Abstract
We characterized three subsets of NK cells in blood, and two subsets in mucosal tissues. SIVmac251 infection increased total and CD16+ NK cells in the blood. In the rectum, we observed a significant increase in total and NKG2A+ NK cells during SIV infection. In contrast, the NKp44+ subset significantly depleted in acute infection and continued to decline in frequency during chronic phase. During SIV infection, blood CD16 and mucosal NKG2A+ subsets had increased cytotoxic potential. Intriguingly, the NKp44+ NK cell subtype that likely mediates mucosal homeostasis via the production of cytokines, acquired cytotoxicity. Antiretroviral therapy significantly increased the frequency of mucosal NKG2A+ NK cells and peripheral CD16+ NK cells. However, it failed to restore the normal frequency of NKp44+ NK cells in the rectum. Thus, SIVmac251 infection causes changes in the distribution and function of NK cells and antiretroviral therapy during chronic infection only partially restores NK homeostasis and function.
Introduction
Understanding of the innate and adaptive immunological mechanisms that curtail HIV infection and dissemination will facilitate the development of more effective vaccines or therapies to combat HIV. The early phase of HIV infection is critical as the outcomes of the interaction between the virus and the immune system during this phase is likely to determine whether the virus is eliminated, locally controlled, or disseminates to the rest of the body (Naranbhai, Altfeld et al., 2013; Ansari, Mayne et al., 2011). While it is clear that there is a rapid activation of innate immune responses after viral exposure (Chang & Altfeld, 2010; Haase, 2010) details on innate cell types and their role in the immune responses to HIV remains unclear.
NK cells are part of the innate immune system, which is the body’s initial defense against viral infections (Reeves, Evans et al., 2010; Moretta, Bottino et al., 2002). They kill virus-infected and neoplastic cells, via multiple mechanisms, including degranulation of cytotoxic granules and activation of death receptors (Cooper, Fehniger et al., 2001). NK cells also secrete a wide range of cytokines and chemokines that are pro-inflammatory or anti-inflammatory and are involved in regulating the adaptive immune responses (Farag & Caligiuri, 2006; Cooper, Fehniger et al., 2001). Increasing evidence supports an important role of NK cell subsets, in controlling HIV and SIV infection, both directly and indirectly (Ackerman, Dugast et al., 2012; Fauci, Mavilio et al., 2005). Recent studies have demonstrated a significant association between slower HIV-I disease progression and the presence of NK cell killer immunoglobulin-like receptor gene (KIR3DS1) with its ligand HLA-B alleles (Bw4-80lle) (Boulet, Kleyman et al., 2008; Martin, Gao et al., 2002). Furthermore, Alter et al., showed HIV-I adaptation to NK cell mediated immune pressure (Alter, Heckerman et al., 2011). NK cells in conjunction with antibodies, can also kill target cells by antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cell-mediated virus inhibition (ADCVI) (Chung, Rollman et al., 2008). A recently concluded Phase III HIV vaccine trial (RV144), demonstrated 31% protection from infection, and an inverse correlation between the risk of HIV acquisition and ADCC (Bonsignori, Pollara et al., 2012). Therefore, a better understanding of the mechanism of NK cells and HIV interactions may be important for the development of HIV vaccines.
Our lab and others have shown that SIV infection of Rhesus macaques is a valuable tool to mimic natural HIV infection in humans (Pegu, Vaccari et al., 2012; Morgan, Marthas et al., 2008; Ansari, Mayne et al., 2011). The majority of HIV infections are acquired through sexual transmission, thus HIV enters the body through the vaginal, rectal, or penile mucosa. In the initial phase, a small number of founder viruses may infect target cells in the mucosa and expand locally. Within few days however, the virus disseminates systemically and a persistent infection is established (Haase, 2005; Hladik & Hope, 2009; Yu & Vajdy, 2010). Recent reports suggest that the role of innate immune components, present at the mucosal site during this early phase of the infection, may determine the disease outcome (Borrow, 2011; Ansari, Mayne et al., 2011).
Although, NK cell subsets have been described in human mucosal tissues, most of the studies on NK cell responses during HIV infection are limited to human peripheral blood (Sips, Sciaranghella et al., 2012; Alter, Teigen et al., 2005). This is mainly due to practical difficulties in obtaining appropriate samples (vaginal or rectal mucosa) from humans. Recent transcriptional studies have shown a high degree of homology between Rhesus macaques and human NK cells (Hong, Rajakumar et al., 2013). Thus, macaques are a powerful and relevant model to study how SIV/HIV infection changes NK cells frequency and function.
Accurate identification of NK cells subsets in the blood and tissues of nonhuman primates is complex and requires several surface markers (Pereira, Johnson et al., 2008). Rhesus macaque peripheral blood NK cells are generally defined as CD3−CD20−CD8αα+ and NKG2A+. NK cells can be further subdivided into three groups depending on the degree of CD56 and CD16 receptors expression (Reeves, Gillis et al., 2010; Hong, Rajakumar et al., 2013; Ansari, Mayne et al., 2011).
The majority of naïve Rhesus macaques blood NK cells are CD16+/CD56− (CD16 subset) and considered to be more cytotoxic, whereas the smaller CD16−/CD56+ (CD56) subset produces more cytokines. In contrast, the CD16−CD56− (DN subset) may have both properties (Webster & Johnson, 2005; Pereira, Johnson et al., 2008; Ansari, Mayne et al., 2011).
Classifications of mucosal NK cell subsets are rather complicated. According to the recent classification, gut associated NK cells in human and mouse are grouped in the innate lymphoid cell group1 (ILC- group 1) (Walker, Barlow et al., 2013). However, two distinct NK cell subsets were recently defined in the mucosal tissues of the Rhesus macaque depending on NKp44 and NKG2A expression (Reeves, Rajakumar et al., 2011). In naïve animal’s NKG2A+ NK cells are more cytotoxic and NKp44+ NK cells produce cytokines. NKp44+ NK cells closely resemble the NK22 cells reported in human mucosal tissues (Cella, Fuchs et al., 2009) and they produce LIF, BAFF, and cytokines including IL17 and IL22 (Reeves, Rajakumar et al., 2011). Thus, the NKp44+ subset may be important in maintaining gut mucosal integrity and regulating B-cell function.
