Abstract
Objective
To conduct an open label, multi-dose Phase I/II clinical study in spontaneous canine cancers and evaluate the pharmacokinetics, safety, and efficacy of the hyaluronan-based cisplatin formulation (HA-Pt).
Animals
13 dogs with heterogeneous, naturally occurring cancers.
Procedures
The dogs received up to four injections of 10-30 mg/m2 HA-Pt into the tumor or peritumoral sub-mucosa at three-week intervals. Blood sample (2 mL) was collected from the jugular catheter at 0.5, 1, 2, 4, and 24 hours following drug administration. A complete blood count and renal profile with urinalysis were conducted prior to and one week after each treatment. Tumor measurements were collected three weeks following each administration to assess response.
Results
Of the 13 dogs with heterogeneous, naturally occurring cancers, 23% had complete response and 15% had partial response or stable disease. Among the dogs that received drug with low diaquated content, the complete response rate for SCC was 3/7 (43%). Myelosuppression and cardiac toxicity were observed for 38% and 19% of the dogs, respectively. The formulation did not cause nephrotoxicity, the dose-limiting toxicity of standard cisplatin, in any dogs.
Conclusions and Clinical Relevance
The HA-Pt formulation demonstrated positive response in spontaneous canine squamous cell carcinomas. It did not cause nephrotoxicity in any patients. Canine oral SCC is very homogenous in progression and drug response to human HNSCC, and these results could be useful in developing human treatments.
Hyaluronan-cisplatin nanoconjugate (HA-Pt) is a novel hyaluronan-cisplatin nanoparticle formulation that traffics cisplatin to tumors and draining lymphatics after intra- and peri-tumoral injection. In mouse xenografts of squamous cell carcinoma (SCC) of the head and neck and breast cancer, the formulation has shown improved tumor control over conventionally administered cisplatin.1,2 Complementing this enhanced local efficacy, the pharmacokinetic profile of this formulation is superior to intravenously administered cisplatin with a reduced Cmax but greater area-under-the-curve (AUC), resulting in reduced nephrotoxicity, enhanced local efficacy, and extended systemic tumor cell exposure.3,4 In a pilot trial of HA-Pt in dogs with spontaneous soft tissue sarcomas, the formulation resulted in similarly favorable tumor and plasma distributions.5
Prior to the canine clinical trial, the toxicity and efficacy of HA-Pt in rodents has been reported.1,3,4,6-8 HA-Pt is highly water soluble (solubility of cisplatin: 1 mg/mL; solubility of HA-Pt: >10 mg/mL) and is readily injectable via a 27-ga needle. HA-Pt nanoparticles are approximately 20-25 nm in size and have an in vitro release half-life of 10 hours in phosphate buffered saline.3,4 HA-Pt has a similar anti-proliferative activity as cisplatin in multiple cancer cell lines including head and neck squamous cell carcinoma (e.g. MDA-1986 and JMAR),1 breast cancers (e.g. MCF-7, MDA-MB-231 and 4T1),7 lung cancers (e.g. A549 and Lewis lung carcinoma),6 and melanoma (e.g. B16 and A2058).8 It enters human head and neck squamous cell carcinoma cells via CD44-mediated endocytosis.9
HA-Pt is administered via S.C. injection in rats and mice. A rodent pharmacokinetic study suggested that HA-Pt demonstrated reduced Cmax, prolonged tmax, greater AUC, and extended mean residence time compared to intravenous cisplatin. It showed significantly improved lymph node drainage and penetration after S.C. injection relative to the cisplatin I.V. injection. This is beneficial, as many cancers metastasize to the lymph nodes prior to systemic dissemination. A rodent pathological study indicates that HA-Pt is no more toxic than intravenous cisplatin. The rodent pharmacokinetic result is consistent with the findings from the pilot canine pharmacokinetic study conducted previously. The formulation resulted in superior PK and lymph node disposition in canines with spontaneously occurring soft tissue sarcomas. In addition, the formulation appeared safe in dogs at 96-hours post-dose. Based on advantageous pharmacokinetics and satisfactory toxicology, the efficacy of HA-Pt was evaluated in mouse xenografts of head and neck squamous cell carcinoma, breast cancer, melanoma, and lung cancer. Compared to cisplatin, HA-Pt significantly decreased tumor burden, prolonged survival rate, and reduced systemic toxicity as indicated by weight loss and pathological analysis in mice.1,2,8
Intravenous cisplatin must be administered to dogs with a prolonged fluid diuresis to avoid nephrotoxicity, as with humans.10 A representative protocol includes three hours of diuresis before cisplatin administration and another hour of diuresis afterwards, while other protocols recommend longer durations of diuresis (e.g. 4-6 h).11,12 In addition to saline diuresis, concurrent administration of mannitol or furosemide is often used in veterinary practices to ameliorate cisplatin-induced nephrotoxicity by increased urinary excretion. The characteristics of the HA-Pt formulation could allow neoadjuvant and adjuvant investigation of efficacy in tumors of companion dogs for translation into humans with more convenient weekly injections, and improved efficacy without prolonged diuresis.
Cisplatin chemotherapy has been investigated in dogs with osteosarcoma,13 peritoneal and pleural mesotheliomas,14 transitional cell carcinomas of the urinary bladder,15 mast cell tumors,16 and many other malignancies. The goal of the combined Phase I/II study was to determine the pharmacokinetics and to evaluate the safety and effectiveness of the formulation in spontaneous canine cancers.
Material and Methods
Synthesis of HA-Pt
HA-Pt was synthesized under USP 797 compounding sterile conditions. Typically, 7.5 L of Water for Injectiona was combined with 15 grams of cisplatin.b Subsequently, 30 g of sodium hyaluronatec was added, and the pH was adjusted to 6.5 using 6-N NaOH. After 4 days, the unreacted cisplatin and unwanted reaction products were removed by tangential flow filtrationd with a 10-kDa MWCO PES membrane, followed by concentration to 6-8 mg/mL on a cisplatin basis. The concentrated HA-Pt was terminally sterilized by passage through two sterile 0.22-micron filters.d
ICP-MS Analysis of HA-Pt
The platinum concentration of HA-Pt was determined using an Agilent 7500i inductively coupled plasma mass spectrometrye (ICP-MS). A calibration curve was generated from 1 to 50 ppb platinum (acceptable criterion R2>0.995) and 50-ppb bismuth was used as an internal standard. Unknown samples were interweaved with a 20-ppb platinum quality control; the acceptance criterion was an IS recovery of 80-120%.
DNA-Platinum Adduct Formation
DNA (Type I calf thymus sodium salt, Sigma Aldrich, St. Louis, MO) was dissolved in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0 in H2O) to 1 μg/μL. Cisplatin and HA-Pt were dissolved in H2O to 1 mg/mL. Vehicle (H2O), cisplatin (2.5 μg), or HA-Pt (2.5 μg on cisplatin basis) were added to PBS containing DNA (20 μg) (400 μL total reaction volume) and incubated in a 37°C water bath for 72 hours. DNA was precipitated and rehydrated in water according to Brouwers et al.17 The concentration and purity were determined using 260 and 280 nm absorption. DNA ratios (260 nm/280 nm) between 1.8 and 1.9 were routinely obtained. Samples were digested in 1% nitric acid at 70°C, and platinum concentrations were determined using ICP-MS analysis.
