Abstract
Invasive fungal infections (IFIs) are life-threatening complications of hematological malignancies that must be diagnosed early to allow effective treatment. Few data are available on the performance of serum (1–3)-β-D-glucan (BG) assays for diagnosing IFI in patients with hematological malignancies admitted to the intensive care unit (ICU). In this study, 737 consecutive patients with hematological malignancies admitted to 17 ICUs routinely underwent a BG assay at ICU admission. IFIs were diagnosed using standard criteria applied by three independent specialists. Among the 737 patients, 439 (60%) required mechanical ventilation and 273 (37%) died before hospital discharge. Factors known to alter BG concentrations were identified in most patients. IFIs were documented in 78 (10.6%) patients (invasive pulmonary aspergillosis, n = 54; Pneumocystis jirovecii pneumonia, n = 13; candidemia, n = 13; and fusarium infections, n = 3). BG concentrations (pg/mL) were higher in patients with than without IFI (144 (77–510) vs. 50 (30–125), < 0.0001). With 80 pg/mL as the cutoff, sensitivity was 72%, specificity 65%, and area-under-the-curve 0.74 (0.68–0.79). Assuming a prevalence of 10%, the negative and positive predictive values were 94% and 21%. By multivariable analysis, factors independently associated with BG > 80 pg/mL were IFI, admission SOFA score, autologous bone-marrow or hematopoietic stem-cell transplantation, and microbiologically documented bacterial infection. In conclusion, in unselected critically ill hematology patients with factors known to affect serum BG, this biomarker showed only moderate diagnostic performance and rarely detected IFI. However, the negative predictive value was high. Studies are needed to assess whether a negative BG test indicates that antifungal de-escalation is safe.
Keywords: invasive fungal infection, intensive care units, diagnostic tests, (1–3)-beta-D-glucan assay
INTRODUCTION
Advances in the management of patients with hematological malignancies have led to improved survival, but have also increased the incidence of invasive fungal infections (IFIs). [1] despite the widespread use of prophylactic antifungal agents. Early recognition and treatment of life-threatening IFIs in patients with hematological malignancies is crucial [2]. Both cultures and imaging studies often fail to establish the definitive diagnosis of specific IFIs, leading to prolonged empirical antifungal therapy, whose effectiveness may be limited [1, 3].
IFIs carry a grim prognosis in patients with hematological malignancies admitted to the ICU, particularly those receiving mechanical ventilation or having a history of allogeneic bone-marrow or hematopoietic stem-cell transplantation [4]. In these patients, co-infections [5] and organ dysfunctions are common and may impair the performance of diagnostic biomarkers for IFIs [6, 7].
Plasma (1, 3)-β-D-glucan (BG) antigenemia is among the revised criteria for IFI and probable IFI developed by the European Organization for Research and Treatment of Cancer and Mycoses Study Group (EORTC-MSG) [1, 8]. BG is a cell-wall component of most fungi responsible for IFIs, except Zygomycetes spp. and Cryptococcus spp. The assay relies on activation by BG of factor G in the coagulation cascade in the Limulus amebocyte lysate, which leads to quantifiable transformation of a chromogenic substrate [9]. BG has been evaluated using various study designs, in different settings. In case-control studies comparing patients with proven or probable IFI to healthy controls or patient populations at low risk for IFI, BG assay was 50%–90% sensitive and 70%–100% specific [10–13]. Performance was better in prospective studies of homogeneous populations at high risk for IFI [14, 15] but differed markedly between patients with acute leukemia and recipients of allogeneic hematopoietic stem-cell transplants (HSCT). In an autopsy-based study of 47 critically ill immunocompromised patients (including 17 with hematological malignancies) at risk for invasive aspergillosis (IA), serum BG levels were significantly higher in patients with IA than in those with no IFI [16]. However, overall performance of BG was moderate and 2 patients with bacteremia had serum BG levels higher than 140 pg/mL [16]. In a retrospective study, BG increased the rate of IA detection compared to galactomannan [13]. Importantly, BG was positive in all patients with Pneumocystis jirovecii pneumonia and in 85% of those with fungal bloodstream infections [13]. A metaanalysis that excluded patients with P. jirovecii infection showed a sensitivity of 76.8% (95% confidence interval, [95% CI], 67.1%–84.3%) and a specificity of 85.3% (95% CI, 79.6–89.7) [17]. Another metaanalysis focused on patients with hematological malignancies (n = 1771, including 414 with IFIs) and showed that two consecutive positive BG assays had very good specificity (98.9%; 95%CI, 97.4%–99.5%), positive predictive value (PPV, 83.5% for a prevalence of 10%), and negative predictive value (NPV, 94.6% for a prevalence of 10%) for proven or probable IFI. However, sensitivity was low (49.6%; 95% CI, 34.0%–65.3%) [18]. Performance was similar across the different BG assays [18].
No study has evaluated how BG contributes to the diagnosis of IFI in unselected patients with hematological malignancies admitted to the ICU. These patients usually have organ dysfunctions, which may affect BG performance; [4] and bacterial infections, which complicate the interpretation of BG assay results [13, 16]. Furthermore, over 90% of critically ill hematology patients receive antibacterial agents and up to 50% antifungal agents, which have been reported to modify BG values [19].
The objective of this multicenter database study conducted by the Groupe de Recherche en Réanimation respiratoire Onco-Hématologique (Grrr-OH) was to assess the accuracy of BG for diagnosing IFI in unselected critically ill patients with hematological malignancies.
