Abstract
Importance
Individuals with autism spectrum disorder (ASD) exhibit severe difficulties in social interaction, motor coordination, behavioral flexibility, and atypical sensory processing, with considerable interindividual variability. This heterogeneous set of symptoms recently led to investigating the presence of abnormalities in the interaction across large-scale brain networks. To date, studies have focused either on constrained sets of brain regions or whole-brain analysis, rather than focusing on the interaction between brain networks.
Objectives
To compare the intrinsic functional connectivity between brain networks in a large sample of individuals with ASD and typically developing control subjects and to estimate to what extent group differences would predict autistic traits and reflect different developmental trajectories.
Design, Setting, and Participants
We studied 166 male individuals (mean age, 17.6 years; age range, 7-50 years) diagnosed as having DSM-IV-TR autism or Asperger syndrome and 193 typical developing male individuals (mean age, 16.9 years; age range, 6.5-39.4 years) using resting-state functional magnetic resonance imaging (MRI). Participants were matched for age, IQ, head motion, and eye status (open or closed) in the MRI scanner. We analyzed data from the Autism Brain Imaging Data Exchange (ABIDE), an aggregated MRI data set from 17 centers, made public in August 2012.
Main Outcomes and Measures
We estimated correlations between time courses of brain networks extracted using a data-driven method (independent component analysis). Subsequently, we associated estimates of interaction strength between networks with age and autistic traits indexed by the Social Responsiveness Scale.
Results
Relative to typically developing control participants, individuals with ASD showed increased functional connectivity between primary sensory networks and subcortical networks (thalamus and basal ganglia) (all t ≥ 3.13, P < .001 corrected). The strength of such connections was associated with the severity of autistic traits in the ASD group (all r ≥ 0.21, P < .0067 corrected). In addition, subcortico-cortical interaction decreased with age in the entire sample (all r ≤ −0.09, P < .012 corrected), although this association was significant only in typically developing participants (all r ≤ −0.13, P < .009 corrected).
Conclusions and Relevance
Our results showing ASD-related impairment in the interaction between primary sensory cortices and subcortical regions suggest that the sensory processes they subserve abnormally influence brain information processing in individuals with ASD. This might contribute to the occurrence of hyposensitivity or hypersensitivity and of difficulties in top-down regulation of behavior.
Introduction
Brain abnormalities in autism spectrum disorder (ASD) are present at different scales of anatomical organization, ranging from cortical layers1,2 and minicolumns3,4 to large-scale distributed brain networks.5–9 There is increasing consensus that these abnormalities reflect atypical interactions across multiple neural systems, rather than a problem affecting isolated brain regions.10–18 Abnormalities in the development and interaction across brain networks could arise from early disruptions of local neuronal circuitry, signaled by abnormal laminar organization2 and reduced size of cortical minicolumns.3,19 The latter in particular is likely to reflect disrupted functional segregation between minicolumns, giving rise to local overconnectivity between minicolumns.3,11 Excessive local information processing would positively reinforce and stabilize local physical connections while at the same time negatively affect the development of efficient long-range connections due to delays in information transfer between distant brain regions, failure to differentiate signal from noise, and reduced synchrony in the activity of distant clusters of minicolumns11,20–23 (see the initial figure in the study by Belmonte et al22 for a graphical depiction of the effect of local overconnectivity coupled with long-range underconnectivity). At the network level, the cascading causal effect of local overconnectivity on long-range disconnectivity could result in decreased functional integration within networks and functional segregation between networks,24–26 as well as persistent subcortico-cortical overconnectivity.8,27
Within this perspective, functional neuroimaging studies focused on 2 levels of anatomical organization. Examining the interaction between specific brain regions with functional magnetic resonance imaging (fMRI), functional connectivity studies have shown that ASD is associated with abnormal connectivity within cortico-cortical networks supporting language,28–30 working memory,31,32 visual attention,33 face recognition,34,35 salience detection,7 and social cognition.36–39 Abnormal subcortico-cortical connectivity has also been evidenced by studies18,27,40–43 focusing on the basal ganglia and the thalamus. Considering the topological properties of the whole-brain network, graph theoretical studies44–47 consistently reported alterations in the efficiency of information transfer both at the local and the global level in ASD. While these investigations have contributed to characterize the disconnection model of ASD,11,13,15,48 one largely underexamined domain regards the investigation of between-network interactions in ASD.
To date, few studies have examined between-network interactions in ASD, reporting reduced connectivity between the saliency network and a medial temporal lobe network in young adults with ASD49 and between a frontoparietal network and a cingulate gyrus network in children with ASD.50 While these studies provide initial evidence about abnormalities in between-network interactions in ASD, they focused on a limited number of networks selected a priori49 and did not analyze the interaction with sensory networks.50
Herein, we aimed to systematically explore the interaction between brain networks in individuals with ASD using independent component analysis (ICA)51–53 on resting-state fMRI (rs-fMRI). This technique allows one to extract functional networks that resemble brain networks recruited during task performance.54–56 We quantify interactions between brain networks using the temporal correlation of their spontaneous activity at rest, and we estimate to what extent group differences would predict autistic traits and reflect different developmental trajectories. Our study uses a large sample of participants selected from the Autism Brain Imaging Data Exchange (ABIDE), a recently launched publicly available database of 1112 structural and rs-fMRI data sets acquired on 539 participants with ASD and 573 age-matched controls,57 aggregated from 17 international sites.
