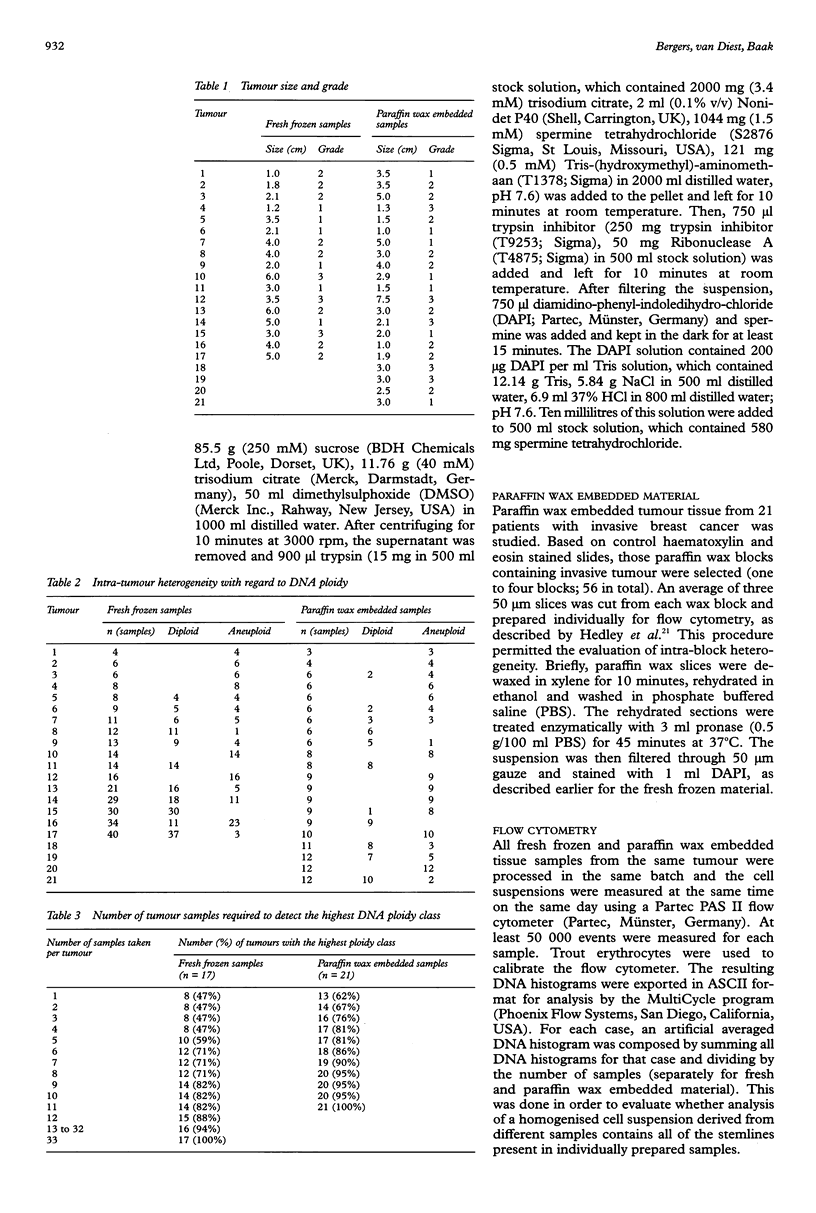
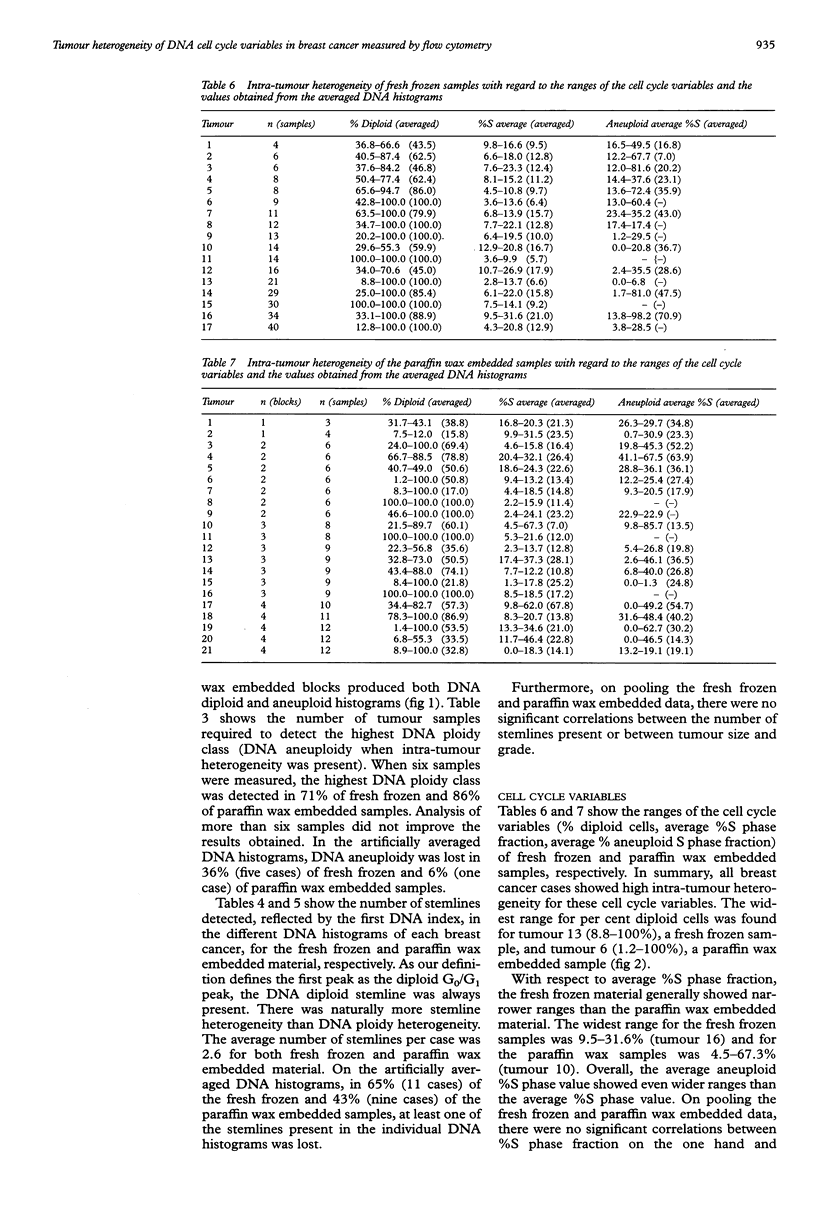
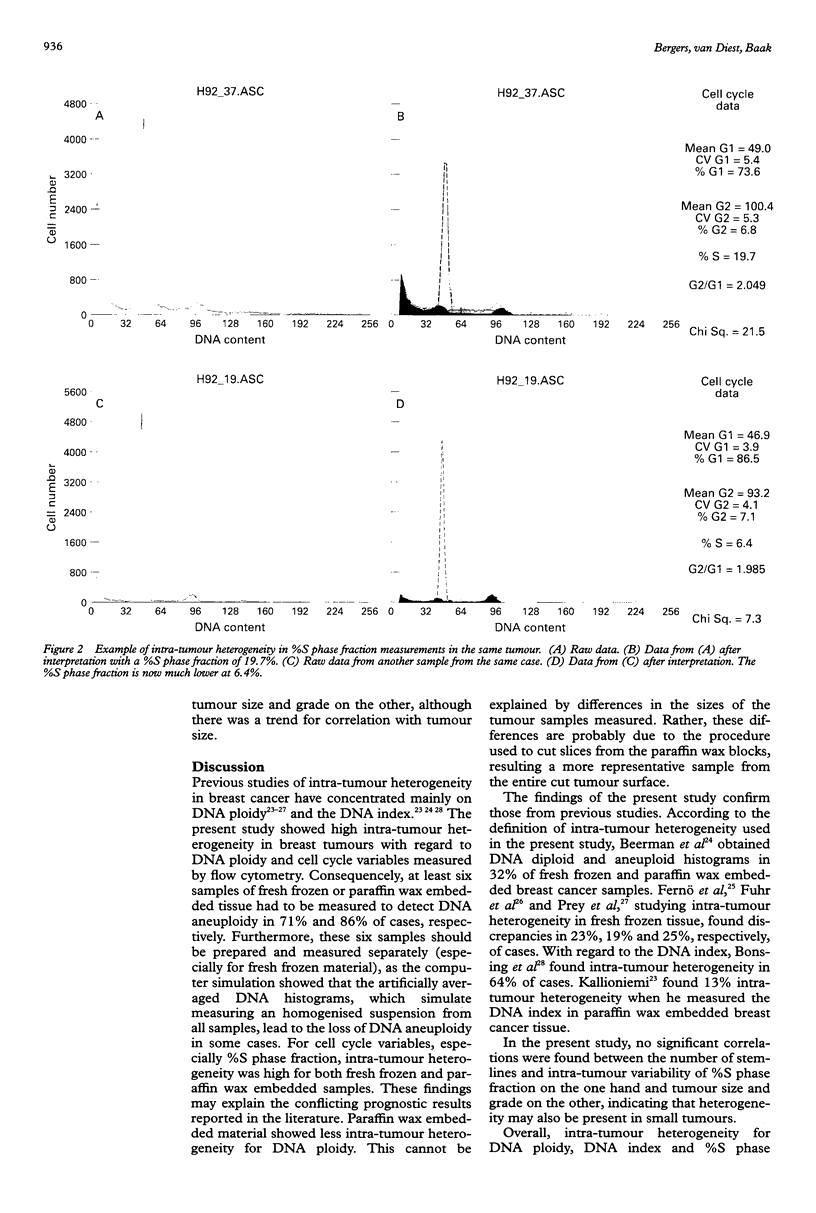
Abstract
AIM/BACKGROUND: Conflicting results have been reported concerning the prognostic value of DNA flow cytometric variables (DNA ploidy, DNA index, %S phase fraction) in breast cancer. Selection bias and differences in treatment may have contributed to these conflicting prognostic results. Differences in tissue processing, the number of nuclei measured, DNA histogram/cell cycle analysis, and intra-tumour heterogeneity may also have played a role. The aim of the present study was to assess intra-tumour heterogeneity of DNA flow cytometric variables in breast cancer. METHODS: Fresh frozen specimens (n = 274) (0.3 x 0.3 x 0.3 cm) of 17 breast cancers and 167 slices, 50 microns thick, of 58 paraffin wax embedded blocks of 21 breast cancers were studied. All samples were prepared individually for DNA flow cytometry. DNA histograms were interpreted by semi-automated cell cycle analysis (MultiCycle) by two observers to avoid biased interpretation. An artificial averaged DNA histogram of each case was composed to simulate a sample prepared from whole tumour tissue. RESULTS: With regard to DNA ploidy, classified as diploid or aneuploid, the fresh frozen and paraffin wax embedded breast cancers showed intra-tumour heterogeneity in 53% and 38% of cases, respectively. For fresh frozen and paraffin wax embedded material, respectively, six samples had to be measured to detect the highest DNA ploidy class in 71% and 86% of cases. Averaged DNA histograms showed a loss of DNA aneuploidy in 36% and 6% of fresh frozen and paraffin wax embedded samples, respectively. High intra-tumour heterogeneity (wide ranges) was found for the %S phase fraction. Average %S phase fraction and average aneuploid %S phase fraction had the widest ranges at 9.5-31.6% and 0.0-62.7%, respectively. There was no correlation between the number of stemlines and intra-tumour %S phase