RESULTS
Patients
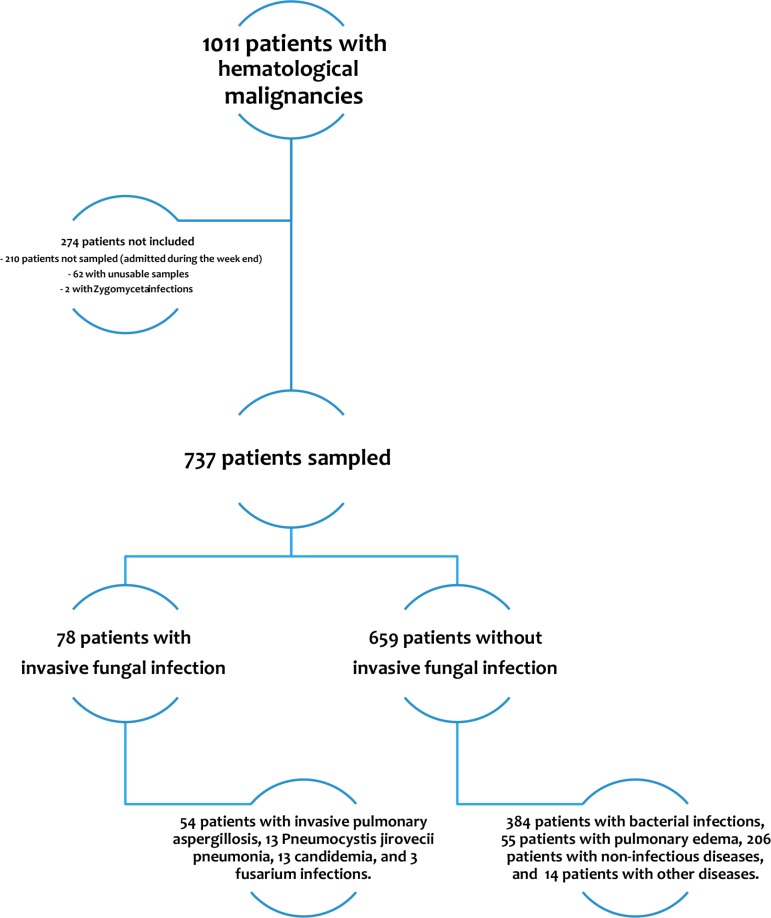
As shown in Figure 1, of 1011 patients with hematological malignancies admitted to the study ICUs, 801 were admitted on weekdays and 737 were included in the study. These 737 patients showed no significant differences with the 274 patients who were not included. Two patients with Zygomycetes infections were not included.
Figure 1. Patient flowchart (5 patients had both candidemia and another IFI).
Table 1 reports the main patient characteristics. Acute leukemia and non-Hodgkin lymphoma were the most common malignancies and 80% of patients had received chemotherapy in the past week, whereas only 174 (23.6%) were in partial or complete remission. The malignancy had been diagnosed in the past 2 weeks in 283 (38.4%) patients. Of the 184 (25%) bone-marrow transplant (BMT) or HSCT recipients, 81 had received autologous and 103 allogeneic transplants. Neutropenia at ICU admission was found in 208 (28%) patients. The main reason for ICU admission was acute respiratory failure (n = 398, 54%), and mechanical ventilation was provided to 439 (60%) patients. Hospital mortality was 37%.
Table 1. Patient characteristics at ICU admission.
| IFI n = 78 | No IFI n = 659 | P value | |
|---|---|---|---|
| Age | 58 (48–64) | 61 (50–70) | 0.08 |
| Male gender | 46 (59.0%) | 397 (60.2%) | 0.83 |
| Underlying malignancy | 209 (31.7%) | 0.37 | |
| Non-Hodgkin lymphoma | 23 (29.5%) | 180 (27.3%) | |
| Acute myeloid leukemia | 20 (25.6%) | 90 (13.7%) | |
| Myeloma | 7 (9.0%) | 40 (6.1%) | |
| Acute lymphocytic leukemia | 9 (11.5%) | 57 (8.6%) | |
| Chronic lymphocytic leukemia | 5 (6.4%) | 13 (2.0%) | |
| Chronic myeloid leukemia | 2 (2.6%) | 20 (3.0%) | |
| Myelodysplastic syndrome | 1 (1.3%) | 14 (2.1%) | |
| Hodgkin lymphoma | 3 (3.8%) | 36 (5.5%) | |
| Other malignancies | 8 (10.3%) | ||
| Newly diagnosed malignancy ¥ | 22 (28.2%) | 261 (39.6%) | 0.06 |
| Partial or complete remission | 19 (24.4%) | 155 (23.5%) | 0.88 |
| BMT / HSCT | 0.02 | ||
| No | 49 (62.8%) | 504 (76.5%) | |
| Autologous | 11 (14.1%) | 70 (10.6%) | |
| Allogeneic | 18 (23.1%) | 85 (12.9%) | |
| Chemotherapy in the last 7 days | 72 (92.3%) | 519 (78.8%) | 0.004 |
| Neutropenia | 34 (43.6%) | 174 (26.4%) | 0.002 |
| Reasons for ICU admission £ | |||
| Acute respiratory failure | 69 (88.5%) | 329 (49.9%) | < 0.0001 |
| Shock | 52 (66.7%) | 308 (46.7%) | 0.001 |
| Acute renal failure | 9 (11.5%) | 146 (22.2%) | 0.03 |
| Sepsis | 78 (100%) | 398 (60.4%) | < 0.0001 |
| Systemic antifungal agents | 59 (76%) | 224 (34%) | < 0.0001 |
| Life-sustaining therapies | |||
| Mechanical ventilation (invasive or noninvasive) | 65 (83.3%) | 374 (56.8%) | 0.02 |
| Vasopressors | 52 (66.7%) | 308 (46.7%) | 0.001 |
| Renal replacement therapy | 22 (28.6%) | 167 (26.0%) | 0.73 |
| Outcomes | |||
| Length of ICU stay | 9 (5–17) | 5 (3–10) | < 0.0001 |
| ICU mortality | 32 (41.0%) | 158 (24.0%) | 0.002 |
| Length of hospital stay | 9 (5–17) | 5 (3–10) | < 0.0001 |
| Hospital mortality | 42 (53.8%) | 231 (35.1%) | 0.002 |
| BG concentrations (pg/mL) were higher in patients with than without IFI (144 (77–510) vs. 50 (30–125), < 0.0001) | 144 (77–510) | 50 (30–125) | < 0.0001 |
Hematological malignancy diagnosed in the past 2 weeks.
