Abstract
Circulating endothelial progenitor cells (EPCs) are reduced in hypertension, which inversely correlates with its mortality. DOCA–salt hypertension features elevated ET-1 and oxidative stress. We tested the hypothesis that ET-1 induces EPC dysfunction by elevating oxidative stress through the ETA/NADPH oxidase pathway in salt-sensitive hypertension. Both ETA and ETB receptors were expressed in EPCs, but only ETA receptors were significantly increased in EPCs of DOCA-salt rats. EPC number and function were reduced in DOCA-salt rats compared with Sham controls, both were reversed by in vivo blockade of ETA receptors or NADPH oxidase. The enzymatic activity of NAPDH oxidase and its subunits gp91phox, p22phox and Rac1 were augmented in EPCs of DOCA-salt rats, with concomitantly decreased antioxidant enzymes MnSOD, CuZnSOD, and GPx-1. ROS level was elevated in EPCs from DOCA-salt rats, accompanied by increased EPC telomerase inactivation, senescence and apoptosis, which were rescued by ETA or NADPH oxidase blockade. Cell therapy of normal or treated DOCA EPCs, but not untreated DOCA EPCs, significantly increased capillary density and blood perfusion in ischemic hindlimbs of DOCA-salt rats. p53 and Bax/Bcl-2 ratios were increased in EPCs of DOCA-salt rats, which were reversed by ETA antagonist or NADPH oxidase inhibitor or superoxide scavenger (PEG-SOD). Finally, in ETB-deficient rats, plasma ET-1 was elevated and EPC number and telomerase activity were diminished. These results demonstrate, for the first time, that ET-1 contributes to EPC reduction and dysfunction via an ETA/NADPH oxidase pathway-induced oxidative stress in salt-sensitive hypertension.
Keywords: Endothelin-1, oxidative stress, NADPH oxidase, endothelial progenitor cell, hypertension
Introduction
Endothelial dysfunction contributes to the pathogenesis and progression of hypertension and is an independent predictor of cardiovascular risk1. The balance between endothelial injury and recovery is important to reduce cardiovascular incidences1, 2. Endothelial progenitor cells (EPCs) can differentiate into mature endothelial cells and regenerate the injured endothelium 2, 3. There has been accumulating evidence for reduced availability and impaired function of EPCs in the presence of cardiovascular risks 3–5. Among them, hypertension is a strong predictor of EPC migratory impairment 5, 6. Both clinical and animal studies have indicated that hypertension is inversely correlated with EPC reduction and/or dysfunction 7–9. However, investigations of in vivo EPC biology under hypertensive condition are limited and the mechanisms underlying EPC dysfunction in hypertension remains poorly understood.
Deoxycorticosterone acetate (DOCA)–salt hypertension is a low renin, salt-sensitive model with elevated arterial endothelin-1 (ET-1) and oxidative stress 10. Elevated arterial ET-1 levels in DOCA-salt rats led to NADPH oxidase activation and superoxide formation via the ETA receptors 10, resulting in endothelial dysfunction. In contrast, ETB receptors may protect against vascular injuries in this setting 11. Circulating EPCs are an important back up for endothelium integrity and function, so the effect of ET-1 on EPCs is a critical issue. In this study, we tested the hypothesis that ET-1 induces oxidative stress in EPCs via an ETA/NADPH oxidase pathway and critically contribute to EPC dysfunction in DOCA-salt hypertension. Our findings may provide a mechanistic basis for restoring EPC number and function to combat endothelial dysfunction in hypertension.
Methods
All methods and data analysis are described in the online supplemental materials (please see http://hyper.ahajournals.org).
Results
Characterization of bone marrow-derived EPCs
Bone marrow derived EPCs used in the present study were characterized by flow cytometry, Dil-acLDL/lectin double staining and Western blot, which were presented in the online supplemental materials (Table S1, Table S2, and Fig S1, please see http://hyper.ahajournals.org).
ETA and ETB receptor expression in EPCs
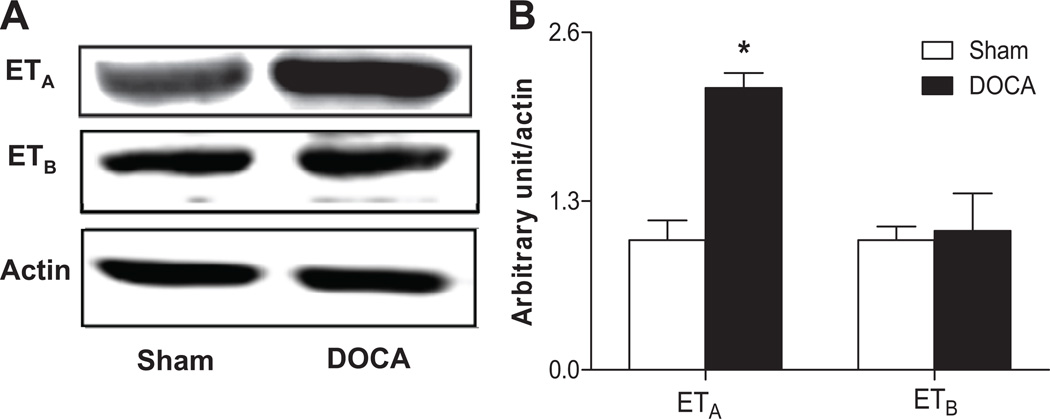
The mRNA levels of ETA and ETB receptors were detected in normal EPCs (1.00 ± 0.28 vs. 1.27 ± 0.68, P=0.732, n=4–6), which were further confirmed by immunohistochemistry (Fig. S2). Furthermore, the expression of ETA receptors in EPCs of DOCA-salt rats was significantly increased, compared to Sham controls, whereas there were no significant differences in the expression of ETB receptors in EPCs between DOCA-salt and Sham rats (Fig. 1A–1B).