The wide heterogeneity of symptoms associated with ASD led us to hypothesize the presence of abnormal patterns of interaction between multiple brain networks, ranging from sensory and motor processing to higher-order cognitive functions. We also hypothesized that group differences in between-network interaction would be associated with the degree of autistic traits and with delayed or arrested development of cortico-cortical interactions and persistent subcortico-cortical connectivity.
Methods
Included Participants From the ABIDE Database
From the ABIDE database, we included all male individuals with a DSM-IV-TR diagnosis of either autism or Asperger syndrome, collectively referred to as the ASD group and typically developing (TD) control subjects. Participant inclusion criteria were as follows: (1) the data sets included a T1-weighted image (an rs-fMRI acquisition of ≥180 time points with near full-brain coverage), (2) a full-scale IQ higher than 70, and (3) a mean framewise displacement (FD)58 of less than 0.34, corresponding to 2 SDs above the whole-sample mean. These criteria yielded 359 participants (166 ASD and 193 TD) from 8 sites, matched by age (t357 = 0.86, P = .39), full-scale IQ (t357 = −0.93, P = .35), mean FD (t357 = 1.67, P = .09), and eye status (open or closed) in the scanner (χ21 = 0.05, P = .81). Demographic information for the final sample (N = 359) is summarized in Table 1. Further details about demographics, diagnostic criteria, and a selection flowchart for the final sample are provided in eFigure 1, eFigure 2, and eTable 1 in the Supplement. Institutional review board approval was provided by each data contributor. Detailed recruitment and assessment protocols and inclusion criteria are available on the ABIDE website. The ABIDE data set was made public in August 2012 and can be accessed at: http://fcon_1000.projects.nitrc.org/indi/abide/.
Table 1. Participants demographics.
| Mean (SD) [Range] | ||
|---|---|---|
| Autism Group (N = 166) | Control Group (N = 193) | |
| Age, years | 17.6 (7.6) [7-50] | 16.9 (6.6) [6.5-39.4] |
| Full-scale IQ | 109.6 (16.16) 71-148 | 111 (13.1) [73-146] |
| ADI-R Social (N=93) | 19.7(5.3)[7-28] | N/A |
| ADI-R Verbal (N=94) | 15.6(4.5)[2-25] | N/A |
| ADI-R Repetitive Behavior (N=93) | 5.8(2.58)[0-12] | N/A |
| ADOS Total (N=171) | 10.7(5.3)[0-22] | N/A |
| ADOS Communication (N=170) | 3.5(1.9)[0-8] | N/A |
| ADOS Social (N=171) | 7.1(3.8)[0-14] | N/A |
| ADOS Repetitive Behaviour (N=142) | 1.7(1.6)[0-8] | N/A |
| SRS (NASD = 111; NTD = 108) | 89.4 (32.4) [6-164] | 22.2 (18.1) [0-103] |
Abbreviations: SD = standard deviation; ADI-R = Autism Diagnostic Interview-Revised 113; ADOS = Autism Diagnostic Observation Schedule 114; SRS = Social Responsiveness Scale 75, 76. Participants from the following sites from ABIDE were included in the final sample of 359 participants: Leuven (sample 1), NYU, OLIN, PITT, Stanford, SDSU, USM, Yale. The number of participants for whom raw scores in ADOS, ADI-R and SRS are available in the current version of the Composite Phenotypic File (Phenotypic_V1_0b.csv) is reported in brackets.
Independent Component Analysis
Image processing was carried out using FSL63–65 and in-house written software (https://github.com/sblnin/rsfnc). Computations were performed on the Millipede cluster at the University of Groningen (Groningen, the Netherlands) to take advantage of parallel computing for processing a data set of this magnitude. After preprocessing of the rs-fMRI data (detailed in the eMaterials in the Supplement), spatially independent components (ICs) were extracted using FSL MELODIC software.66 The number of components was estimated by the MELODIC algorithm. Temporally concatenated probabilistic ICA53,66 was carried out 25 times on randomized subsets of 112 participants (7 in the TD group plus 7 in the ASD group for each of the 8 sites). The resulting spatial components were entered in a meta-ICA67 to extract robust and reproducible resting-state networks (RSNs).
Components Selection
The meta-ICA estimated 52 spatial components. Among these, we selected RSNs according to their spatial distribution, consistency with previous rs-fMRI studies,7,54,67–69 and resemblance to functional networks recruited by task-based fMRI experiments.54,55,70 This selection was complemented by calculating for each spatial component the reproducibility across the 25 temporally concatenated probabilistic ICAs and the overlap with gray matter (eMaterials and eFigures 3, 4, 5, 6, and 7 in the Supplement). This led to the identification of 19 RSNs that were the focus of subsequent analyses (Figure 1 and Table 2). Excluded components are shown in eFigure 3 in the Supplement.
Figure 1. Components selected for FNC analysis.
The metaICA on 359 participants (166 ASD + 193 TD) extracted 52 independent components (IC), 19 of which were selected for FNC analyses using a semi-supervised procedure detailed in the eMaterials. For each IC, we indicate the component order in the results of the metaICA, reflecting the amount of variance explained by that IC (in decreasing order), along with an anatomical labeling. Abbreviations are listed in Table 2. Discarded ICs are shown in eFigure 3. The similarity of these RSNs with those previously found in Biswal et al. (2010)63 and in Smith et al. (2009)54 was quantified by means of spatial correlation, and is reported in eFigure 7.