Highly active anti-retroviral therapy (HAART) effectively suppresses HIV replication, restores CD4+ T-cell counts in blood, and reduces HIV morbidity. However, little is known about NK cell frequency and function in blood and tissues during HAART therapy. Indeed, only few studies addressed the effect of HAART on mucosal NK cell subsets (Costiniuk & Angel, 2012; Mela, Steel et al., 2007). Peripheral blood NK cells from patients treated with HAART showed durable activation of NK cells, and also reported a decrease in the expression of FcR-γ 111 receptor and reduced ADCC signaling (Lichtfuss, Cheng et al., 2012; Leeansyah, Zhou et al., 2010). Another study has shown a significant increase of NK cells in colonic lamina propria after HAART treatment in humans (Mela, Steel et al., 2007).
In this study, we characterized the NK cell subsets and their phenotypic and functional alterations during acute and chronic SIV infection in peripheral blood and rectal mucosa and also studied the changes of NK cell subsets after anti-retroviral therapy. During the acute phase of SIV infection, we observed a rapid increase of cytotoxic NK cells (CD16 NK cells) in the blood and NKG2A+ NK cell subsets in the gut. Mucosal NKp44+ cell subsets rapidly decreased in the acute phase and further decreased during the chronic phase. Mucosal NKG2A+ and NKp44+ NK cells altered their phenotypic and functional characteristics during SIV infection. This may lead to alteration of the gut homeostasis and subsequent chronic immune activation and disease progression. HAART treatment significantly increased CD16 NK cells in the blood and NKG2A+ NK cell subsets in the gut. However, mucosal NKp44+ NK cell subsets continue to decline, even after ART treatment in the chronically infected macaques.
Materials and Methods
Animals and antiretroviral therapy
We studied a total of thirty-two Indian Rhesus macaques. Twelve animals were vaccinated with ALVAC-SIV and / or HPV-SIV and gp120 and twenty animals were unvaccinated. All animals were challenged with repeated low doses of SIVmac251 either intra-rectally or intra-vaginally. Seven of the thirty-two animals were followed longitudinally during the acute and chronic phases. At 6 months post infection, these seven animals were treated daily with Raltegravir (100mg/day/BID), Zerit (Stavudine) (1.2mg/kg/BID) orally, PMPA (20mg/kg/day), and Emtricitabine (FTC) (50mg/kg/day) subcutaneously. The prior treatment of these macaques is summarized in Supplemental Table 1. Naïve samples were collected prior to vaccination and SIV infection. Post infection samples were obtained 2 weeks post infection (acute) and 21–41 weeks post infection (chronic). Vaccination did not reduce SIV viral burden or CD4 loss in the acute or chronic phase. All of the Rhesus macaques were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International, and the study was reviewed and approved by the Animal Care and Use Committee at Advanced BioScience Laboratories (Rockville, MD).
Cell culture and flow cytometry
Mononuclear cells from blood and tissues were isolated as described previously (Vargas-Inc, Demberg et al., 2011; Reeves, Rajakumar et al., 2011). For the phenotypic characterization, 2×106 cells were used, and flow cytometry staining was carried out for cell surface and intracellular molecules using standard protocols. NK cell functions were analyzed using 3×106 cells after stimulation with phorbol myristate acetate (50 ng/mL) and ionomycin (1μg/mL) or 721.221 cells a MHC-devoid human cell line that are MICA/MICB-negative, at an effector-target ratio of 5:1. Anti-CD107a (eBioH4A3) was added directly to each of the tubes at a concentration of 20 μL/mL, and Golgi Plug™ (Brefeldin A) and Golgi Stop™ (Monensin) were added at final concentrations of 6 μg/mL. All samples were then cultured for 12 hours at 37°C in 5% CO2. Unstimulated (medium alone) samples were used as the negative controls. After incubation, cells were washed and stained for the surface and then fixed and permeabilized with a Cytofix/Cytoperm Kit™ (BD Biosciences), and stained for the specific intracellular molecules. The following anti-human fluorochrome-conjugated mAbs known to cross-react with rhesus macaques were used for the staining: V450 anti–IFN-γ (B27), PE-Cy7 anti-CD56 (NCAM 16.2), Alexa Fluor 700 anti-CD3 (SP34-2), Allophycocyanin-Cy7 anti CD3 (SP34-2), PerCP Cy5.5 anti-CCR6, V450 anti-Caspase 3 (C92-605), Alexa Fluor 700 anti-Ki67 (B56) (all from BD Biosciences, San Jose, CA); PE-Cy5 anti-CD107a (eBioH4A3), Alexa 488 anti-IL17 (eBio64DEC17), eFluor 605NC anti-CD20 (2H7), and eFluor 605NC anti-CD8a (RPA-T8) (all from eBioscience, San Diego, CA); PE anti-NKG2A (Z199), ECD anti-CD16 ( 3G8), PE-Cy5 anti-NKp46 (BAB281)(Beckman Coulter, Fullerton, CA), Fluorescein anti CCR5 (CTC5) (R and D) and allophycocyanin anti-NKp44 (P44-8) and PerCP/Cy5.5 anti–TNF-a (Mab11) (BioLegend, San Diego, CA). The yellow and aqua LIVE/DEAD viability dyes (Invitrogen) were used to exclude dead cells. At least 500,000 singlet events (PBMCs) or 50,000 CD3− singlet events (mucosal mononuclear cells) were acquired on a LSR II (BD Biosciences) and analyzed using FlowJo Software (TreeStar).
SIV RNA and DNA measurements
A real-time nucleic acid sequence-based amplification assay was used to measure SIV viral RNA in plasma(Romano, Williams et al., 1997). Plasma was clarified by centrifugation at 2,300g for 3 minutes. Nucleic acid was isolated as described previously and then analyzed by real-time nucleic acid sequence-based amplification as described previously. The copy number was expressed per 100 μl of plasma. The real-time nucleic acid sequence-based amplification assay had a lower limit of sensitivity of 50 copies of RNA (Romano, Williams et al., 1997).
Statistical analysis
All statistical and graphical analyses were done using Prism Version 5.0 software (GraphPad Software). Nonparametric Wilcoxon matched pairs and Mann-Whitney tests were used where indicated, and P < 0.05 was assumed to be significant.
