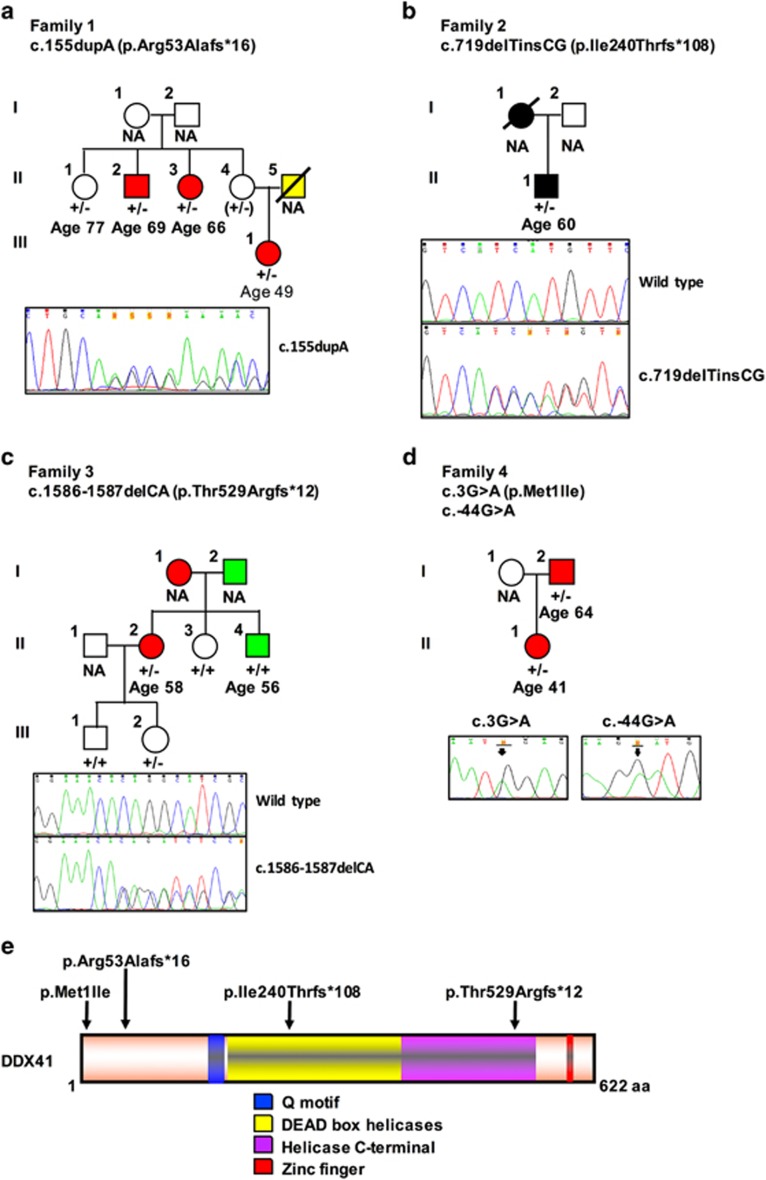
Myelodysplasia (MDS) and acute myeloid leukemia (AML) are mostly sporadic hematopoietic stem cell clonal disorders. However, there are rare occurrences of familial MDS/AML where there are two or more affected cases in the same family. To date, germline heterozygous mutations have been identified in 10 genes (RUNX1, CEBPA, TERC, TERT, GATA2, SRP72, ANKRD26, ACD, ETV6 and DDX41)1, 2, 3, 4, 5, 6, 7, 8, 9, 10 associated with familial MDS/AML. Over the last 15 years we have accrued 78 families in which there are at least two cases of bone marrow failure and at least one of whom has MDS or AML. We have undertaken a combination of whole-exome and targeted sequencing to characterize these families. The targeted sequencing uses a newly designed familial MDS/AML gene panel that includes the above 10 listed genes. This analysis has enabled us to identify four families harboring heterozygous germline DDX41 (DEAD-box helicase 41) variants (Figures 1a–d); three families have novel frameshift variants (c.155dupA, c.1586_1587delCA and c.719delTinsCG) and the fourth family has a recurrent missense variant in the initiation codon (c.3G>A, rs141601766) described previously by Lewinsohn et al.11 Collectively, these four families comprise seven cases of MDS and two cases of AML (age range, 40–70 years). These patients did not have any extra-hematopoietic features and therefore represent ‘pure' MDS/AML (Table 1).