Table 2. Localization of the 19 Resting State Networks (RSN) used for FNC analysis.
| IC number | Spatial location | Abbreviation |
|---|---|---|
| IC 1 | Primary visual cortex | V1 |
| IC 3 | Cerebellum posterior | pCRB |
| IC 5 | dorsal primary sensory cortex + dorsal primary motor cortex + medial premotor cortex | dSI + dM1 + mPMC |
| IC 8 | posterior Fusiform gyrus + Primary Visual Cortex | pfus + V1 |
| IC 9 | subgenual BA32 + basal orbitofrontal cortex | BA32(sg) + bOFC |
| IC 10 | BA32 + BA9 + BA10 | BA32+9+10 |
| IC 13 | Cerebellum anterior | CRB anterior |
| IC 15 | dorsal precuneus | dPcun |
| IC 16 | primary auditory cortex + posterior superior temporal gyrus (including a portion of planum polare) + parietal operculum + posterior insular cortex | TE + pSTG + opPAR + pIC |
| IC 17 | Basal Ganglia + Thalamus | BG+Th |
| IC 19 | Occipital pole | OCC pole |
| IC 21 | Intraparietal sulcus + RH ventral premotor cortex | IPS + vPMC(RH) |
| IC 23 | Dorsal anterior insula + middle frontal gyrus + medial cingulate + preSMA | Saliency |
| IC 24 | Superior temporal sulcus + LH inferior frontal gyrus | STS + IFG(LH) |
| IC 25 | LH inferior parietal cortex + LH middle frontal gyrus + LH middle temporal gyrus + LH frontal pole + LH preSMA (Left Fronto-Temporo-Parietal network) | LH FTP |
| IC 27 | BA32 + retrosplenial cortex + precuneus + angular gyrus (Default Mode Network) | DMN |
| IC 29 | ventral primary sensory cortex + ventral primary motor cortex + posterior insular cortex | vSI + vM1+pIC |
| IC 30 | Inferior frontal gyrus/sulcus + LH posterior intraparietal sulcus | IFG + pIPS(LH) |
| IC 33 | Temporal pole + basolateral Amygdala + Pons | TP+Amy+Pons |
The meta–independent component analysis on 359 participants (166 autism spectrum disorder group and 193 typically developing group) extracted 52 independent components (ICs), 19 of which were selected for functional network connectivity analyses using a semisupervised procedure detailed in the eMaterials in the Supplement. For each IC, we indicate the component order in the results of the meta–independent component analysis, reflecting the amount of variance explained by that IC (in decreasing order), along with an anatomical labeling. The expanded IC abbreviations are listed in Table 2. Discarded ICs are shown in eFigure 3 in the Supplement. The similarity of these resting-state networks with those previously found by Smith et al54 and Biswal et al67 was quantified by means of spatial correlation and is shown in eFigure 7 in the Supplement.
Functional Network Connectivity
Each RSN’s summary time course was estimated at the participant level by spatial regression of the full set of 52 components from the meta-ICA against each participant’s preprocessed rs-fMRI data.71 Although we focused our analyses on the 19 identified RSNs, we used the full set of components for spatial regression to account for potential effects of noise captured by the non-RSN components (n = 33). Each RSN summary time course was then band-pass filtered (0.08-0.009 Hz).72 We calculated functional network connectivity (FNC)73–75 using the Pearson correlation coefficient between each and every other summary time course. This resulted in an FNC matrix with the dimensions of 19 times 19 (RSNs) times 359 (participants). Group differences in FNC were estimated for each pair of RSNs in a general linear model that included age, IQ, and eye status at scan. Seven covariates were added to capture the mean FNC differences across sites and one to capture the global mean. Finally, the mean participant FD was added as a covariate to minimize the effects of motion.57,76 Inference was carried out using nonparametric permutation testing (FSL randomize [20 000 permutations]). The significance threshold was corrected for multiple comparisons using false discovery rate (FDR).77 In addition, we repeated the analyses using data despiking78 and a more stringent group matching for motion (P = .36) to assess whether group differences in FNC could depend on residual differences in motion between groups (see eMaterials in the Supplement). We then focused on RSN pairs showing significant group differences in FNC after correction to investigate their association with autistic traits and with different developmental trajectories.
Association Between FNC and the Social Responsiveness Scale
We examined whether FNC group differences could predict autistic traits, measured using the Social Responsiveness Scale (SRS).61,62 We correlated SRS raw scores with FNC in the whole sample and in each group separately, after groupwise demeaning of SRS scores and regressing out age, full-scale IQ, site of acquisition, eye status at scan, and mean FD. This was performed separately for each pair of RSNs with a significant group difference in FNC. The SRS scores were groupwise demeaned to prevent that correlations with FNC across groups could be confounded by group differences in SRS scores. Inference was carried out using FSL randomize, and the final results were corrected for multiple comparisons using the FDR. This analysis was restricted to the 67% of ASD (n = 111) and 56% of TD (n = 108) participants for whom SRS data were available (Table 1).
Association Between FNC and Age
We examined whether group differences in FNC would be associated with different neurodevelopmental trajectories. We correlated age with FNC in the whole sample and in each group separately, after regressing out full-scale IQ, mean FD, site of acquisition, and eye status (open or closed) at scan. We then tested the hypotheses of decreased negative correlation of FNC with age in ASD for subcortico-cortical interactions and of decreased positive correlation of FNC with age in ASD for cortico-cortical interactions. As in the previous analysis, inference was carried out using nonparametric permutation testing (FSL randomize [20 000 permutations]), and the results were corrected with the FDR.