Results
Phenotypic and functional characterization of NK cells subsets in the blood and mucosa of healthy un-infected macaques
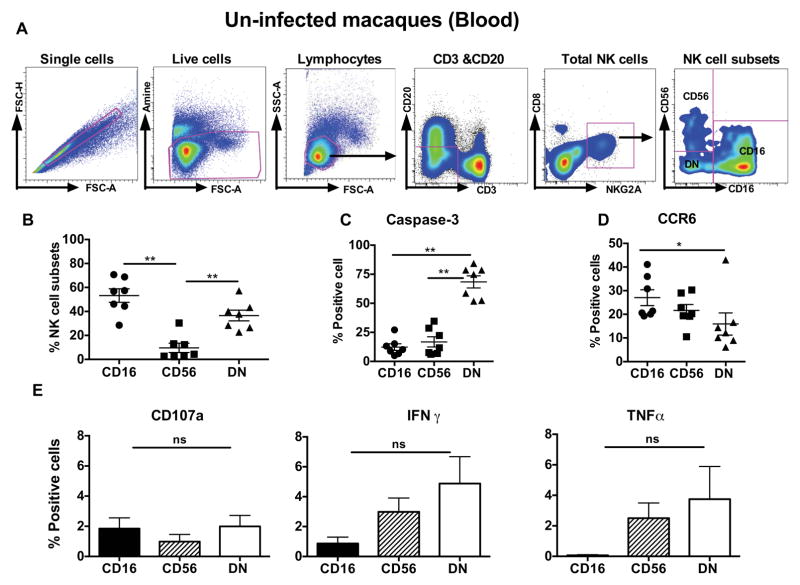
Recent studies have defined macaque peripheral blood NK cell as CD3-CD8αα+ NKG2A+ (Total NK cells) (Xu, Wang et al., 2012; Reeves, Rajakumar et al., 2011; Reeves, Gillis et al., 2010). Three different NK cell subsets are identified based on the expression of surface receptors CD56 and CD16 (Fig. 1A). The predominant peripheral blood NK cell subsets are the CD16+ CD56− (CD16 subset) and the CD56− and CD16− (DN) cells, while the least frequent subset is the CD56+CD16− (CD56 subset) (Fig. 1B). We characterized the activation/ apoptotic potential, homing and cytokine production of each subset in the blood. The DN subset expresses the highest levels of the active form of caspase 3, suggesting a higher rate of turnover (Fig. 1C). The CD16 subset expresses the highest level of the CCR6, a gut homing marker (Ito, Carson et al., 2011) (Fig. 1D). The CD16 subset produced higher CD107a and low IFN-γ and TNF-α where as the CD56 subset produced higher IFN-γ and TNF-α when stimulated with 721.221 cells. This indicates that CD16 subset is potentially more cytotoxic while cytokine production may be a key feature of the CD56 NK subset in naïve rhesus macaque peripheral blood (Fig. 1E).
Figure 1. Phenotypical and functional characterization of NK cell subsets in peripheral blood of naïve Rhesus macaques.
(A). Representative flow cytometric gating strategy to identify NK cell subsets in peripheral blood of naïve rhesus macaques. Macaque’s blood NK cells were gated as CD3-CD20- CD8αα+ NKG2A+ cells. They were further delineated by expression of CD16 and CD56 receptors. (B). Relative distribution of different NK cell subsets CD16 (CD56−CD16+), CD56 (CD56+CD16) and DN (CD56−CD16−) in the peripheral blood. (C) Expression of Caspase-3 or (D) chemokine receptor CCR6 was measured on blood NK cell subsets. (E) Peripheral blood mononuclear cells were stimulated with 721.122 cells (E: T ratio at 5:1) for 12 hours and measured CD107a expression, IFN-γ and TNF-α production on CD16, CD56 and DN NK cell subsets in naive macaques. The data represents seven naïve animals. Only significant P values are shown. Mann-Whitney test used to compare two groups and; 1way ANOVA used to compare multiple groups (*P < .05; **P < .01; ***P < .001). (ns: non significant).
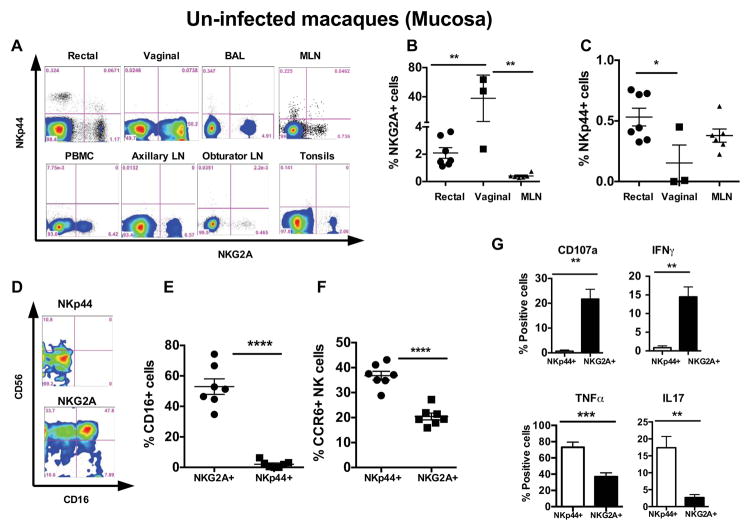
Next, we studied the mucosal NK cell subsets in naïve macaques. Human mucosal NK cell subsets have been reclassified as an innate lymphoid cell Group1 (Walker, Barlow et al., 2013). However, two different NK cell subpopulations have been recently reported at the mucosal tissues of rhesus macaques (Reeves, Gillis et al., 2010). Using a comparable gating strategy (Supplemental Figure 1), we observed two distinct NK subpopulations; NKG2A+ NKp44− (NKG2A+ subset) and NKp44+NKG2A− (NKp44+ subset) in the mucosal tissues (Fig. 2A). The NKG2A+ cells were the most frequent in mucosal tissues. Interestingly, the highest NKG2A+ frequency was observed in vaginal tissues followed by mesenteric lymph nodes, bronchoalveolar lavage (BAL), and rectal biopsies (Fig. 2A and 2B), whereas NKp44+ NK cells were predominantly present in the rectal biopsies and mesenteric lymph nodes (Fig. 2A and 2C). We did not find NKp44+ NK cells in peripheral blood, auxiliary, inguinal, or obturator lymph nodes in naive Rhesus macaques (Fig. 2A). Most of the gut associated NKG2A+ NK cells expressed the classical NK cell marker CD56, and more than 50% of cells expressed CD16. However, NKp44+ cells expressed low amounts of CD56 and no CD16 in naïve animals (Fig. 2D and 2E). Although frequencies of NKp44+ NK cells were lower than NKG2A+ cells, they expressed higher levels of CCR6 than NKG2A+ cells (Fig. 2F). We did not observe a significant difference in proliferation or CCR5 expression between these subsets (data not shown). Since NK cells exhibit either cytotoxic, cytokine producing capabilities, or both, we stimulated mucosal NK cells with mitogens and measured intracellular cytokine production and expression of the degranulation marker CD107a. CD107a or LAMP-1 has been described as a functional marker for NK cells. CD107a on NK cells correlate with target cell lysis. Therefore expression of CD107a can be use to estimate cytotoxic capability of NK cells (Alter, Malenfant et al., 2004). Following PMA and ionomycin stimulation, NKG2A+ cells showed higher CD107a expression and significantly elevated IFN-γ production indicating a potential cytotoxic role. NKp44+ cells produced higher amounts of IL17 and TNFα upon mitogenic stimulation, however they produce very little IFN-γ and no expression of CD107a (Fig. 2G). These data suggest that NKp44+ cells have a regulatory role in gut mucosal tissues rather than a cytotoxic role.
