Abstract
Species extinctions are accelerating globally, yet the mechanisms that maintain local biodiversity remain poorly understood. The extinction of species that feed on or are fed on by many others (i.e. ‘hubs’) has traditionally been thought to cause the greatest threat of further biodiversity loss. Very little attention has been paid to the strength of those feeding links (i.e. link weight) and the prevalence of indirect interactions. Here, we used a dynamical model based on empirical energy budget data to assess changes in ecosystem stability after simulating the loss of species according to various extinction scenarios. Link weight and/or indirect effects had stronger effects on food‐web stability than the simple removal of ‘hubs’, demonstrating that both quantitative fluxes and species dissipating their effects across many links should be of great concern in biodiversity conservation, and the potential for ‘hubs’ to act as keystone species may have been exaggerated to date.
Keywords: Carbon flux, centrality, energy budget, quantitative food web, robustness, secondary extinction, sequential deletion, species loss
Introduction
Biodiversity loss is a major threat to Earth's ecosystems (Barnosky et al. 2011) and it is crucial to identify and conserve influential ‘keystone’ species or nodes, whose loss would cause the cascading extinctions of many other species (Dunne et al. 2002; Jordán 2009). Theoretical and empirical approaches to studying the importance of interactions among species in maintaining biodiversity recognise that there is a bi‐directional component to every interaction that gauges the separate impacts of one species on the dynamics of another (May 1972; Tang et al. 2014). For every direct interaction, the consumer will have a negative effect on the resource and the resource will have a positive effect on the consumer. Simple binary measures of interaction strength have been used to identify ‘hubs’, i.e. highly connected nodes with a high‐degree centrality (Dunne et al. 2002; Memmott et al. 2004; Dunne & Williams 2009). Selective removal of nodes with the most trophic links in a network typically causes more secondary extinctions than random removal of nodes (Dunne et al. 2002; Memmott et al. 2004; Dunne & Williams 2009). However, this index of node importance based on degree centrality ignores two major components of food webs: the strength or weight of the links and indirect effects (Scotti et al. 2007). This can lead to an inaccurate ranking of species importance, such that removing the most‐connected nodes does not necessarily identify the most destructive extinction sequence (Allesina & Pascual 2009).
There has been a tradition of binary descriptions in many network studies, reflecting the relative ease of data collection, but there is a growing appreciation that this qualitative network structure is often uninformative (Jordán et al. 2006). An increasing number of studies now consider weighted networks in ecology (Jordán et al. 2006; Borrett 2013; Ulanowicz et al. 2014), which can dramatically alter the conclusions about node importance (Scotti et al. 2007; Jordán 2009). In many quantitative food webs, link weights (i.e. the strength of trophic interactions) have been estimated based on the biomass, numbers of individuals or carbon flows between species or compartments (Moore et al. 1993; Jordán et al. 2006; Borrett 2013). These quantitative approaches are arguably more robust than binary methods, but are not without criticism (Paine 1980). For example, controlled removal studies have demonstrated empirically that material flow does not always translate directly to the impacts that interaction strength purports to capture (Woodward et al. 2005).
Indirect effects describe the impact of one species on another that is mediated by a third species (Montoya et al. 2009), and their importance for the maintenance of structure, stability and biodiversity in food webs has been increasingly emphasised (Bukovinszky et al. 2008; Woodward et al. 2008; Sanders et al. 2013). Both empirical (Sanders et al. 2013) and theoretical (Stouffer & Bascompte 2011; Säterberg et al. 2013) studies show that secondary extinctions can occur even when a species is more than one trophic link away from the primary extinction. Recent studies that used a static structural approach showed that direct and indirect indices provide quite different rankings of node importance in networks (Scotti et al. 2007; Jordán 2009) and we do not know which of the indices performs best at identifying keystone species for maintaining biodiversity.
There are two main approaches to simulating the cascading extinctions that occur after primary removals: topological and dynamical analyses (Eklöf & Ebenman 2006). Both approaches have strengths and weaknesses: the former considers only binary network structure and so is easier to parameterise, whereas the latter takes into account both link structure and changes in species abundance through time (Curtsdotter et al. 2011). In topological models, secondary extinctions emerge from bottom‐up cascades (Eklöf & Ebenman 2006). In a natural system, the loss of species can also cause top‐down extinction cascades (Elmhagen & Rushton 2007; Säterberg et al. 2013), meaning that the full range of indirect effects is not covered and food‐web robustness is often overestimated (Curtsdotter et al. 2011). We chose the dynamical approach here and we simulated natural communities using parameter values derived from empirical data, which should provide more realistic outcomes than can be derived from simulating purely artificial communities (Curtsdotter et al. 2011). Furthermore, both top‐down and bottom‐up effects are possible in the dynamical approach, therefore extinction cascades in both directions could be detected. We expect the assessment of node importance indices using the dynamical approach should bring new insights into the magnitude and extent of secondary extinctions.
We compared the performance of four different ranking scenarios (considering direct effects only, both direct and indirect effects, weighted direct effects only and weighted direct and indirect effects) at identifying taxa that maintain biodiversity. Considering the importance of link weight and indirect effects, we expect the nodes with large carbon flux or dissipating their effects across many nodes would be influential. We hypothesised that the indices considering weighted links and/or indirect effects would perform better than the more traditional measures: i.e. more secondary extinctions will be caused in deletion sequences ordered by link weight, direct plus indirect effects or both.
Materials and Methods
Quantitative food webs
We analysed 20 of the 50 aquatic food webs from a recently published database (see Table 1; Salas & Borrett 2011; Borrett 2013). The extraction criteria were as follows: (1) small networks (containing no more than 10 nodes) which are easily collapsed were excluded; (2) each dataset was from a distinct study system to avoid pseudoreplication of similar networks from the same location (e.g. we randomly chose one web from Florida Bay Dry Season and Florida Bay Wet Season).
Table 1.