Anti-proliferation
The in vitro anti-proliferation assay was conducted to evaluate the potency of the drug against cancer cells prior to further in vivo studies. The MDA-1986 human head and neck squamous cell carcinoma cell line was a gift from Jeffrey Myers, MD, PhD, of The University of Texas, MD Anderson Cancer Center, Houston. The MDA-1986 cells were maintained in Dulbecco's Modified Eagle Mediumf with 4.5g/L of glucose, 4.5g/L of L-glutamine and 10% fetal bovine serumg in a humidified incubator at 37°C and 5% CO2. Metabolic activity was determined using Resazurin Blue, compared to vehicle treated controls, as previously reported.1
Toxicity of HA-Pt in Rodents
Toxicity was evaluated by complete blood count (CBC), renal and liver function tests. Throughout the period of the study, rodents were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility at the University of Kansas. All experimental procedures were approved by the University of Kansas Institutional Animal Care and Use Committee.
Female Sprague-Dawley ratsh were randomly divided into three groups of five animals each and received a single dose of I.V. cisplatin (6 mg/kg), HA-Pt (6 mg/kg) or saline. Animals were housed individually, and 24-hour urine samples were collected 5 and 30 days after treatment. Urine samples were centrifuged (2500× g, 4 °C, 10 minutes), and supernatants frozen at −80 °C until creatinine analysis.i
Balb/C miceh were randomly divided into three groups of three mice each and received a single dose of I.V. cisplatin (10 mg/kg), HA-Pt (10 mg/kg) or saline. Venal blood samples were collected 24 hours post dosing, centrifuged, and sent to Kansas State Veterinary Diagnostic Laboratory for CBC and liver function test.
Dogs and Treatment
An open label, multiple dose/dosage phase I/II trial of HA-Pt was conducted at the University of Missouri Veterinary Medical Teaching Hospital (MU-VMTH). The clinical trial protocol was approved by the University of Missouri Institutional Animal Care and Use Committee. Dogs of any age, sex, or breed presented to the MU-VMTH oncology service with the a histological diagnosis of anal sac carcinoma, oral squamous cell carcinoma, oral melanoma, nasal carcinoma, or digital squamous cell carcinoma with a performance score of 0 or 1 (0: normal activity; 1: restricted activity; 2: compromised activity; 3: disabled; 4: dead) and without comorbid conditions that would limit life expectancy to less than nine weeks were offered enrollment in the trial. Dogs less than 10 kg were excluded to allow for safe collection of pharmacokinetic samples.
Eligible dogs had to meet the following criteria: a tumor > 2 cm in longest dimension; fully staged with CBC, chemistry profile, thoracic radiographs (SCC of mouth or forepaw and melanoma), abdominal ultrasound (SCC of hindpaw or anal sac carcinoma), and fine needle aspiration and cytology of the draining lymph node. Dogs must not have had NSAIDs (nonsteroidal anti-inflammatory drugs) administered within seven days of starting the trial or during the trial. In addition, dogs were excluded from eligibility if they had received immunotherapy, radiation therapy, or another chemotherapy within 3 weeks of the trial, had grade 1 or higher renal dysfunction, persistent neutropenia or thrombocytopenia, or a significant infection of their tumor that could not be readily managed.
Following complete staging, client consent was obtained to enroll each dog in the study. Once enrolled in the study, longest tumor diameters of all target lesions were recorded. An indwelling jugular catheter was placed to facilitate collection of blood samples. Dogs received 10 to 30 mg/m2 intra-tumoral injections through 1 to 3 pre-placed 22-ga spinal needles once every three weeks for up to four planned doses. Dogs were sedated or anesthetized for each treatment, and a 2-mL blood sample was collected from the jugular catheter at 0.5, 1, 2, 4, and 24 hours following drug administration. Blood samples were spun, plasma was collected and frozen within two hours. A CBC and renal profile with urinalysis were conducted prior to and one week after each treatment. Some dogs experiencing apparent adverse effects of drug administration had complete biomedical profiles performed. Tumor measurements were collected three weeks following each administration to assess response.
Dogs were withdrawn from the study if they experienced grade 3 nephrotoxicity, grade 3 local reaction, progression of disease while on study, or client request to withdraw from study. Necropsy was requested from all dogs dying during or after the study period to identify tumor response and evidence of systemic toxicity.
Pharmacokinetics and Modeling of HA-Pt in Canines with Spontaneous Cancers
Serum samples of 12 dogs were diluted 20-fold in 1% HNO3, and vortexed prior to ICP-MS analysis using method described in section “ICP-MS Analysis of HA-Pt”. The pharmacokinetics were modeled using SAAM II.j
Results
Synthesis of HA-Pt
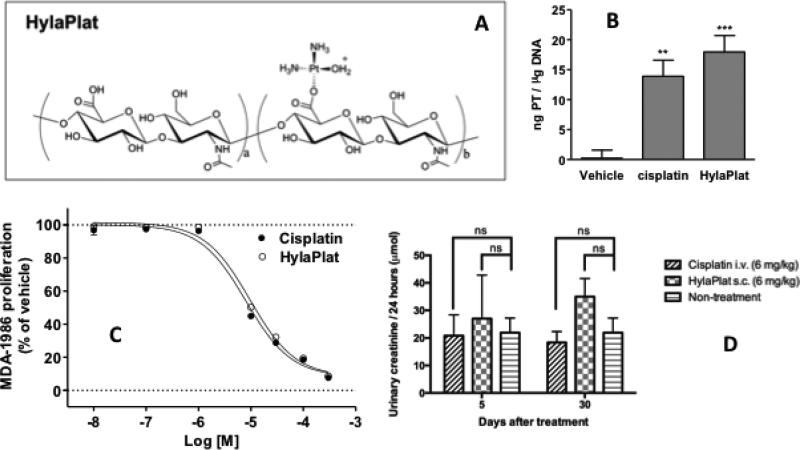
HA-Pt was successfully synthesized, and filled into 5-mL ready-to-use sterile syringes. A typical batch usually produces 12-15 grams of the HA-Pt conjugate (2 grams on cisplatin basis) with a cisplatin loading degree of 12-18%wt and a cisplatin concentration of 6-8 mg/mL. The chemical structure of HA-Pt is shown in Figure 1A.
Figure 1.