Results
ICA and Components Selection
The number of ICs estimated in the 25 temporally concatenated probabilistic ICAs ranged from 22 to 30 (median, 27). The subsequent meta-ICA extracted 52 ICs, among which we selected 19 RSNs for further analyses (Figure 1 and Table 2). These 19 RSNs featured significantly higher reproducibility (t50 = 4.90, P < .0000052 by 2 independent-samples t tests) and proportion of gray matter within or outside their spatial extent (t50 = 1.95, P < .03) compared with the 33 discarded components (eFigure 3 in the Supplement). The spatial distribution of most of our 19 RSNs was consistent with that of RSNs identified in previous work7,54,67–69 (eFigure 7 in the Supplement), including sensory networks (IC1, IC5, IC16, IC19, and IC29), fronto-temporo-parietal networks (IC24 and IC25), subcortical structures (IC17), cerebellum (IC3 and IC13), paralimbic regions (IC9 and IC33), saliency79 (IC23), and default-mode network (IC10, IC15, and IC27).
Group Differences in FNC
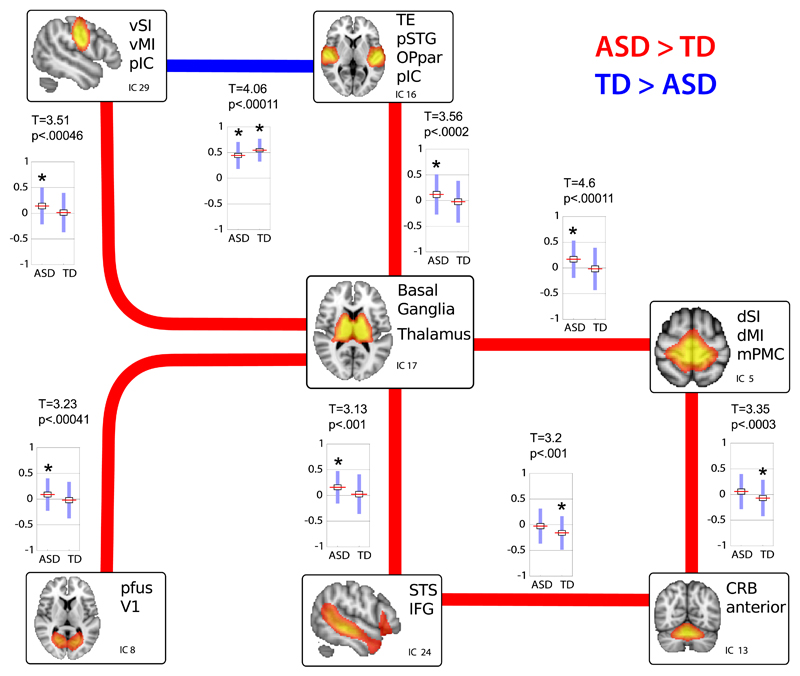
Relative to the TD group, the ASD group exhibited a significantly increased (P < .001, q[FDR] = 0.05) positive interaction between the RSN encompassing basal ganglia and thalamus (IC17) with several cortical networks (with q[FDR] indicating the upper bound in the expected proportion of false positives) (Figure 2). Most of these cortical RSNs included regions in the primary somatosensory (IC5 and IC29), auditory (IC16), and visual (IC8) cortices, as well as the superior temporal sulcus (STS) and left inferior frontal gyrus (IFG) (IC24). An anterior cerebellar RSN (IC13) was also overconnected with the STS and left IFG (IC24) and with dorsal somatosensory and motor cortices (IC5). The ASD group showed decreased FNC only in the interaction between ventral sensorimotor cortices (IC29) and temporoparietal regions centered on the primary auditory cortex (IC16). Results from further analyses performed using data despiking and a more stringent group matching for motion make it unlikely that these group differences depended on differences in motion between groups (eMaterials, eFigure 8, and eTable 2 in the Supplement). Additional analyses on the effect of the sample size are reported in the eMaterials and eFigure 9 in the Supplement.
Figure 2. Group differences in between-network connectivity.
Group differences in FNC strength are shown as lines (red indicates increased FNC in ASD with respect to TD participants, blue the reverse situation) together with boxplots of the Pearson correlation values per each group. Boxplots report the mean (red line), standard deviation (blue bars) and standard error of the mean (black rectangle around the mean) of group-level FNC values. Stars above the boxplots indicate those cases in which the mean FNC was found significantly different from zero (at q(FDR)=.05 - see also eTable 5). Results were obtained by comparing the between-network functional connectivity of 166 ASD and 193 TD participants, using nonparametric permutation testing (20,000 permutations) and correcting the final results with q(FDR)=.05, leading to a final threshold of p<0.001. Converting the correlation scores to Z values using Fisher r to Z transformation yielded almost identical results (see eFigure 5).
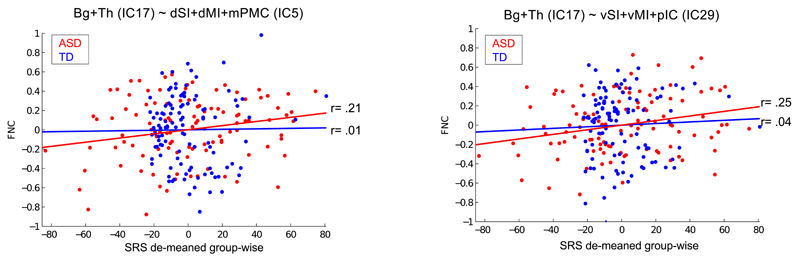
Association Between FNC Abnormalities and the SRS
In the ASD group, autistic traits measured with the SRS scores were positively associated with FNC between the subcortical RSN (IC17) and both dorsal IC5 (r = 0.21) and ventral IC29 (r = 0.25) primary somatosensory and motor cortices (P < .0067 for both, q[FDR] = 0.05) (Table 3, Figure 3, eFigure 10, and eTable 4 in the Supplement). Conversely, the strength of cortico-cortical interaction between auditory (IC16) and ventral somatosensory (IC29) cortices was negatively associated with autistic traits in TD controls only (r = −0.11, P < .0006, q[FDR] = 0.05).