Fig 1. Expressions of ET receptors in EPCs.
A. The expressions of ETA and ETB receptors in Sham and DOCA rat cultured bone marrow-derived EPCs. B. Quantitative analysis of band density. Values were expressed as mean±SEM, which were normalized to Sham rats, n=5–6, *P<0.05 vs. Sham rats (By a Student’s 2-tailed unpaired t test). The n values shown represented individual rat.
Activation of NADPH oxidase and increased ROS in EPCs of DOCA-salt rats
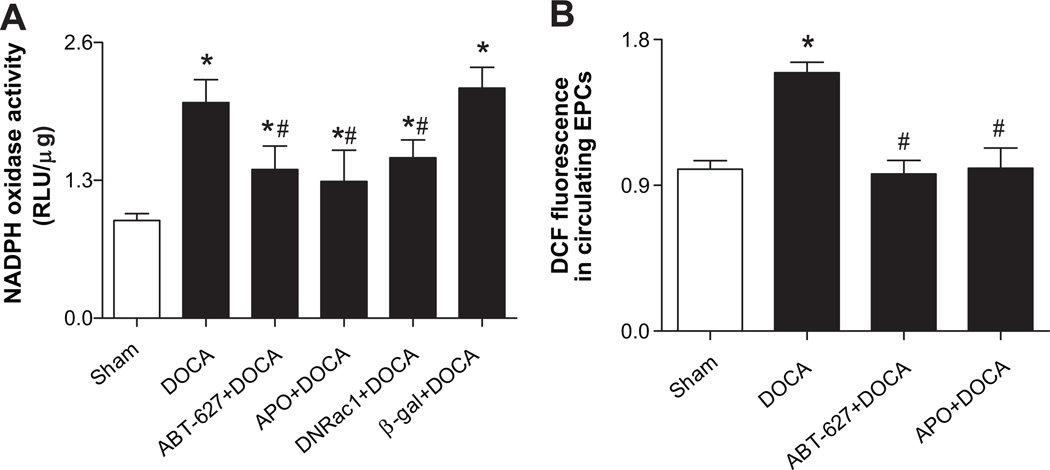
Rac1, gp91phox, and p22phox proteins were significantly increased in EPCs of DOCA-salt rats compared to Sham controls (Fig. S3A). Consistently, NADPH oxidase activity was significantly increased in EPCs from DOCA-salt rats compared to Sham rats. In vivo treatment with ETA antagonist (ABT-627) or NADPH oxidase inhibitors (Apocynin) blunted the activation of NADPH oxidase (Fig. 2A). When EPCs of DOCA-salt rats were transfected with the dominant negative Rac1 (DNRac1), which inhibits the key NADPH oxidase subunit Rac1, their NADPH activities were significantly decreased. The same effect was not observed in β-gal transfected EPCs (Fig. 2A). Meanwhile, the intracellular ROS in DOCA-derived CD34+/Flk-1+ progenitor cells was significantly elevated compared with that in Sham rat cells, which was blunted after 4-weeks of treatment with ABT-627 or Apocynin (Fig. 2B).
Fig 2. Activation of NADPH oxidase and increased ROS in EPCs of DOCA-salt rats.
A. NADPH oxidase activity in EPCs of DOCA-salt rats was significantly increased, which was inhibited by 4-week treatment with ETA antagonist (ABT-627, 5 mg·kg −1·d−1) or NADPH oxidase inhibitor (Apocynin, APO, 1.5 mmol/L). DNRac1 blunted NADPH oxidase activity in EPCs of DOCA-salt rats. EPCs were transfected with AdDNRac1 or Adβ-gal at a titer of 500 multiplicity of infection (MOI) for 24 hours. NADPH oxidase activity was measured by a lucigenin-enhanced chemiluminescence assay. The enzyme activity was expressed as relative light units (RLU)/µg protein. Values were expressed as mean±SEM, which were normalized to Sham rats, n=5–6, *P<0.05 vs. Sham rats, #P<0.05 vs. DOCA rats or β-gal+DOCA group. The n values shown represented individual rat. β-gal: β-galactosidase (reporter gene); DNRac1: dominant-negative Rac1, inhibiting endogenous NADPH oxidase subunit Rac1. B. Mean fluorescence of DCF in freshly isolated circulating CD34+/Flk-1+ progenitor cells by flow cytometry. CM-H2DCFDA, a membrane-permeable probe, enters the cells and produces a green fluorescent signal (DCF fluorescence) after intracellular oxidation by ROS. Values were normalized to Sham rats and expressed as mean±SEM, n=6–8, *P<0.05 vs. Sham rats, #P<0.05 vs. DOCA rats. All data were analyzed by one-way ANOVA with Bonferroni's post-test.
