and amphibians to phototoxicity, the invertebrates were significantly different compared with other groups. Nano‐TiO2 was significantly more toxic to invertebrates after exposure to light compared with other groups, resulting in a greater phototoxicity ratio (Kruskal‐Wallis followed by post hoc multiple comparison). On the other hand, the Spearman rank correlation test was not statistically significant for phylogeny and phototoxicity ratio (decreased or increased phototoxicity of nano‐TiO2 from species on the lower organism stadium, such as bacteria, toward more complex organisms, such as amphibians). Also, there was no correlation between phototoxicity ratio and irradiation intensity, duration of irradiation, or received insolation.
Indeed, when exclusive Cladocera data were analyzed against all other taxa (Figure 1), the statistical difference was highly significant (Mann‐Whitney U test, p < 0.01). Because of the clear need for data segregation, separate descriptive statistics were performed for Cladocera and non‐Cladocera phototoxicity ratio values (Table 2). On average, nano‐TiO2 was 20 times more toxic to non‐Cladocera and 1867 times more toxic to Cladocera (median values, 3.3 and 24.7, respectively) after illumination.
Figure 1.
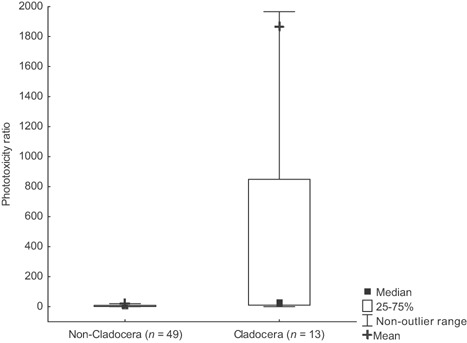
Comparison of phototoxicity ratio values between Cladocera and non‐Cladocera species.
Table 2.
Descriptive statistics of phototoxicity ratio (PR) values
| PR | Valid n | Mean | Median | Minimum | Maximum | 25% quartile | 75% quartile | Standard deviation | Standard error |
| Cladocera | 12 | 1867.56 | 24.75 | 1.00 | 16 778.5 | 9.88 | 848.48 | 46 02.74 | 1276.57 |
| Non‐Cladocera | 49 | 20.09 | 3.33 | 0.84 | 347.02 | 1.50 | 9.49 | 54.50 | 7.79 |
Significant statistical difference was observed between “true” phototoxicity data and data obtained in the absence of sunlight or SSR (Figure 2). Once the data were corrected by dividing data obtained in the absence of sunlight or SSR with a median phototoxicity ratio or a 75% quartile phototoxicity ratio value, there was no longer statistical difference compared with the data obtained in the presence of sunlight or SSR (Figure 2). However, we do not claim that values for the median phototoxicity ratio and the 75% phototoxicity ratio quartile are definite, because they will change over time as more data points become available from future studies.
Figure 2.
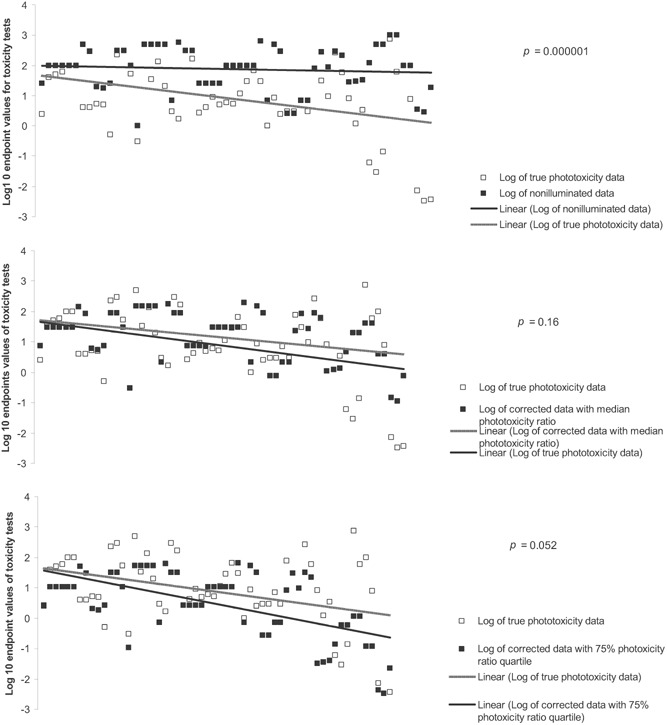
Comparison of uncorrected data (top), corrected data with median phototoxicity ratio (middle), and corrected data with 75% phototoxicity ratio quartile (bottom) obtained in the absence of sunlight or simulated solar radiation (SSR) with true data obtained in the presence of sunlight or SSR. Cladocera and non‐Cladocera data were corrected separately with the group corresponding median or 75% quartile values.