Original names and structural properties of the 20 empirical food webs examined in the study
| Food web | Original name | S a | C b | C w c |
|---|---|---|---|---|
| Bothnian Bay | Bothnian Bay | 12 | 0.222 | 0.184 |
| Baltic Sea | Baltic Sea | 15 | 0.173 | 0.184 |
| Ems Estuary | Ems Estuary | 15 | 0.196 | 0.169 |
| Swartkops | Swartkops Estuary | 15 | 0.169 | 0.121 |
| Crystal River | Crystal River (control) | 21 | 0.186 | 0.070 |
| Benguela | Northern Benguela Upwelling | 24 | 0.208 | 0.101 |
| Neuse Estuary | Neuse Estuary (late summer 1998) | 30 | 0.138 | 0.062 |
| Georges Bank | Georges Bank | 31 | 0.354 | 0.162 |
| Gulf of Maine | Gulf of Maine | 31 | 0.345 | 0.148 |
| Narragansett | Narragansett Bay | 32 | 0.154 | 0.093 |
| Atlantic Bight | Middle Atlantic Bight | 32 | 0.366 | 0.156 |
| New England | Southern New England Bight | 33 | 0.347 | 0.154 |
| Chesapeake | Chesapeake Bay | 36 | 0.094 | 0.068 |
| St. Marks | St. Marks Seagrass, site 1 (Feb.) | 51 | 0.103 | 0.086 |
| Graminoids | Graminoids (wet) | 66 | 0.182 | 0.033 |
| Cypress | Cypress (wet) | 68 | 0.118 | 0.060 |
| Lake Oneida | Lake Oneida (pre‐ZM) | 74 | 0.223 | 0.072 |
| Bay of Quinte | Bay of Quinte (pre‐ZM) | 74 | 0.211 | 0.056 |
| Mangroves | Mangroves (wet) | 94 | 0.152 | 0.036 |
| Florida Bay | Florida Bay (wet) | 125 | 0.124 | 0.029 |
Number of taxa.
Binary directed connectance, L/S 2; where L is the number of trophic links.
weighted directed connectance (see Materials and Methods for calculation method).
The data for each food web include a list of taxa, the carbon biomass of each taxon (g C m−2), the carbon per unit time of import, export and respiration of each taxon (g C m−2 day−1) and the carbon flux between a pair of taxa (g C m−2 day−1). The dataset was archived in the ‘enaR’ package in R (Borrett & Lau 2014). Nodes represent species, trophic guilds, functional groups or non‐living components of the system in which matter is stored. Initially unbalanced food webs, i.e. energy entering a taxon does not exactly balance the output, were balanced using the AVG2 algorithm using established procedures in Matlab 7.12.0 (Allesina & Bondavalli 2003). Our focal food webs exhibit a wide range of network complexity, indicated by taxon richness (S = 12–125), binary directed connectance (C = 0.094–0.366) and weighted directed connectance (C w = 0.029–0.184), but all of these were within the range reported for other recently described catalogues (see Table 1; cf. Williams & Martinez 2000; Ings et al. 2009). Binary directed connectance is a qualitative descriptor based on binary networks, which measures the proportion of possible links between taxa that are realised; weighted directed connectance C w is a quantitative descriptor based on Shannon's entropy (Banašek‐Richter et al. 2009; see Appendix S1 for details).
Food‐web dynamics
The model was constructed based on energy budgets that index the carbon fluxes entering and leaving each taxon. The imports and exports via animal migration and water flows are considered to be in balance and not to influence the food‐web dynamics, similar to many other dynamical models (Moore et al. 1993; Hudson & Reuman 2013). In general, the taxa in the system can be divided into four categories (see Fig. S1): producers, consumers, decomposers and non‐living compartments (i.e. detritus).
The change in biomass of producers can be described as:
| (1) |
Here, ‘herbi’ are herbivorous taxa, r is the maximum specific or intrinsic growth rate and G i is the growth model, following . Here, ‘pro’ are producer taxa and K is the carrying capacity. The value of K is considered as the total initial producer biomass in the community multiplied by a term 10k0. The carrying capacity coefficient k 0 was set to follow the distribution U[0, 3] (after Hudson & Reuman 2013). Φij is the functional response when taxon j consumes taxon i (see below for more details), and d is the specific death rate. The biomass of producer taxon i is increased by photosynthesis and decreased by intertaxon competition, consumption and non‐predatory death.
The change in biomass of consumers (including herbivores and predators) can be depicted as:
| (2) |
Here, ‘res’ means resource taxon, ‘pred’ means predator taxon, a is the assimilation efficiency and x is the respiration rate. The biomass of consumer taxon i is increased by assimilation of consumed resources and decreased by predation and respiration.
The change in biomass of decomposers can be depicted as:
| (3) |
Here, ‘det’ are detrital taxa. The biomass of decomposer taxon i is increased by assimilation of consumed detritus and decreased by predation and respiration.
In some food webs, detritus has been divided into separate compartments. For example, there are three detrital taxa in the Florida Bay ecosystem: water particulate organic carbon (POC), benthic POC and dissolved organic carbon. The change in biomass of each detrital taxa can be described as:
| (4) |
Here, ‘con’ are consumer taxa and ‘dec’ are decomposer taxa, p ji is the proportion of converted detritus i to the total amount of detritus converted from taxon j, e = (1 − a) is the egestion rate and c ji is the conversion coefficient from detrital taxon j to detrital taxon i. Here we consider that the amount of faeces, i.e. the unassimilated fraction of prey killed, is proportional to the amount of predation (Moore et al. 1993; de Ruiter et al. 1995; Moore & de Ruiter 2012). The biomass stored in detrital taxon i is increased by the dead bodies of producer taxa, the faeces of consumer taxa and the conversion from other detritus, and decreased by consumption of decomposer taxa and conversion into other detritus. The meanings and calculations of the parameters listed above (except the functional response Φ which was given below) can be found in Table 2.
Table 2.
Details of the parameters used in the model
| Symbol | Meaning | Value | Unit | |
|---|---|---|---|---|
| r i | Maximum specific or intrinsic growth rate |
|
day−1 | |
| K | Carrying capacity |
|
g C m−2 | |
| d i | Natural specific death rate |
|
day−1 | |
| a i | Assimilation efficiency |
|
Proportion (unitless) | |
| x i | Respiration rate | R i/B i | day−1 | |
| p ji | Proportion of converted detritus i in all the converted detritus from producer or consumer taxon j |
|
Proportion (unitless) | |
| e i | Egestion rate |
|
Proportion (unitless) | |
| c ji | Conversion coefficient from detritus j to detritus i | F ji/B j | day−1 |
Our data (see Table 1) contain the values of GPP i (gross primary production), R i (respiration), B i (biomass) and F ij (carbon flux when taxon j consumes taxon i). k 0 is an undetermined parameter. Considering that carrying capacity K was within three orders of magnitude of total primary producer biomass in the community being simulated (Hudson & Reuman 2013), we assumed k 0 follows the distribution U[0, 3]. We ran 1000 separate simulations for each food web, using different values of k 0, chosen randomly from this distribution.