variability on the one hand and tumour size and grade on the other. CONCLUSIONS: High intra-tumour heterogeneity for breast cancer was found for DNA ploidy, the DNA index and %S phase fraction as measured by flow cytometry, which may explain the conflicting prognostic results reported in the literature. To detect aneuploid cells, six samples may have to be prepared and measured separately. Measurement of these variables may be more reliable in paraffin wax sections because the thick slices provide a more representative sample. Prospective studies are required to determine whether the highest %S phase fraction value or the average value is more useful in the clinical context.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baildam A. D., Zaloudik J., Howell A., Barnes D. M., Turnbull L., Swindell R., Moore M., Sellwood R. A. DNA analysis by flow cytometry, response to endocrine treatment and prognosis in advanced carcinoma of the breast. Br J Cancer. 1987 May;55(5):553–559. doi: 10.1038/bjc.1987.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisch H., Göhde W., Linden W. A. Analysis of PCP-data to determine the fraction of cells in the various phases of cell cycle. Radiat Environ Biophys. 1975 Jun 13;12(1):31–39. doi: 10.1007/BF02339807. [DOI] [PubMed] [Google Scholar]
- Beerman H., Smit V. T., Kluin P. M., Bonsing B. A., Hermans J., Cornelisse C. J. Flow cytometric analysis of DNA stemline heterogeneity in primary and metastatic breast cancer. Cytometry. 1991;12(2):147–154. doi: 10.1002/cyto.990120208. [DOI] [PubMed] [Google Scholar]
- Benson N. A., Braylan R. C. Evaluation of sensitivity in DNA aneuploidy detection using a mathematical model. Cytometry. 1994 Jan 1;15(1):53–58. doi: 10.1002/cyto.990150109. [DOI] [PubMed] [Google Scholar]
- Bergers E., Montironi R., van Diest P. J., Prete E., Baak J. P. Interlaboratory reproducibility of semiautomated cell cycle analysis of flow cytometry DNA-histograms obtained from fresh material of 1,295 breast cancer cases. Hum Pathol. 1996 Jun;27(6):553–560. doi: 10.1016/s0046-8177(96)90161-6. [DOI] [PubMed] [Google Scholar]
