Abstract
Background:
Adverse drug events (ADEs) occurring in hospital inpatients can have serious implications. The ability to identify and prioritize patients at higher risk of ADEs could help pharmacists to optimize their impact as members of the patient care team.
Objective:
To identify risk factors, patient characteristics, and medications associated with a higher likelihood of ADEs in adult inpatients through an overview of reviews on this topic.
Data Sources:
Systematic reviews and narrative reviews or guidelines identified through a search of MEDLINE and the Cochrane Database of Systematic Reviews (limited to articles published from 1995 to June 4, 2015), as well as a grey literature search.
Study Selection and Data Extraction:
For inclusion in this overview, a review had to discuss patient characteristics or risk factors associated with ADEs, medications associated with ADEs, or drug–drug interactions associated with ADEs, in adult inpatients. Articles retrieved by the literature search were screened for eligibility by a single reviewer.
Data Synthesis:
Eleven articles were deemed eligible for inclusion in this overview: 4 systematic reviews and 7 narrative reviews or guidelines. Their results were described narratively. Older age and polypharmacy were the most frequently cited risk factors associated with ADEs in hospital inpatients. Renal impairment, female sex, and decline in cognition were also frequently reported as being associated with ADEs. Medication classes reported to be associated with ADEs during the hospital stay included anticoagulants, anti-infectives/antibiotics, antidiabetic agents, analgesics (including opioids and nonsteroidal anti-inflammatory drugs), and cardiovascular drugs (including antihypertensive agents, diuretics, and digoxin). Two publications reported on preventable ADEs in hospital inpatients; the medications associated with preventable ADEs were consistent with those reported above.
Conclusions:
The risk factors, patient characteristics, and medication classes highlighted in this overview may help clinicians to prioritize patient populations who may be at higher risk of ADEs.
Keywords: adverse drug event, systematic review, review, risk factors, medications, hospital
RÉSUMÉ
Contexte :
Les événements indésirables liés aux médicaments (EIM) touchant les patients hospitalisés peuvent avoir de graves conséquences. La capacité d’identifier les patients qui présentent un haut risque d’EIM et de les prioriser pourrait aider les pharmaciens à optimiser l’influence qu’ils exercent comme membres de l’équipe de soins aux patients.
Objectif :
Identifier les facteurs de risque, les caractéristiques des patients ou les médicaments associés à un potentiel plus élevé d’EIM chez les patients adultes hospitalisés à l’aide d’une synthèse des comptes rendus sur le sujet.
Sources des données :
Des analyses systématiques et des revues narratives ou des lignes directrices trouvées à l’aide d’une recherche dans MEDLINE et la Cochrane Database of Systematic Reviews (se limitant aux articles publiés entre 1995 et le 4 juin 2015) et d’une recherche dans la littérature grise.
Sélection des études et extraction des données :
Afin d’être admissible à la présente synthèse, un compte rendu devait aborder les caractéristiques des patients ou les facteurs de risque associés aux EIM, les médicaments associés aux EIM ou les interactions médicament-médicament associées aux EIM chez le patient adulte hospitalisé. L’admissibilité des articles trouvés grâce à la recherche documentaire n’a été évaluée que par une seule personne.
Synthèse des données :
Les résultats des comptes rendus retenus ont été décrits de manière narrative. Onze articles ont été admis dans la présente synthèse : quatre analyses systématiques et sept revues narratives ou lignes directrices. L’âge avancé et la polypharmacie représentaient les facteurs de risque associés aux EIM les plus souvent mentionnés chez le patient adulte hospitalisé. L’insuffisance rénale, le sexe féminin et le déclin cognitif étaient eux aussi fréquemment indiqués comme étant des facteurs liés aux EIM. Parmi les classes de médicaments signalées comme étant associées aux EIM pendant le séjour à l’hôpital, on comptait : les anticoagulants, les anti-infectieux et les antibiotiques, les antidiabétiques, les analgésiques (notamment les opioïdes et les anti-inflammatoires non stéroïdiens) et les agents cardiovasculaires (notamment les antihypertenseurs, les diurétiques et la digoxine). Deux publications abordaient les EIM évitables chez le patient hospitalisé; les médicaments associés aux EIM faisaient partie de ceux mentionnés ci-dessus.