DISCUSSION
The fact that the Cladocera taxon was more sensitive to nano‐TiO2 phototoxicity cannot be explained by the intensity of irradiation or received insolation during testing, because such correlation was not statistically significant (Spearman rank correlation test). In Cladocera‐related experiments, median irradiation and insolation were even smaller than in experiments with other species. The original publications from which data were derived provided no evidence that Cladocera were exposed to any specific grade, type, or size of nano‐TiO2 particles to which other taxa were not exposed. Ultraviolet sensitivity of the taxon has to be ruled out as well, because appropriate exposure controls to UV were included and no increase in toxicity was detected. Although UV is toxic and lethal to Cladocera at higher exposure doses, numerous protection mechanisms prevent hazardous occurrences at lower doses 15. Both UV and nano‐TiO2 toxicity are based on ROS, and oxidative stress was indicated in Cladocera exposed to either UV 15, 16 or nano‐TiO2 17. However, this does not necessarily mean that UV and nano‐TiO2 have the same toxicity mechanism. Whereas generation of ROS and consequently oxidative stress following exposure to UV radiation requires endogenous photosensitizer molecules, generation of ROS by nano‐TiO2 under UV radiation is a direct photochemical process, and the substantial ROS production can readily damage or kill cells or organisms such as Cladocera. Why Cladocera are more sensitive to irradiated nano‐TiO2 remains unclear, and more targeted research is needed. However, one possible explanation for the high sensitivity of Cladocera to nano‐TiO2 phototoxicity is that photoinduced ROS on the surface of Cladocera carapace may interfere with the respiratory gas exchange. In fact, surface attachment of nano‐TiO2 to Cladocera carapace has been observed in previous studies 18, 19, and the inner wall of the carapace is a major site of respiratory gas exchange for Cladocera 20.
Sunlight is composed of visible, UV, and infrared spectra. Some of the analyzed studies reported irradiance values exclusively within the UV spectrum, whereas others reported values for the full spectrum. Thus, insolation data also were presented based on reported irradiance spectrum. When the data were segregated into what appeared to be insolation values for the full spectrum, the mean insolation was 25.9 Wh/m2, and the median was 5 Wh/m2. The studies that supposedly only reported values for the UV spectrum had a mean insolation of 8.72 Wh/m2, with a median of 2.83 Wh/m2. For the purpose of comparison, a solar constant (irradiance of the sun when positioned at 1 astronomical unit compared with Earth at zenith) measured at the outer surface of Earth's atmosphere is approximately 1360 W/m2 21. A significant amount of the solar constant is lost by the time sunlight reaches a location on the Earth's surface, depending on atmosphere, latitude, and time of day. For example, average insolation of the visible spectrum during a decade of measurements over Europe is between 5 Wh/m2 and 302 Wh/m2 in winter and between 285 Wh/m2 and 430 Wh/m2 in summer 22. Therefore, both the mean and median (25.9 Wh/m2 and 5 Wh/m2, respectively) insolation used in the studies reporting only values for full spectrum are much less than the insolation values over Europe.