Table 3. Association between FNC and SRS scores (group-wise demeaned).
| Network pair | ASD | TD | whole sample | group difference (z) |
|---|---|---|---|---|
| Bg+Th ~ dSI/MI/mPMC | 0.21a | 0.01 | 0.09 c | 1.43d |
| Bg+Th ~ vSI+vMI+pIC | 0.25a | 0.04 | 0.13 c | 1.51d |
| TE+pSTG+PARop+pIC ~ vSI+vMI+pIC | -0.04 | -0.11b | -0.07c | .54 |
| CRB anterior ~ STS+LH IFG | 0.13 | -0.07 | 0.06c | 1.5d |
Values in the 'ASD', 'TD' and 'whole sample' columns report the Pearson correlation coefficient between FNC and group-wise demeaned SRS scores, after regressing out FIQ, site of acquisition, eyes open/closed at scan, and mean FD. The corresponding scatterplots are presented in Figure 3 and eFigure 8. We report in this table only RSN interactions where results were significant. The complete results and scatterplots for all examined RSN interactions are presented in eTable2 and eFigure 8.
Abbreviations: Bg+Th (IC17): Basal Ganglia + Thalamus; dSI+dMI+mPMC (IC5): dorsal primary somatosensory + primary motor cortex + medial premotor cortex; vSI+vMI+pIC (IC29): ventral primary somatosensory + primary motor cortex + posterior insular cortex; TE+pSTG+PARop+pIC (IC16): primary auditory cortex + posterior superior temporal gyrus + parietal operculum + posterior insular cortex; CRB anterior (IC13): anterior Cerebellum; STS+LH IFG (IC24): superior temporal sulcus + left inferior frontal gyrus.
p<.0067 (corrected with q(FDR)=.053)
p<.0006 (corrected with q(FDR)=.05)
p<.007 (corrected with q(FDR)=.05)
p<.077 uncorrected
Figure 3. Correlation between somatosensory-subcortical FNC and group-wise demeaned SRS scores.
These scatterplots illustrate the association between FNC and SRS (after group-wise demeaning) in ASD (red) and TD (blue) participants for the interaction between the subcortical RSN and the two RSNs centered around the ventral (IC29) and dorsal (IC5) primary somatosensory and motor cortex. The association between SRS scores and FNC was found significant only for the ASD group after correction with q(FDR)=.053. These results were confirmed by repeating the analysis using robust regression (p<.024, q(FDR)=.05). The lines in the scatterplot represent the linear fit witihin each group: red for ASD, blue for TD. Detailed statistics for these within-group correlations, as well as for the correlation analysis in the entire sample, are reported in the Table 3. Scatterplots and statistics for the correlation of SRS with other FNC scores are reported in eFigure 8 and eTable 2.
Association Between FNC Abnormalities and Age
Functional network connectivity between the subcortical RSN (IC17) and networks encompassing primary visual (IC8), auditory (IC16), and ventral somatosensory (IC29) regions significantly decreased with age in TD participants (P < .009, q[FDR] = 0.05) (Table 4). While the effect was maintained in the entire sample of ASD plus TD groups (P < .012, q[FDR] = 0.05), in the ASD group the negative association between age and subcortico-cortical FNC was weaker than in the TD group and not significant (Table 4, eFigure 11, and eTable 5 in the Supplement). However, this difference did not yield significant group interactions. Conversely, FNC between anterior cerebellum (IC13) and dorsal somatosensory and premotor cortices (IC5) significantly increased with age in TD participants (P < .0077, q[FDR] = 0.05) and in the entire sample (P < .0009, q[FDR] = 0.05). Finally, FNC between anterior cerebellum (IC13) and STS plus left IFG (IC24) significantly increased with age in the ASD group (P < .0008, q[FDR] = 0.05) and in the entire sample (P < .0014, q[FDR] = 0.05).
Table 4. Association Between Functional Network Connectivity and Agea.
| Network pair | ASD | TD | whole sample | group difference (z) |
|---|---|---|---|---|
| Bg+Th ~ pfus + V1 | -0.06 | -0.13b | -0.10c | 0.69 |
| Bg+Th ~ TE+pSTG+PARop+pIC | -0.06 | -0.13b | -0.09c | 0.67 |
| Bg+Th ~ vSI+vMI+pIC | -0.04 | -0.18b | -0.11c | 1.31 |
| CRB anterior ~ STS+LH IFG | 0.18a | 0.08 | 0.14c | 0.87 |
| CRB anterior ~ dSI+dMI+mPMC | 0.14 | 0.11b | 0.12c | 0.27 |
Values in the 'ASD', 'TD' and 'whole sample' columns report the Pearson correlation coefficient between FNC and age, after regressing out FIQ, site of acquisition, eyes open/closed at scan, and mean FD. We report in this table only RSN interactions where results were significant. The complete results and scatterplots for all examined RSN interactions are presented in eTable3 and eFigure 9.