Antioxidant enzyme in the EPCs of DOCA-salt rats
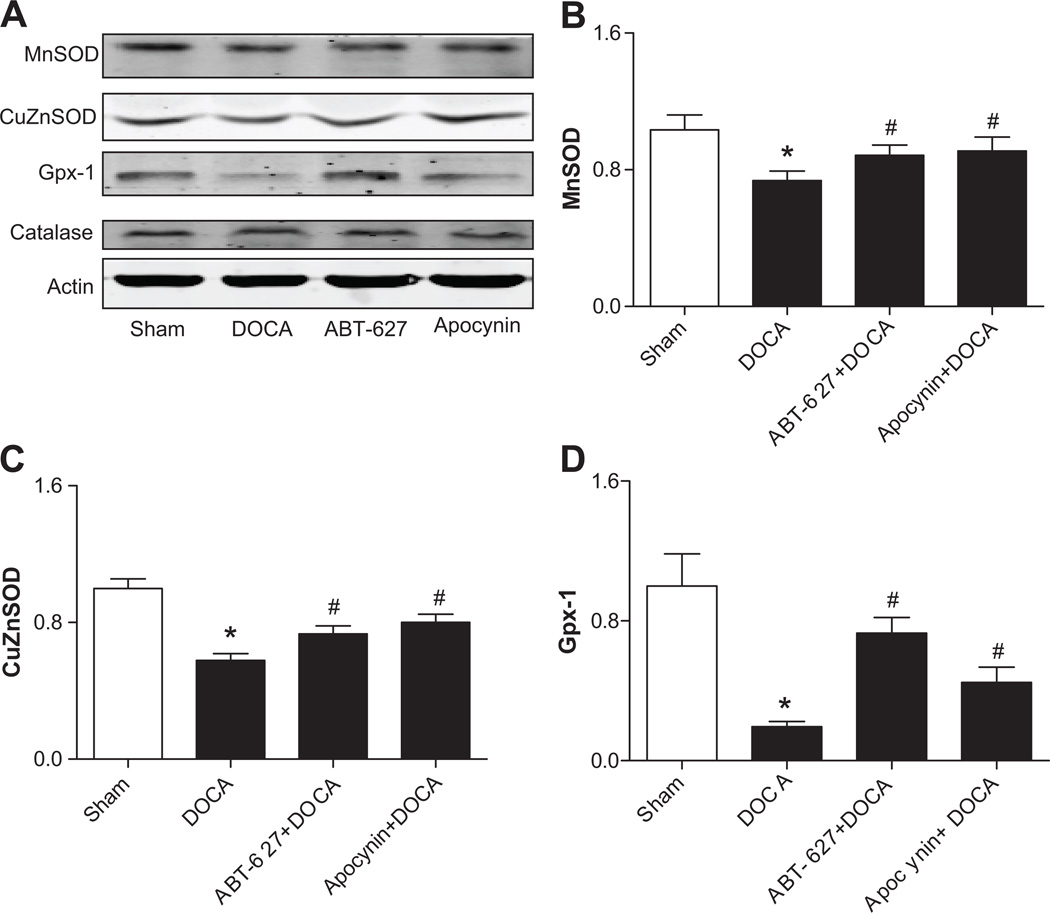
The expressions of major antioxidant enzymes were measured in EPCs from Sham and DOCA-salt rats. Except for catalase, the expressions of MnSOD, CuZnSOD, and GPx-1 in EPCs from DOCA-salt rats were significantly decreased when compared to those from Sham rats. In vivo blockade of ABT-627 or Apocynin for 4 weeks significantly reversed the expressions of decreased antioxidant (Fig. 3).
Fig 3. Antioxidant enzyme in the EPCs of DOCA-salt rats.
A. Expressions of intracellular antioxidant enzymes in EPCs were determined by Western blot analysis. B–D. The statistical analysis of Western blot. Values were expressed as mean±SEM, which were normalized to Sham rats, n=5–6, *P<0.05 vs. Sham rats, #P<0.05 vs. DOCA rats. The n values shown represented individual rat.
EPC dysfunctions in DOCA-salt rats
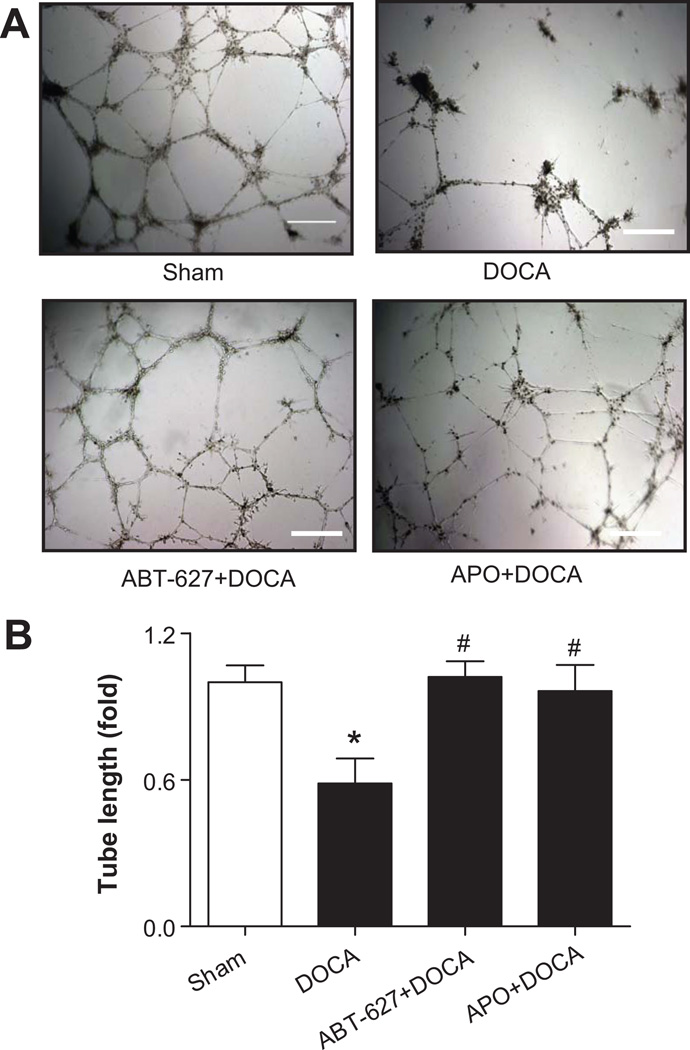
The tube formation capacity of EPCs was significantly impaired in DOCA-salt rats compared to Sham controls. In vivo treatment with ABT-627 or Apocynin significantly preserved EPC tube formation capacity (Fig. 4A–4B). In addition, the adhesion activity of EPCs from DOCA-salt rats was decreased by 50%~60% compared with to Sham controls, which was reversed by in vivo blockade of ETA receptors (n=4–8, P<0.05), but not by Apocynin (n=3 in Apocynin group and n=5 in DOCA group, P=0.7234).