Some patients were admitted for more than one reason.
IFI, invasive fungal infection; BMT, bone marrow transplant; HSCT, hematopoietic stem-cell transplant; ICU, intensive care unit; BG, (1–3)-β-D-glucan.
IFIs were documented in 78 (10.6%) patients, including 54 with IPA (5 proven, 31 probable, and 18 possible), 13 with proven P. jirovecii pneumonia, 13 with candidemia, and 3 with fusarium infections; 5 patients had both candidemia and another IFI. Mortality in patients without IFI was 35.1%, rising to 64.8% in patients with invasive aspergillosis (OR 3.41 (1.91–6.10)).
(1–3)-β-D-glucan (BG) assay results
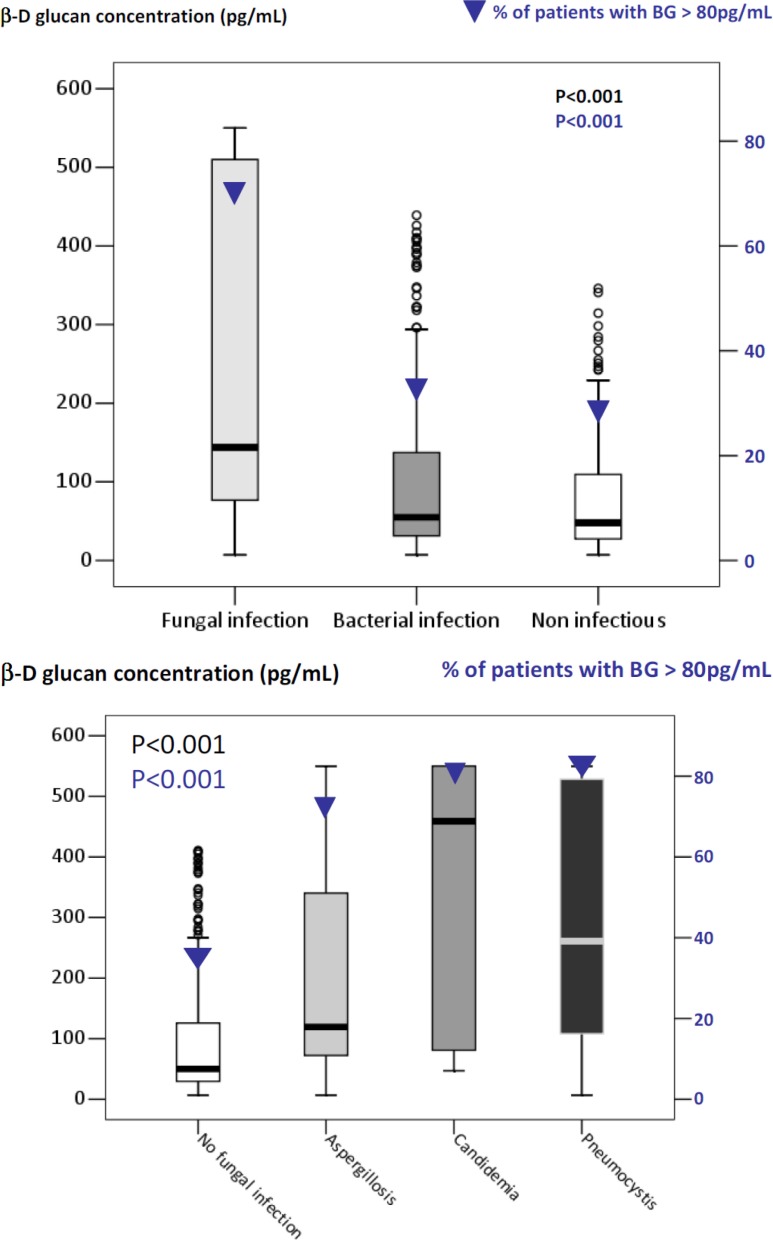
Median (25th–75th) BG concentration was 62 (37–120) pg/mL, and 32% of patients had BG concentrations above the manufacturer's cutoff (80 pg/mL). BG concentrations were higher in patients with than without IFI (144 [77–510] vs. 50 [30–125], P < 0.0001). Of the 78 patients with IFIs, 74.4% had BG > 80 pg/mL (Figure 2A). However, BG was also > 80 pg/mL in 38.3% of patients with bacterial infections and 30.7% with noninfectious diseases (P < 0.0001). Figure 2B shows BG results according to type of IFI. BG was < 80 pg/mL in 2 patients with Pneumocystis pneumonia, 3 patients with candidemia, and 30% of patients with IPA. BG was > 80 pg/mL in 35% of patients without IFIs.