Conclusion :
Connaître les facteurs de risque, les caractéristiques des patients et les classes de médicaments mis en évidence dans la présente synthèse peut aider les cliniciens à accorder la priorité aux populations de patients qui pourraient présenter un plus grand risque d’EIM.
Keywords: événement indésirable lié à un médicament, analyse systématique, revue, facteurs de risque, médicaments, hôpital
INTRODUCTION
The occurrence of adverse drug events (ADEs) in hospital inpatients can have serious implications, including disability, death, prolonged hospital stay, and increased costs.1
The Canadian Adverse Events Study, published in 2004, found that 37% of adverse events occurring in Canadian hospitals were preventable.2 Twenty-four percent of these preventable adverse events were associated with drug or fluid administration.2
An adverse event has been defined by the World Health Organization3 as “[a]ny untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment”; such events can be characterized as either preventable or nonpreventable. An adverse drug reaction (ADR) is a type of ADE that involves “suspicion of a causal relationship between the drug and the occurrence” and that “occurs at doses normally used in humans for the prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function”.3
The ability to identify and prioritize patients at higher risk of ADEs could help pharmacists to maximize their impact as members of the patient care team. The objective of this overview of reviews was to identify risk factors, patient characteristics, and medications associated with a higher likelihood of ADEs in adult inpatients.
METHODS
An overview of reviews was thought to be the most appropriate method to meet our objective, because we were interested in summarizing the evidence for a very broad research question. MEDLINE and the Cochrane library were searched, in collaboration with an information specialist, up to June 4, 2015, using keywords such as “adverse drug event”, “hospital”, and “review”. The full search strategy is available in Appendix 1 (www.cjhp-online.ca/index.php/cjhp/issue/view/116/showToc). Additionally, a grey literature search was performed using the Grey Matters guide of the Canadian Agency for Drugs and Technologies in Health.4 Publication type was limited to narrative reviews, systematic reviews, and guidelines published in 1995 or later.
For the purposes of this overview, a systematic review was defined as a review that used systematic methods to identify, select, and synthesize studies. Eligible reviews had to report on at least one of the following: patient characteristics or risk factors associated with ADEs, medications associated with ADEs, or drug–drug interactions associated with ADEs. Only reviews reporting on ADEs occurring in hospital inpatients and those that combined inpatient and outpatient data were included. Reviews reporting exclusively on ADEs leading to hospital admission and those occurring exclusively in outpatients were excluded. Publications were also excluded if they reported on pediatric patients only or assessed only one specific drug, condition, or type of ADE. Publications reporting on drug-related problems, medication-related problems, or medication errors were selected only if a separate analysis specific to ADEs was performed.
The titles and abstracts of all articles retrieved through the literature search were screened for eligibility by a single reviewer (S.M.). The full texts of all potentially relevant articles were retrieved and screened by the same reviewer to confirm eligibility.