Figure 1.
(a–d) Families with MDS–AML with variants in DDX41, their age at diagnosis and their respective Sanger sequencing traces. Affected individuals are colored as follows: red, MDS; yellow, CML; black, AML; and green, other non-hematological cancer. (e) Schematic of DDX41 protein showing the heterozygous variants identified in this study. CML, chronic myeloid leukemia.
Table 1. Characteristics and family history of index cases.
| Family | Case | Age (years) | Diagnosis | Relationship to index | Nucleotide | Amino acid |
|---|---|---|---|---|---|---|
| 1 | I-1 | NA | Asymptomatic | Grandmother | NA | NA |
| I-2 | NA | Asymptomatic | Grandfather | NA | NA | |
| II-1 | 77 | Asymptomatic | Maternal aunt | c.155dupA | p.Arg53Alafs*16 | |
| II-2 | 69 | MDS | Maternal uncle | c.155dupA | p.Arg53Alafs*16 | |
| II-3 | 66 | MDS | Maternal aunt | c.155dupA | p.Arg53Alafs*16 | |
| II-4 | NA | Asymptomatic | Mother | NA | NA | |
| II-5 | NA | CML | Father | NA | NA | |
| III-1 | 49 | MDS | Index case | c.155dupA | p.Arg53Alafs*16 | |
| 2 | I-1 | NA | AML | Mother | NA | NA |
| I-2 | NA | Asymptomatic | Father | NA | NA | |
| II-1 | 60 | AML | Index case | c.719delTinsCG | p.Ile240Thrfs*108 | |
| 3 | I-1 | NA | MDS | Mother | NA | NA |
| I-2 | NA | Stomach cancer | Father | NA | NA | |
| II-1 | NA | Asymptomatic | Husband | NA | NA | |
| II-2 | 58 | MDS | Index case | c.1586-1587delCA | p.Thr529Argfs*12 | |
| II-3 | NA | Asymptomatic | Sister | NV | NV | |
| II-4 | 56 | Tongue cancer | Brother | NV | NV | |
| III-1 | NA | Asymptomatic | Son | NV | NV | |
| III-2 | NA | Asymptomatic | Daughter | c.1586-1587delCA | p.Thr529Argfs*12 | |
| 4 | I-1 | NA | Asymptomatic | Mother | NA | NA |
| I-2 | 64 | MDS | Father | c.3G>A | p.Met1Ile | |
| c.-44G>A Met1Ile | NA | |||||
| II-1 | 41 | MDS | Index case | c.3G>A | p.Met1Ile | |
| c.-44G>A Met1Ile | NA |
Abbreviations: AML, acute myeloid leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; NA, not available; NV, does not have the variant.
At present, little is known about DDX41 function and its role in hematopoiesis. However, Polprasert et al.10 showed that the protein encoded by DDX41 interacts directly with spliceosomal proteins and inactivation of tumor suppressors can occur once this interaction is disrupted. It is known that members of the DEAD/H box RNA helicase family can act as oncogenes or tumor suppressors in other cancers, depending on the specific protein interactions.12 In addition, alterations in DDX41 can cause exon skipping or exon retention in the RNA-splicing process resulting in alteration of specific genetic isoforms.10
Kirwan et al.3 demonstrated that familial MDS/AML patients with germline variants in TERT and TERC have significantly shorter telomeres compared with controls. To determine whether our group of ‘pure' MDS/AML patients with germline DDX41 variants have a similar impact on telomere length, we measured peripheral blood telomere length by monochrome multiplex quantitative PCR method13 in our patients. Slightly shorter telomere length was found in this group of patients harboring germline DDX41 variants compared with age-matched controls (P<0.05, Supplementary Figure S1). It will be important to investigate telomere length in additional patients with DDX41 variants to substantiate these observations.