Figure 2. β-D-glucan concentrations (box plots, Y axis on the left) and proportion of patients with β-D-glucan > 80 pg/mL (▼, Y axis on the right) according to final diagnoses.
(A) β-D-glucan was > 80 pg/mL not only in 74.4% of patients with invasive fungal infections, but also in 38.3% with bacterial infections and 30.7% with noninfectious diseases (P < 0.0001). (B) β-D-glucan was < 80 pg/mL in 2 patients with Pneumocystis pneumonia, 3 patients with candidemia, and 30% of patients with invasive pulmonary aspergillosis. On the opposite, β-D-glucan was > 80 pg/mL in 30% of patients without fungal infections. Patients with Fusarium infection had β-D-glucan concentrations of 76 pg/mL [75–77].
Diagnostic performance of the (1–3)-β-D-glucan (BG) assay
Figure 3 displays the performance characteristics of the BG assay. The manufacturer's cutoff of 80 pg/mL was associated with the best performance, with 72% sensitivity, 65% specificity, and a ROC-AUC of 0.74 (0.68–0.79). The sensitivity analyses showed no differences in performance characteristics in patients with IA (Supplementary Figure S1) or with acute respiratory failure, or allogeneic BMT/HSCT (data not shown). Additional sensitivity analyses were performed to assess the performance of a BDG > 80 pg/ml in different subgroups. It did not disclose any difference patients without neutropenia (AUC = 0.752 (0.678–0.826)), those not needing mechanical ventilation (AUC 0.778 (0.693–0.862)), or vasopressors (AUC 0.796 (0.715–0.877)).
Figure 3. Performance of (1–3)-β-D-glucan for the diagnosis of invasive fungal infection (area under the ROC curve according to BG value).
Sensitivity and sensitivity are reported for (1–3)-β-D-glucan cutoffs of 60 pg/mL, 80 pg/mL, and 100 pg/mL. The best cutoff was 80 pg/mL.
Furthermore, antifungal treatment at ICU admission did not affect BG assay performance (ROC-AUCs: all patients, 0.74 [0.68–0.79]; patients without antifungal therapy, 0.72 [0.61–0.83]; and patients with antifungal therapy, 0.73 [0.66–0.80]). With a 10% prevalence of IFI, NPV was 94% and PPV 21% (Supplementary Figure S2). The contribution of a BG > 80 pg/mL to estimating the pre-test probability of IFI was fair. For instance, a 40% pre-test probability of IFI was increased to 58% by a positive BG assay and decreased to 22% by a negative BG assay (Supplementary Table S1 and Supplementary Figure S3).
Factors affecting (1–3)-β-D-glucan (BG) assay results
Table 2 reports the multivariable analysis of factors independently associated with a positive BG (> 80 pg/mL). IFI was associated with a positive BG (OR, 4.42 [95%CI, 2.57–7.75]), as well as admission SOFA score, autologous BMT/HSCT, and microbiologically documented bacterial infection. When neutropenia was forced into the final model, this variable was not selected and did not change the results.
Table 2. Multivariable analysis of factors associated with a (1–3)-β-D-glucan concentration > 80 pg/mL.
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| SOFA score at ICU admission | 1.06 per point | 1.02–1.11 | 0.009 |
| Autologous BMT/HSCT | 1.56 | 1.07–2.29 | 0.02 |
| Invasive fungal infection | 4.42 | 2.57–7.75 | < 0.0001 |
| Documented bacterial infection | 1.41 | 0.96–2.07 | 0.08 |
Hossmer Lemeshow Chi-square: 11.07; df = 8, P = 0.20.
Estimated clinical impact of the (1–3)-β-D-glucan (BG) assay
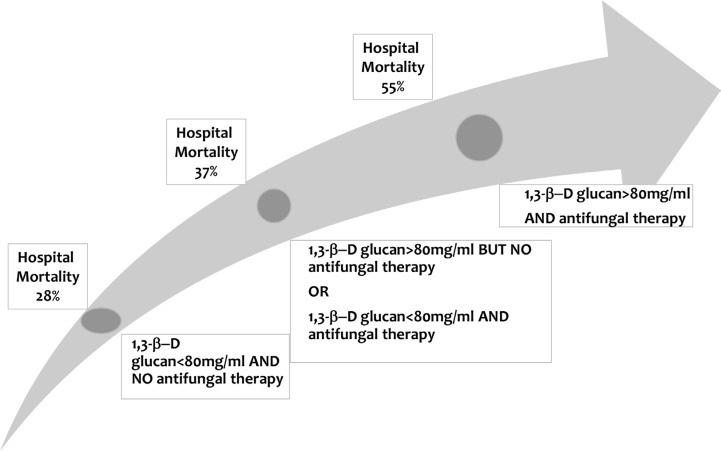
Among the 78 patients with a final diagnosis of IFI, only 59 (76%) received systemic antifungals during the ICU stay; thus, 19 (24%) patients with IFI were left untreated. Had the BG assay results been communicated to the managing physicians and treatment given when (and only when) BG was > 80 pg/mL, then 12 of these 19 patients would have received antifungals but 15 of the 59 treated patients with IFIs would have had their antifungals withdrawn. Among the 659 patients without IFI, 145 would have received antifungal therapy and 135 would have had their antifungal therapy withdrawn. Figure 4 reports the relation between hospital mortality, BG concentrations, and antifungal therapy. Antifungal agents were administered to 46% of patients with BG > 80 pg/mL and to 33% of patients with BG < 80 pg/mL. Figure 4 shows significant differences in hospital mortality across patient groups defined by BG concentrations and use of systemic antifungals.