A) Chemical structure of HA-Pt. B) DNA-Pt adduct formation with HA-Pt. DNA was incubated with drug (2.5 μg on cisplatin basis) for 72 hours, followed by DNA precipitation and platinum analysis by ICP-MS. Mean ± S.E.M. from four independent experiments (n=2 / experiment; n=8 total) are shown (vehicle 0.22 ± 1.34; cisplatin 13.90 ± 2.69; HA-Pt 17.97 ± 2.72 ng / μg DNA). Both treatments resulted in significant DNA-Pt adduct formation compared to vehicle (one-way ANOVA p<0.0001; Tukey's Multiple Comparison Test **p<0.01, ***p<0.001 vs vehicle). Cisplatin and HA-Pt did not differ significantly from each other. C) Anti-proliferative activity of HA-Pt in MDA-1986 human head and neck squamous cell carcinoma cell line. Cells were treated with increasing concentrations of compound for 72 hours, and cell proliferation was quantified using Resazurin Blue. Data from at least three separate experiments performed in duplicate was analyzed by non-linear regression. Representative curves are shown. Growth inhibition potencies (mean IC50 ± S.E.M.), 8.2 ± 1.1 μM for cisplatin and 8.4 ± 0.6 μM for HA-Pt, were not significantly different (student's t-test, p>0.05, n=8). D) Creatinine excretion over 24 hours in rats at 5- and 30-day post-dose of cisplatin I.V. (6 mg/kg) or HA-Pt S.C. (6 mg/kg) or with no treatment. Two-way ANOVA statistical analysis and Sidak's multiple comparisons test were performed (ns: non-significant, p>0.05, n=5).
DNA-Pt Adduct Formation
The formation of DNA-Pt adducts is a favorable indicator of potential efficacy of platinum chemotherapeutics.18 It has been well accepted that cisplatin exerts its therapeutic efficacy through the binding to DNA bases, preferentially the highly nucleophilic N-7 positions of adenine and guanine bases.18 We measured the formation of the DNA-Pt adducts after incubating DNA with HA-Pt to ensure conjugation of cisplatin to a large linear polymer would not impede the pharmacodynamics of the active drug once it was liberated. The positive control cisplatin and HA-Pt resulted in significantly more platinum bound to DNA than vehicle treatment. The difference in Pt-DNA adduct formation between cisplatin and HA-Pt was insignificant (Figure 1B, p>0.05).
In Vitro Anti-proliferative Activity of HA-Pt
The effect of HA-Pt on the growth of the MDA-1986 cell line was tested (Figure 1C). Cisplatin and HA-Pt both inhibited cell growth over 80% compared to PBS. IC50's were 8.2 and 8.4 μM, respectively.
Toxicology of HA-Pt in Rodents
HA-Pt and cisplatin treated rats showed similar levels of creatinine excretion and urinary creatinine at 5 and 30 days post-treatment (Figure 1D). Two-way ANOVA statistical analysis and Sidak's multiple comparisons test suggested statistically insignificant difference between the treated and untreated groups.
The CBCs revealed similar results among the treatment groups (Table 1), which suggests the tolerability of 10 mg/kg cisplatin and HA-Pt in mice was similar and no major hematological toxicity was present compared to the control group. There were no significant differences in levels of liver enzymes (alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP)) between any two of the three treatment groups (cisplatin vs. control, cisplatin vs. HA-Pt, and HA-Pt vs. control). Based on the aforementioned liver function testing, no major hepatotoxicity was observed in mice treated with 10 mg/kg cisplatin or HA-Pt.
Table 1.
CBC and liver function testing of balb/c mice treated with cisplatin (I.V., 10 mg/kg single dose), HA-Pt (S.C., 10 mg/kg single dose) and saline.
| Parameters | Cisplatin I.V. (n=3) | HA-Pt S.C. (n=3) | Saline I.V. (n=3) |
|---|---|---|---|
| Leukocyte count (K/μL) | 2.00±0.10 | 1.47±0.83 | 2.45±1.34 |
| Segmented neutrophil concentration (K/μL) | 1.03±0.75 | 0.73±0.42 | 0.65±0.35 |
| Band neutrophil concentration (K/μL) | 0 | 0.03±0.06 | 0 |
| Lymphocyte concentration (K/μL) | 0.93±0.64 | 0.57±0.25 | 1.75±1.06 |
| Monocyte concentration (K/μL) | 0 | 0.07±0.12 | 0 |
| Eosinophil concentration (K/μL) | 0 | 0.03±0.06 | 0.05±0.07 |
| Basophil concentration (K/μL) | 0 | 0 | 0 |
| Erythrocyte concentration (M/μL) | 10.9±1.2 | 10.7±0.4 | 9.8±0.1 |
| Hemoglobin (g/dL) | 17.3±1.9 | 17.1±0.7 | 15.5±0.1 |
| Hematocrit (calculated, %) | 58.7±1.9 | 58.4±2.1 | 50.0±7.1 |
| Hematocrit (spun, %) | 52.3±3.2 | 53.4±1.6 | 46.5±2.1 |
| Mean cell volume (fL) | 54.2±4.2 | 52.0±4.3 | 50.8±8.1 |
| Mean cell hemoglobin (pg) | 15.8±0.1 | 15.9±0.1 | 15.9±0.1 |
| Mean cell hemoglobin concentration (g/dL) | 29.4±2.5 | 29.2±0.4 | 31.5±5.0 |
| Cell hemoglobin concentration mean | 27.2±3.4 | 27.2±0.4 | 25.0* |
| Cellular hemoglobin content | 14.6±0.6 | 14.8±0.1 | 14.1* |
| RBC distribution width | 13.1±0.3 | 13.5±0.6 | 13.2* |
| Platelet (electronic) (K/uL) | 854±55 | 834±131 | - |
| Mean platelet volume | 8.0±1.6 | 7.0±0.5 | 10.9* |
| Absolute reticulocyte (M/uL) | 0.18±0.10 | 0.24±0.04 | 0.22* |
| Reticulocyte cellular hemoglobin | 16.3±0.5 | 16.0±0.2 | 15.7* |
| Reticulocyte mean cell volume | 62.3±3.4 | 61.2±0.8 | 65.1* |
| Plasma protein by refractometry (g/dL) | 7.6±0.7 | 8.1±0.1 | 6.6±0.8 |
| ALT (U/L) | 64±44 | 61±5 | 78±42 |
| AST (U/L) | 482±329 | 396±181 | 576±324 |
| ALP (U/L) | 95±22 | 89±10 | 89±20 |
The standard deviations of the values were missing due to the blood coagulation that occurred in two mice.
Pharmacokinetics (PK) of HA-Pt in Canines with Spontaneous Cancers
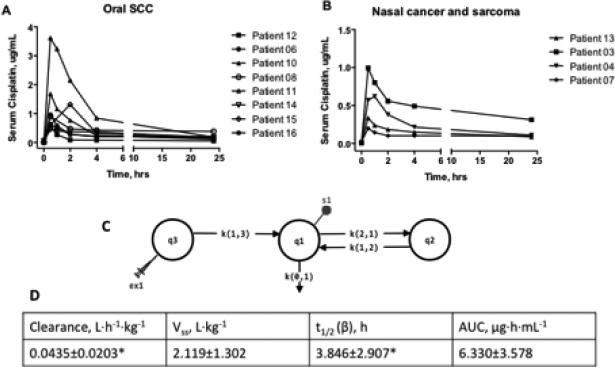
Of the sixteen dogs that received HA-Pt treatments, nine had oral squamous cell carcinomas, four had nasal cancers, one had sarcoma, and two had anal sac adenocarcinoma. Serum platinum levels were measured by ICP-MS in 12 dogs. The serum drug concentration vs. time curves are shown in Figures 2A and 2B. For clarity, dogs with different types of cancers were plotted separately.