Abbreviations: Bg+Th (IC17): Basal Ganglia + Thalamus; pfus + V1 (IC8): posterior fusiform gyrus + primary visual cortex; TE+pSTG+PARop+pIC (IC16): primary auditory cortex + posterior superior temporal gyrus + parietal operculum + posterior insular cortex; vSI+vMI+pIC (IC29): ventral primary somatosensory + primary motor cortex + posterior insular cortex; STS+LH IFG (IC24): superior temporal sulcus + left inferior frontal gyrus; CRB anterior (IC13): anterior Cerebellum.
p<.0008 (corrected with q(FDR)=.05)
p<.009 (corrected with q(FDR)=.05)
p<.012 (corrected with q(FDR)=.05)
Discussion
Increased Subcortico-Cortical FNC
Relative to TD controls, in participants with ASD a subcortical RSN encompassing basal ganglia and thalamus showed increased functional connectivity with 5 cortical RSNs, most of which included primary sensory cortices (results at P < .05 uncorrected are presented in eFigures 12, 13A, 13B, and 14 in the Supplement). Our findings concur with previous studies in ASD that reported increased functional connectivity between regions in the primary sensory cortices and in the striatum,27,41,43,80,81 as well as increased40 thalamo-cortical connectivity (but see Nair et al42 for thalamo-cortical underconnectivity). A comparison of our results with those reported in the inaugural ABIDE article57 is provided in the eMaterials in the Supplement.
The evidence of subcortico-cortical overconnectivity mostly targeting primary sensory cortices provides a framework to conceptualize the presence of sensory abnormalities in ASD. A growing clinical and experimental literature reports atypical sensory processing in ASD,82–86 qualified as hyporeactivity or hyperreactivity to sensory stimuli82,84 and enhanced sensory perceptual processing and discrimination.87,88 Accordingly, the newly released DSM-5 manual89 now also includes hyporeactivity or hyperreactivity to sensory stimulation as a diagnostic criterion, acknowledging that sensory abnormalities are central in the symptomatology of ASD. Our results suggest that the presence of atypical sensory processing in ASD could stem from an abnormal, possibly excessive influence of basic sensory features of the environment on information processing in the brain, which could override higher-order cognitive processes in determining the relevance of different sources of information for behavior. At the neural level, this situation could be engendered by an abnormally high sensory input from subcortical nuclei to the cortex, reflected indirectly by our findings of subcortico-cortical overconnectivity even at rest. A similar conjecture by Belmonte and colleagues10 proposed that in ASD the impairment of low-level mechanisms for filtering irrelevant and unwanted sensory stimuli would prompt the development of compensatory mechanisms that operate at a later, less efficient stage of processing. The recently reported7 overconnectivity within the saliency network in ASD might reflect the development of compensatory mechanisms aimed at counteracting the overwhelming amount of sensory input reaching the cortex due to impaired gating circuits at the subcortical level.7,90,91
It is remarkable that the RSN pairs where we detect group differences in FNC are likely to reflect, at least in part, the activity of projections from deep cerebellar nuclei to the cerebral cortex and from the striatum to the thalamus. The hypothesis of an imbalance in the ratio of excitatory to inhibitory activity in ASD had been proposed by Rubenstein and Mezernich,92 and even earlier studies93–95 consistently reported loss of Purkinje cells in the cerebellum. There is now a growing body of evidence suggesting that the disruption of γ-aminobutyric acid–ergic signaling contributes to the pathophysiology of ASD.23,96–98 Importantly, stereotyped behaviors appear to be related to dysfunctions in γ-aminobutric acid signaling,99 while insistence on sameness is associated with caudate overgrowth.100 Additionally, very recent evidence from in vivo proton magnetic resonance spectroscopy showed a decreased ratio of γ-aminobutric acid to creatinine in the cerebellum and in the primary sensory and motor cortices of individuals with ASD.101,102 Therefore, the increased interaction we observed between subcortical and cortical regions might reflect an abnormally low inhibitory activity, rather than an abnormally high excitatory activity. For instance, the cortico-cerebellar overconnectivity that we detected could stem from a disinhibition of the deep cerebellar nuclei due to the loss of Purkinje cells.22 This conjecture, however, awaits testing with methods different from fMRI because the fMRI signal may confuse excitation and inhibition.103
Association Between FNC Abnormalities and Autistic Traits Measured With SRS
We observed that in ASD subcortico-cortical overconnectivity was related to increased severity of autistic traits as measured with the SRS. Scores on the SRS clearly distinguish individuals with ASD from TD controls at the group level (in our sample, t174* = 19.00, P < 1.412e-44, with the asterisk indicating the df adjusted for unequal variance between groups). At the same time, this measure reflects that autistic traits (1) are present in a continuous gradient of severity in the general population,61 (2) have an increased likelihood to manifest in family members of ASD participants with a negative diagnosis of ASD,62,104,105 and (3) express variability both between and within groups.106 Resting-state fMRI has been shown to capture variability in autistic traits indexed by SRS in neurotypical adults.107 We show that FNC between subcortical and primary somatosensory and motor networks, which is abnormally high in individuals with ASD, was correlated to the severity of autistic traits in the whole sample, as well as within the ASD group. This suggests that this FNC measure is able to capture variability both between and within group described by the SRS scores.