Fig 4. EPC dysfunction in DOCA-salt rats.
A. Representative microscopic views of tube formation, defined as a tube-like structure exhibiting a length four times its width (magnifications 40×). Bar=200 µm. B. Statistical analysis of tube length. Image-Pro plus 5.1 was used to determine the total length of the tube-like structures in images. Values were normalized to Sham rats and expressed as mean±SEM, n=5–8, *P<0.05 vs. Sham rats, #P<0.05 vs. DOCA rats (By a one-way ANOVA with Bonferroni's post-test).
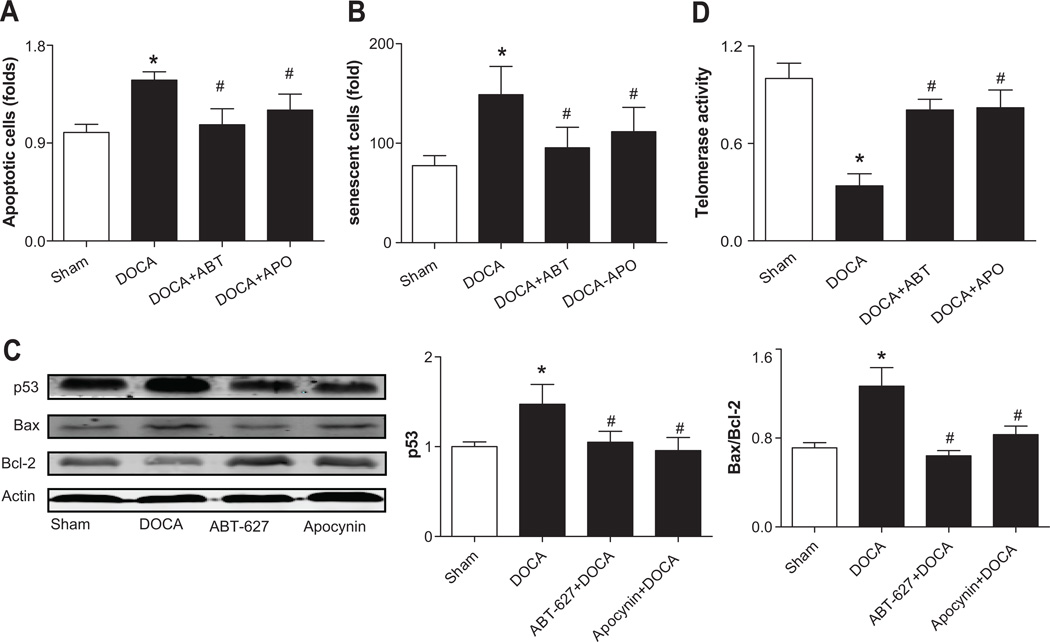
Increased EPC apoptosis and senescence in DOCA-salt rats
Both apoptosis and senescence were significantly increased in EPCs from DOCA-salt rats, which were prevented in rats treated with ABT-627 or Apocynin (Fig. 5A–5B). The expressions of apoptosis related proteins were detected in EPCs. p53 expression in EPCs from DOCA-salt rats was significantly increased and accompanied by up-regulation of pro-apoptotic Bax and down-regulation of anti-apoptotic Bcl-2, as compared to Sham controls. The ratio of Bax to Bcl-2 (Bax/Bcl-2) was significantly increased by ~2 folds in the EPCs of DOCA-salt rats. Above effects were reversed by chronic blockades of ETA receptors or NADPH oxidase (Fig. 5C and Supplement Fig. S3C–S3D). The ROS scavenger, PEG-SOD (100 U/ml, 24 hours) can reverse these effects (Supplemental Fig. S3B). Consistently, the telomerase activity in the EPCs of DOCA-salt rats was decreased by approximately 70% compared to Sham rats, which was rescued following in vivo treatment with ABT-627 or Apocynin for 4 weeks (Fig. 5D).
Fig 5. Increased EPC senescence and apoptosis in DOCA-salt rats.