Figure 2.
A) Pharmacokinetics of HA-Pt treated dogs with oral squamous cell carcinomas. B) Pharmacokinetics of HA-Pt treated dogs with nasal cancers or sarcomas. C) A three-compartmental model of HA-Pt pharmacokinetics. D) Pharmacokinetic parameters calculated by fitting plasma concentration vs time data to a three-compartmental model. Each dog was modeled individually. *Patients 07, 08, 10, 11, and 16 were excluded due to the incomplete elimination phase.
PK Modeling
The serum concentration vs time data was modeled using a three-compartmental model as an injection site/plasma/tissue three-compartment model properly represented the physiological distribution after a locally injected polymer-based chemotherapeutic is administered. Models using one or two compartments failed to reflect the complexity of the pharmacokinetics of an intratumorally administered polymer-drug conjugate, resulting in unacceptable data fitting. Figure 2C demonstrates the three-compartmental model with an injection site compartment (q3), a serum compartment (q1), and a tissue compartment (q2). The syringe represents the bolus dosing of HA-Pt (ex1), which is associated with the injection site compartment, while the dot (s1) represents the sampling event associated with the serum compartment. Three transfer constants, k(1,3), k(1,2) and k(2,1), and one loss constant, k(0,1), were assigned to the model. The k(1,3) defined the drug absorption process allowing the transfer of HA-Pt from the injection site compartment to the serum compartment. A reversible k(3,1) was not necessary as a sink condition was assumed. Due to the concentration gradient between the injection site and the central serum compartments, drug redistribution back to the injection site was not expected. A loss constant was designated by k(0,1) which represented the renal clearance of either the unchanged cisplatin or its metabolites. Drug transfer and equilibrium between plasma and tissue compartments were represented by k(1,2) and k(2,1). Post-injection drug diffused from the tissue at the injection site to the blood capillaries and the lymphatic vessels. Eventually the drug molecules entered the systemic circulation and distributed into organs. It is likely that the highly perfused organs, such as liver and kidneys, established equilibrium sooner than organs with low blood flow.
The pharmacokinetic data of eleven tumor-bearing dogs were successfully fitted to the three-compartmental model. Patient 15 was excluded as insufficient serum samples were available to model the terminal phase. Except for one dog, patient 04, who had a Cmax at 1 hour, all other dogs exhibited a Cmax at 0.5 hour post drug administration, which indicates a rapid distribution of the HA-Pt from the injection site to the systemic circulation. The Cmax varied by 18-fold among dogs, although the dose received varied by only 2-fold.
The elimination process varied greatly between patients, whereas Cmax was fairly uniform. Specifically, patients 03, 04, 06, 11, 13, and 14 had an elimination half-life in the range of 0.5 to 8.5 hours; whereas patients 07, 08, 10, 11, and 16 had extended half-lives from 51 to more than 1000 hours. This may be partially attributed to the incomplete drug elimination at 24 hours and the different renal health of each dog. Thus, additional blood sampling time points at 48 and 72 hours post-dosing may be beneficial for modeling of the elimination phase. Due to travel distance and owner trial compliance considerations, samples past 24 hours were not requested in this trial, but may be requested in future trial designs.
The volumes of distribution at steady state (Vss) were fairly similar for all dogs after normalizing for patient weight. The clearance was also reported per body weight, as the patients’ weights varied greatly from 11.5 to 40.7 kg. Furthermore, the AUC was calculated as 6.3±3.6 μg·h·mL−1. The drug was eliminated from the body relatively rapidly after injections, which resulted in an average elimination half-life of 3.8±2.9 hours. Patients 07, 08, 10, 11, and 16 were excluded from the clearance and t1/2 (β) calculations due to the incomplete drug elimination at 24-hour post dose. However, the Vss and AUC were included in the calculation as they did not significantly differ from the rest of the dogs, who had more complete drug excretion by 24 hours.
Clinical Chemistry of HA-Pt in Tumor Bearing Canines
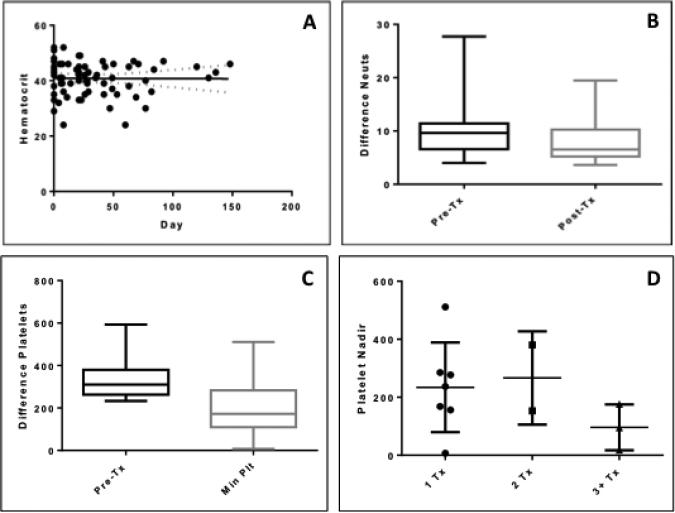
All patients received pre- and post-treatment (every 1 to 3 weeks for up to 148 days) examinations, including CBCs and clinical chemistry. Hematocrit was normal over time (Figure 3A), suggesting HA-Pt did not affect the ability of the bone marrow to produce normal numbers of red blood cells at the end of the study (Figure 3A). However, the neutrophil counts decreased significantly post treatment from day 4 to 148 (Figure 3B), suggesting that the ability of bone marrow to produce normal numbers of the most abundant types of white blood cells may be compromised. Cisplatin is known to induce bone marrow toxicity in dogs,19 and the unbound cisplatin released from the polymer may deposit in bone marrow and affect its ability to produce neutrophils. The statistical analysis was performed using a nonparametric Wilcoxon matched-pairs signed-rank test that compares before and after, or matched subjects. In addition, the platelet counts at pre- and post-dose suggested a significant decrease (Figure 3C), indicating myelosuppression induced by the treatment; however, the decrease was not related to the number of doses (Figure 3D). The statistical analysis of the difference in platelet counts between pre-dose and nadir (minimum platelet counts) was performed using the Wilcoxon matched-pairs signed-rank test. The statistical analysis of the platelet nadirs after 1-, 2-, or 3- treatments was performed using a nonparametric Kruskal-Wallis test that compares three or more unmatched groups. A p value of 0.4308 indicated that the differences in the platelet nadirs after the first, second, and the third dose were statistically insignificant.
Figure 3.