Concerning the nature of the association between SRS and FNC that we report herein, studies91,108,109 in sensorimotor gating in ASD proposed that difficulties in inhibiting repetitive behaviors could stem from problems in filtering out irrelevant sensory stimuli. Deficits in sensorimotor gating in ASD appear to be rooted in structural abnormalities in fronto-striatal and cerebellar circuits108 and are strongly associated with the presence of repetitive behaviors.91 The association we have identified between SRS scores and subcortico-cortical connectivity involving somatosensory and motor cortices is compatible with the idea of a relationship between sensory abnormalities and repetitive behaviors. However, this hypothesis should be corroborated by future studies investigating the association between subcortical-sensorimotor overconnectivity and direct measures of sensory symptoms in ASD84 and by task-based fMRI studies specifically probing these sensory and motor processes.
Association Between FNC Abnormalities and Age
Consistent with prior literature,110 examining the developmental trajectory of between-network overconnectivity revealed that subcortico-cortical connectivity significantly decreased with age in the sample of participants. This suggests that during development cortical processing becomes decreasingly determined by processes elicited by sensory stimuli, emotions, and interoceptive feelings.110 The relationship between subcortico-cortical FNC was negative within each group and did not significantly differ across groups, although the correlation was significant only for TD participants. Therefore, while our results concur with previous studies27,40,41,43,80,81 in reporting a persistent subcortico-cortical overconnectivity across different age groups in ASD, such overconnectivity in the ASD participants we examined decreased with age at a rate that failed to show significant differences from that recorded in the TD participants.
Limitations
Our study has several limitations. First, the correlation approach in functional connectivity does not provide directional information. Such information will be crucial to determine whether the observed subcortico-cortical hyperconnectivity reflects cortical compensatory mechanisms aimed at regulating the information flow from sensory organs, increased information flow from the thalamus to the cortex, or both.
Second, the weak association between FNC and SRS potentially reflects the wide interindividual variability in ASD. Gathering richer phenotypical information is needed to yield a multivariate characterization of the association between phenotypic and neuroimaging parameters.111,112
Third, our group differences in the FNC center around an RSN encompassing basal ganglia and thalamus. Given the neuroanatomical heterogeneity of different structures within this RSN, it is remarkable that ICA does not further decompose this network. This limits the level of detail that can be achieved using spatially independent components and highlights the complementary role of region-based and network-based functional connectivity studies.113,114
Conclusions
We report that hyperconnectivity between subcortical regions and sensory cortices is a central feature in ASD. This hyperconnectivity was related to the degree of autistic traits in the examined sample of individuals with ASD. We propose that such hyperconnections could relate to abnormal sensory processing in that they represent an alteration of the normal equilibrium between sensory information stemming from the thalamus and top-down influence from higher-order cortices.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grants 056-13-013 and 056-13-017 from the Hersenen & Cognitie–Maatschappelijke Innovatie (HCMI) in Gezondheidszorg, Educatie en Veiligheid and by grants 051-07-003 and 400-08-089 from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek. Dr Keysers was supported by ERC-StG grant 312511 from the European Research Council of the European Commission.
Role of the Funder/Sponsor: The Nederlandse Organisatie voor Wetenschappelijk Onderzoek had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Cerliani had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Mennes and Thomas contributed equally to this work. Drs Thioux and Keysers contributed equally to this work as senior authors.
Study concept and design: Cerliani, Thomas, Keysers.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Cerliani, Mennes, Keysers.
Critical revision of the manuscript for important intellectual content: Cerliani, Mennes, Di Martino, Thioux, Keysers.
Statistical analysis: Cerliani, Thomas.
Obtained funding: Keysers.
Study supervision: Cerliani, Thioux, Keysers.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank all the members of the Autism Brain Imaging Data Exchange Consortium (ABIDE;http://fcon_1000.projects.nitrc.org/indi/abide/) and the International Neuroimaging Data-Sharing Initiative (INDI) team (http://fcon_1000.projects.nitrc.org/) supporting the ABIDE effort.