Functional forms
The functional response Φij was set to follow either a nonlinear form or a linear form. The nonlinear form was set as follows (see Hudson & Reuman 2013):
| (5) |
Here, y j is the maximum consumption rate of taxon j and ωij is the preference of taxon j for taxon i. For a consumer j, . F ij is the carbon flux from taxon i to taxon j, which was contained in the empirical data. Given that , we can calculate ωij as:
| (6) |
H j is the half‐saturation density, which was one order of magnitude either side of the mean of all biomasses in the community being simulated (Hudson & Reuman 2013). That means . Here, b is a coefficient following the distribution U[−1, 1]. q j is the predator interference coefficient, which was randomly chosen from 0 to 100 (Hudson & Reuman 2013). h is the hill exponent that regulates the shape of the curve from Holling Type II (h = 1) to Holling Type III (h = 2). We chose the value of h randomly from 1 to 2.
Notice that F ij = Φij B j, combining eqn 5, and we can calculate the value of y j by:
| (7) |
We ran 1000 simulations for each food web. The values of parameter b, q and h for each simulation were chosen randomly from their ranges, i.e. U[−1, 1] for b, U[0, 100] for q and U[1, 2] for h.
To increase the generality of our model, we also applied the linear functional response, i.e. the Holling Type I. The linear form of the functional response Φij is as follows:
| (8) |
Here, f ij is the feeding rate coefficient when taxon j consumes taxon i. The value of f ij can be obtained by:
| (9) |
Sequential node deletions
Following the framework of Scotti et al. (2007), but using the whole food‐web dynamical model, we compared four different rankings based on the presence or absence of information on indirect effects and weighted links. Here, we ordered nodes by their: (1) maximum unweighted direct effect (Max.D); (2) maximum unweighted direct plus indirect effect (Max.DI); (3) maximum weighted direct effect (Max.wD) and (4) maximum weighted direct plus indirect effect (Max.wDI). Unweighted direct effect is defined as the degree centrality of a node (i.e. the number of its direct neighbours including both consumers and resources), whereas weighted direct effect of a node is defined as the total amount of its inwards and outwards carbon fluxes. The unweighted direct plus indirect effect is the mean of effects originating from one taxon in a binary network, whereas weighted direct plus indirect effect has the same meaning but in a weighted network.
The method for quantifying the direct plus indirect effects has been used in both undirected (Jordán et al. 2006; Jordán 2009) and directed networks (Scotti et al. 2007). First, we calculated the direct plus indirect effects in unweighted networks. We defined a n,ij as the effect of taxon j on taxon i when i can be reached from j in n steps. The simplest case of calculating a n,ij is when n = 1:
| (10) |
where b ij is the element of the qualitative feeding matrix. Here, a 1,i,j = 1/D i,out, if species j is a consumer and a 1,i,j = 1/D i,in, if species j is a resource. D i,in is the number of resources for taxon i, whereas D i,out is the number of consumers for taxon i. Furthermore, we define the n‐step effect originating from species i by the following formula:
| (11) |
The direct and indirect effects originating from species i up to n steps are considered as:
| (12) |
which represents the sum of effects originating from species i up to n steps averaged over by the maximum number of steps considered. Here, we considered a maximum of five‐step‐long indirect effects, i.e. n = 5. As the strength of indirect effects decreases dramatically with distance (Berlow et al. 2009; Borrett et al. 2010; Stouffer & Bascompte 2011), up to five steps are sufficient to get their precise value (Scotti et al. 2007; Borrett et al. 2010). For a weighted network, all the effects are defined in the same way as above except that the value of b ij is the amount of biomass flowing from taxon i to taxon j.
We simulated taxon loss for each food web by sequentially removing taxa. We used the Adaptive Runge–Kutta method with adaptive step sizes to perform numerical simulations. In each simulation, the empirical biomass data were employed to give the initial biomass values. Thousand days were simulated first, to allow transient dynamics caused by initial effects to settle down and let the system reach steady state (Hudson & Reuman 2013). Then we started the sequential deletions, which can be seen as a stepwise process: 1000 days were simulated after each deletion, and secondary extinctions during this time were recorded. Before adding a new step, the deletion sequences were updated, as the extinctions in the former step would change network structure and carbon fluxes among the surviving taxa. During the simulation, a species was considered to be extinct if its biomass fell to < 10−30 g C m−2 (Berlow et al. 2009). We did not remove any detrital nodes in the extinction sequence (Staniczenko et al. 2010) to guarantee that energy cycling would occur during the simulations, which thus continued until only detrital nodes were left in the web. Note that an established food web may persist for a long period without autotrophs if detrital taxa have accumulated sufficient carbon storage to sustain detritus‐based organisms (see Appendix S2).
Measures of stability
We employed two indices to characterise the stability of food webs: robustness (R 50) and survival area (SA). Robustness was quantified as the proportion of species subjected to primary removals that resulted in 50% of total species loss, which is commonly used in such analyses (Dunne et al. 2002; Dunne & Williams 2009; Curtsdotter et al. 2011). A higher value of R 50 means fewer secondary extinctions and thus higher stability. SA is the area under the curve resulting from plotting the number of surviving taxa, N P, having occurred at a specific number of primary deletions, p. SA is calculated as
| (13) |
where S l is the number of living taxa in the original food web. The value of SA meets the term SA + EA = 1, where EA means extinction area as used in prior studies (Allesina & Pascual 2009; Curtsdotter et al. 2011). Here, we chose SA rather than EA because it exhibits a positive relationship with stability, i.e. a higher value of SA indicates higher stability. All numerical simulations and calculations were carried out in Matlab (version 7.12.0).