RESULTS
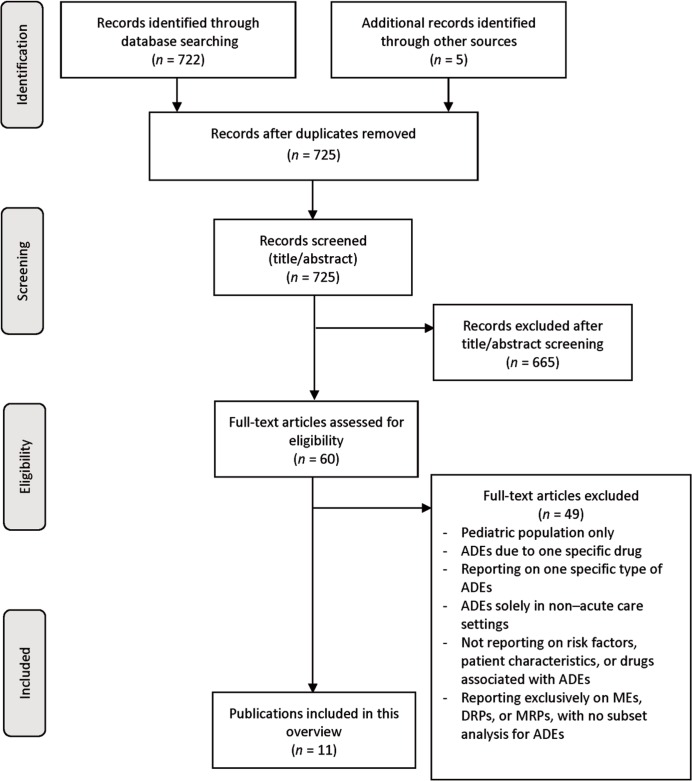
The electronic database search retrieved 722 articles, and an additional 5 articles were identified through the grey literature search. After removal of duplicates and screening of titles and abstracts, 60 full-text articles were retrieved for review (Figure 1). After full-text review, 11 articles were deemed eligible for inclusion.6–16
Figure 1.
FPRISMA flow diagram5 showing the process for selecting articles for inclusion in an overview of reviews and guidelines concerning patient characteristics associated with adverse drug events (ADEs) in hospital. DRP = drug-related problem, ME = medication error, MRP = medication-related problem.
The characteristics of the included reviews are summarized in Table 1. Of the included publications, 4 were systematic reviews6,9,11,15 and 7 were narrative reviews or guidelines.7,8,10,12–14,16 Seven reviews reported on ADEs occurring during the hospital stay only,7–9,11–13,16 2 reported both inpatient and outpatient data,10,14 and 2 reported on ADRs leading to or occurring during admission.6,15
Table 1.
Characteristics of Included Reviews
| Reference | Study Design | Population | Objectives |
|---|---|---|---|
| Wiffen et al. (2002)15 | Systematic review | Patients of all ages | To (1) estimate the incidence of ADRs in patients resulting in hospitalization or occurring in hospital, (2) estimate the burden of ADRs for the United Kingdom, (3) identify risk factors for ADRs, and (4) identify ways by which they can be reduced |
| Kanjanarat et al. (2003)11 | Systematic review | Hospitalized patients of all ages | (1) To describe the drug classes and types of errors and outcomes most frequently associated with preventable ADEs in hospitalized patients and (2) to determine high-risk areas to focus on for “quality improvement” |
| Krähenbühl-Melcher et al. (2007)9 | Systematic review | Hospitalized patients (age not specified) | To describe the frequency of and risk factors for medication errors and ADEs in hospitalized patients and how they can be avoided |
| Alhawassi et al. (2014)6 | Systematic review | Elderly patients in the acute care setting (≥ 65 years) | To estimate the prevalence of and risk factors for ADRs in the elderly, either leading to hospital admission or occurring during admission |
| Davies et al. (2007)8 | Narrative review | Not defined | To determine the incidence, causative drugs, and prevention strategies for ADRs occurring in hospital |
| Magro et al. (2012)10 | Narrative review | Inpatients and outpatients (age not specified) | To report on the incidence, characteristics of, and prevention strategies for ADRs caused by drug–drug interactions in inpatients and outpatients |
| Petrovic et al. (2012)7 | Narrative review | Older adults (age range not specified) | To summarize strategies for improving recognition and prevention of ADRs |
| Weiss and Elixhauser (2013)12 | Statistical brief | Patients admitted to hospital in 2011 in 32 states in the United States | To report statistics regarding the 4 most common causes of ADEs occurring during hospitalization of patients in 2011 from 32 US states participating in the Healthcare Cost and Utilization Project |
| Weiss et al. (2013)13 | Statistical brief | Patients admitted to hospital in 2011 in 32 states in the United States | To report statistics regarding the origin of ADEs (either originating in hospital or present on admission) in patients hospitalized in 2011 from 32 US states participating in the Healthcare Cost and Utilization Project |
| US Department of Health and Human Services (2014)14 | Report | Inpatient and outpatient data from the United States | To identify the most common preventable ADEs and, in conjunction with federal health agencies, to develop a US national action plan to prevent these specific ADEs |
| Zhu and Weingart (2015)16 | Clinical guidelines and recommendations | Hospitalized patients of all ages | To discuss strategies for preventing ADEs caused by medication errors in hospitalized patients |
ADE = adverse drug event, ADR = adverse drug reaction.