A) Hematocrit test over time. The pre-dose value was collected on day 0. HA-Pt was given on day 1. Patients were monitored until the end of the study. The solid line represents the best-fit line using the linear regression model. The two dashed lines represent the confidence bands that define the 95% confidence interval of the best-fit line. A p value of 0.964 indicates that the slope of the best-fit line was not significantly non-zero, suggesting that the pre- and post-dose values were similar; B) Difference in neutrophil counts between pre- and post-treatment. The pre-dose values were collected on day 0, and the post-dose values were collected on day 4 to 148. Wilcoxon matched-pairs signed rank test indicated a statistically significant difference between pre- and post-treatment values (p=0.002); C) Platelet counts at pre-dose and nadir (minimum platelet counts). Wilcoxon matched-pairs signed rank test indicated a statistically significant difference between pre- and post-treatment values (p=0.001); D) Platelet nadir after one, two, or three plus treatments. Kruskal-Wallis test indicated platelet nadir was not correlated with the number of doses (p>0.05), suggesting that the increase in the number of treatments did not result in a decrease in platelet nadir.
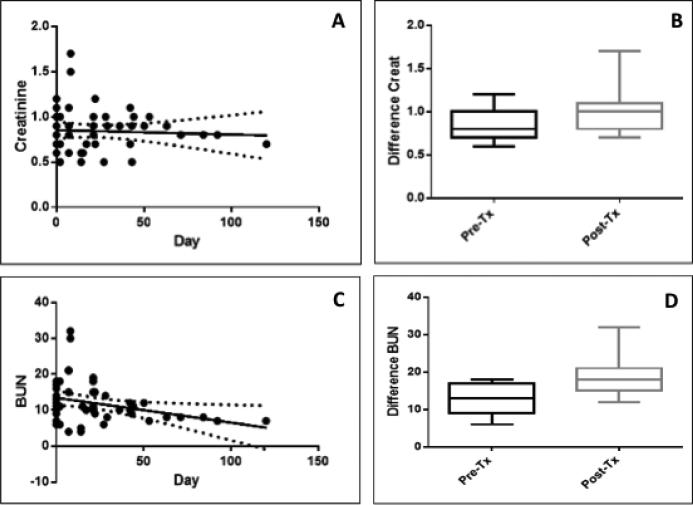
Renal profiles, including serum creatinine and blood urea nitrogen (BUN) levels, were evaluated at pre-dose (day 0) and post-dose (days 2 to 120). Though elevated shortly after treatment, creatinine post-treatment levels returned to approximately the pre-dose levels (Figure 4A) over time. As another indicator of renal damage, BUN levels were determined at both pre- and post-dose (Figure 4C and 4D). Results showed that the post-treatment BUN levels dropped over time (Figure 4C); however, the pre- and post-treatment values were not significantly different (p>0.05). This suggests that HA-Pt did not cause nephrotoxicity over time and the normal renal function was not greatly affected by the drug. In addition, the specific gravity of urine was measured to be 1.031±0.011 and 1.031±0.014 before and after treatment, respectively. The unaltered specific gravity indicated that the kidneys’ ability to concentrate urine is not affected by the treatment, thus kidney injury is not likely a concern.
Figure 4.
A) Creatinine levels in tumor bearing dogs treated with HA-Pt. The solid line indicates the best-fit of the data to a linear regression model. The dashed lines are confidence bands that indicate the 95% confidence interval of the best-fit. The slope of the best-fit was not significantly non-zero (p>0.05), suggesting that the pre- and post-dose creatinine levels did not differ significantly; B) Creatinine levels at pre- and post-dose not statistically different (Wilcoxon matched-pairs signed rank test, p>0.05); C) BUN levels in tumor bearing dogs treated with HA-Pt. The post-dose BUN levels decreased with time (p<0.05); D) The pre- and post-dose BUN levels are not statistically different (Wilcoxon matched-pairs signed rank test, p>0.05).
Liver enzymes were measured during the follow-up monitoring for five patients. One patient had similar pre- and post-treatment ALT values; one demonstrated a slight increase; two had moderate elevation; and one displayed a marked increase. The ALTmax occurred at 114, 60, 99, 28, and 21 days after the first treatment for patients 10, 12, 14, 15, and 16, respectively. Patients 10 and 15 participated in the long-term follow-up for up to 329 and 189 488 and 91 days post treatment, respectively. In both patients, the ALT levels returned to approximately the pre-dose level by the end of the monitoring duration. During the course of the study, three dogs underwent necropsy and one dog (Patient 10) underwent liver biopsy due to elevated ALT. Histology revealed disruption of the hepatocellular cords, loss of hepatocytes, and dissecting fibrous connective tissue in the centrolobular region typical of a toxic injury in the biopsy sample and two of the necropsy specimens. The third necropsy specimen did not reveal these characteristic changes. Only one of the dogs undergoing necropsy had elevated ALT. In addition, serum bilirubin levels were measured to be 0.16±0.09 and 0.74±0.38 mg/dL before and after treatment, respectively. Elevated bilirubin levels suggested the liver's ability to clear bilirubin effectively was compromised, which may result from drug-induced liver damage. We suspect the hepatic injury might be attributed to the presence of the cisplatin hydrolysis product diaqua. To evaluate this assumption, we injected rats with intravenous diaquated cisplatin (cis-[Pt(NH3)2(OH2)2]2+) at 0.1 and 0.25 mg/kg. At the lower dose, no signs of toxicity were observed; however, elevated serum ALT and AST levels were detected. At the higher dose, the animals were found lethargic the day after the injection. Liver function testing revealed elevated levels of serum ALP and ALT. In addition, another rat was injected with four doses of 0.1-mg/kg diaquated cisplatin at four-day intervals and evaluated for liver function on day seventeen. Similarly, elevated ALT and AST were detected. Taken together, the HA-Pt-induced hepatic toxicity might be likely due to the enhanced exposure to the toxic diaquated cisplatin to the liver. Further investigation of the effect of diaquated cisplatin on liver tissues may help elucidate the observation.
Efficacy of HA-Pt in Dogs
The treated dogs ranged from 4.75 to 15.75 years in age (median: 9.25 years). Breeds included four Labrador retrievers, four mixed-bred dogs, two golden retrievers, and one each of American foxhound, Australian shepherd, Basset hound, boxer, miniature Schnauzer, and Pembroke Welsh corgi. One dog was an intact male, 8 were castrated males, and 7 were spayed female. Initial tumor size ranged from 2 cm to 11 cm in longest dimension. A complete response was observed in 3 of 16 dogs (19%) with spontaneous, highly heterogeneous locally advanced cancers and a partial response or stable disease in two others. Excluding the three non-responders with anal sac adenocarcinomas and sarcoma, the complete response rate in dogs with head and neck cancers, including oral SCC and nasal carcinomas was 3/13 (23%). During the scale-up of drug production, changes in the synthesis and purification procedures resulted in the introduction of diaquated impurities, which were not detected before patient treatment. These impurities resulted in reduced tolerability and moderate or severe toxicity in some dogs. Two dogs with anal sac adenocarcinomas experienced acute cardiac death with underlying cardiomyopathy identified by necropsy. Excluding the intermediate lots with confirmed or suspected diaquated content that resulted in not completing the trial, the complete response rate for oral SCC was 3/7 (43%). Of 16 dogs, 1 dog (6%) received 4 injections; 3 dogs (19%) received 3 injections; 3 dogs (19%) received 2 injections; and 9 dogs (56%) received 1 injection. Dogs that had progressive disease or experienced toxicities after the first treatment were not given the second treatment.