Figure 4. Hospital mortality according to (1–3)-β-D-glucan concentrations and antifungal therapy in the ICU.
P < 0.0001 across the four groups.
DISCUSSION
The (1, 3)-β-D-glucan assay has been recommended for diagnosing IFIs in high-risk patients with hematological malignancies [1]. This assay has been used to guide clinical management decisions in conjunction with clinical, microbiological, and radiological findings [8]. However, the diagnostic accuracy and optimal cutoff of the BG assay in unselected critically ill hematology patients remain unclear. We evaluated these two points in a population that reflects everyday clinical practice. Furthermore, our sample size was sufficiently large to allow separate assessments of diagnostic accuracy in high-risk patients (i.e., patients with acute leukemia and prolonged neutropenia after induction/consolidation chemotherapy or allogeneic HSCT) and in various types of fungal infections.
Many of the factors reported to impair the performance of the BG assay are frequently present in critically ill patients. False-negative BG results occur in patients receiving antifungal agents and in those with IFIs due to organisms not detected by the BG assay [10]. However, in our study, BG concentrations were not affected by prophylactic or curative antifungal therapy. Moreover, we did not include patients with IFI due to Zygomycetes, which is not detected by the BG assay. Factors associated with false-positive BG results include blood transfusions or administration of blood products [1] (70% of our patients), renal replacement therapy [7] (27%), β-lactam antibiotics (90%) and bacterial infection (53%) [26]. These factors may explain the limited sensitivity and specificity of the BG assay in critically ill hematology patients in our study.
The contribution of the BG assay to the diagnosis of IFI in our population was modest: sensitivity and PPV were low, and the increase in pre-test probability of IFI was moderate. However, the good NPV of the BG assay suggests a possible role in deciding antifungal agent de-escalation in high-risk patients with low pre-test probability. With antibacterial agents, de-escalation has been found feasible and safe in general ICU patients [27, 28] and in ICU patients with neutropenia [29]. Fungal infections are associated with high morbidity and mortality rates in hematology patients. Establishing a definitive diagnosis of IFI is difficult. The signs and symptoms are nonspecific and may not develop until the disease is advanced or disseminated. Antifungal agents are therefore given prophylactically or pre-emptively in high-risk patients. Withdrawing unnecessary antifungal therapy decreases the risk of toxicity and drug interactions and diminishes healthcare costs. Studies are needed to determine whether the BG assay is effective and safe in guiding decisions to de-escalate antifungal therapy in ICU patients with hematological malignancies.
In our study, among patients with BG > 80 pg/mL who received antifungal agents, 55% died. This rate is significantly higher than mortality rates in patients with either low BG or no antifungal therapy but is nevertheless considerably lower than previously reported in critically ill hematology patients with IFIs. Overall mortality in patients with IFIs was 53.8%. This suggests that the physicians in the 17 participating ICUs, who were blinded to BG results, maintained a high level of suspicion regarding IFI. This possibility is consistent with an autopsy study showing that the proportion of patients who received antifungal agents but had no IFI was larger than the proportion who died from untreated IFI [30]. This point further supports the need for studies of antifungal agent withdrawal in patients with no proof of IFI and low BG levels.
This study has several limitations. First, BG was assayed only once, at ICU admission. Serial measurements of fungal cell-wall biomarkers may be more effective both for diagnosing IFI and for assessing the therapeutic response. However, this study is the first to assess the diagnostic accuracy of the BG assay in a large cohort of critically ill hematology patients. Second, we did not focus on a single fungal infection. However, we analyzed the patients with IA separately and found that the performance of the BG assay in this subgroup was similar to that in the overall population. Nevertheless, whereas the BG assay was previously reported to be consistently positive in patients with P. jirovecii pneumonia, 2 such patients had a negative BG assay in our study. A careful assessment of pre-test probabilities of each etiology is essential, and the BG assay should be interpreted in the light of a broad array of clinical, laboratory, and imaging findings. Last, etiological diagnoses were made by consensus among three independent experts, based on the most recent diagnostic criteria for IFI. However, these criteria may lack sensitivity, and some patients with high BG concentrations who were classified as not having IFI may have had undiagnosed IFI. In patients with febrile neutropenia and lung infiltrates, combining multiple diagnostic tests, including PCR assays, provided only modest increases in the sensitivity and PPV predictive value of the BG assay [19]. Moreover, BG levels were higher in patients with IFI proven by autopsy than in patients without IFI [16].
In conclusion, the diagnostic usefulness of BG concentrations in critically ill patients with hematological malignancies is limited by low sensitivity and specificity. However, the good NPV may warrant studies to assess the usefulness of the BG assay for identifying patients whose antifungal treatment can be safely withdrawn.