Statistical procedures
We conducted 1000 Monte‐Carlo simulations for each web and for each node‐ordering index, with four parameters (h, k 0, b and q) varying randomly in each replicate (1000 replicates × 20 webs × 4 indices = 80 000 simulations). We separately compared the effects of the four indices (Max.D, Max.DI, Max.wD and Max.wDI) on R 50 and SA using a linear mixed effects model (LME) with a maximum‐likelihood estimator (function ‘lme’ with ‘method = ML’ within the ‘nlme’ package in R 3.2.3). Food‐web identity was included in the model as a random factor to correct for differences between study systems. Post‐hoc comparisons were applied using the Tukey HSD test at α = 0.05 level of significance (function ‘glht’ within the ‘multcomp’ package). As robustness and connectance are logarithmically related (Dunne et al. 2002), we explored the relationship between stability and log transformations of the measures of complexity (i.e. S, C and C w), using the functions ‘lm’ and ‘cor’ in the ‘stats’ package.
Results
With the nonlinear functional response, the four deletion orders produced significantly different values of R 50 (Fig. 1a, LME: F 3,57 = 13.07, P < 0.001). Deletion orders Max.DI, Max.wD and Max.wDI had significantly lower values of R 50 than order Max.D (Tukey test, see Table S1). There was no significant difference in R 50 among the three deletion orders Max.DI, Max.wD and Max.wDI (Tukey test, Table S1). The four deletion orders also produced significantly different values of SA (Fig. 1b, LME: F 3,57 = 12.072, P < 0.001). Again, the three new indices led to significantly lower values of SA than Max.D (Tukey test, Table S1). Using a linear functional response led to significantly lower stability than the nonlinear form (LME: F 1,79 = 98.974, P < 0.001 for R 50; F 1,79 = 101.338, P < 0.001 for SA). The comparison of the four deletion orders produced similar results to the nonlinear functional response, however, with significantly different values of R 50 (Fig. S2a, LME: F 3,57 = 12.520, P < 0.001) and SA (Fig. S2b, LME: F 3,57 = 25.048, P < 0.001), whereas Max.DI, Max.wD and Max.wDI led to significantly lower stability than Max.D (Tukey test, Table S1).
Figure 1.
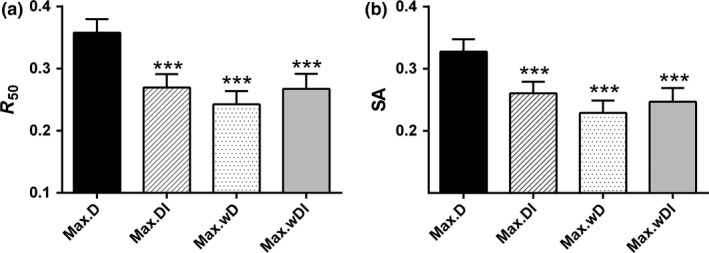
Stability of the 20 food webs to species loss in four deletion sequences (mean ± SEM). Here, stability is represented by (a) robustness, R 50, the fraction of taxa that have to be removed to induce ≥ 50% total taxon loss, and (b) survival area, SA, the area under the curve resulting from plotting the number of surviving taxa. The stars directly above the error bars denote significant differences in stability between the focal deletion orders and the control order (Max.D): *** P < 0.001.
Further analyses showed that different values of the four free parameters (h, k 0, b and q) in the nonlinear functional form did not alter our major conclusion for both R 50 and SA (Figs S3–S6), i.e. deletions in Max.D led to significantly higher stability than the three new indices in all groups (Tukey test). With the linear functional response, the change in the only free parameter (k 0) also did not alter this conclusion (Fig. S7).
There was no significant difference in the connectivity of nodes that went secondarily extinct compared with the average value of those that remained (Fig. 2a; t 19 = 0.31, P = 0.762 for Max.D; t 19 = 0.44, P = 0.667 for Max.DI; t 19 = 1.65, P = 0.115 for Max.wD and t 19 = 1.44, P = 0.167 for Max.wDI). There was a significant difference in the link weight of nodes that went secondarily extinct compared with the average value of those that remained (Fig. 2b; t 19 = − 14.47, P < 0.001 for Max.D; t 19 = − 12.66, P < 0.001 for Max.DI; t 19 = − 19.03, P < 0.001 for Max.wD and t 19 = − 18.40, P < 0.001 for Max.wDI). Most (54–71%) secondary extinctions were caused by indirect effects (the pink, yellow and purple groups in Fig. 2c). Bottom‐up cascades, which are the only cause of collateral losses in the topological approach, accounted for about 40% of secondary extinctions (the red and pink groups in Fig. 2c).
Figure 2.
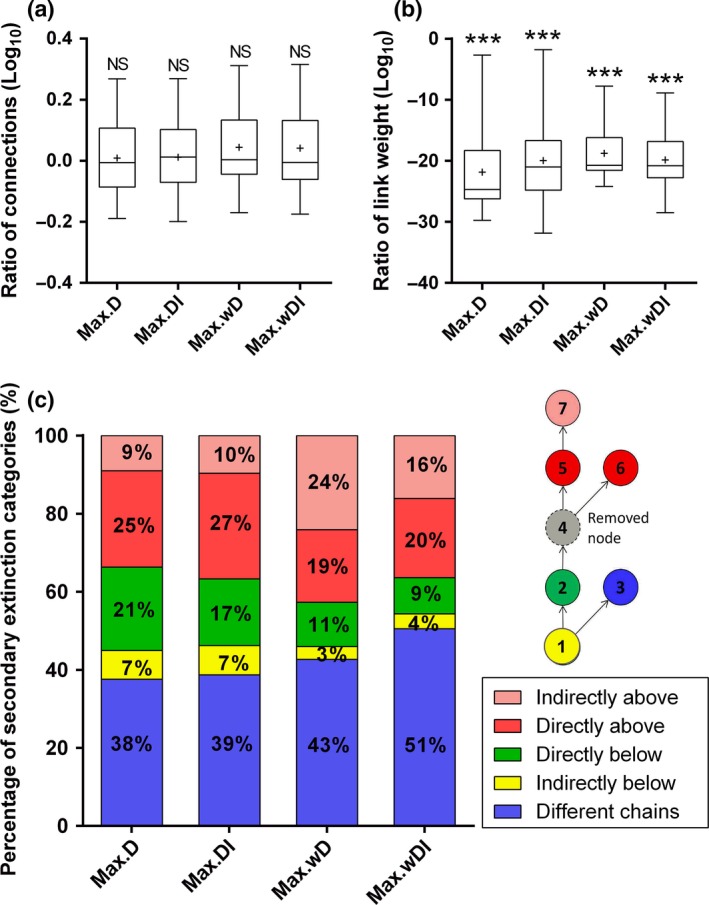
(a–b) Comparison of the types of nodes that went secondarily extinct with those surviving. The nodes going secondarily extinct were significantly different (denoted by stars) from the surviving nodes in (a) number of links or (b) link weight if the confidence intervals around the logarithm of the ratio between the value of the secondarily extinct nodes and the average value of all surviving nodes did not overlap with zero. (c) Trophic categories of nodes that caused the secondary extinctions. The percentage of secondary extinctions for each trophic category across the 1000 simulations of all 20 food webs is shown, along with an illustration of what each of the trophic categories imply.