A. Quantitative analysis of apoptotic cells. Values were expressed as mean±SEM, n=6. *P<0.05 vs. Sham rats, #P<0.05 vs. DOCA rats. B. Quantitative analysis of senescent cells. Values were normalized to Sham rats and expressed as mean±SEM, n=4–6, *P<0.05 vs. Sham rats, #P<0.05 vs. DOCA rats. C. The expressions of p53 and the Bax/Bcl-2 ratio in EPCs of DOCA-salt rats with or without chronic treatments of ETA receptor blockade (ABT-627, 5 mg·kg −1·d−1) or NADPH oxidase inhibitor (Apocynin, APO, 1.5 mmol/L). The n values shown represented individual rat. Values were expressed as mean±SEM, n=4–5. *P<0.05 vs. Sham rats, #P<0.05 vs. DOCA rats. Bax/Bcl-2: the ratio of Bax to Bcl-2. D. Telomerase activity in EPCs of DOCA-salt rats, as measured by the TRAP assay. Values were expressed as mean±SEM, n=6. *P<0.05 vs. Sham rats, #P<0.05 vs. DOCA rats. All data were analyzed by one-way ANOVA with Bonferroni's post-test.
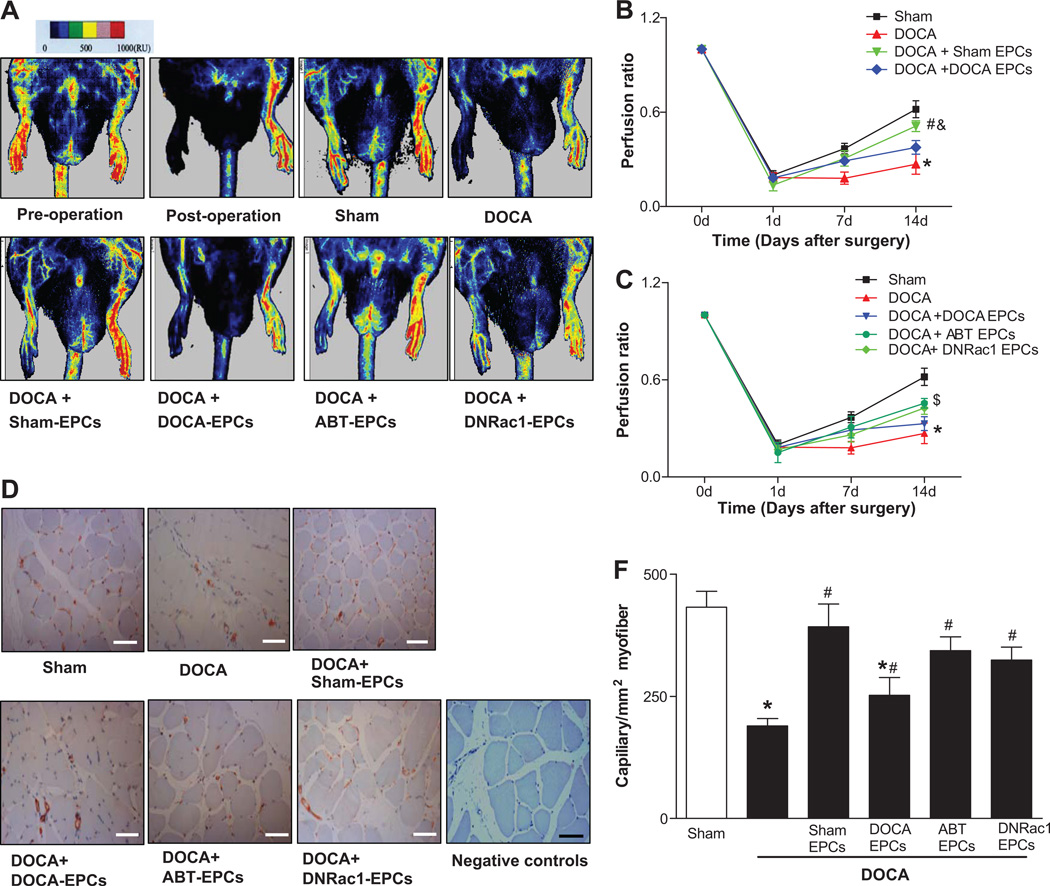
EPC therapy restored impaired angiogenesis in DOCA-salt hypertensive rats
Serial blood flow measurements by laser Doppler showed that limb perfusion recovery was severely delayed and impaired in DOCA-salt rats compared with Sham rats (Fig. 6A). The ratio of perfusion in ischemia relative to that in non-ischemic hindlimb was 0.18 ± 0.04 for DOCA rats vs. 0.29 ± 0.02 for sham rats at Day 7, and 0.27±0.06 % vs. 0.62±0.05% at Day 14, respectively (Fig. 6B). To determine the effects of EPCs on blood flow reperfusions after DOCA-salt regiment, 1×107 EPCs were injected intramuscularly into anterior tibial muscles 24 hours after femoral artery excision. The Sham EPCs implanted group significantly improved blood flow perfusion in DOCA-salt ischemia limbs from Day 7 until Day 14 (0.51 ± 0.03 vs. 0.27 ± 0.06). Implantation with EPCs from ABT-627 treated DOCA rats or EPCs treated with DNRac1 significantly accelerate delayed reperfusion in ischemic hind limbs from Day 7 to Day 14. In contrast, implantation of DOCA EPCs seems improved the ischemic limb reperfusion at Day 7, but they failed to improve the reperfusion in DOCA-salt ischemia limb at Day 14 (0.33 ± 0.04 vs. 0.27 ± 0.06, P=0.849) (Fig.6B–6C).
Fig 6. The Effects of EPC therapy on hindlimb ischemia in DOCA-salt rats.