The most likely culprits for TiO2 phototoxicity are UVA and UVB spectrum because those photons would have enough quantum energy (UVA, 3.10–3.94 eV per photon; UVB, 3.94–4.43 eV per photon) 23 versus energy of visible light photon (1.6–3.4 eV) to overcome the band gap. When UVA and UVB level approximations were performed on the studies reporting full spectrum and combined with studies directly reporting UVA and UVB, mean and median values were 5.64 W/m2 and 1.7 W/m2, respectively, for UVA and 0.243 W/m2 and 0.015 W/m2, respectively, for UVB. The actual UV spectrum insolation over Europe is, on average, 0.7 Wh/m2 to 37.7 Wh/m2 in winter and 34 Wh/m2 to 64.2 Wh/m2 in summer for UVA; for UVB, the average is 0.001 Wh/m2 to 1.08 Wh/m2 in winter and 0.77 Wh/m2 to 2.05 Wh/m2 in summer 22. The mean and the median values for UVA and UVB used in toxicity studies are well within the range of UVA and UVB values over Europe 22. Therefore, the current experimental setups represent realistic and natural conditions, and the obtained results should not be doubted. Thus, levels used in experimental setups are credible for the purpose of risk assessment, since they do not exceed natural conditions. It is important to note, however, that approximation to the UVA and UVB values were based on the assumption that all of the irradiation lamps spectra used in the phototoxicity studies fully corresponded to sunlight spectrum. An early study in 1965 suggested that this might not be the case 24. Although the technology has advanced significantly over the years, there is no absolute guarantee that all of the studies had the proper irradiation lamps. Furthermore, a significant amount of irradiation at sea level altitude is lost because of reflection and adsorption in the water column, according to the Beer‐Lambert law
where z is depth, e is natural logorithm, k is attenuation coefficient, and I 0 is the energy of the sunlight at the surface of the water. Although attenuation in pure water might not affect energy of UV light, reflectance of the water surface may, thus reducing the actual UV energy to which aquatic organisms are exposed. However, an opposite effect may occur in shallow waters because of strong scattering of light, thus multiplying the UV exposure levels 25. Shading effects of macrophyte vegetation may also affect the level of available light. Therefore, although UV insolation levels currently used in nano‐TiO2 phototoxicity studies are credible at the water surface level, it is still not clear whether they are credible for risk assessment below the water surface. The fact that different studies used different exposure time and different irradiances only suggest that current scientific community does not really have a standardized toxicity test to check for the phototoxicity effects of nanoparticles. Therefore, we strongly recommend that universal agreement on irradiation time and irradiance in a standard nanomaterial phototoxicity test is necessary.
A validation test of phototoxicity ratio correction (Figure 2) showed that after correction with a median phototoxicity ratio value the corrected data are no longer statistically different from the real data obtained in the presence of sunlight or SSR. Data correction for the 75% quartile of the phototoxicity ratio was still not significantly different from the real data (p = 0.052) but in general generated much lower endpoints (higher toxicity), as expected. However, the value of 75% quartile application is that, compared with the median phototoxicity ratio, it can more successfully prevent false toxicity underestimation. The use of phototoxicity ratio does not mean that the newly corrected data are the true representation of endpoints from toxicity tests, but rather that they are likely as close as possible. The true correction of data can be achieved only by defining a function through regression analysis. However, because many variables— such as particle size, hydrodynamic diameter, crystal structure, illumination time, irradiance, insolation, species, and organic matter content in test media—will likely influence the phototoxicity of nano‐TiO2 (even if their effect is not statistically significant, they will contribute certain percentage of variability), generating such a function will be difficult. In addition, its use in practice will likely not be feasible. Therefore, the use of a phototoxicity ratio is an oversimplified method that can provide an approximate correction with lots of versatility.
One recent study 13 deployed a similar methodology to the present phototoxicity ratio approach to determine which is more toxic in the environment, nanosized or dissolved metals. Toxicity ratio was calculated between median lethal dose, LC50, EC50, and IC50 values of dissolved and nanoparticulated metals to provide corrections for threshold values in existing regulatory standards. Therefore, the ratio metric approach—whether toxicity ratio, phototoxicity ratio, or nano‐ratio—is an inexpensive, straightforward method that mitigates uncertainties for the purpose of risk assessment and management, assuming enough literature is available.