Two of the 11 publications focused on ADEs or ADRs in elderly patients,6,7 1 study commented on drug–drug interactions often associated with ADEs,10 and 2 investigated preventable ADEs.11,14
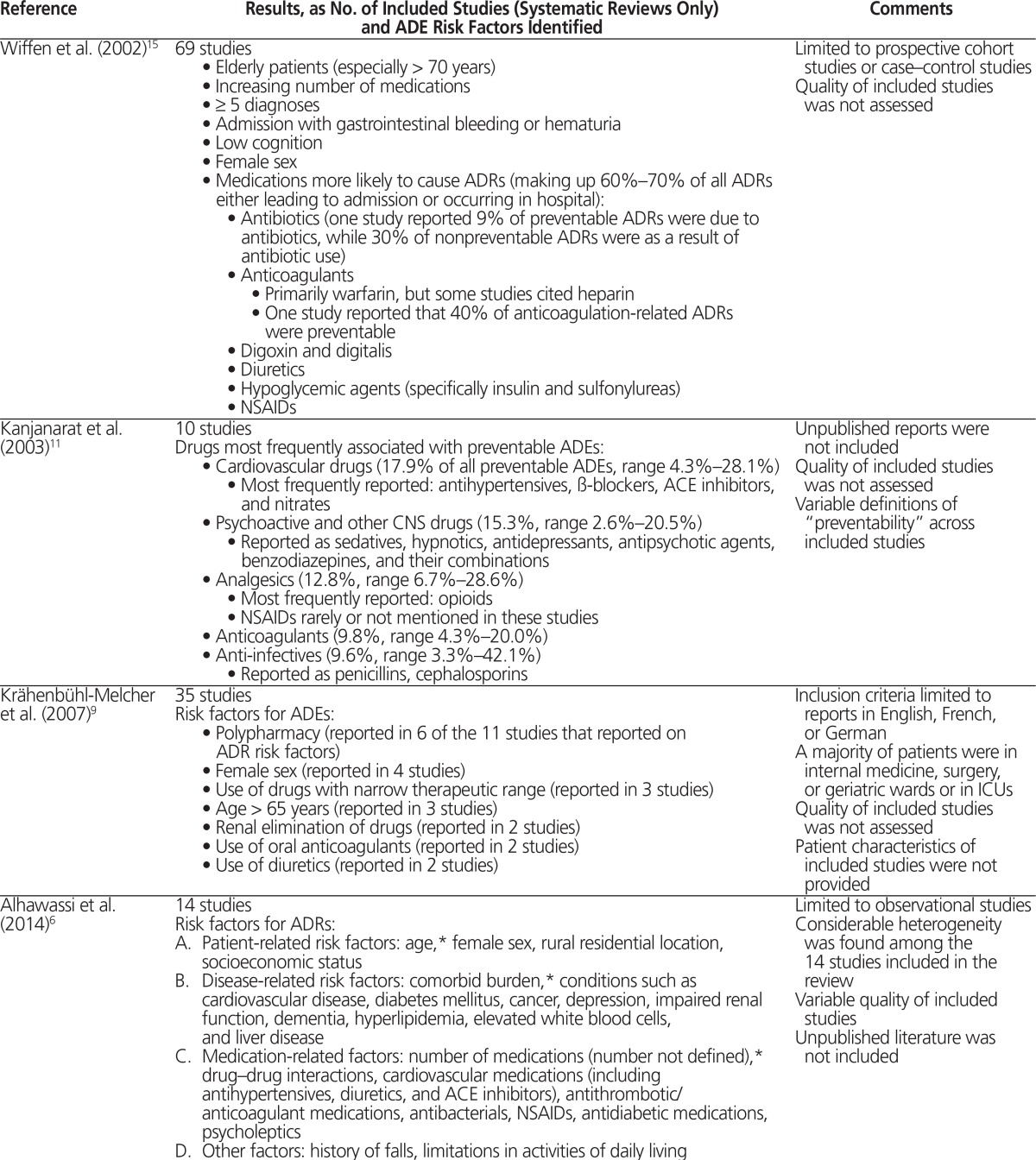
Several risk factors were frequently cited as being associated with ADEs in adult inpatients, as summarized in Table 2.6–16 Of note, older age6–10,12,14–16 and polypharmacy6–10,14–16 were the most frequently cited risk factors among the included reviews. However, the included reviews did not consistently and clearly define these risk factors. For example, 2 publications defined “elderly” as age over 65 and age over 70, respectively.9,15 In one publication that identified polypharmacy as a risk factor for ADEs, this term was defined as 5 or more medications.7 Renal impairment,6–8 female sex,6,8,9,15 and decline in cognition6,7,15 were also frequently reported as being associated with ADEs. Drug– drug interactions were cited as risk factors for ADEs in 2 reviews,6,10 and one of these10 reported specifically on ADRs caused by drug–drug interactions; the risk factors for adverse drug interactions were consistent with those reported in other reviews, and included polypharmacy and older age.10
Table 2.
Summary of Risk Factors for Adverse Drug Events Identified in Included Reviews
| Reference | Results, as No. of Included Studies (Systematic Reviews Only) and ADE Risk Factors Identified | Comments |
|---|---|---|
| Wiffen et al. (2002)15 | 69 studies
|
Limited to prospective cohort studies or case–control studies Quality of included studies was not assessed |
| Kanjanarat et al. (2003)11 | 10 studies Drugs most frequently associated with preventable ADEs:
|
Unpublished reports were not included Quality of included studies was not assessed Variable definitions of “preventability” across included studies |
| Krähenbühl-Melcher et al. (2007)9 | 35 studies Risk factors for ADEs:
|