Figure 2. Tissue distribution and functional characteristics of rectal mucosal tissue NK cell subsets in naïve rhesus macaques.
(A) Flow cytometry plots comparing the tissue distribution of NKG2A+ and NKp44+ NK cell subsets in a representative naïve Rhesus macaque using a gating strategy shown in Supplemental Figure 1. Frequency of (B) NKG2A+ NK subset and (C) NKp44+ subset in rectal mucosa, vaginal tissues and mesenteric lymph nodes (MLN). (D) Flow cytometry plots comparing CD56 and CD16 receptor expression on NKp44+ and NKG2A+ NK cell subsets in rectal mucosal tissues of uninfected macaques. (E) CD16 receptor expression on NKp44+ and NKG2A+ in the gut in rectal mucosal tissues of seven uninfected macaques. (F) Differential distribution of chemokine receptor 6 (CCR6) in rectal mucosal NK cell subsets in naïve macaques. (G) Intracellular cytokines production of TNFα, IFNγ, and IL17 and CD107a expression measured in the rectal mucosal NK cell subsets of naïve macaques after 12hrs of phorbol 12-myristate 13-acetate/ionomycin stimulation. The data represents three to seven naïve animals. Only significant P values are shown. Mann-Whitney test used to compare two groups and; Kruskal-Wallis tests used to compare multiple groups (*P < .05; **P < .01; ***P < .001).
Blood and gut NK cell subsets in Rhesus macaques during acute and chronic SIV infection
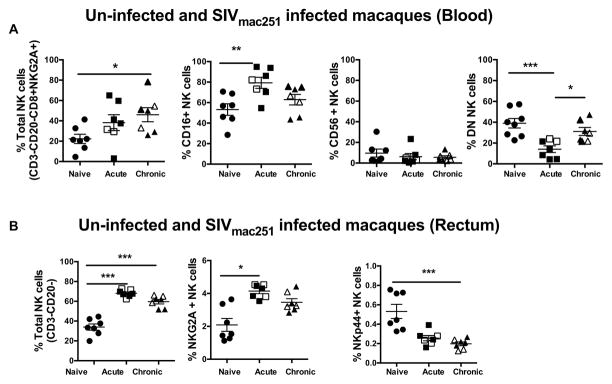
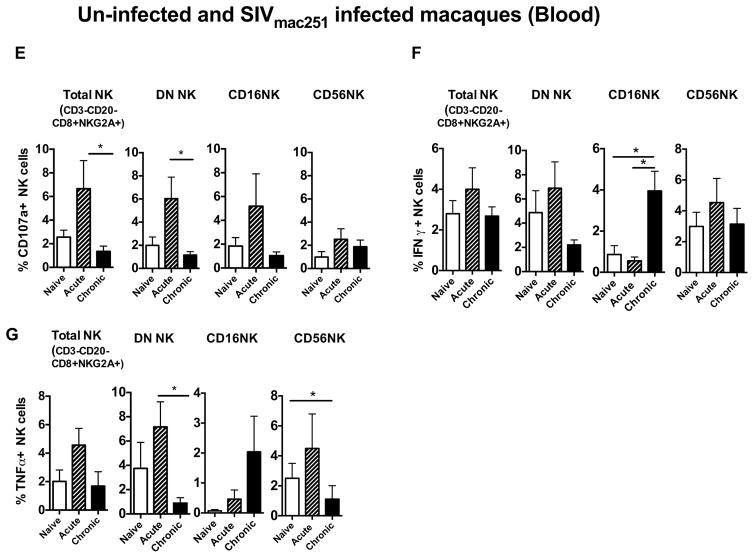
We studied the changes in the frequency of NK cell subsets in the blood and rectal mucosa during the acute and chronic phases of SIV infection. During the acute phase, total NK cells (CD3−CD20−CD8αα+NKG2A+) in the blood were increased compared to naïve animals and continued to increase during the chronic phase (Fig. 3A). The CD16+ NK cell subset was increased significantly in acute infection and then began to decline during the chronic phase but remained higher than the pre-infection level (Fig. 3A). The CD56 subset showed a slight decrease, however it was not significant. Interestingly, the DN subset was significantly reduced during the acute phase, but gradually increased to pre infection level in the chronic phase (Fig. 3A). Of note, no correlation was observed between CD4 T cell count, viral load or frequency of CD4 Ki67+ in the blood and CD16 NK cell subset (supplemental Figure 2) or any other peripheral NK cell subset (Data not shown).
Figure 3. Dynamics of NK cell subsets during acute and chronic phases of SIV infection in the peripheral blood and rectal mucosal tissues of Rhesus macaques.
Dot plots represent the frequency of different NK cell subpopulations in the (A) peripheral blood and (B) rectal mucosal tissues of Rhesus macaques during acute and chronic SIV infections. Previously vaccinated animals are indicated with open symbols. There were no differences in viral loads or CD4 counts between vaccinated and unvaccinated animals (Supplemental Table 1) (C) Bar graphs represent the average and standard deviation for the levels of CCR6 expression on subsets of NK cell in peripheral blood and (D) rectal mucosa during acute and chronic SIV infection. Kruskal-Wallis tests were used for comparisons of each group. Data represents the seven animals per group. Only significant P values are shown (*P < .05; **P < .01; ***P < .001).