Across all 20 food webs, the stability indicated by R 50 and SA under the four deletion orders with the nonlinear functional response varied significantly with S and C w, but rarely with C (Table 3 and Fig. 3). More specifically, R 50 and SA decreased with increasing Log S (except SA in Max.D) and with decreasing Log C w (Table 3). There was no significant effect of the different deletion orders on the slopes of Log R 50 vs. Log S (Two‐way anova: F 3,72 = 0.31, P = 0.821) and Log C w (F 3,72 = 0.07, P = 0.977). Similarly, there was no significant effect of the different deletion orders on the slopes of Log SA vs. Log S (F 3,72 = 0.34, P = 0.795) and Log C w (F 3,72 = 0.09, P = 0.968). The same patterns emerged from the dynamical models based on a linear functional response (Fig. S8). Although food‐web stability increased with decreasing food‐web size, we found that the inevitable decrease in the size of a food web during the deletion process seldom affected our conclusion (see Appendix S3).
Table 3.
Stability of food webs under four different species deletion sequences as a function of three measures of food‐web complexity
| Stability | Deletion sequences | Log S | Log C | Log C w | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slope | P | r 2 | Slope | P | r 2 | Slope | P | r 2 | ||
| R 50 | Max.D | − 0.067 | 0.043 | 0.21 | 0.201 | < 0.001 | 0.69 | 0.096 | 0.008 | 0.33 |
| Max.DI | − 0.092 | 0.002 | 0.41 | 0.081 | 0.141 | 0.12 | 0.076 | 0.040 | 0.21 | |
| Max.wD | − 0.072 | 0.022 | 0.26 | 0.065 | 0.238 | 0.08 | 0.085 | 0.016 | 0.28 | |
| Max.wDI | − 0.094 | 0.007 | 0.34 | 0.112 | 0.065 | 0.18 | 0.106 | 0.008 | 0.33 | |
| SA | Max.D | − 0.053 | 0.083 | 0.16 | 0.186 | < 0.001 | 0.71 | 0.082 | 0.013 | 0.30 |
| Max.DI | − 0.083 | 0.001 | 0.45 | 0.096 | 0.035 | 0.22 | 0.078 | 0.010 | 0.31 | |
| Max.wD | − 0.064 | 0.030 | 0.23 | 0.065 | 0.209 | 0.09 | 0.081 | 0.015 | 0.29 | |
| Max.wDI | − 0.081 | 0.012 | 0.30 | 0.107 | 0.051 | 0.20 | 0.095 | 0.009 | 0.33 | |
Linear regressions of robustness, R 50 (the fraction of species that have to be removed to induce ≥ 50% total species loss), and survival area, SA (the area under the curve resulting from plotting the number of survival taxa), of 20 food webs to species loss following four deletion sequences as a function of the logarithm of taxon richness (S), binary directed connectance (C) and weighted directed connectance (C w). Significant results (P < 0.05) are shown in bold.
Figure 3.
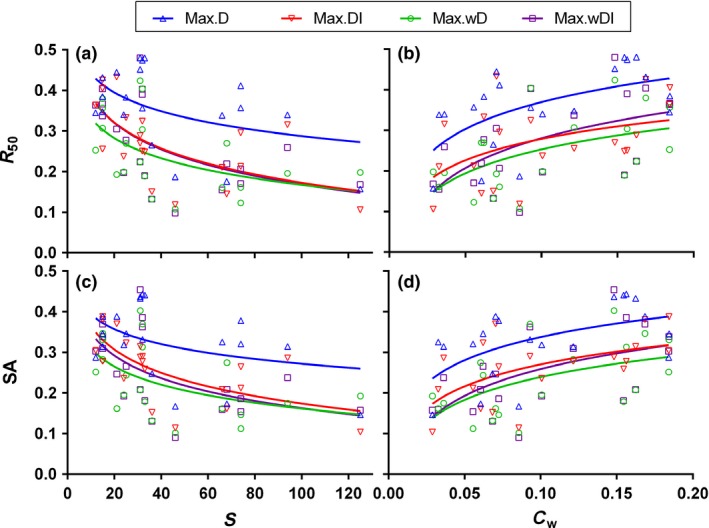
Stability in nonlinear functional response simulations indicated by robustness, R 50 (top panels), and survival area, SA (bottom panels), as a function of the taxon richness, S (left panels), and weighted directed connectance, C w (right panels), of each food web. Logarithmic fits to the four datasets are shown, with different colours and markers indicating different deletion orders.