Inclusion criteria limited to reports in English, French, or German A majority of patients were in internal medicine, surgery, or geriatric wards or in ICUs Quality of included studies was not assessed Patient characteristics of included studies were not provided |
| Alhawassi et al. (2014)6 | 14 studies Risk factors for ADRs:
|
Limited to observational studies Considerable heterogeneity was found among the 14 studies included in the review Variable quality of included studies Unpublished literature was not included |
| Davies et al. (2007)8 |
|
— |
| Magro et al. (2012)10 | Risk factors for adverse drug interactions:
|
Inpatient and outpatient data were not separated Reported only on ADEs resulting from drug–drug interactions |
| Petrovic et al. (2012)7 |
|
Use of GerontoNet ADR Risk Score not validated in a North American population |
| Weiss and Elixhauser (2013)12 |
|
Reported on ADEs occurring within a 1-year span in the United States Conclusions based on ADEs occurring in patients of all ages (including pediatric patients) |
| Weiss et al. (2013)13 |
|
Reported on ADEs occurring within a 1-year span in the United States Patient characteristics not provided |
| US Department of Health and Human Services (2014)14 | Populations most vulnerable to ADEs:
Drug classes most commonly implicated in ADEs occurring in hospital inpatients:
|
ADEs identified from a pool of inpatient and outpatient data for Medicare beneficiaries in the United States Action plan for each drug class was developed according to the following 4-step approach: surveillance, evidence-based prevention tools, incentives and oversight, and research |
| Zhu and Weingart (2015)16 | Populations at high risk for ADEs:
Drug classes associated with ADEs in hospitalized patients:
|
— |
ACE = angiotensin-converting enzyme, ADE = adverse drug event, ADR = adverse drug reaction, CNS = central nervous system, eGFR = estimated glomerular filtration rate, ICU = intensive care unit, NSAID = nonsteroidal anti-inflammatory drug, PK = pharmacokinetics.