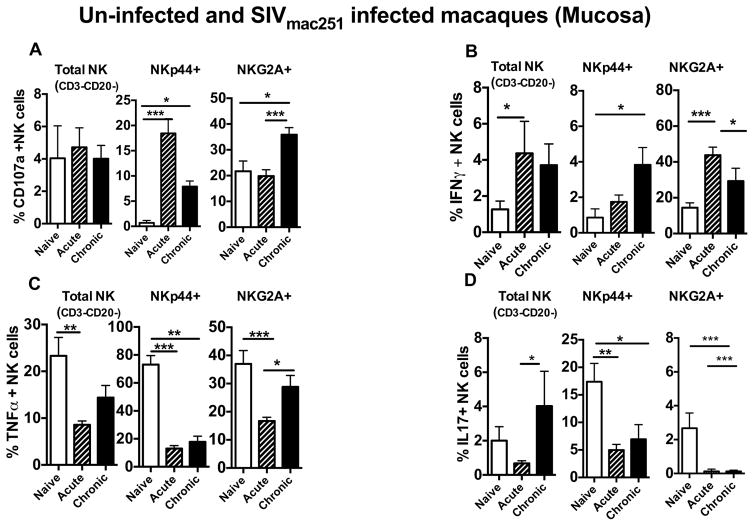
We further examined the mucosal NK cells responses during acute and chronic SIV infection. Similar to the total peripheral blood NK cells, total NK cells (CD3-CD20-) in the gut also increased significantly post SIV infection and peeked during the acute phase. Further analysis of subsets showed that the NKG2A+ subset increased in acute SIV infection yet returned to the baseline levels in the chronic phase. Interestingly, NKp44+ cell subsets were severely depleted during acute infection and continued to decline in the chronic phase (Fig. 3B). Loss of NKp44+, cells that produce IL17 and potentially IL22, may lead to a loss of intestinal integrity causing microbial translocation and an increase in immune activation.
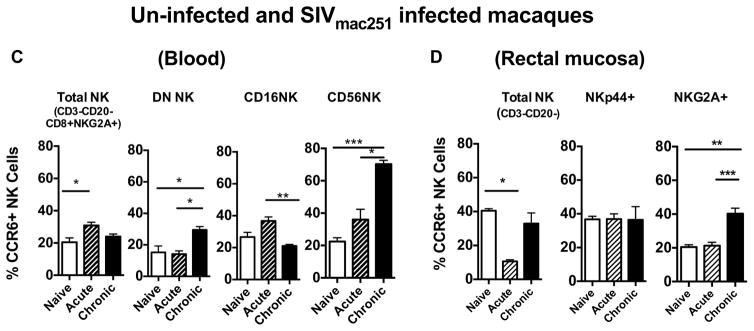
Chemokine receptor 6 (CCR6) expression on NK cells during SIV infection
CCR6 is the receptor for CCL20 ligand, which is primarily expressed in the gut. The CCR6/CCL20 combination plays an important role in gut immunity (Ito, Carson et al., 2011). CCR6 expression on NK cells may indicate a possible gut homing signature. Thus, we next examined the expression of CCR6 on NK subsets during the SIV infection in the peripheral blood and the gut. We observed increased expression of CCR6 on the CD56 subset of NK cells in the blood during the acute phase and a significant elevation during the chronic phase as well (Fig. 3C). Similarly, we observed increased CCR6 expressions of DN NK cells in the blood. The CD16 subset showed a slight increase of CCR6 on their surface during acute infection but was reduced significantly in the chronic phase. In the rectal mucosa, the NKp44+ subset did not show alteration of CCR6 expression on their surface; however the NKG2A+ subset had significantly increased CCR6 expression during the chronic phase (Fig. 3D).
Functional alteration of blood and gut NK cell subsets during acute and chronic SIV infection
NK cells have both effector and regulatory functions. A recent study on human gut mucosal NK cells showed two different NK cell populations present in the intraepithelial layer (IEL) and lamina propria (LP). Spontaneous controllers showed a stable IEL NK cell subset, suggesting a potential role of gut NK cells in virus control (Sips, Sciaranghella et al., 2012). Therefore, the alteration of NK cell functions may affect viral control and dissemination of the infection. We studied the functional profiles of mucosal NK cell subsets during acute and chronic infections. We stimulated total rectal mucosal lymphocytes with PMA and ionomycin for 12 hours and analyzed their functional changes during acute and chronic infections.
Interestingly, NKp44+ NK cell subsets demonstrated higher levels of CD107a production in the acute phase of the infection, which declined in the chronic phase but remained significantly higher than naïve animals (Fig 4A). Expression of CD107a by NKp44+ cells was surprising and suggests that the NKp44+ NK cell subset acquired cytotoxic capabilities when exposed to SIV (Fig. 4A). In addition, the NKp44+ subset demonstrated significantly increased production of Th1 cytokine IFN-γ during all stages of SIV infection (Fig 4B). Conversely, TNF-α and IL17 production was significantly decreased in the NKp44+ subset, which could indicate a reduction in their ability to regulate mucosal homeostasis (Fig. 4C and 4D). NKG2A+ NK cells, the dominant NK cell subset in the gut, also had increased expression of CD107a during the chronic phase (Fig. 4A). Production of IFN-γ from NKG2A+ cells were significantly increased during the acute phase but reduced during progression to the chronic phase (Fig. 4C and 4D). TNF-α production was significantly reduced in NKG2A+ cells during acute SIV infection but increased during progression to the chronic phase (Fig. 4C). NKG2A+ cells produced very low amounts of IL17 in naïve animals and a further significant decrease was observed in SIV infection (4D).
Figure 4. Alteration of functional capability during acute and chronic SIV infection in rectal mucosal NK cell subsets.
Bar graphs represent the average and standard deviation for (A) expression of CD107a (B) and levels of IFN-γ, (C) TNF-α and (D) IL17 secretion following a 12 hour stimulation with phorbol 12-myristate-13-acetate (PMA)/ionomycin for the subsets of NK cells in rectal mucosa of naïve, SIV acute and SIV chronically infected rhesus macaques. Peripheral blood mononuclear cells were stimulated with 721.122 cells at E:T ratio of 5:1 for 12 hours and measured (E) CD107a, (F) IFN-γ and (G) TNF-α production on Total, CD16, CD56 and DN NK cells subsets in naïve, acute and chronically SIV infected animals. Data represents the seven animals per group. Kruskal-Wallis tests were used for comparisons of each group. Only significant P values are shown (*P < .05; **P < .01; ***P < .001).
Naïve and infected blood NK cell subsets were analyzed for functional changes after stimulation with 721.221 cells, as described in the Material and Methods. We measured expression of the degranulation marker CD107a and production of IFN-γ, and TNF-α by different NK cell subsets. During the acute phase, CD107a expression was increased in all subsets. Interestingly, CD107a expression on the DN subset was significantly reduced in the chronic phase (Fig. 4E). IFN-γ production was elevated in CD16 subsets during the chronic phase (Fig. 4F) whereas; TNF-α production was transiently elevated during the acute phase in DN and CD56 subsets (Fig. 4G).