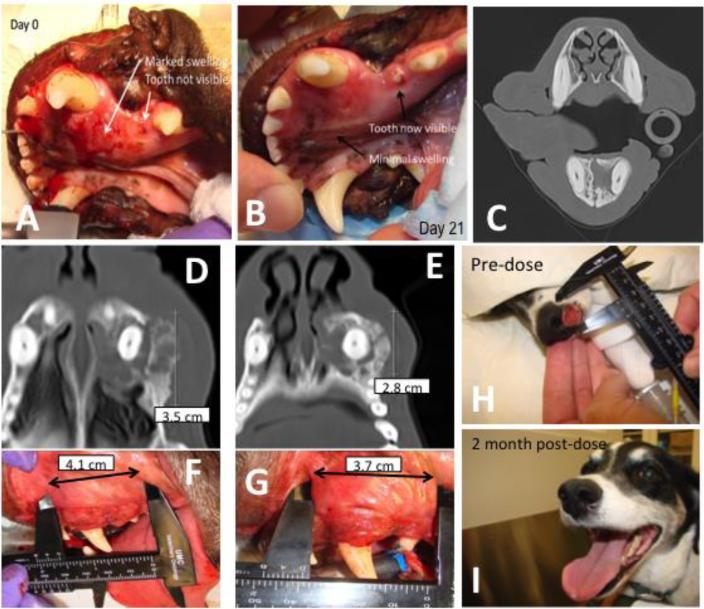
A dog (Patient 01) with oral SCC had marked swelling in its mouth at diagnosis (Fig 5A-C). It received 30 mg/m2 HA-Pt as a single intratumoral injection. Seven days post-injection, the swelling and inflammation was greatly reduced. After another two weeks, the swelling was further improved and the dog had complete tumor response (Fig 5B). One tooth that was previously invisible due to the tumor burden became visible after a single treatment. She remains in a complete remission 910 days after the start of treatment (Fig 5C).
Figure 5.
A-C) A dog (Patient 01) with an oral squamous cell carcinoma was given 30 mg/m2 of HA-Pt into the tumor. Three weeks later, the tumor had shrunk into the bone. The dog has received 4 doses (First: 30 mg/m2, second: 30 mg/m2, third: 20 mg/m2, fourth: 10 mg/m2) at 3-week intervals without any toxicity, and the tumor is in a complete remission for 910 days. The dark, lucent areas around the tooth in the CT image of Fig 5C indicated the tumor-free status, which was confirmed by biopsy. D-G) A dog (Patient 10) with oral squamous cell carcinoma was treated with four doses of HA-Pt (First: 10 mg/m2, second: 10 mg/m2, third: 10 mg/m2, fourth: 12.5 mg/m2) at 3-week intervals. The tumor entered a stable partial remission and the inflammation has reversed for 196 days. H&I) A dog (Patient 15) with nasal planum squamous cell carcinoma was given a single injection of 10 mg/m2 HA-Pt. The tumor has receded and no recurrence has been diagnosed 4 months after treatment.
Another dog (Patient 10) with spontaneous oral SCC received four injections of local HA-Pt (First: 10 mg/m2, second: 10 mg/m2, third: 10 mg/m2, fourth: 12.5 mg/m2) at 3-week intervals. The drug was administered intra-tumorally. The dog had partial response at the completion of the treatment (Figure 5D-G). The inflammation was significantly reduced and the computed tomography scan demonstrated bone sclerosis and resolution of the contrast enhancing mass in the region of the tumor. The mass remained stable in size with no further gingival bleeding or erythema for 196 days. At that time, progression was identified, and the dog has subsequently undergone radiation therapy with complete remission of the tumor. The tumor mas not recurred in 329 days.
A third dog (Patient 15) diagnosed with nasal planum SCC received a single 10 mg/m2 HA-Pt injection intralesionally. The dog showed a complete response at two months post-treatment. The pre-dose and post-dose images were shown in Figure 5H and 5I. The dog remains in a complete remission 198 days after the single treatment.
Discussion
In the Phase I/II clinical trial, the pharmacokinetics of HA-Pt in tumor-bearing dogs was evaluated. The dogs were dosed at 10-30 mg/m2, which equals approximately 0.3 to 1.0 mg/kg. The Vss was determined to be 2.119±1.302 L/kg. The clearance was 0.0435±0.0203 L/h/kg. The elimination half-life was 3.846±2.907 hours, excluding patients who did not demonstrate a complete elimination phase within 24 hours. Vaden et al conducted a pharmacokinetic study of intravenous cisplatin in dogs in the 1980s. The dogs were given 1 mg/kg cisplatin as a bolus injection. The volume of distribution, clearance, and elimination half-life were determined to be 0.45 L/kg, 8.6 mL/kg/min and 38 minutes, respectively.20 The greater Vss of intratumoral HA-Pt compared to the intravenous cisplatin was likely due to the differences in the route of administration, slower clearance of the drug, and superior lymphatic and tissue penetration.3,4 Intravenous cisplatin was cleared from the body at a much higher rate than the polymer drug conjugate because small molecules like cisplatin undergo rapid renal excretion when administered as an intravenous bolus injection. In comparison, large molecules may undergo a more complex process of clearance. Lymphatically targeted HA-Pt may preferentially distribute to the lymphatics before it enters the systemic circulation. Interactions between hyaluronan and its receptors or other cell-surface proteins in the body may also slow down the systemic clearance of the drug. Furthermore, the elimination half-life of intravenous cisplatin was shorter than intratumoral HA-Pt in all animals except for one. The relatively short Tmax indicates a rapid distribution of the HA-Pt from the injection site to the systemic circulation. This is likely due to the rich blood supply in the oral and nasal mucosa, which expedites the systemic entrance of the drug. This is consistent with pharmacokinetics of large molecule protein therapeutic where subcutaneous absorption rate is highly dependent on injection site, and can be due to a combination of increased lymphatic flow due to high hydrostatic pressure in confined tissues and richness of blood perfusion in the tissue space.21 Since these nanoparticle carriers have a distribution of sizes, it is expected that uptake could occur by both lymphatic and capillary routes. The variation in Cmax may be due to the heterogeneity of the tumors and their surrounding environments.22 In other words, it suggests a distinct “leakiness” of local blood capillaries and lymphatic vessels that affected the serum kinetics of the drug molecules. Patient to patient variability in tumor morphology and heterogeneity within the same tumor can strongly affect the distribution of drug from the injection site and absorption by blood vessels.23,24 Incorporation of the vasoconstrictor epinepherin was required in Intradose's cisplatin gel formulation for intratumor dosing of cisplatin to prevent rapid variable absorption of small Pt species, which could occur in highly perfused tissues,25 yet high patient variability in pharmacokinetics was cited by FDA in their rejection of the NDA.26 In the case of our formulation, release of cisplatin species is retarded by chemical conjugation to the carrier. Although the rate of release is dependent on both pH and presence of chloride ions,3 which could vary with the perfusion and morphology of the injection site, these are expected to be less variable than a diffusion only controlled system.