PATIENTS AND METHODS
Patients
We studied patients from the Grrr-OH database described in a previous publication [4]. Consecutive adults (> 18 years) with hematological malignancies admitted to 17 ICUs in France and Belgium over a 16-month period in 2010–2011 were included into the database (ClinicalTrials.gov Identifier: NCT01172132). As described previously, [4] we excluded patients who had been disease-free for more than 5 years or were admitted to the ICU only to undergo a procedure under optimal safety conditions. The study was approved by the appropriate ethics committees in France and Belgium. All patients or relatives gave their informed consent to study participation.
Data collection
In each center, an investigator recorded the study data on a standardized electronic case-report form. In all 17 centers, a senior intensivist and a senior hematologist were available around the clock and made decisions together. All diagnostic strategies and antifungal treatment decisions were based on clinical judgment, according to standard practice in the Grrr-OH research group.
The data in the tables and figures were collected prospectively. The Sepsis-Related Organ Failure Assessment (SOFA) score was computed at admission [20] to estimate the risk of death based on organ dysfunctions. Reasons for ICU admission were recorded based on the main symptoms at ICU admission [21]. Acute respiratory failure was defined as previously reported [22, 23] and neutropenia as a neutrophil count < 500/mm3 [24]. Prespecified criteria were applied to define the causes of pulmonary involvement; [5] for possible or probable IA, the most recent definitions were used [1]. Candidemia was defined as recovery of any Candida species from at least one blood culture [25]. Invasive fusarium infections were documented by blood culture or skin biopsy. Confirmed Pneumocystis pneumonia was presence of P. jirovecii by Gomori-Grocott or toluidine-blue stain, or a positive immunofluorescence test, on bronchoalveolar lavage (BAL) fluid or induced sputum. Mucormycoses were documented as previously described [1].
Etiological diagnosis
Etiologic diagnoses were these that required the need for ICU admission and present at the time the patients was sampled for BG dosage. They were made by consensus among the managing physicians (intensivists, hematologists, and consultants), according to recent definitions [4, 5]. Only IFIs present at ICU admission or in the first 5 days of ICU admission were considered. For the study, the diagnosis in each patient was reevaluated by three independent experienced intensivists (EA, FV, VL) who were blinded to the initial diagnosis and had access to all the clinical and microbiological data. They disagreed with the managing physicians for 87 patients, 70 with non-fungal etiologies and 17 with IFIs. Of these 17 patients, 6 had microbiological samples that became positive after their death (3 candidemia and 3 Aspergillus in the lower respiratory tract), 5 were reclassified as having probable instead of possible invasive pulmonary aspergillosis [IPA], 2 with a PCR-based diagnosis of Pneumocystis pneumonia were reclassified as having Pneumocystis colonization, and 4 with possible IPA were reclassified as having no identifiable cause (2 had pulmonary findings consistent with leukemic infiltrates but no CT scan and were quickly discharged from the ICU and 2 had normal CT scans). These disagreements were resolved by consensus between the main investigator and the investigator at the relevant study center.
(1–3)-β-D-glucan assay
All patients admitted on weekdays had an arterial and/or venous blood sample taken at admission for a BG assay. The serum was processed within 6 h of collection then stored at −20°C until batch testing was performed. The BG assay Fungitell® (Associates of Cape Cod Inc., Falmouth, MA, USA) was used as recommended by the manufacturer, with the ELx808™ Absorbance Microplate Reader and Gen5™ Data Analysis Software (Biotek Instruments, Winooski, VT, USA). The BG concentration in each sample was calculated automatically using a calibration curve established from standard solutions containing 31.25 pg/mL to 500 pg/mM of BG. We evaluated the manufacturer-recommended BG cutoff of 80 pg/mL and other cutoff values. All materials used for the assay were glucan-free. Each sample was assayed in triplicate, and the mean of the three values was used for the study. When one of the three values differed markedly from the other two, it was discarded, provided the other two results had a coefficient of variation ≤ 20% for BG concentrations below 200 pg/mL; for higher BG concentrations, the assay was repeated if the coefficient of variation was > 100%. The assay results were not reported to the managing physicians.
Statistical analysis
Continuous variables were described as medians (25th–75th quartiles) and compared using the Mann-Whitney U test. Categorical variables were described as percentages and compared using the two-tailed χ2 test or Fisher's exact test, as appropriate. The following parameters of diagnostic performance and their 95%CIs were calculated: sensitivity, specificity, PPV, NPV, and Cohen's kappa. The discriminatory power of BG was evaluated by computing areas under the receiver-operating characteristic curves (ROC-AUCs), which were compared as described by Hanley and McNeil.
We performed logistic regression analyses to identify variables significantly associated with BG concentrations > 80 pg/mL, by estimating the odds ratio (OR) with the 95%CI. Variables yielding P values lower than 0.20 by univariate analyses or considered clinically relevant were entered into a backward stepwise logistic regression model. We checked that continuous variables were log-linear. The covariates were entered into the model with critical entry and removal P values of 0.20 and 0.1, respectively. Multicollinearity and interactions were tested. The Hosmer-Lemeshow test was used to check goodness-of-fit of the logistic regression model. Last, because neutropenia is a major clinical risk factor and confounder for fungal infection in patients with hematological malignancies, the predefined statistical analysis plan involved forcing this variable into the final model in the event it was not selected. All tests were two-sided, and P values lower than 0.05 were considered statistically significant. Statistical tests were done using the SPSS 13 software package (IBM, Armonk, NY, USA).