A. Representative images of hindlimb blood flow analyzed by Laser Doppler Perfusion Imaging (LDPI) 14 days after transplantation. Color-coded images representing blood flow distribution. Low or no perfusion is displayed as dark blue, whereas highest perfusion is displayed as yellow or red. Seven-day cultured bone marrow-derived EPCs were used for cell therapy. Pre-operation: an intact hindlimb blood flow before femoral ligation artery surgery, Post-operation: the hindlimb blood flow right after surgery, Sham: Sham rats, DOCA: DOCA rats, DOCA+Sham-EPCs: DOCA rats treated with EPCs from Sham rats, DOCA+DOCA-EPCs: DOCA rats treated with EPCs from DOCA rats, DOCA+ABT-EPCs: DOCA rats treated with EPCs from ABT-627 rats, DOCA+DNRac1-EPCs: DOCA rats treated with DNRac1 EPCs. B–C. Quantitative analysis of blood flow in hindlimb expressed as perfusion ratio of ischemic hindlimb to non-ischemic limb. A two-way Repeated-measures ANOVA with Bonferroni's post-test was used to analyze blood flow perfusion in hindlimb ischemia models, and a significant overall difference was detected (P<0.05), n=5 for each group. The perfusion ratio of ischemic to non-ischemic limb of Sham and DOCA-salt rats represent the baseline, were the same for B and C (*P<0.05 DOCA rats vs. Sham rats). B. DOCA rats that received DOCA-EPCs failed to improve the reperfusion in their ischemia hind limb (P=0.8490). DOCA rats that received Sham-EPCs showed significant improvement of reperfusion in ischemic limbs compared with that of DOCA rats receiving DOCA-EPCs (#P<0.05 vs. DOCA+ DOCA-EPCs, &P<0.05 vs. Sham rats). C. DOCA rats that received EPCs after ABT-627 treatment or EPCs transfected with Ad-DNRac1 both showed significant improvement of reperfusion in ischemic limbs ($P<0.05 vs. DOCA+DOCA EPCs). D. Representative images of CD31-positive capillaries 14 days after EPC implantation. CD31-positive stainings (red) are identified as capillaries. Sham: Sham rats, DOCA: DOCA rats, DOCA+Sham-EPCs: DOCA rats treated with EPCs from Sham rats, DOCA+DOCA-EPCs: DOCA rats treated with EPCs from DOCA rats, DOCA+ABT-627-EPCs: DOCA rats treated with EPCs from ABT-627 rats, DOCA+DNRac1-EPCs: DOCA rats treated with DNRac1 EPCs, Negative controls: no primary antibodies. Bar=100 µm. E. Quantitative analysis of capillary. *P<0.05 vs. Sham, #P<0.05 vs. DOCA or DOCA+DOCA EPCs, by a one-way ANOVA with Bonferroni's post-test, n=5 each group.
The capillary density of ischemia hindlimb was significantly deceased in DOCA-salt rats compared with Sham rats at Day 14. EPCs from Sham rats significantly increased the capillary number in ischemia hindlimb compared with the untreated DOCA rats (Figure 6D and 6E). Meanwhile, EPCs from ABT-627 treated DOCA rat or EPCs treated with DNRac1 restored capillary density in DOCA rats as well. To investigate whether implanted EPCs incorporate into existing vessels to participate in angiogenesis in hindlimb ischemia, BrdU labeled EPCs were injected intramuscularly into anterior tibial muscle. Some BrdU-positive cells (red fluorescence) integrated into the CD31 positive vessels (green fluorescence), whereas the remaining BrdU-positive cells were found in perivascular spaces or matrix between muscle fibers surrounding the vessels (Supplement Fig. 4S).
The effects of ETA receptor antagonist, NADPH oxidase inhibitor, or diuretic on blood pressure and circulating EPCs in DOCA-salt rats
Average systolic blood pressure (SBP) in DOCA-salt rats began to increase on Day 5 following the DOCA regimen compared to Sham controls (Fig. S5A) accompanied with a significantly elevated level of circulating CD34+/Flk-1+ progenitor cells (Fig. S5B). On Day 28, average SBP was further increased in DOCA-salt rats compared to Sham controls (Fig. S5A). However, the level of circulating CD34+/Flk-1+ progenitor cells in DOCA-salt rats was reduced by 50% (Fig. S5B). In vivo blockade of ETA receptors, inhibition of NADPH oxidase or diuretic (all in drinking water) for 4 weeks significantly lowered blood pressure and reserved circulating EPCs in DOCA-salt rats (Fig. S5C–S5D).
EPC number and telomerase activity in ETB receptor-deficient rats
The circulating CD34+/Flk-1+ progenitor cell level was significantly decreased in ETB receptor deficient (ETB−/−) rats compared to ETB+/+ rats (Fig. S6A). The telomerase activity in EPCs was also significantly reduced in ETB−/− rats (Supplemental Fig. S6B), paralleled with elevated SBP (129±0.9 vs. 151±1.2 mmHg, n=6, P<0.05) and plasma ET-1 levels (3.61±0.14 vs. 5.17±0.26 pg/ml, n=6, P<0.05).
Direct effects of ETA receptor antagonist and NADPH oxidase inhibitor on EPCs
ET-1-induced EPC reduction and NADPH oxidase activation were reversed by pretreatment with the ABT-627 or Apocynin. Transfection of EPCs with DNRac1, which inhibits the key NADPH oxidase subunit Rac1, blunted ET-1 induced EPC reduction and NADPH oxidase activity (Fig. S7A–S7C). Consistently, ROS level and apoptosis in normal EPCs was significantly increased following ET-1 treatment in vitro, which were abolished by pretreatment of ABT-627 or Apocynin (Fig. S7D–S7E). Telomerase activity was markedly reduced in ET-1 treated normal EPCs, which was rescued by ABT-627 or Apocynin pretreatment (Fig. S7F).