Discussion
In the last few decades, the influence of random loss of nodes (‘error’) and selective loss of the most‐connected nodes (‘attack’) has been investigated in many real‐world networks, e.g. the Internet (Albert et al. 2000). All of these networks exhibit high fragility against the removal of the most‐connected nodes (i.e. ‘hubs’), which in an ecological context suggests these nodes would represent keystone species that play an important role in maintaining biodiversity (Dunne et al. 2002; Memmott et al. 2004; Dunne & Williams 2009). However, this conclusion is drawn based on topological approaches that always underestimate the amount of secondary extinctions (Curtsdotter et al. 2011). Recent studies using a static structural approach have found that node ordering would be altered when considering link weight or indirect effects (Jordán et al. 2006; Scotti et al. 2007; Bauer et al. 2010), suggesting the possibility of more useful centrality indices. Using a food‐web dynamical model derived from empirical energy budget data, we found that network stability was significantly lower when deletions were ordered by indirect effects, link weight or both, compared with the ordering by unweighted degree centrality (see Fig. 1). Furthermore, poorly connected nodes faced the same extinction risk as highly connected nodes, whereas nodes with low link weight were more likely to go extinct secondarily (see Fig. 2a,b), indicating the failure of degree centrality and the importance of weighted indices. Over 50% of secondarily extinct nodes were not directly connected to the removed nodes (see Fig. 2c), emphasising the need to consider indirect effects. These findings suggest that indices considering link weight and indirect effects are better descriptors of centrality in food webs than the traditional binary, direct‐link measure. Moreover, our findings were robust to different forms of the functional response and different values of the hill exponent, carrying capacity coefficient, half‐saturation coefficient and predator interference coefficient, suggesting a high level of generality.
The uneven distribution of interaction strengths in food webs (O'Gorman et al. 2010) provides a cautionary note when interpreting results derived from analyses of simple binary networks (Banašek‐Richter et al. 2009). It is generally assumed that specialised species tend to have strong connections, whereas generalised species have weak interactions (Wootton & Emmerson 2005) and hence weaker net effects (Montoya et al. 2009; O'Gorman et al. 2010) and different contributions to network structure and stability relative to poorly connected species. We found that species removals ordered by link weight led to a > 30% reduction in network stability relative to direct unweighted orderings. This conclusion is important because it suggests that studies focused solely on direct, unweighted indices for identifying key species in food webs may have severely overestimated the relative importance of degree centrality and, although successfully identifying topologically important nodes, they may fail to detect functionally important ones. Notice that considering weights did not add anything to the conclusion as long as indirect effects were considered, which might be caused by the significant correlation between Max.DI and Max.wDI in 18 of the 20 food webs (Spearman rank correlation = 0.557 ± 0.037; mean ± SEM).
Most empirical studies only contain qualitative food‐web data due to logistical constraints in collecting quantitative information on link weights, although the situation is improving (Ings et al. 2009). Our results show that food‐web stability is significantly lower after removals ordered by both unweighted direct and indirect effects than by direct effects alone. Thus, in the absence of quantitative data, an understanding of indirect effects will give a more realistic view of species importance than in a network constructed solely from direct‐link information. This is perhaps unsurprising, given that indirect effects can often be stronger than direct effects in food webs (Werner & Peacor 2003; Salas & Borrett 2011). Trophic cascades and apparent competition are the best known examples of indirect effects (Montoya et al. 2009). Indirect effects have also been regarded as important drivers of secondary extinctions in a recent empirical study (Sanders et al. 2013). In our study, indirect effects accounted for over 50% of the secondary extinctions (Fig. 2c). This implies that not only neighbouring links but also neighbours of neighbours need to be considered to better understand how species losses propagate. For example, in the well‐studied Chesapeake ecosystem, zooplankton have the most direct links to other taxa, whereas bacteria in sediment POC have the strongest direct plus indirect and weighted effects (see Table S2 for the other food webs).
The hypothesis that diversity may give rise to ecosystem stability has led to more than half a century of heated debate in ecology (May 1972; Tilman et al. 2006). Many experiments have shown that higher diversity is associated with a reduction in temporal variability (i.e. increased temporal stability; Tilman et al. 2006; Cardinale et al. 2009), but the opposite may be true when considering other measures of stability (Donohue et al. 2013). Our study demonstrated a negative relationship between species richness and the robustness of food webs to secondary extinction under all four of the deletion scenarios, suggesting that even species‐rich ecosystems can be vulnerable to cascading extinctions. A possible explanation for this is density compensation, i.e. population densities decrease with increasing species richness because of the increased intensity of interspecific competition (Borrvall & Ebenman 2008; Kaneryd et al. 2012). Our data showed a strong negative correlation between average biomass densities and taxon richness (Fig. S9, Pearson correlation coefficient r = − 0.95, P < 0.001), which supported this hypothesis. As a consequence, species are more easily excluded because they are closer to their extinction threshold, a finding consistent with previous dynamical analyses (Borrvall & Ebenman 2008; Kaneryd et al. 2012).
We found a strong positive relationship between weighted directed connectance, C w, and food‐web stability, but a surprisingly weak relationship with binary directed connectance, C. This stands in contrast to earlier topological analyses (Dunne et al. 2002; Dunne & Williams 2009) conducted on binary networks of direct links. When topological approaches are used, highly connected communities are robust to species loss because species with many binary links are unlikely to become isolated and thus go extinct. Using a dynamical approach, however, highly connected nodes face a similar extinction risk as poorly connected nodes (Fig. 2a). In this case, the density of weighted carbon flows plays an increasingly important role, where the loss of a particularly strong link may result in a node receiving insufficient energy to persist in the network, even if it retains several weak connections to other nodes. Therefore, the nodes with lower link weight would have a significantly higher risk of going secondarily extinct (Fig. 2b). This is also consistent with recent findings that increasing the energy threshold for consumer secondary extinction would nullify the previously positive relationship between robustness and binary directed connectance (Thierry et al. 2011; Bellingeri & Bodini 2013). The dynamical approach, through the weighting of links refines our understanding of the factors affecting network stability in ways that topological analyses cannot do because they assign equal importance to all connections in the network (Eklöf & Ebenman 2006; Curtsdotter et al. 2011).
Moreover, in the topological approach, nodes are considered to be extinct only when they lose all their resources, so all secondary extinctions emerge from bottom‐up cascades (Eklöf & Ebenman 2006). In dynamical approaches, however, a node cannot persist if it receives insufficient energy, even though it still has resources. This is in agreement with a recent study (Bellingeri & Bodini 2013), which investigated the effects of the thresholds of minimum energy requirement for species survival on the robustness of food webs. Top‐down effects and other effects mediated by exploitative and apparent competition can also play an important role (Elmhagen & Rushton 2007; Säterberg et al. 2013). In our study, bottom‐up effects only accounted for about half of all the secondary extinctions (Fig. 2c), highlighting the potential for dynamical analyses to identify a significant proportion of secondary extinctions that would otherwise be missed with topological approaches.