- Bergers E., van Diest P. J., Baak J. P. Reproducibility of semi-automated cell cycle analysis of flow cytometric DNA-histograms of fresh breast cancer material. Anal Cell Pathol. 1995 Jan;8(1):1–13. [PubMed] [Google Scholar]
- Bonsing B. A., Beerman H., Kuipers-Dijkshoorn N., Fleuren G. J., Cornelisse C. J. High levels of DNA index heterogeneity in advanced breast carcinomas. Evidence for DNA ploidy differences between lymphatic and hematogenous metastases. Cancer. 1993 Jan 15;71(2):382–391. doi: 10.1002/1097-0142(19930115)71:2<382::aid-cncr2820710219>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bosari S., Lee A. K., Tahan S. R., Figoni M. A., Wiley B. D., Heatley G. J., Silverman M. L. DNA flow cytometric analysis and prognosis of axillary lymph node-negative breast carcinoma. Cancer. 1992 Oct 1;70(7):1943–1950. doi: 10.1002/1097-0142(19921001)70:7<1943::aid-cncr2820700722>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Clark G. M., Dressler L. G., Owens M. A., Pounds G., Oldaker T., McGuire W. L. Prediction of relapse or survival in patients with node-negative breast cancer by DNA flow cytometry. N Engl J Med. 1989 Mar 9;320(10):627–633. doi: 10.1056/NEJM198903093201003. [DOI] [PubMed] [Google Scholar]
- Cornelisse C. J., van de Velde C. J., Caspers R. J., Moolenaar A. J., Hermans J. DNA ploidy and survival in breast cancer patients. Cytometry. 1987 Mar;8(2):225–234. doi: 10.1002/cyto.990080217. [DOI] [PubMed] [Google Scholar]
- Ellis I. O., Galea M., Broughton N., Locker A., Blamey R. W., Elston C. W. Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology. 1992 Jun;20(6):479–489. doi: 10.1111/j.1365-2559.1992.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Fernö M., Baldetorp B., Ewers S. B., Idvall I., Olsson H., Sigurdsson H., Killander D. One or multiple samplings for flow cytometric DNA analyses in breast cancer-prognostic implications? Cytometry. 