Incomplete restoration of NK cell subsets in blood and rectal mucosa of infected macaques treated with ART
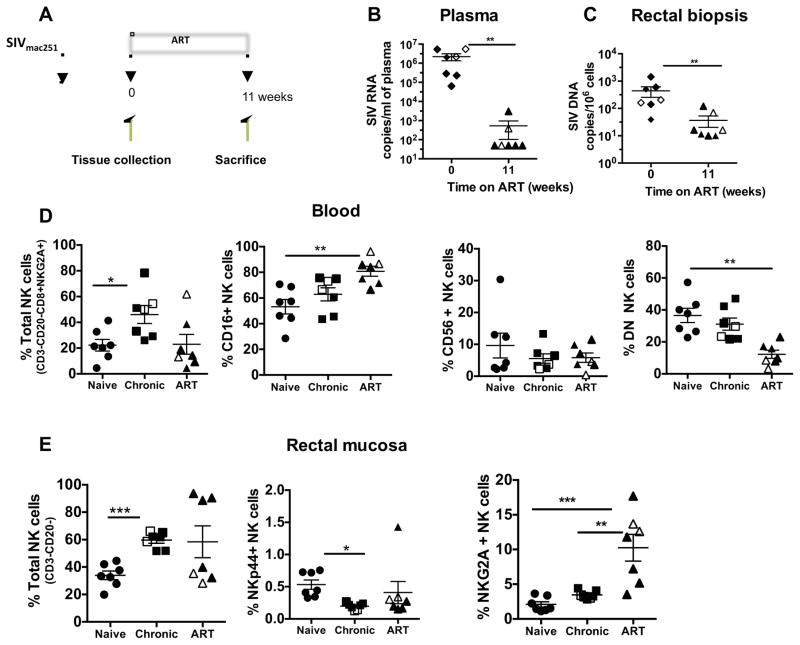
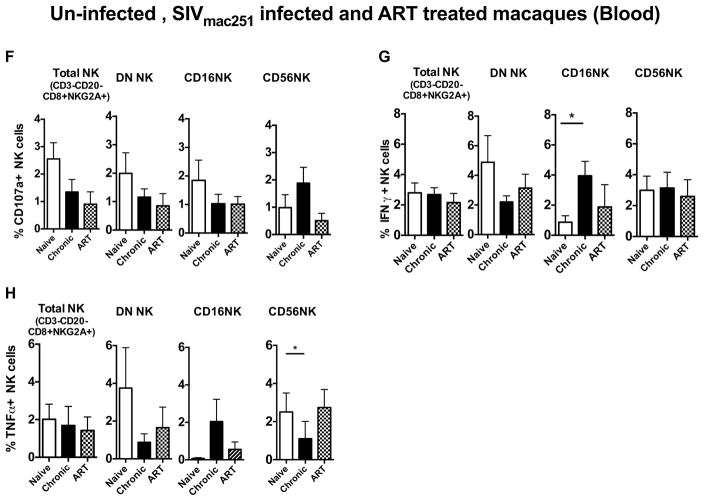
ART therapy can lead to partial restoration of the peripheral and mucosal immune system. However, the effect of ART on the innate immune system, particularly NK cell subsets, has not been extensively studied. Therefore, in this study, seven chronically infected Rhesus macaques were treated with antiretroviral treatment for 11 weeks (Fig 5A). ART significantly reduced plasma viral load (Fig 5B), as well as SIV DNA in rectal biopsies (Fig 5C). The effect of ART on NK cells in blood and rectal mucosa were studied by multi-parametric flow cytometry. The CD16 NK cell subset in the peripheral blood significantly increased after ART treatment. However, DN population (Fig 5D) demonstrated a significantly lower frequency when compared to naive animals. In rectal mucosa, the NKG2A+ NK subset was significantly elevated after ART treatment (Fig 5E). However, the NKp44+ NK cell subset remained depleted despite ART treatment (Fig 5E). No ART mediated functional changes were observed in any blood NK cell subsets stimulated with 721.221 cells (Fig. 5F–H).
Figure 5. The effects of ART treatment on peripheral blood and gut NK cell subsets in SIV chronically infected Rhesus macaques.
(A) Diagram representing ART treatment schedule. Chronically infected seven rhesus macaques were treated with ART as described in the Material and Methods. Samples were collected pre-ART, and 11 weeks post treatment from peripheral blood and gut tissues and the NK cell responses were evaluated. (B) Plasma viral load (SIV RNA) and (C) SIV viral load (SIV DNA) in rectal mucosa. The dot plots represent the changes of (D) blood NK cell subsets and (E) rectal mucosal NK cell subsets during pre- and post-ART treatment. Prior vaccinated animals are indicated in open squares or open triangles. As indicated in Supplemental Table 1, there were no differences in viral loads or CD4 counts between vaccinated and unvaccinated animals. Bar graphs represent the average and standard deviation for (F) expression of CD107a (G) and levels of IFN-γ and (H) TNF-α production by peripheral blood NK cell subsets following a 12 hour stimulation with 721.122 cells at a E:T ratio of 5:1. Kruskal-Wallis tests were used for comparisons of each group. Only significant P values are shown (*P < .05; **P < .01; ***P < .001).
Discussion
NK cells are a heterogeneous population that plays an important role in many viral infection and are involved in controlling SIV ad HIV infection (Ansari, Mayne et al., 2011; Naranbhai, Altfeld et al., 2013). They are part of the innate defense systems in the body and influence the development of adaptive immune responses.
We assessed peripheral blood NK cells in macaques based on a previously reported gating strategy (Reeves, Gillis et al., 2010). We found that the CD16 subset is the most abundant NK cell population in the peripheral blood of naïve animals. This is consistent with findings in the peripheral blood of humans (Reeves, Gillis et al., 2010). Our results also indicate a cytotoxic role for CD16 cells and a cytokine producing role for CD56 and DN subsets that express the highest levels of IFNγ and TNFα after in vitro stimulation.
The chemokine receptor CCR6 expressed by lymphocytes, including NK cells, binds to the chemokine ligand CCL20 and is an important receptor involved in regulating mucosal immunity37. Here, we observed higher CCR6 expression on CD16 NK cells compared to other NK subsets in naïve animals. Reeves et al., (2010) showed that the CD16 NK cell subset did not express lymph node homing markers such as CCR7 and CD62L (Reeves, Gillis et al., 2010). Therefore, we can speculate that the CD16 NK subset may be capable of migration to mucosal sites.