As we enter the age of the sixth mass extinction (Barnosky et al. 2011), we need efficient indices to quantify the relative importance of species to develop new management policies for prioritising key populations to be conserved (Waldron et al. 2013). Our study contributes towards potential solutions and may help ecologists to outline a better conservation policy based on the functional importance of species, rather than qualitative metrics such as rarity or ‘hubs’. By quantifying link weights (or in the absence of quantitative link data, by considering indirect effects) we can improve the accuracy of keystone species identification (Jordán et al. 2008). The extent to which our methods help in detecting more accurate indices remains to be seen, but we posit that it will improve the designs of subsequent experiments or dynamical simulation studies.
Data Accessibility
All data used in this study are available in the ‘enaR’ package (Borrett & Lau 2014) in R (https://cran.r-project.org/web/packages/enaR/index.html).
Authorship
HZ and GW were responsible for research design. LZ drafted the main text and prepared the figures. SRB processed the data. LZ, HZ and JCM developed the modelling framework. LZ and WT performed numerical simulation. EOG, LZ and AM analysed the results. All authors were involved in discussions and editing.
Supporting information
Acknowledgements
This study was financially supported by the National Special Water Programs (Nos. 2009ZX07210‐009, 2015ZX07203‐011, 2015ZX07204‐007), Department of Environmental Protection of Shandong Province (SDHBPJ‐ZB‐08), the China Scholarship Council (No. 201206730022), the Chinese Natural Science Foundation (No. 39560023), NERC grants NE/L011840/1 and NE/I009280/2 and Imperial College London's Grand Challenges in Ecosystems and the Environment initiative.
[The copyright line for this article was changed on 15‐July after original online publication.]
References
- Albert, R. , Jeong, H. & Barabási, A. (2000). Error and attack tolerance of complex networks. Nature, 406, 378–382. [DOI] [PubMed] [Google Scholar]
- Allesina, S. & Bondavalli, C. (2003). Steady state of ecosystem flow networks: a comparison between balancing procedures. Ecol. Model., 165, 221–229. [Google Scholar]
- Allesina, S. & Pascual, M. (2009). Googling food webs: can an eigenvector measure species' importance for coextinctions? PLoS Comput. Biol., 5, e1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banašek‐Richter, C. , Bersier, L. , Cattin, M. , Baltensperger, R. , Gabriel, J. , Merz, Y. et al (2009). Complexity in quantitative food webs. Ecology, 90, 1470–1477. [DOI] [PubMed] [Google Scholar]
- Barnosky, A.D. , Matzke, N. , Tomiya, S. , Wogan, G.O. , Swartz, B. , Quental, T.B. et al (2011). Has the Earth/'s sixth mass extinction already arrived? Nature, 471, 51–57. [DOI] [PubMed] [Google Scholar]
- Bauer, B. , Jordán, F. & Podani, J. (2010). Node centrality indices in food webs: rank orders versus distributions. Ecol. Complex., 7, 471–477. [Google Scholar]
- Bellingeri, M. & Bodini, A. (2013). Threshold extinction in food webs. Theor. Ecol.‐Neth., 6, 143–152. [Google Scholar]
- Berlow, E.L. , Dunne, J.A. , Martinez, N.D. , Stark, P.B. , Williams, R.J. & Brose, U. (2009). Simple prediction of interaction strengths in complex food webs. Proc. Natl Acad. Sci. USA, 106, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrett, S.R. (2013). Throughflow centrality is a global indicator of the functional importance of species in ecosystems. Ecol. Indic., 32, 182–196. [Google Scholar]
- Borrett, S.R. & Lau, M.K. (2014). enaR: an R package for ecosystem network analysis. Methods Ecol. Evol., 5, 1206–1213. [Google Scholar]
- Borrett, S.R. , Whipple, S.J. & Patten, B.C. (2010). Rapid development of indirect effects in ecological networks. Oikos, 119, 1136–1148. [Google Scholar]
- Borrvall, C. & Ebenman, B. (2008). Biodiversity and persistence of ecological communities in variable environments. Ecol. Complex., 5, 99–105. [Google Scholar]
- Bukovinszky, T. , van Veen, F.F. , Jongema, Y. & Dicke, M. (2008). Direct and indirect effects of resource quality on food web structure. Science, 319, 804–807. [DOI] [PubMed] [Google Scholar]
- Cardinale, B.J. , Srivastava, D.S. , Duffy, J.E. , Wright, J.P. , Downing, A.L. , Sankaran, M. et al (2009). Effects of biodiversity on the functioning of ecosystems: a summary of 164 experimental manipulations of species richness. Ecology, 90, 854. [Google Scholar]
- Curtsdotter, A. , Binzer, A. , Brose, U. , de Castro, F. , Ebenman, B. , Eklöf, A. et al (2011). Robustness to secondary extinctions: comparing trait‐based sequential deletions in static and dynamic food webs. Basic Appl. Ecol., 12, 571–580. [Google Scholar]
- Donohue, I. , Petchey, O.L. , Montoya, J.M. , Jackson, A.L. , McNally, L. , Viana, M. et al (2013). On the dimensionality of ecological stability. Ecol. Lett., 16, 421–429. [DOI] [PubMed] [Google Scholar]
- Dunne, J.A. & Williams, R.J. (2009). Cascading extinctions and community collapse in model food webs. Philos. Trans. R. Soc. B, 364, 1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne, J.A. , Williams, R.J. & Martinez, N.D. (2002). Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol. Lett., 5, 558–567. [Google Scholar]
- Eklöf, A. & Ebenman, B.O. (2006). Species loss and secondary extinctions in simple and complex model communities. J. Anim. Ecol., 75, 239–246. [DOI] [PubMed] [Google Scholar]
- Elmhagen, B. & Rushton, S.P. (2007). Trophic control of mesopredators in terrestrial ecosystems: top‐down or bottom‐up? Ecol. Lett., 10, 197–206. [DOI] [PubMed] [Google Scholar]