SUPPLEMENTARY MATERIALS TABLE AND FIGURES
Abbreviations list
- 95%CI
95% confidence interval
- AUC
area under the curve
- BAL
bronchoalveolar lavage
- BG
(1–3)-β-D-glucan
- BMT
bone marrow transplant
- CT
computed tomography
- EORTC
European Organization for Research and Treatment of Cancer Mycoses Study Group
- Grrr-OH
Groupe de Recherche en Réanimation respiratoire Onco-Hématologique (oncology-hematology respiratory research group)
- HM
hematological malignancies
- HSCT
hematopoietic stem-cell transplant
- ICU
intensive care unit
- IFI
invasive fungal infection
- MSG
Mycoses Study Group
- NPV
negative predictive value
- OR
odds ratio
- PCR
polymerase reaction chain
- PPV
positive predictive value
- ROC
receiver-operating characteristic
- SOFA
Sequential Organ Failure Assessment
Footnotes
FUNDING
This work was supported by Grant PHRC [AOM #08235] from the French Ministry of Health and French Society for Critical Care; a grant from Pfizer (Aspire 2012); and a grant from Associates of Cape Cod. None of the funding sources had any role in designing or conducting the study, analyzing or interpreting the study data, or drafting or deciding to publish the manuscript.
Affiliations
| Department | Hospital | City | Country | |
|---|---|---|---|---|
| Elie Azoulay, Virginie Lemiale | ICU | CHU | Paris | France |
| Nicolas Guigue, Stéphane Bretagne | Mycology | CHU | Paris | France |
| Michael Darmon | ICU | CHU | Saint-Etienne | France |
| Djamel Mokart | ICU | CHU | Marseille | France |
| Achille Kouatchet | ICU | CHU | Angers | France |
| Julien Mayaux | ICU | CHU | Paris | France |
| François Vincent | ICU | CHU | Montfermeil | France |
| Martine Nyunga | ICU | CHU | Roubaix | France |
| Fabrice Bruneel, | ICU | CHU | Versailles | France |
| Antoine Rabbat | ICU | CHU | Paris | France |
| Christine Lebert | ICU | CHU | La Roche Sur Yon | France |
| Anne-Pascale Meert, | ICU | CHU | Bruxelles | Belgium |
| Dominique Benoit | ICU | CHU | Ghent | Belgium |
| Frédéric Pene | ICU | CHU | Paris | France |
Summary
A single (1, 3)-β-D-glucan assay at ICU admission of patients with hematological malignancies makes only a modest contribution to the diagnosis of invasive fungal infection but, when negative, may indicate that prophylactic/pre-emptive antifungals can be withdrawn safely.
Authors' contributions
EA had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
EA conceived and designed the study; collected, analyzed, and interpreted the data; and wrote the manuscript
NG designed the study; collected, analyzed, and interpreted the data; and wrote the manuscript
MD collected, analyzed, interpreted data and revised the manuscript for important intellectual content
DM collected, analyzed, interpreted data and revised the manuscript for important intellectual content
VL conceived and designed the study, included patients and help in data interpretation.
AK collected, analyzed, interpreted data and revised the manuscript for important intellectual content
JM collected, analyzed, interpreted data and revised the manuscript for important intellectual content
FV collected, analyzed, interpreted data and revised the manuscript for important intellectual content
MN collected, analyzed, interpreted data and revised the manuscript for important intellectual content
FB collected, analyzed, interpreted data and revised the manuscript for important intellectual content
AR collected, analyzed, interpreted data and revised the manuscript for important intellectual content
SB collected, analyzed, interpreted data and revised the manuscript for important intellectual content
CL collected, analyzed, interpreted data and revised the manuscript for important intellectual content
APM collected, analyzed, interpreted data and revised the manuscript for important intellectual content
DB collected, analyzed, interpreted data and revised the manuscript for important intellectual content
FP collected, analyzed, interpreted data and revised the manuscript for important intellectual content
CONFLICTS OF INTEREST
M. Darmon lectured for MSD, Gilead, Bristol-Myers Squibb, and Astellas. His institution received grant support from MSD and Astute Medical.
E Azoulay lectured for MSD, Astellas, Gilead and Alexion. His institution received research grants from Pfizer, Gilead, MSD, Cubist and Fisher & Paeckle.
All other authors declare no conflicts of interest.