Mucosal NK cell subsets are more complicated in classification. A recent study has shown two different NK cell subsets present in mucosal tissues which include NKG2A+ and NKp44+ NK cell subset, (Reeves, Rajakumar et al., 2011). The NKG2A+ NK subset is similar to blood NK cells and exhibits cytotoxic properties and is the dominant NK cells in the gut and many other mucosal tissues. Interestingly, we observed that the frequency of NKG2A+ NK cells was higher in vaginal tissues and in the lung than any other tissue. NKp44+ NK cells are cytokine producing cells and may represent the human NK22 counterpart. Other researches also suggest that this subset is a part of innate lymphoid cell group (Xu, Wang et al., 2012). In naïve animals, NKp44+ NK cells produce IL17 and TNFα and are present mainly in mucosal tissues, such as rectal mucosa, gut tissues, or mucosal associated lymphoid tissues like mesenteric lymph nodes, indicating that this subset may be associated with mucosal immunity. Interestingly, CCR6 expression is also higher in the NKp44+ NK subset suggesting that this subset may circulate between inductive and effector sites within mucosal sites.
The initial interactions of HIV/SIV with the immune system during the acute phase is critical to the outcome of the infection (Borrow, 2011). This phase determines whether the virus will be eliminated, or becomes established and the individual progresses to a chronic infection. We evaluated both peripheral blood and mucosal NK cell responses during the acute and chronic phases of the infection. Consistent with NK cell responses during acute HIV (Alter, Teigen et al., 2005) and SIV infections, we also showed that CD16+, the cytotoxic NK cell subset in the blood, increased significantly during the acute phase and declined during the chronic phase of infection. CD16+ cells also showed significantly higher IFNγ production during the chronic phase. This early expansion of the CD16+ NK cell subset may be important for initial viral control. We also observed that the frequency of multifunctional DN NK cells in the blood is reduced during the acute phase with a slight increase during the chronic phase of infection. DN NK cells expressed higher levels of lymph node-homing marker CCR7 and CD62L (Reeves, Rajakumar et al., 2011). Thus, initial reduction of DN cells in the blood may be due to trafficking to the lymph nodes during acute infection. The CD56 subset, which expressed CCR5, CCR6, CXCR3, and CXCR4 (Reeves, Gillis et al., 2010; Reeves, Evans et al., 2010) decreased during chronic phase but did not show significant changes during both acute and chronic infection. However, TNFα production of this subset significantly reduced during the chronic phase. Interestingly, CCR6 expression in CD56 subset was significantly increased during the chronic phase of infection. This may suggest a possible recruitment of this NK cell subset to the mucosal tissues as infection progresses.
The frequency of cytotoxic mucosal NK cell subset (NKG2A+) was transiently increased during acute infection in the rectal mucosa, where the virus primarily replicates. The cells also significantly increased production of IFNγ during the acute phase of infection. This may improve the anti-viral status in the gut before the induction of adaptive immune cells. With progression to the chronic phase of infection, we observed a loss of frequency and a significant reduction of IFNγ production, which indicates the suppression of NK cell function during the chronic stages of the infection. However, the cytotoxic capability remained significantly increased. In addition, the NKp44+ NK cell subset was significantly reduced during the acute and chronic phase of infection. This subset also exhibited a significant reduction of IL17 and TNFα producing cells during disease progression. IL17, together with TNFα, has been shown to be an important cytokine in the host defense (Hartupee, Liu et al., 2007; Xu, Wang et al., 2012). Previous studies have shown that the IL17 producing cells were important for mucosal epithelial integrity during SIV and HIV infection (Brenchley, Paiardini et al., 2008; Hartigan-O’Connor, Hirao et al., 2011; Reeves, Rajakumar et al., 2011). Therefore, the loss of IL17 producing NKp44+ cells during infection in the gut may have a significant impact on gut homeostasis during SIV infection in macaques. Furthermore, NKp44+ cells significantly changed their functional capacity. The reduced IL17 production and TNFα production occurred in conjunction with a significant increase in the production of CD107a and IFNγ production during infection. Taken together, our study has shown that rapid changes occur both in the frequency and in the function of different NK cell subsets during the course of SIV infection.
HAART therapy restores CD4+ T-cell counts in blood and has reduced the incidence of AIDS in HIV-infected individuals. However, little is known about NK cell responses during HAART therapy. There are only a few reports about the mucosal NK cell subset during HAART therapy (Beaumier, Harris et al., 2009). Recent reports from HAART treated patient’s peripheral blood NK cells showed continued activation of NK cells and decreased expression of FcR γ and reduced ADCC signaling. In this study, we showed that with ART treatment, the CD16 NK cell subset significantly increased in the blood. However, we did not observe restoration of functional capability of blood NK cells after ART. Interestingly, the frequency of the cytotoxic NKG2A+ NK cell subset in the rectal mucosa was also significantly elevated. Nevertheless, NKp44+ NK cell subsets continuously declined during ART. Thus, we observed a continued alteration of NK cell subsets in chronically infected macaques treated with ART. Further studies are needed to gain better understanding of the effect of antiretroviral therapy on HIV/SIV infections.
We have studied NK cell subsets in the peripheral blood and mucosal tissues during acute and chronic SIV infection and while we observed no significant associations with virus loads, we do observe significant alterations in NK cells during infection. During the chronic infection, NK cell subsets in both peripheral blood and mucosal tissues increased their cytotoxicity. Mucosal tissues, in particular, lost their cytokine producing NK cell subsets. Interestingly, the expression of chemokine receptor 6 (CCR6) and their trafficking patterns are changed during the infection. Furthermore, ART treatment in animals with chronic SIV infection demonstrated an up regulation of cytotoxic NK cell subsets in blood and rectal mucosa but ART failed to restore cytokine producing NKp44+ cells.
Supplementary Material
Representative gating strategy to identify NKG2A+ and NKp44+ NK cells among live mononuclear cells in tissues in rectal mucosa specimens. B cells and T-cells were excluded by using CD3 and CD20 markers and remaining CD3-CD20- cells further divided into two groups depending on the expression of NKp44 and NKG2A markers.
The absolute CD4+ T-cell count (A) viral load (B) the frequency of Ki67+ CD8+ T cells (C) and the frequency of Ki67+ CD4+ T-cells during acute infection do not correlate with frequency of CD16 NK cell subset in the blood.
Absolute CD4+ T-cells (A) Geometric mean of CD4+ T-cells (B) Frequency of CD4+ T-cells and CD16 NK cells after 11 weeks of ART treatment. There was no correlation between frequency of CD4+ T-cells and CD16 NK cells (C).