In conclusion, the present study found that nano‐TiO2 is phototoxic to aquatic species, because the phototoxicity ratio values were substantially greater than 1 for the majority of analyzed studies. Existing literature on the subject is likely credible for the purpose of risk assessment because the insolation levels used in experimental setups did not exceed UV levels under natural conditions at the water surface. A significant difference was observed between the phototoxicity ratios of 2 analyzed groups: aquatic species belonging to order Cladocera, and all other aquatic species. The order Cladocera is very sensitive and prone to nano‐TiO2 phototoxicity, at least in laboratory‐based toxicity tests. A median phototoxicity ratio value and a 75% quartile were chosen as the most practical approach for correcting nano‐TiO2 toxicity endpoints obtained in the absence of sunlight or SSR. Using a median phototoxicity ratio value in correction is a more conservative approach, whereas using the 75% quartile lowers the chance of underestimating toxicity and may be favored by risk assessors when analyzing previously published data. The values for the phototoxicity ratio are not definite and may change as more data become available in the future.
Data Availability
All of the data used for calculation and statistical analysis are presented in Table 1 of the present study, with corresponding References.
Acknowledgment
The present study was supported by a Marie Curie FP7 Career Integration Grant within the 7th European Union Framework Programme – Project No PCIG13‐GA‐2013‐618006.
REFERENCES
- 1. Weir A, Westerhoff P, Fabricius L, von Goetz N. 2012. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ Sci Technol 46:2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jovanović B. 2015. Critical review of public health regulations of titanium dioxide, a human food additive. Integr Environ Assess Manag 11:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clément L, Hurel C, Marmier N. 2013. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants: Effects of size and crystalline structure. Chemosphere 90:1083–1090. [DOI] [PubMed] [Google Scholar]
- 4. Jovanović B, Palić D. 2012. Immunotoxicology of nonfunctionalized engineered nanoparticles in aquatic organisms with special emphasis on fish: Review of current knowledge, gap identification, and call for further research. Aquat Toxicol 118:141–151. [DOI] [PubMed] [Google Scholar]
- 5. Menard A, Drobne D, Jemec A. 2011. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ Pollut 159:677–684. [DOI] [PubMed] [Google Scholar]
- 6. Minetto D, Libralato G, Volpi Ghirardini A. 2014. Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: An overview. Environ Int 66:18–27. [DOI] [PubMed] [Google Scholar]
- 7. Nam D‐H, Lee B‐C, Eom I‐C, Kim P, Yeo M‐K. 2014. Uptake and bioaccumulation of titanium‐ and silver‐nanoparticles in aquatic ecosystems. Mol Cell Toxicol 10:9–17. [Google Scholar]
- 8. Shi H, Magaye R, Castranova V, Zhao J. 2013. Titanium dioxide nanoparticles: A review of current toxicological data. Part Fibre Toxicol 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carp O, Huisman CL, Reller A. 2004. Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32:33–177. [Google Scholar]
- 10. Fu PP, Xia Q, Hwang H‐M, Ray PC, Yu H. 2014. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J Food Drug Anal 22:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma H, Brennan A, Diamond SA. 2012. Photocatalytic reactive oxygen species production and phototoxicity of titanium dioxide nanoparticles are dependent on the solar ultraviolet radiation spectrum. Environ Toxicol Chem 31:2099–2107. [DOI] [PubMed] [Google Scholar]
- 12. Ma H, Brennan A, Diamond SA. 2012. Phototoxicity of TiO2 nanoparticles under solar radiation to two aquatic species: Daphnia magna and Japanese medaka. Environ Toxicol Chem 31:1621–1629. [DOI] [PubMed] [Google Scholar]