Cisplatin is known to induce nephrotoxicity in experimental animal models including mice, rats, and dogs. In humans, at a dose of 50-100 mg/m2, approximately 30% of patients develop acute renal failure during or after the therapy.27 A toxicology study conducted in cisplatin treated dogs revealed that the nephrotoxicity was initiated by proximal tubular impairment as the proximal reabsorption rates decreased significantly after intravenous administration of cisplatin in dogs.28 The prolonged localization of cisplatin, platinum metabolites, or cisplatin hydrolytic products in the kidney is attributed to the continuous excretion and active secretion by the kidney. The most striking morphological changes in the kidney occur in the pars recta of the proximal tubules of the lumen, which is the most active site of secretion.29 The underlying mechanism of cisplatin-induced renal toxicity has not been clearly understood. However, evidence suggests renal cytotoxicity is likely due to the inhibition of DNA synthesis and compromised self-recovery of the pars recta of the proximal tubules in the kidney.30,31 Our clinical results regarding the potential nephrotoxicity of HA-Pt in both rodents and dogs suggested that HA-Pt did not affect the normal renal function based on creatinine and BUN evaluation. It is likely attributed to the fact that cisplatin is bound to a natural carrier and slowly released from the carrier in vivo, modifying the kinetics and the renal deposition of the drug and preventing irreversible damages to the kidneys.
Cisplatin rarely causes hepatic toxicity. Our previous rodent study demonstrated that neither cisplatin nor HA-Pt induced an elevation of liver enzymes such as ALT, AST, and ALP. Surprisingly, HA-Pt clinical trials revealed hepatic toxicity in dogs. Post-dose, 40% of the dogs evaluated had moderately elevated levels of ALT, and 20% dogs showed greatly elevated levels. We believe the liver toxicity may be attributed to the altered pharmacokinetics and deposition of HA-Pt compared to cisplatin,3,4 as well as the rapid release of diaquated platinum in the liver. In contrast to cisplatin which is rapidly excreted by the kidney, the polymer-based HA-Pt is too large to be cleared via the kidney. Alternatively, HA-Pt may be broken down by the liver as the carrier hyaluronan is naturally metabolized by the liver. As the liver digests the polymer, aquated free platinum molecules are liberated from the polymer backbone in the forms of cis-[Pt(NH3)2(OH2)Cl]+ and cis-[Pt(NH3)2(OH2)2]2+. Subsequently, the aquated platinum enters hepatic cells and causes DNA damage to the cells, resulting in elevated liver enzymes released by the necrotic cells. In addition, one dog developed acute toxicity after anal sac injection of HA-Pt and died within a few hours. The toxicity is traced to the presence of diaqua in batches. Diaqua not only causes safety concerns, significant amounts of diaqua in batches may also reduce the efficacy of the drug as the highly charged diaqua molecules may not enter tumor cells as efficiently as cisplatin, rendering patients with insufficient exposure to drug. Cleare et al. reported similar findings of diaqua toxicity in rodents and suggested that diaqua may interfere with a vital nervous system function at the neuromuscular junction.32 Subsequent studies eliminated diaqua contamination by including an additional purification step by dialysis. To resolve the toxic diaqua issue, we are developing a second-generation HA-Pt that demonstrates a longer drug release half-life and is free of diaquated cisplatin while preserving the effectiveness of HA-Pt. HA-Pt achieved a higher rate (23%) of complete response in canine head and neck cancers compared to cisplatin (7%).33 In addition, the elevated liver enzymes levels may be due to the increased update of the polymer-drug conjugate by the liver which is not non-common for relatively large delivery carriers.
A relatively small Phase I/II clinical trial was conducted in tumor-bearing dogs to evaluate the pharmacokinetics, safety, and efficacy of HA-Pt. HA-Pt demonstrated biphasic pharmacokinetics in dogs after an intratumoral injection. HA-Pt achieved a 23% (3/13) complete response in dogs with locally advanced heterogeneous head and neck cancers. HA-Pt did not cause nephrotoxicity, which is the primary toxicity for platinum drugs in both dogs and humans, although hepatotoxicity appeared in some tumor-bearing dogs, which was attributed to the increased deposition of HA-Pt in the liver as well as the release of the toxic diaquated cisplatin from the polymer. A second-generation HA-Pt with improved hepatic safety and equivalent anti-cancer effectiveness is being developed.
Acknowledgments
Supported by the National Institute of Health, American Cancer Society, K-INBRE, Kansas EPSCoR, and HylaPharm LLC
Abbreviations
- SCC
Squamous cell carcinoma
- HNSCC
Head and neck squamous cell carcinoma
- CBC
Complete blood count
- AUC
Area under the curve
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ALP
Alkaline phosphatase
- ICP-MS
Inductively coupled plasma mass spectrometry
- PK
Pharmacokinetics
- Cmax
Plasma maximum concentration
- t1/2
Half-life
- Vss
Volume of distribution at steady state
- BUN
Blood urea nitrogen
- CT
Computed tomography
- IV
Intravenous
- SC
Subcutaneous
- WFI
Water for injection
- HA
Sodium hyaluronate
- MWCO
Molecular weight cutoff
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- PBS
Phosphate buffered saline
- HA-Pt (hyaluronan-cisplatin nanoconjugate)
Cisplatin-based hyaluronan nanoparticle
Footnotes
Presented by abstract form at the American Association of Pharmaceutical Scientists Annual Meeting, San Diego, California, November 4, 2014
ML Forrest, WC Forrest, Aires, and Cai have ownership interest in HylaPharm, which has licensed technologies from the University of Kansas.