References
- 1.Courchesne E, Mouton PR, Calhoun ME, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 2.Stoner R, Chow ML, Boyle MP, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370(13):1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58(3):428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 4.Casanova M, Trippe J. Radial cytoarchitecture and patterns of cortical connectivity in autism. Philos Trans R Soc Lond B Biol Sci. 2009;364(1522):1433–1436. doi: 10.1098/rstb.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 7.Uddin LQ, Supekar K, Lynch CJ, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70(8):869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Supekar K, Uddin LQ, Khouzam A, et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013;5(3):738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zielinski BA, Anderson JS, Froehlich AL, et al. scMRI reveals large-scale brain network abnormalities in autism. PLoS One. 2012;7(11):e49172. doi: 10.1371/journal.pone.0049172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belmonte MK, Cook EH, Jr, Anderson GM, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9(7):646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- 11.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 13.Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16(11):559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64(7):945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13(1):85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Res. 2007;156(2):117–127. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casanova MF. The neuropathology of autism. Brain Pathol. 2007;17(4):422–433. doi: 10.1111/j.1750-3639.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casanova MF. White matter volume increase and minicolumns in autism. Ann Neurol. 2004;56(3):453. doi: 10.1002/ana.20196. [DOI] [PubMed] [Google Scholar]
- 21.Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003;17(3):651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- 22.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubenstein JL. Annual Research Review. Development of the cerebral cortex: implications for neurodevelopmental disorders. J Child Psychol Psychiatry. 2011;52(4):339–355. doi: 10.1111/j.1469-7610.2010.02307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudie JD, Shehzad Z, Hernandez LM, et al. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 2012;22(5):1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishman I, Keown CL, Lincoln AJ, Pineda JA, Müller RA. Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry. 2014;71(7):751–760. doi: 10.1001/jamapsychiatry.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller RA. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padmanabhan A, Lynn A, Foran W, Luna B, O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci. 2013;7:814. doi: 10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 29.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones TB, Bandettini PA, Kenworthy L, et al. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49(1):401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62(3):198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belmonte MK, Gomot M, Baron-Cohen S. Visual attention in autism families: “unaffected” sibs share atypical frontal activation. J Child Psychol Psychiatry. 2010;51(3):259–276. doi: 10.1111/j.1469-7610.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- 34.Kleinhans NM, Richards T, Sterling L, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 35.Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 37.Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135(pt 9):2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104(1):160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 41.Di Martino A, Kelly C, Grzadzinski R, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nair A, Treiber JM, Shukla DK, Shih P, Müller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136(pt 6):1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delmonte S, Gallagher L, O’Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front Hum Neurosci. 2013;7:430. doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Sigman M. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia. 2011;49(2):254–263. doi: 10.1016/j.neuropsychologia.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Boersma M, Kemner C, de Reus MA, et al. Disrupted functional brain networks in autistic toddlers. Brain Connect. 2013;3(1):41–49. doi: 10.1089/brain.2012.0127. [DOI] [PubMed] [Google Scholar]
- 46.Peters JM, Taquet M, Vega C, et al. Brain functional networks in syndromic and non-syndromic autism: a graph theoretical study of EEG connectivity. BMC Med. 2013;11:54. doi: 10.1186/1741-7015-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudie JD, Brown JA, Beck-Pancer D, et al. Altered functional and structural brain network organization in autism. Neuroimage Clin. 2012;2:79–94. doi: 10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 49.von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 2013;8(6):694–701. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bos DJ, van Raalten TR, Oranje B, et al. Developmental differences in higher-order resting-state networks in Autism Spectrum Disorder. Neuroimage Clin. 2014;4:820–827. doi: 10.1016/j.nicl.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKeown MJ, Makeig S, Brown GG, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laird AR, Eickhoff SB, Rottschy C, Bzdok D, Ray KL, Fox PT. Networks of task co-activations. Neuroimage. 2013;80:505–514. doi: 10.1016/j.neuroimage.2013.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mennes M, Kelly C, Colcombe S, Castellanos FX, Milham MP. The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cereb Cortex. 2013;23(1):223–229. doi: 10.1093/cercor/bhs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Martino A, Yan CG, Li Q, et al. The Autism Brain Imaging Data Exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 60.Lord C, Rutter M, Goode S, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 61.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 62.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 63.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 64.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1suppl):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 66.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 67.Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laird AR, Fox PM, Eickhoff SB, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 73.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demirci O, Stevens MC, Andreasen NC, et al. Investigation of relationships between fMRI brain networks in the spectral domain using ICA and Granger causality reveals distinct differences between schizophrenia patients and healthy controls. Neuroimage. 2009;46(2):419–431. doi: 10.1016/j.neuroimage.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30(8):2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 78.Jo HJ, Gotts SJ, Reynolds RC, et al. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. J Appl Math. 2013;2013 doi: 10.1155/2013/935154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2:34. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Martino A, Zuo XN, Kelly C, et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(8):623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- 83.Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 84.Minshew NJ, Hobson JA. Sensory sensitivities and performance on sensory perceptual tasks in high-functioning individuals with autism. J Autism Dev Disord. 2008;38(8):1485–1498. doi: 10.1007/s10803-007-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J Autism Dev Disord. 2009;39(1):1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- 86.Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69(5 Pt 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 88.Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos Trans R Soc Lond B Biol Sci. 2009;364(1522):1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 90.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 91.Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61(4):482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 92.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bailey A, Luthert P, Dean A, et al. A clinicopathological study of autism. Brain. 1998;121(pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 94.Kemper TL, Bauman M. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57(7):645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 95.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 96.Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev. 2012;36(9):2044–2055. doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tyzio R, Nardou R, Ferrari DC, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343(6171):675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 98.Pizzarelli R, Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011;2011:297153. doi: 10.1155/2011/297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chao HT, Chen H, Samaco RC, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468(7321):263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Langen M, Bos D, Noordermeer SD, Nederveen H, van Engeland H, Durston S. Changes in the development of striatum are involved in repetitive behavior in autism. Biol Psychiatry. 2014;76(5):405–411. doi: 10.1016/j.biopsych.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Gaetz W, Bloy L, Wang DJ, et al. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014;86:28–34. doi: 10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 104.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57(6):655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 105.Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167(11):1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lai MC, Lombardo MV, Chakrabarti B, Baron-Cohen S. Subgrouping the autism “spectrum”: reflections on DSM-5. PLoS Biol. 2013;11(4):e1001544. doi: 10.1371/journal.pbio.1001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Di Martino A, Shehzad Z, Kelly C, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166(8):891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McAlonan GM, Daly E, Kumari V, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125(pt 7):1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- 109.Madsen GF, Bilenberg N, Cantio C, Oranje B. Increased prepulse inhibition and sensitization of the startle reflex in autistic children. Autism Res. 2014;7(1):94–103. doi: 10.1002/aur.1337. [DOI] [PubMed] [Google Scholar]
- 110.Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9(10):1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 112.Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn Sci. 2012;16(3):181–188. doi: 10.1016/j.tics.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 114.Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.