REFERENCES
- 1.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265–73. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 3.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR. Infectious Diseases Society of A. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay E, Mokart D, Pene F, Lambert J, Kouatchet A, Mayaux J, Vincent F, Nyunga M, Bruneel F, Laisne LM, Rabbat A, Lebert C, Perez P, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium—a groupe de recherche respiratoire en reanimation onco-hematologique study. J Clin Oncol. 2013;31:2810–8. doi: 10.1200/JCO.2012.47.2365. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay E, Mokart D, Lambert J, Lemiale V, Rabbat A, Kouatchet A, Vincent F, Gruson D, Bruneel F, Epinette-Branche G, Lafabrie A, Hamidfar-Roy R, Cracco C, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–46. doi: 10.1164/rccm.201001-0018OC. [DOI] [PubMed] [Google Scholar]
- 6.Mohr JF, Sims C, Paetznick V, Rodriguez J, Finkelman MA, Rex JH, Ostrosky-Zeichner L. Prospective survey of (1 – > 3)-beta-D-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J Clin Microbiol. 2011;49:58–61. doi: 10.1128/JCM.01240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato A, Takita T, Furuhashi M, Takahashi T, Maruyama Y, Hishida A. Elevation of blood (1 – > 3)-beta-D-glucan concentrations in hemodialysis patients. Nephron. 2001;89:15–9. doi: 10.1159/000046037. [DOI] [PubMed] [Google Scholar]
- 8.Marchetti O, Lamoth F, Mikulska M, Viscoli C, Verweij P, Bretagne S. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 2012;47:846–54. doi: 10.1038/bmt.2011.178. [DOI] [PubMed] [Google Scholar]
- 9.Obayashi T, Yoshida M, Tamura H, Aketagawa J, Tanaka S, Kawai T. Determination of plasma (1 – > 3)-beta-D-glucan: a new diagnostic aid to deep mycosis. J Med Vet Mycol. 1992;30:275–80. doi: 10.1080/02681219280000361. [DOI] [PubMed] [Google Scholar]
- 10.Obayashi T, Negishi K, Suzuki T, Funata N. Reappraisal of the serum (1 – > 3)-beta-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864–70. doi: 10.1086/588295. [DOI] [PubMed] [Google Scholar]
- 11.Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I. Utility of galactomannan enzyme immunoassay and (1, 3) beta-D-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol. 2009;47:129–33. doi: 10.1128/JCM.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, Finkelman MA, Rex JH. Multicenter clinical evaluation of the (1 – > 3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–9. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 13.Persat F, Ranque S, Derouin F, Michel-Nguyen A, Picot S, Sulahian A. Contribution of the (1 – > 3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2008;46:1009–13. doi: 10.1128/JCM.02091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- 15.Senn L, Robinson JO, Schmidt S, Knaup M, Asahi N, Satomura S, Matsuura S, Duvoisin B, Bille J, Calandra T, Marchetti O. 1,3-Beta-D-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin Infect Dis. 2008;46:878–85. doi: 10.1086/527382. [DOI] [PubMed] [Google Scholar]
- 16.De Vlieger G, Lagrou K, Maertens J, Verbeken E, Meersseman W, Van Wijngaerden E, Obayashi T, Negishi K, Suzuki T, Funata N. Beta-D-glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically ill patients with symptoms of respiratory infection: an autopsy-based study Reappraisal of the serum (1 – > 3)-beta-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. J Clin Microbiol. 2011;49:3783–7. doi: 10.1128/JCM.00879-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52:750–70. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 18.Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, Marchetti O. beta-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3) Clin Infect Dis. 2012;54:633–43. doi: 10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]
- 19.Maschmeyer G, Carratala J, Buchheidt D, Hamprecht A, Heussel CP, Kahl C, Lorenz J, Neumann S, Rieger C, Ruhnke M, Salwender H, Schmidt-Hieber M, Azoulay E. Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients (allogeneic SCT excluded): updated guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO) Ann Oncol. 2014;26:21–33. doi: 10.1093/annonc/mdu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 21.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azoulay E, Lemiale V, Mokart D, Pene F, Kouatchet A, Perez P, Vincent F, Mayaux J, Benoit D, Bruneel F, Meert AP, Nyunga M, Rabbat A, et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. 2014;40:1106–14. doi: 10.1007/s00134-014-3354-0. [DOI] [PubMed] [Google Scholar]
- 23.Hernu R, Wallet F, Thiolliere F, Martin O, Richard JC, Schmitt Z, Wallon G, Delannoy B, Rimmele T, Demaret C, Magnin C, Vallin H, Lepape A, et al. An attempt to validate the modification of the American-European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med. 2013;39:2161–70. doi: 10.1007/s00134-013-3122-6. [DOI] [PubMed] [Google Scholar]
- 24.Mokart D, Darmon M, Resche-Rigon M, Lemiale V, Pene F, Mayaux J, Rabbat A, Kouatchet A, Vincent F, Nyunga M, Bruneel F, Lebert C, Perez P, et al. Prognosis of neutropenic patients admitted to the intensive care unit. Intensive Care Med. 2015;41:296–303. doi: 10.1007/s00134-014-3615-y. [DOI] [PubMed] [Google Scholar]
- 25.Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, Fontanet A, Bretagne S, Dromer F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002-2010) Intensive Care Med. 2014;40:1303–12. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert O, Toubas D, Strady C, Cousson J, Delmas C, Vernet V, Villena I. Reactivity of (1 – > 3)-beta-d-glucan assay in bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis. 2011;30:1453–60. doi: 10.1007/s10096-011-1244-8. [DOI] [PubMed] [Google Scholar]
- 27.Leone M, Bechis C, Baumstarck K, Lefrant JY, Albanese J, Jaber S, Lepape A, Constantin JM, Papazian L, Bruder N, Allaouchiche B, Bezulier K, Antonini F, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014;40:1399–408. doi: 10.1007/s00134-014-3411-8. [DOI] [PubMed] [Google Scholar]
- 28.Garnacho-Montero J, Gutierrez-Pizarraya A, Escoresca-Ortega A, Corcia-Palomo Y, Fernandez-Delgado E, Herrera-Melero I, Ortiz-Leyba C, Marquez-Vacaro JA. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40:32–40. doi: 10.1007/s00134-013-3077-7. [DOI] [PubMed] [Google Scholar]
- 29.Mokart D, Slehofer G, Lambert J, Sannini A, Chow-Chine L, Brun JP, Berger P, Duran S, Faucher M, Blache JL, Saillard C, Vey N, Leone M. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40:41–9. doi: 10.1007/s00134-013-3148-9. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S, Nadrous HF, Peters SG, Tefferi A, Litzow MR, Aubry MC, Afessa B. Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest. 2005;128:1385–92. doi: 10.1378/chest.128.3.1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.