Discussion
The present study demonstrates for the first time that in DOCA-salt hypertension: 1) both ETA and ETB receptors are expressed in rat EPCs, and the expression of ETA receptors is significantly increased; 2) in vivo EPC angiogenesis is significantly impaired; 3) ETA-mediated NADPH oxidase activation leads to decreased EPC number and function; and 4) blockade of the ETA/NADPH oxidase pathway rescues EPC number and function.
Seven-day cultured EPCs used in present study are heterogeneous populations containing progenitor cells with the potency differentiating into endothelial cells, as we have shown recently4. A number of clinical and animal studies have shown that the number and function of EPCs are decreased in hypertension. We showed that EPCs from DOCA-salt rats failed to promote capillary formation and blood flow recovery, whereas EPC from DOCA-salt rats with ETA receptors or NADPH oxidase blockade significantly restored peripheral perfusion. Of note, while the capillary density in DOCA-EPCs implanted hind limbs was enhanced, blood reperfusion was not proportionally improved following DOCA-EPC implantation on Day 14, suggesting that the newly formed capillaries upon DOCA-EPC implantation are not functional, leading to impaired angiogenesis and reduced reperfusion. We previously demonstrated that EPCs from DOCA-salt mice secretes thrombospondin-1 (TSP-1, a well-documented anti-angiogenic factor) inhibiting Matrigel tube formations, which can be reversed by antioxidant treatments 12. Thus, it is possible that altered EPC paracrine functions impair their ability in angiogenesis in DOCA-salt hypertension.
An important finding in the present study is the presence of ET receptors on rat early-cultured EPCs. ET-1, as a major humoral contributor to the development of DOCA-salt hypertension, exerts its biological effects through binding to two G protein-coupled membrane receptors in mature vascular system, namely ETA and ETB subtypes 13. Decreased circulating EPC number and telomerase activity were found in ETB receptor- deficiency (ETB−/−) rats who possess a phenotype of increased plasma ET-1 levels (due to the lack of ET-1 clearance), increased ROS level and hypertension 14. These data indicate that elevated ET-1 in ETB−/− rats may inactivate EPC telomerase activity and reduce EPC number through the unmasked effect on ETA receptors. Our study provide first evidence that both ETA and ETB receptors are expressed in rat EPCs. More importantly, the up-regulated expression of ETA receptors, but not ETB receptors, suggests that ETA receptors may be the therapeutic target for EPC dysfunction in salt-sensitive hypertension. It has been reported that protein kinase C (PKC) induces ETA receptors at a transcriptional level in rat fibroblasts 15 or in cardiac cells from DOCA-salt hypertensive 16. However, whether these possibilities exist in EPCs remain unknown.
NADPH oxidase is a complex enzyme with multiple membrane and cytosolic subunits, and pharmacological interventions are rather limited and often difficult for specific inhibition of the enzyme subunits17. To this end, we transfected EPCs with the dominant negative Rac1 (DNRac1) by adenoviral vectors in order to abrogate endogenous Rac1 expression, a key GTPase component of the NADPH oxidase complex1, 10. Our results show that excessive intracellular ROS in EPCs were dominantly generated by activated NADPH oxidase in DOCA-salt rats. Furthermore, blockades of ETA receptors with ABT-627 significantly inhibited NADPH oxidase activity in EPCs from DOCA-salt rats. These evidences from the present study suggest that ETA/NADPH oxidase is a potential pathway involving in EPC dysfunction in DOCA-salt hypertension.
Increased apoptotic and senescence EPCs were observed in DOCA-salt rats, suggesting that some molecules controlling cell survival and cell cycle may be the downstream targets in ETA/NADPH oxidase pathway. Telomerase protects EPCs from senescence and improve their survival and regenerative properties against excessive ROS 18,8, 9, 18, whereas, the activity of telomerase in EPCs from DOCA-salt rats was markedly decreased, which partially contribute to impaired EPC angiogenic functions. Additionally, the apoptosis-related molecules (p53 and Bax) were up-regulated in DOCA EPCs, which promote EPC apoptosis in this hypertensive setting. Blockades of the ETA receptors or NADPH oxidase significantly blunted telomerase activity and inhibited p53 expression and Bax/Bcl-2 ratio. Moreover, PEG-SOD, a membrane-permeable superoxide scavenger, reversed the above changes. Together, oxidative stress induced by ETA/NADPH oxidase activation lead to EPC apoptosis and senescence via blunting telomerase activity and down-regulated p53 expression and Bax/Bcl-2 ratio in DOCA-salt hypertension.