VWR, Radnor, PA
Qilu chemical Co., Zibo, China
Lifecore Biomedical, Chaska, MN
Spectrum Laboratories, Rancho Dominguez, CA
Agilent, Santa Clara, CA
DMEM, Corning, Manassas, VA
Fisher Scientific, Waltham, MA
Charles River Laboratories, Raleigh, NC
Sigma Aldrich, St Louis, MO
The Epsilon Group LLC, Charlottesville, VA
References
- 1.Cai S, Xie Y, Davies NM, et al. Carrier-based intralymphatic cisplatin chemotherapy for the treatment of metastatic squamous cell carcinoma of the head & neck. Ther Deliv. 2010;1:237–245. doi: 10.4155/tde.10.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen SM, Rockefeller N, Mukerji R, et al. Efficacy and toxicity of peritumoral delivery of nanoconjugated cisplatin in an in vivo murine model of head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139:382–387. doi: 10.1001/jamaoto.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai S, Xie Y, Davies NM, et al. Pharmacokinetics and disposition of a localized lymphatic polymeric hyaluronan conjugate of cisplatin in rodents. J Pharm Sci. 2010;99:2664–2671. doi: 10.1002/jps.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai S, Xie Y, Bagby TR, et al. Intralymphatic chemotherapy using a hyaluronancisplatin conjugate. J Surg Res. 2008;147:247–252. doi: 10.1016/j.jss.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venable RO WD, Gustafson DL, Hansen RJ, Ehrhart EJ, 3rd, Cai S, Cohen MS, Forrest ML. Effects of intratumoral administration of a hyaluronan-cisplatin nanoconjugate to five dogs with soft tissue sarcomas. Am J Vet Res. 2012;73:1969–1976. doi: 10.2460/ajvr.73.12.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Aillon KL, Cai S, et al. Pulmonary delivery of cisplatin-hyaluronan conjugates via endotracheal instillation for the treatment of lung cancer. Int J Pharm. 2010;392:156–163. doi: 10.1016/j.ijpharm.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Cai S, Xie Y, et al. A novel intralymphatic nanocarrier delivery system for cisplatin therapy in breast cancer with improved tumor efficacy and lower systemic toxicity in vivo. Am J Surg. 2009;198:781–786. doi: 10.1016/j.amjsurg.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Q, Aires DJ, Cai S, et al. In vivo efficacy of nano hyaluronan-conjugated cisplatin for treatment of murine melanoma. J Drugs Dermatol. 2014;13:283–287. [PMC free article] [PubMed] [Google Scholar]
- 9.Cai S, Alhowyan AA, Yang Q, et al. Cellular uptake and internalization of hyaluronan-based doxorubicin and cisplatin conjugates. J Drug Target. 2014:1–10. doi: 10.3109/1061186X.2014.921924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogilvie GK, Krawiec DR, Gelberg HB, et al. Evaluation of a short-term saline diuresis protocol for the administration of cisplatin. Am J Vet Res. 1988;49:1076–1078. [PubMed] [Google Scholar]
- 11.Barabas K, Milner R, Lurie D, et al. Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol. 2008;6:1–18. doi: 10.1111/j.1476-5829.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Sarraf M, Fletcher W, Oishi N, et al. Cisplatin hydration with and without mannitol diuresis in refractory disseminated malignant melanoma: a southwest oncology group study. Cancer Treat Rep. 1982;66:31–35. [PubMed] [Google Scholar]
- 13.Dawe J. Osteosarcoma in a 6-year-old Newfoundland dog: limb-sparing surgery and cisplatin chemotherapy. Can Vet J. 2007;48:1169–1171. [PMC free article] [PubMed] [Google Scholar]
- 14.Seo KW, Choi US, Jung YC, et al. Palliative intravenous cisplatin treatment for concurrent peritoneal and pleural mesothelioma in a dog. J Vet Med Sci. 2007;69:201–204. doi: 10.1292/jvms.69.201. [DOI] [PubMed] [Google Scholar]
- 15.Knapp DW, Henry CJ, Widmer WR, et al. Randomized trial of cisplatin versus firocoxib versus cisplatin/firocoxib in dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 2013;27:126–133. doi: 10.1111/jvim.12013. [DOI] [PubMed] [Google Scholar]
- 16.Spugnini EP, Vincenzi B, Citro G, et al. Evaluation of Cisplatin as an electrochemotherapy agent for the treatment of incompletely excised mast cell tumors in dogs. J Vet Intern Med. 2011;25:407–411. doi: 10.1111/j.1939-1676.2011.0678.x. [DOI] [PubMed] [Google Scholar]
- 17.Brouwers EE, Tibben MM, Pluim D, et al. Inductively coupled plasma mass spectrometric analysis of the total amount of platinum in DNA extracts from peripheral blood mononuclear cells and tissue from patients treated with cisplatin. Anal Bioanal Chem. 2008;391:577–585. doi: 10.1007/s00216-008-2034-8. [DOI] [PubMed] [Google Scholar]
- 18.Chaney SG, Sancar A. DNA repair: enzymatic mechanisms and relevance to drug response. J Natl Cancer Inst. 1996;88:1346–1360. doi: 10.1093/jnci/88.19.1346. [DOI] [PubMed] [Google Scholar]
- 19.Dernell WS, Withrow SJ, Straw RC, et al. Adjuvant chemotherapy using cisplatin by subcutaneous administration. In ViVo. 1997;11:345–350. [PubMed] [Google Scholar]
- 20.Vaden SL, Page RL, Williams PL, et al. Effect of hyperthermia on cisplatin and carboplatin disposition in the isolated, perfused tumour and skin flap. Int J Hyperthermia. 1994;10:563–572. doi: 10.3109/02656739409009358. [DOI] [PubMed] [Google Scholar]
- 21.Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14:559–70. doi: 10.1208/s12248-012-9367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao A, Zhao X, Phillips WT, et al. Theoretical study of the influence of a heterogeneous activity distribution on intratumoral absorbed dose distribution. Med Phys. 2005;32:200–8. doi: 10.1118/1.1833151. [DOI] [PubMed] [Google Scholar]
- 23.Gaustad JV, Brurberg KG, Simonsen TG, et al. Tumor vascularity assessed by magnetic resonance imaging and intravital microscopy imaging. Neoplasia. 2008;10:354–62. doi: 10.1593/neo.08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasparini G, Weidner N, Maluta S, et al. Intratumoral microvessel density and p53 protein: correlation with metastasis in head-and-neck squamous-cell carcinoma. Int J Cancer. 1993;55:739–44. doi: 10.1002/ijc.2910550507. [DOI] [PubMed] [Google Scholar]
- 25.Mok TS, Kanekal S, Lin XR, et al. Pharmacokinetic study of intralesional cisplatin for the treatment of hepatocellular carcinoma. Cancer. 2001;91:2369–77. [PubMed] [Google Scholar]
- 26. [October 27, 2015]; http://www.fda.gov/ohrms/dockets/ac/01/briefing/3782b1_06_FDA%20IntraDose%20ClinPharm.pdf.
- 27.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–379. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 28.Daugaard G. Cisplatin nephrotoxicity: experimental and clinical studies. Dan Med Bull. 1990;37:1–12. [PubMed] [Google Scholar]
- 29.Jacobs C, Kalman SM, Tretton M, et al. Renal handling of cisdiamminedichloroplatinum(II). Cancer Treat Rep. 1980;64:1223–1226. [PubMed] [Google Scholar]
- 30.Townsend DM, Deng M, Zhang L, et al. Metabolism of Cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol. 2003;14:1–10. doi: 10.1097/01.asn.0000042803.28024.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safirstein R, Winston J, Moel D, et al. Cisplatin nephrotoxicity: insights into mechanism. Int J Androl. 1987;10:325–346. doi: 10.1111/j.1365-2605.1987.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 32.Cleare MJ, Hoeschele JD. Studies on the Antitumor Activity of Group VIII Transition Metal Complexes. Part 1. Platinum (II) Complexe. Bioinorg Chem. 1973;2:187–210. [Google Scholar]
- 33.Knapp DW, Richardson RC, Bonney PL, et al. Cisplatin therapy in 41 dogs with malignant tumors. J Vet Intern Med. 1988;2:41–46. doi: 10.1111/j.1939-1676.1988.tb01976.x. [DOI] [PubMed] [Google Scholar]