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Teresa Habina for manuscript editing, Katherine McKinnon for flow cytometric support and M. Colonna for helpful discussions. FTC and PMPA was a generous gift from Gilead Sciences, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ackerman ME, Dugast AS, Alter G. Emerging concepts on the role of innate immunity in the prevention and control of HIV infection. Annu Rev Med. 2012;63:113–130. doi: 10.1146/annurev-med-050310-085221. [DOI] [PubMed] [Google Scholar]
- Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. Journal of Immunological Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, Yu XG, Lichterfeld M, Basgoz N, Rosenberg ES, Altfeld M. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- Ansari AA, Mayne AE, Takahashi Y, Pattanapanyasat K. Incorporation of innate immune effector mechanisms in the formulation of a vaccine against HIV-1. Advances in Experimental Medicine and Biology. 2011;780:143–159. doi: 10.1007/978-1-4419-5632-3_12. [DOI] [PubMed] [Google Scholar]
- Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nature Medicine. 2009;15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. Journal of Virology. 2012;86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P. Innate immunity in acute HIV-1 infection. Curr Opin HIV AIDS. 2011;6:353–363. doi: 10.1097/COH.0b013e3283495996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS. 2008;22:1487–1491. doi: 10.1097/QAD.0b013e3282ffde7e. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. Journal of Infectious Diseases. 2010;202(Suppl 2):S297–S301. doi: 10.1086/655657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A, Rollman E, Johansson S, Kent SJ, Stratov I. The utility of ADCC responses in HIV infection. Curr HIV Res. 2008;6:515–519. doi: 10.2174/157016208786501472. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- Costiniuk CT, Angel JB. Human immunodeficiency virus and the gastrointestinal immune system: does highly active antiretroviral therapy restore gut immunity? Mucosal. Immunol. 2012;5:596–604. doi: 10.1038/mi.2012.82. [DOI] [PubMed] [Google Scholar]
- Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Reviews. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- Hartigan-O’Connor DJ, Hirao LA, McCune JM, Dandekar S. Th17 cells and regulatory T cells in elite control over HIV and SIV. Curr Opin HIV AIDS. 2011;6:221–227. doi: 10.1097/COH.0b013e32834577b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. Journal of Immunology. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6:20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- Hong HS, Rajakumar PA, Billingsley JM, Reeves RK, Johnson RP. No monkey business: why studying NK cells in non-human primates pays off. Front Immunol. 2013;4:32. doi: 10.3389/fimmu.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Carson WF, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317:613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeansyah E, Zhou J, Paukovics G, Lewin SR, Crowe SM, Jaworowski A. Decreased NK Cell FcRgamma in HIV-1 infected individuals receiving combination antiretroviral therapy: a cross sectional study. PLoS One. 2010;5:e9643. doi: 10.1371/journal.pone.0009643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, Kramski M, Hearps AC, Cameron PU, Lewin SR, Crowe SM, Jaworowski A. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. Journal of Immunology. 2012;189:1491–1499. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O’Brien SJ, Carrington M. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- Mela CM, Steel A, Lindsay J, Gazzard BG, Gotch FM, Goodier MR. Depletion of natural killer cells in the colonic lamina propria of viraemic HIV-1-infected individuals. AIDS. 2007;21:2177–2182. doi: 10.1097/QAD.0b013e3282f08b72. [DOI] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L. What is a natural killer cell? Nat. Immunol. 2002;3:6–8. doi: 10.1038/ni0102-6. [DOI] [PubMed] [Google Scholar]
- Morgan C, Marthas M, Miller C, Duerr A, Cheng-Mayer C, Desrosiers R, Flores J, Haigwood N, Hu SL, Johnson RP, Lifson J, Montefiori D, Moore J, Robert-Guroff M, Robinson H, Self S, Corey L. The use of nonhuman primate models in HIV vaccine development. PLoS Med. 2008;5:e173. doi: 10.1371/journal.pmed.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranbhai V, Altfeld M, Karim SS, Ndung’u T, Karim QA, Carr WH. Changes in Natural Killer cell activation and function during primary HIV-1 Infection. PLoS One. 2013;8:e53251. doi: 10.1371/journal.pone.0053251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegu P, Vaccari M, Gordon SN, Keele BF, Doster MN, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras GD, Alam M, Fenizia C, Lifson J, Stablein D, Tartaglia J, Michael NL, Kim J, Venzon D, Franchini G. Antibodies with high avidity to the gp120 envelope protein in protection from SIVmac251 acquisition in an immunization regimen that mimics the RV-144 Thai Trial. Journal of Virology. 2012 doi: 10.1128/JVI.02544-12. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LE, Johnson RP, Ansari AA. Sooty mangabeys and rhesus macaques exhibit significant divergent natural killer cell responses during both acute and chronic phases of SIV infection. Cell Immunol. 2008;254:10–19. doi: 10.1016/j.cellimm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Reeves RK, Evans TI, Gillis J, Johnson RP. Simian immunodeficiency virus infection induces expansion of alpha4beta7+ and cytotoxic CD56+ NK cells. Journal of Virology. 2010;84:8959–8963. doi: 10.1128/JVI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, Kuzmichev YV, Carville A, Johnson RP. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011;118:3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano JW, Williams KG, Shurtliff RN, Ginocchio C, Kaplan M. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunological Investigations. 1997;26:15–28. doi: 10.3109/08820139709048912. [DOI] [PubMed] [Google Scholar]
- Sips M, Sciaranghella G, Diefenbach T, Dugast AS, Berger CT, Liu Q, Kwon D, Ghebremichael M, Estes JD, Carrington M, Martin JN, Deeks SG, Hunt PW, Alter G. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 2012;5:30–40. doi: 10.1038/mi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Inc, Demberg T, Robert-Guroff M. A CD8alpha(−) subpopulation of macaque circulatory natural killer cells can mediate both antibody-dependent and antibody-independent cytotoxic activities. Immunology. 2011;134:326–340. doi: 10.1111/j.1365-2567.2011.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat. Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Webster RL, Johnson RP. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology. 2005;115:206–214. doi: 10.1111/j.1365-2567.2005.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 2012;5:658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Vajdy M. Mucosal HIV transmission and vaccination strategies through oral compared with vaginal and rectal routes. Expert Opin Biol Ther. 2010;10:1181–1195. doi: 10.1517/14712598.2010.496776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative gating strategy to identify NKG2A+ and NKp44+ NK cells among live mononuclear cells in tissues in rectal mucosa specimens. B cells and T-cells were excluded by using CD3 and CD20 markers and remaining CD3-CD20- cells further divided into two groups depending on the expression of NKp44 and NKG2A markers.
The absolute CD4+ T-cell count (A) viral load (B) the frequency of Ki67+ CD8+ T cells (C) and the frequency of Ki67+ CD4+ T-cells during acute infection do not correlate with frequency of CD16 NK cell subset in the blood.
Absolute CD4+ T-cells (A) Geometric mean of CD4+ T-cells (B) Frequency of CD4+ T-cells and CD16 NK cells after 11 weeks of ART treatment. There was no correlation between frequency of CD4+ T-cells and CD16 NK cells (C).