Although ETA receptor antagonism or NADPH oxidase inhibition could preserve the EPC number and its resistance to oxidative stress in DOCA-salt rats, the question remained regarding their direct anti-hypertensive effect on EPCs. To address this issue, we treated the DOCA-salt rats with diuretic trichlormethiazide (TCM), a non-selective blood pressure lowering agent, for 4 weeks and found that TCM failed to rescue circulating EPC number in DOCA-salt rats despite its blood pressure lowering effect. A recent study also showed that diuretics did not reverse the EPC number in spontaneously hypertensive rats (SHR) when the blood pressure is reduced19. Additionally, it is important to note that although the systolic blood pressure was markedly reduced in DOCA-salt rats following in vivo ETA receptor blockade or NADPH oxidase inhibition, it was significantly higher (i.e. approximately 30 mmHg) compared with Sham rats. In contrast, the number of circulating EPC in the two groups of pharmacologic intervention maintained the similar level as that of Sham rats. Moreover, inhibition of ETA receptors or NADPH oxidase also ameliorated ET-1 induced EPC reduction in vitro, without the compounding effect of the blood pressure. Finally, we found that the circulating EPCs were increased at the beginning of the blood pressure elevation, which suggests that high blood pressure itself may not decrease circulating EPCs in DOCA-salt rats, and the elevation in the number of EPCs in the beginning of DOCA-salt regimen may be due to increased compensation of EPC mobilization from the bone marrow. Thus, inhibition of ET-1/ETA-NADPH oxidase induced oxidative stress, more than blood pressure reduction, may account for the preservation of circulating EPC number in DOCA-salt rats.
Perspectives
The present study demonstrates, for the first time, that activation of the ETA/NADPH oxidase pathway critically contributes to EPC reduction and dysfunction in salt-sensitive hypertension. Oxidative stress induced telomerase inactivation, senescence and apoptosis may represent important cellular mechanisms underlying ET-1 induced EPC reduction and dysfunction in low renin salt-sensitive hypertension. Hypertension with exacerbated systemic oxidative stress impairs EPC function, resulting in loss of their regenerative capacity with obvious implications for endothelial dysfunction. Our finding on how ETA/NADPH oxidase pathway affects EPC function may provides a mechanistic basis for EPC genetic modification and therapeutic rejuvenation in hypertension.
Supplementary Material
Novelty and Significance.
What is new?
The present study is the first to 1) identify the expressions of ET-1 receptors on EPCs; 2) delineate how ETA/NADPH oxidase pathway contributes to EPC dysfunction in salt-sensitive hypertension; 3) report that circulating EPCs are decreased in ETB receptor deficient mice, accompanied with increased ROS.
What is relevant?
As EPCs constitute an important system to maintain endothelial cell homeostasis and integrity, this study provided new mechanistic insights into EPC pathophysiology in salt-sensitive hypertension.
Summary
Activation of the ETA/NADPH oxidase pathway critically contributes to EPC reduction and dysfunction in salt-sensitive hypertension, in part via oxidative stress induced EPC telomerase inactivation, senescence and apoptosis.
Acknowledgments
We would like to thank Drs. Eric Marrotte and Xiao-Ling Dai for their technical supports.
Sources of Funding
This work was supported in part by NIH R01 GM077352 and the American Heart Association Grant-in-Aid 0855601G (Dr. AF Chen). Dr. Dan-Dan Chen is the receipt of the AHA Postdoctoral Fellowship 0720114Z and the National Science Foundation of China (NSFC) grant 30900519.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None.
References
- 1.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 3.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 4.Marrotte E, Chen DD, Hakim JS, Chen AF. Restoration of endothelial progenitor cell function with manganese superoxide dismutase accelerates wound healing in diabetic mice. J Clin Invest. 2010;120:4207–4219. doi: 10.1172/JCI36858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 6.Zeoli A, Dentelli P, Brizzi MF. Endothelial progenitor cells and their potential clinical implication in cardiovascular disorders. J Endocrinol Invest. 2009;32:370–382. doi: 10.1007/BF03345729. [DOI] [PubMed] [Google Scholar]
- 7.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Luscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 8.Imanishi T, Hano T, Nishio I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens. 2005;23:97–104. doi: 10.1097/00004872-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005;23:1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura Y, Hashimoto N, Taira S, Kuro T, Kitano R, Ohkita M, Opgenorth TJ, Takaoka M. Different contributions of endothelin-A and endothelin-B receptors in the pathogenesis of deoxycorticosterone acetate-salt-induced hypertension in rats. Hypertension. 1999;33:759–765. doi: 10.1161/01.hyp.33.2.759. [DOI] [PubMed] [Google Scholar]
- 12.Xie XH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF. GTP Cyclohydrolase I/BH4 Pathway Protects EPCs via Suppressing Oxidative Stress and Thrombospondin-1 in Salt-ensitive Hypertension. Hypertension. 2010;56:1137–1144. doi: 10.1161/HYPERTENSIONAHA.110.160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest. 2000;105:925–933. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pachter JA, Mayer-Ezell R, Cleven RM, Fawzi AB. Endothelin (ETA) receptor number and calcium signalling are up-regulated by protein kinase C-beta 1 overexpression. Biochem J. 1993;294:153–158. doi: 10.1042/bj2940153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fareh J, Touyz RM, Schiffrin EL, Thibault G. Altered cardiac endothelin receptors and protein kinase C in deoxycorticosterone-salt hypertensive rats. J Mol Cell Cardiol. 2000;32:665–676. doi: 10.1006/jmcc.2000.1110. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Chu Y, Fink GD, Engelhardt JF, Heistad DD, Chen AF. Endothelin-1 stimulates arterial VCAM-1 expression via NADPH oxidase-derived superoxide in mineralocorticoid hypertension. Hypertension. 2003;42:997–1003. doi: 10.1161/01.HYP.0000095980.43859.59. [DOI] [PubMed] [Google Scholar]
- 18.Murasawa S, Llevadot J, Silver M, Isner JM, Losordo DW, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106:1133–1139. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Fukuda N, Yao EH, Matsumoto T, Kobayashi N, Suzuki R, Tahira Y, Ueno T, Matsumoto K. Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension. Am J Hypertens. 2008;21:72–77. doi: 10.1038/ajh.2007.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.