1992;13(3):241–249. doi: 10.1002/cyto.990130305. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Taylor I. W. Clinical and biological significance of aneuploidy in human tumours. J Clin Pathol. 1984 Sep;37(9):961–974. doi: 10.1136/jcp.37.9.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr J. E., Frye A., Kattine A. A., Van Meter S. Flow cytometric determination of breast tumor heterogeneity. Cancer. 1991 Mar 1;67(5):1401–1405. doi: 10.1002/1097-0142(19910301)67:5<1401::aid-cncr2820670521>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Joensuu H., Toikkanen S., Klemi P. J. DNA index and S-phase fraction and their combination as prognostic factors in operable ductal breast carcinoma. Cancer. 1990 Jul 15;66(2):331–340. doi: 10.1002/1097-0142(19900715)66:2<331::aid-cncr2820660222>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Blanco G., Alavaikko M., Hietanen T., Mattila J., Lauslahti K., Koivula T. Tumour DNA ploidy as an independent prognostic factor in breast cancer. Br J Cancer. 1987 Nov;56(5):637–642. doi: 10.1038/bjc.1987.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi O. P., Blanco G., Alavaikko M., Hietanen T., Mattila J., Lauslahti K., Lehtinen M., Koivula T. Improving the prognostic value of DNA flow cytometry in breast cancer by combining DNA index and S-phase fraction. A proposed classification of DNA histograms in breast cancer. Cancer. 1988 Nov 15;62(10):2183–2190. doi: 10.1002/1097-0142(19881115)62:10<2183::aid-cncr2820621019>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P. Comparison of fresh and paraffin-embedded tissue as starting material for DNA flow cytometry and evaluation of intratumor heterogeneity. Cytometry. 1988 Mar;9(2):164–169. doi: 10.1002/cyto.990090211. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Hietanen T., Mattila J., Lehtinen M., Lauslahti K., Koivula T. Aneuploid DNA content and high S-phase fraction of tumour cells are related to poor prognosis in patients with primary breast cancer. Eur J Cancer Clin Oncol. 1987 Mar;23(3):277–282. doi: 10.1016/0277-5379(87)90071-x. [DOI] [PubMed] [Google Scholar]