- Hudson, L.N. & Reuman, D.C. (2013). A cure for the plague of parameters: constraining models of complex population dynamics with allometries. Proc. Biol. Sci., 280, 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ings, T.C. , Montoya, J.M. , Bascompte, J. , Blüthgen, N. , Brown, L. , Dormann, C.F. et al (2009). Ecological networks–beyond food webs. J. Anim. Ecol., 78, 253–269. [DOI] [PubMed] [Google Scholar]
- Jordán, F. (2009). Keystone species and food webs. Philos. Trans. R. Soc. B, 364, 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordán, F. , Liu, W.C. & Davis, A.J. (2006). Topological keystone species: measures of positional importance in food webs. Oikos, 112, 535–546. [Google Scholar]
- Jordán, F. , Okey, T.A. , Bauer, B. & Libralato, S. (2008). Identifying important species: linking structure and function in ecological networks. Ecol. Model., 216, 75–80. [Google Scholar]
- Kaneryd, L. , Borrvall, C. , Berg, S. , Curtsdotter, A. , Eklöf, A. , Hauzy, C. et al (2012). Species‐rich ecosystems are vulnerable to cascading extinctions in an increasingly variable world. Ecol. Evol., 2, 858–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, R.M. (1972). Will a large complex system be stable? Nature, 238, 413–414. [DOI] [PubMed] [Google Scholar]
- Memmott, J. , Waser, N.M. & Price, M.V. (2004). Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B, 271, 2605–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya, J.M. , Woodward, G. , Emmerson, M.C. & Solé, R.V. (2009). Press perturbations and indirect effects in real food webs. Ecology, 90, 2426–2433. [DOI] [PubMed] [Google Scholar]
- Moore, J.C. & de Ruiter, P.C. (2012). Energetic Food Webs: An Analysis of Real and Model Ecosystems. Oxford University Press, Oxford. [Google Scholar]
- Moore, J.C. , De Ruiter, P.C. & Hunt, H.W. (1993). Influence of productivity on the stability of real and model ecosystems. Science, 261, 906–908. [DOI] [PubMed] [Google Scholar]
- O'Gorman, E.J. , Jacob, U. , Jonsson, T. & Emmerson, M.C. (2010). Interaction strength, food web topology and the relative importance of species in food webs. J. Anim. Ecol., 79, 682–692. [DOI] [PubMed] [Google Scholar]
- Paine, R.T. (1980). Food webs: linkage, interaction strength and community infrastructure. J. Anim. Ecol., 49, 667–685. [Google Scholar]
- de Ruiter, P.C. , Neutel, A. & Moore, J.C. (1995). Energetics, patterns of interaction strengths, and stability in real ecosystems. Science, 269, 1257–1260. [DOI] [PubMed] [Google Scholar]
- Salas, A.K. & Borrett, S.R. (2011). Evidence for the dominance of indirect effects in 50 trophic ecosystem networks. Ecol. Model., 222, 1192–1204. [Google Scholar]
- Sanders, D. , Sutter, L. & Veen, F.J. (2013). The loss of indirect interactions leads to cascading extinctions of carnivores. Ecol. Lett., 16, 664–669. [DOI] [PubMed] [Google Scholar]
- Säterberg, T. , Sellman, S. & Ebenman, B. (2013). High frequency of functional extinctions in ecological networks. Nature, 499, 468–470. [DOI] [PubMed] [Google Scholar]
- Scotti, M. , Podani, J. & Jordán, F. (2007). Weighting, scale dependence and indirect effects in ecological networks: a comparative study. Ecol. Complex., 4, 148–159. [Google Scholar]
- Staniczenko, P. , Lewis, O.T. , Jones, N.S. & Reed‐Tsochas, F. (2010). Structural dynamics and robustness of food webs. Ecol. Lett., 13, 891–899. [DOI] [PubMed] [Google Scholar]
- Stouffer, D.B. & Bascompte, J. (2011). Compartmentalization increases food‐web persistence. Proc. Natl Acad. Sci. USA, 108, 3648–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S. , Pawar, S. & Allesina, S. (2014). Correlation between interaction strengths drives stability in large ecological networks. Ecol. Lett., 17, 1094–1100. [DOI] [PubMed] [Google Scholar]
- Thierry, A. , Beckerman, A.P. , Warren, P.H. , Williams, R.J. , Cole, A.J. & Petchey, O.L. (2011). Adaptive foraging and the rewiring of size‐structured food webs following extinctions. Basic Appl. Ecol., 12, 562–570. [Google Scholar]
- Tilman, D. , Reich, P.B. & Knops, J.M.H. (2006). Biodiversity and ecosystem stability in a decade‐long grassland experiment. Nature, 441, 629–632. [DOI] [PubMed] [Google Scholar]
- Ulanowicz, R.E. , Holt, R.D. & Barfield, M. (2014). Limits on ecosystem trophic complexity: insights from ecological network analysis. Ecol. Lett., 17, 127–136. [DOI] [PubMed] [Google Scholar]
- Waldron, A. , Mooers, A.O. , Miller, D.C. , Nibbelink, N. , Redding, D. , Kuhn, T.S. et al (2013). Targeting global conservation funding to limit immediate biodiversity declines. Proc. Natl Acad. Sci. USA, 110, 12144–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, E.E. & Peacor, S.D. (2003). A review of trait‐mediated indirect interactions in ecological communities. Ecology, 84, 1083–1100. [Google Scholar]
- Williams, R.J. & Martinez, N.D. (2000). Simple rules yield complex food webs. Nature, 404, 180–183. [DOI] [PubMed] [Google Scholar]
- Woodward, G. , Speirs, D.C. & Hildrew, A.G. (2005). Quantification and resolution of a complex, size‐structured food web. Adv. Ecol. Res., 36, 85–135. [Google Scholar]
- Woodward, G. , Papantoniou, G. , Edwards, F. & Lauridsen, R.B. (2008). Trophic trickles and cascades in a complex food web: impacts of a keystone predator on stream community structure and ecosystem processes. Oikos, 117, 683–692. [Google Scholar]
- Wootton, J.T. & Emmerson, M. (2005). Measurement of interaction strength in nature. Annu. Rev. Ecol. Evol. Syst., 36, 419–444. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are available in the ‘enaR’ package (Borrett & Lau 2014) in R (https://cran.r-project.org/web/packages/enaR/index.html).