Identified as independent risk factors for ADRs in elderly patients.
Medication classes reported to be associated with ADEs during the hospital stay included anticoagulants,6,9,11,12,14–16 anti-infectives/antibiotics,6,8,11–13,15,16 antihyperglycemic agents6,8,13–16 analgesics (including opioids/narcotics8,11–14,16 and nonsteroidal anti-inflammatory drugs6,8,13,15), and cardiovascular drugs (including antihypertensive agents, diuretics, and digoxin).6,8,9,11,15 Two publications reported on preventable ADEs in hospital inpatients; the medication classes were consistent with those reported above (including anticoagulants, anti-infectives, anti-hyperglycemic agents, cardiovascular drugs, and opioids).11,14
DISCUSSION
Several risk factors, patient characteristics, and medications associated with ADEs were identified in this overview of systematic reviews, narrative reviews, and guidelines.
Not surprisingly, older age and polypharmacy were consistently associated with ADEs. Age-related pharmacodynamics and pharmacokinetic changes were cited as factors contributing to the frequent association of older age with ADEs.6–8,15 Pharmacists could optimize care by considering patient age in the selection and dosing of medications and by ensuring discontinuation of medications when appropriate. In addition to the lack of clear definitions for polypharmacy and older age among the included reviews, there was also a lack of agreement among the studies as to whether these characteristics were independent risk factors for ADEs. Independent causal associations are difficult to assess, because of the nature of the studies included in the reviews; potential confounding factors such as comorbid conditions, renal and hepatic function, and cognitive function are examples of the many important considerations when assessing older age and polypharmacy as independent risk factors for ADEs. Renal impairment was also frequently cited as being associated with ADEs, and drug–drug interactions were identified as another potential source of ADEs. These factors represent 2 other potential areas of focus for pharmacists, who can take steps to ensure appropriate drug dosing in renal impairment and screening for clinically significant drug–drug interactions.
Several medication classes were implicated in ADEs. Targeting patients who are taking anticoagulants, cardiovascular drugs, antibiotics, antihyperglycemic agents, and analgesics may be one approach to efficiently allocate clinicians’ time and resources; however, the volume of drugs that fall within these classes may prevent this from being a feasible approach. Nevertheless, being cognizant of these drug classes, optimizing doses, ensuring the appropriateness of drug therapy, and ensuring that appropriate monitoring is in place may be ways in which pharmacists can prioritize their services.
This overview had several potential limitations. The selection of articles for inclusion was performed by a single reviewer. Articles were not assessed for overlap of primary studies within the included reviews. Only 2 of the included reviews focused on preventable ADEs; all of the other reviews did not differentiate between preventable and nonpreventable ADEs. If these reviews were to be used for prioritization of pharmacist resources, this is an important consideration, as there may be little benefit in targeting patients at risk of nonpreventable ADEs. Evidence related to the effectiveness of interventions aimed at preventing ADEs in hospital inpatients would be needed to optimize pharmacist resources. Finally, this review was not restricted to Canadian studies, and health care delivery in other countries differs from that in Canada.