- 13. Notter DA, Mitrano DM, Nowack B. 2014. Are nanosized or dissolved metals more toxic in the environment? A meta‐analysis. Environ Toxicol Chem 33:2733–2739. [DOI] [PubMed] [Google Scholar]
- 14. Moan J. 2001. Visible light and UV radiation In Brune D, Hellborg R, Persson BRR, Pääkkönen R, eds, Radiation at Home, Outdoors and in the Workplace. Scandinavian Science Publisher, Oslo, Norway, pp 69–85. [Google Scholar]
- 15. Rautio M, Tartarotti B. 2010. UV radiation and freshwater zooplankton: Damage, protection, and recovery. Freshw Rev 3:105–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vega MP, Pizarro RA. 2000. Oxidative stress and defence mechanisms of the freshwater cladoceran Daphnia longispina exposed to UV radiation. J Photoch Photobio B 54:121–125. [DOI] [PubMed] [Google Scholar]
- 17. Kim KT, Klaine SJ, Cho J, Kim S‐H, Kim SD. 2010. Oxidative stress responses of Daphnia magna exposed to TiO2 nanoparticles according to size fraction. Sci Tot Environ 408:2268–2272. [DOI] [PubMed] [Google Scholar]
- 18. Dabrunz A, Duester L, Prasse C, Seitz F, Rosenfeldt R, Schilde C, Gabriele ES, Schulz R. 2011. Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna . PLoS ONE 6:e20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma H, Diamond SA. 2013. Phototoxicity of TiO2 nanoparticles to zebrafish (Danio rerio) is dependent on life stage. Environ Toxicol Chem 32:2139–2143. [DOI] [PubMed] [Google Scholar]
- 20. Pirow R, Wollinger F, Paul RJ. 1999. The sites of respiratory gas exchange in the planktonic crustacean Daphnia magna: An in vivo study employing blood haemoglobin as an internal oxygen probe. J Exp Bio 202:3089–3099. [DOI] [PubMed] [Google Scholar]
- 21. Kopp G, Lean JL. 2011. A new, lower value of total solar irradiance: Evidence and climate significance. Geophys Res Lett 38:L01706. [Google Scholar]
- 22. Häder D‐P, Lebert M, Schuster M, Ciampo Ld, Helbling EW, McKenzie R. 2007. ELDONET—A decade of monitoring solar radiation on five continents. Photochem Photobio 83:1348–1357. [DOI] [PubMed] [Google Scholar]
- 23. Inernational Organization for Standardization. 2007. Space environment (natural and artificial)—Process for determining solar irradiances. 2138:2007(E). Geneva, Switzerland.
- 24. Monash S. 1965. Composition of sunlight and of a number of ultraviolet lamps. Arch Dermatol 91:495–496. [DOI] [PubMed] [Google Scholar]
- 25. Garcia‐Pichel F, Bebout BM. 1996. Penetration of ultraviolet radiation into shallow water sediments: High exposure for photosynthetic communities. Mar Ecol‐Prog Ser 131:257–262. [Google Scholar]
- 26. Binh CTT, Tong T, Gaillard J‐F, Gray KA, Kelly JJ. 2014. Common freshwater bacteria vary in their responses to short‐term exposure to nano‐TiO2 . Environ Toxicol Chem 33:317–327. [DOI] [PubMed] [Google Scholar]
- 27. Tong T, Shereef A, Wu J, Binh CTT, Kelly JJ, Gaillard J‐F, Gray KA. 2013. Effects of material morphology on the phototoxicity of nano‐TiO2 to bacteria. Environ Sci Tech 47:12486–12495. [DOI] [PubMed] [Google Scholar]
- 28. Clemente Z, Castro VL, Jonsson CM, Fraceto LF. 2014. Minimal levels of ultraviolet light enhance the toxicity of TiO2 nanoparticles to two representative organisms of aquatic systems. J Nanopart Res 16:1–16. [Google Scholar]
- 29. Dalai S, Pakrashi S, Kumar RSS, Chandrasekaran N, Mukherjee A. 2012. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol Res 1:116–130. [Google Scholar]
- 30. Adams LK, Lyon DY, Alvarez PJJ. 2006. Comparative eco‐toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40:3527–3532. [DOI] [PubMed] [Google Scholar]
- 31. Kim S, An Y‐J. 2012. Effect of ZnO and TiO2 nanoparticles preilluminated with UVA and UVB light on Escherichia coli and Bacillus subtilis . Appl Microbiol Biotechnol 95:243–253. [DOI] [PubMed] [Google Scholar]