- Keyhani-Rofagha S., O'Toole R. V., Farrar W. B., Sickle-Santanello B., DeCenzo J., Young D. Is DNA ploidy an independent prognostic indicator in infiltrative node-negative breast adenocarcinoma? Cancer. 1990 Apr 1;65(7):1577–1582. doi: 10.1002/1097-0142(19900401)65:7<1577::aid-cncr2820650721>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Muss H. B., Kute T. E., Case L. D., Smith L. R., Booher C., Long R., Kammire L., Gregory B., Brockschmidt J. K. The relation of flow cytometry to clinical and biologic characteristics in women with node negative primary breast cancer. Cancer. 1989 Nov 1;64(9):1894–1900. doi: 10.1002/1097-0142(19891101)64:9<1894::aid-cncr2820640923>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- O'Reilly S. M., Camplejohn R. S., Barnes D. M., Millis R. R., Allen D., Rubens R. D., Richards M. A. DNA index, S-phase fraction, histological grade and prognosis in breast cancer. Br J Cancer. 1990 May;61(5):671–674. doi: 10.1038/bjc.1990.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owainati A. A., Robins R. A., Hinton C., Ellis I. O., Dowle C. S., Ferry B., Elston C. W., Blamey R. W., Baldwin R. W. Tumour aneuploidy, prognostic parameters and survival in primary breast cancer. Br J Cancer. 1987 Apr;55(4):449–454. doi: 10.1038/bjc.1987.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prey M. U., Meyer J. S., Stone K. R., McDivitt R. W. Heterogeneity of breast carcinomas determined by flow cytometric analysis. J Surg Oncol. 1985 May;29(1):35–39. doi: 10.1002/jso.2930290111. [DOI] [PubMed] [Google Scholar]
- Uyterlinde A. M., Baak J. P., Schipper N. W., Peterse H., Matze E., Meijer C. J. Further evaluation of the prognostic value of morphometric and flow cytometric parameters in breast-cancer patients with long follow-up. Int J Cancer. 1990 Jan 15;45(1):1–7. doi: 10.1002/ijc.2910450102. [DOI] [PubMed] [Google Scholar]