CONCLUSION
A wide variety of patient characteristics, such as older age, polypharmacy, female sex, renal impairment, and decline in cognition, have been associated with ADEs in hospital inpatients. In terms of specific drug classes, anticoagulants, cardiovascular drugs, antibiotics, antihyperglycemic agents, and analgesics have been commonly cited as being associated with ADEs. Prioritization of clinical pharmacy resources according to these risk factors, and further research on interventions aimed at preventing ADEs, could help pharmacists to optimize their impact as members of the patient care team.
Footnotes
Competing interests: None declared.
Funding: None received.
References
- 1.Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4(Suppl 1):S73–7. doi: 10.4103/0976-500X.120957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678–86. doi: 10.1503/cmaj.1040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glossary of terms used in pharmacovigilance. Uppsala (Sweden): Uppsala Monitoring Centre; 2011. [cited 2015 Dec 28]. Available from: http://who-umc.org/Graphics/24729.pdf. [Google Scholar]
- 4.CADTH Information Services . Grey matters: a practical deep-web search tool for evidence-based medicine. Ottawa (ON): Canadian Agency for Drugs and Technology in Health; 2014. [cited 2015 Jul 6]. Available from: https://www.cadth.ca/resources/finding-evidence/grey-matters-practical-search-tool-evidence-based-medicine. [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079–86. doi: 10.2147/CIA.S71178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrovic M, van der Cammen T, Onder G. Adverse drug reactions in older people: detection and prevention. Drugs Aging. 2012;29(6):453–62. doi: 10.2165/11631760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Davies EC, Green CF, Mottram DR, Pirmohamed M. Adverse drug reactions in hospitals: a narrative review. Curr Drug Saf. 2007;2(1):79–87. doi: 10.2174/157488607779315507. [DOI] [PubMed] [Google Scholar]
- 9.Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30(5):379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- 10.Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug–drug interactions. Expert Opin Drug Saf. 2012;11(1):83–94. doi: 10.1517/14740338.2012.631910. [DOI] [PubMed] [Google Scholar]
- 11.Kanjanarat P, Winterstein AG, Johns TE, Hatton RC, Gonzalez-Rothi R, Segal R. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750–9. doi: 10.1093/ajhp/60.17.1750. [DOI] [PubMed] [Google Scholar]
- 12.Weiss AJ, Elixhauser A. Healthcare Cost and Utilization Project Statistical Brief 164: Characteristics of adverse drug events originating during the hospital stay, 2011. Rockville (MD): Agency for Healthcare Research and Quality; 2013. [cited 2014 Jun 23]. Available from: www.hcup-us.ahrq.gov/reports/statbriefs/sb164.pdf. [PubMed] [Google Scholar]
- 13.Weiss AJ, Elixhauser A, Bae J, Encinosa W. Healthcare Cost and Utilization Project Statistical Brief 158: Origin of adverse drug events in U.S. hospitals, 2011. Rockville (MD): Agency for Healthcare Research and Quality; 2013. [cited 2014 Jun 23]. Available from: www.hcup-us.ahrq.gov/reports/statbriefs/sb158.pdf. [PubMed] [Google Scholar]
- 14.National action plan for adverse drug event prevention. Washington (DC): US Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2014. [cited 2015 Jun 23]. Available from: www.health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. [Google Scholar]
- 15.Wiffen P, Gill M, Edwards J, Moore A. Adverse drug reactions in hospital patients: a systematic review of the prospective and retrospective studies. Bandolier Extra. 2002. Jun, [cited 2016 Jul 13]. Available from: www.medicine.ox.ac.uk/bandolier/Extraforbando/ADRPM.pdf.
- 16.Zhu J, Weingart SN. Prevention of adverse drug events in hospitals. In: Post TW, editor. UpToDate. Waltham (MA): UpToDate; [database]. [updated 2015 Feb 17; cited 2015 Jun 23]. Accessed through institutional subscription. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.