- 32. Angelstorf JS, Ahlf W, Kammer FVD, Heise S. 2014. Impact of particle size and light exposure on the effects of TiO2 nanoparticles on Caenorhabditis elegans . Environ Toxicol Chem 33:2288–2296. [DOI] [PubMed] [Google Scholar]
- 33. Dalai S, Pakrashi S, Chandrasekaran N, Mukherjee A. 2013. Acute toxicity of TiO2 nanoparticles to Ceriodaphnia dubia under visible light and dark conditions in a freshwater system. PLoS ONE 8:e62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bar‐Ilan O, Louis KM, Yang SP, Pedersen JA, Hamers RJ, Peterson RE, Heideman W. 2012. Titanium dioxide nanoparticles produce phototoxicity in the developing zebrafish. Nanotoxicology 6:670–679. [DOI] [PubMed] [Google Scholar]
- 35. Faria M, Navas JM, Soares AMVM, Barata C. 2014. Oxidative stress effects of titanium dioxide nanoparticle aggregates in zebrafish embryos. Sci Tot Environ 470:379–389. [DOI] [PubMed] [Google Scholar]
- 36. Amiano I, Olabarrieta J, Vitorica J, Zorita S. 2012. Acute toxicity of nanosized TiO2 to Daphnia magna under UVA irradiation. Environ Toxicol Chem 31:2564–2566. [DOI] [PubMed] [Google Scholar]
- 37. Li S, Pan X, Wallis LK, Fan Z, Chen Z, Diamond SA. 2014. Comparison of TiO2 nanoparticle and graphene‐TiO2 nanoparticle composite phototoxicity to Daphnia magna and Oryzias latipes . Chemosphere 112:62–69. [DOI] [PubMed] [Google Scholar]
- 38. Mansfield CM, Alloy MM, Hamilton J, Verbeck GF, Newton K, Klaine SJ, Roberts AP. 2015. Photo‐induced toxicity of titanium dioxide nanoparticles to Daphnia magna under natural sunlight. Chemosphere 120:206–210. [DOI] [PubMed] [Google Scholar]
- 39. Marcone GPS, Oliveira ÁC, Almeida G, Umbuzeiro GA, Jardim WF. 2012. Ecotoxicity of TiO2 to Daphnia similis under irradiation. J Hazard Mat 211:436–442. [DOI] [PubMed] [Google Scholar]
- 40. Miller RJ, Bennett S, Keller AA, Pease S, Lenihan HS. 2012. TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS ONE 7:e30321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dasari TP, Pathakoti K, Hwang H‐M. 2013. Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria. J Environ Sci 25:882–888. [DOI] [PubMed] [Google Scholar]
- 42. Tong T, Binh CTT, Kelly JJ, Gaillard J‐F, Gray KA. 2013. Cytotoxicity of commercial nano‐TiO2 to Escherichia coli assessed by high‐throughput screening: Effects of environmental factors. Water Res 47:2352–2362. [DOI] [PubMed] [Google Scholar]
- 43. Bundschuh M, Zubrod JP, Englert D, Seitz F, Rosenfeldt RR, Schulz R. 2011. Effects of nano‐TiO2 in combination with ambient UV‐irradiation on a leaf shredding amphipod. Chemosphere 85:1563–1567. [DOI] [PubMed] [Google Scholar]
- 44. Li S, Wallis LK, Ma H, Diamond SA. 2014. Phototoxicity of TiO2 nanoparticles to a freshwater benthic amphipod: Are benthic systems at risk? Sci Tot Environ 466:800–808. [DOI] [PubMed] [Google Scholar]
- 45. Kim J, Lee S, Kim C‐m, Seo J, Park Y, Kwon D, Lee S‐H., Yoon T‐H, Choi K. 2014. Non‐monotonic concentration‐response relationship of TiO2 nanoparticles in freshwater cladocerans under environmentally relevant UV‐A light. Ecotox Environ Saf 101:240–247. [DOI] [PubMed] [Google Scholar]
- 46. Lee W‐M, An Y‐J. 2013. Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: No evidence of enhanced algal toxicity under UV pre‐irradiation. Chemosphere 91:536–544. [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Wages M, Cox SB, Maul JD, Li Y, Barnes M, Hope‐Weeks L, Cobb GP. 2012. Effect of titanium dioxide nanomaterials and ultraviolet light coexposure on African clawed frogs (Xenopus laevis). Environ Toxicol Chem 31:176–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data used for calculation and statistical analysis are presented in Table 1 of the present study, with corresponding References.