In Family 1 (Figure 1a), a novel heterozygous germline variant c.155dupA (p.Arg53Alafs*16 showed in Figure 1e) in DDX41 was identified in the 49-year-old female index case (III-1) diagnosed with MDS, refractory anemia with excess blasts (RAEB). Sanger sequencing revealed that her maternal uncle and aunt who both developed RAEB also harbor this frameshift variant (individuals II-2 and II-3, respectively). There are two asymptomatic carriers (individuals II-1 and II-4), supporting previous observations that haploinsufficiency for DDX41 shows variable penetrance.11 Further family history included her father (II-5) who died of chronic myeloid leukemia, unlikely to be related to the DDX41 variant.
In Family 2 (Figure 1b), the index case is a 60-year-old male (II-1) with AML harboring a novel heterozygous frameshift variant c.719delTinsCG (p.Ile240Thrfs*108), predicted to cause truncation of the protein and consequent loss of function. His mother died of AML (I-1). Segregation analysis was not possible as there were no family samples available, however the variant allele frequency in the index case is 0.494 indicating a heterozygosity. This variant is located in the DEAD-box domain of DDX41, in a highly conserved motif that includes the ATP-binding site of DDX41 (Figure 1e).
The 58-year-old female index case in Family 3 (II-2 in Figure 1c) with MDS, has a novel frameshift deletion variant c.1586-1587delCA (p.Thr529Argfs*12) in the helicase domain of DDX41 (Figure 1e), which is again predicted to cause truncation of the protein. Her brother has tongue cancer (II-4), her mother has MDS (I-1) and her father has stomach cancer (I-2). In the absence of samples of the index case's parents, Sanger sequencing was undertaken on samples from her siblings and children. The siblings (II-3 and II-4) of the index case do not harbor the variant c.1586-1587delCA, whilst her daughter (III-2) is an asymptomatic carrier. This suggests that the index case and her mother (both with MDS) have disease associated with the DDX41 variant, while the non-hematological cancers seen in her brother (II-4) and father (I-2) are unrelated to DDX41.
The index case of Family 4 (Figure 1d) is a 41-year-old female (II-1) diagnosed with MDS/RAEB. Her father (I-2) was also diagnosed with MDS at the age of 64 years. The heterozygous missense variant c.3G>A (p.Met1Ile—rs141601766, showed in Figure 1e) in DDX41, which segregated with disease in these two individuals, has been reported in The Exome Aggregation Consortium (ExAC) database in 6/117 464 alleles (http://exac.broadinstitute.org/, accessed 31 March 2016). Interestingly, both cases with the c.3G>A variant also carried a linked 5′-untranslated region variant (c.-44G>A showed in Figure 1d) previously observed by Lewinsohn et al.11 They also demonstrated that human embryonic kidney 293 cells (HEK-293) cells ectopically expressing the Met1Ile mutant protein used an alternative translation initiation site yielding a smaller DDX41 protein when compared with the full-length of 70 kDa. Their experiments suggest that this isoform may occur naturally and has an altered location.
The recurrence of the Met1Ile variant in the ExAC database poses an interesting question as to the causative role of DDX41 variants in MDS/AML. Excluding any non-canonical and dubious calls in this database, loss of function (LOF) variants (including Met1Ile) are seen to occur at a cumulative frequency of 1 in 1189 people (46 LOF variants in an average of 109 354 alleles). This is in stark contrast to the few LOF variants reported in RUNX1 (6), CEPBA (0), GATA2 (0) and ETV6 (1). We also note that in a screen of 1034 patients with MDS and secondary AML, 7 patients (1 in 148) had germline LOF variants in DDX41 (ref. 10). These data indicate that rather than establishing a causal Mendelian link between germline LOF DDX41 variants and MDS/AML, it is better to think of them as genetic risk factors. Comparing the frequency of LOF DDX41 variants seen in MDS and secondary AML with the frequency seen in ExAC we obtain an odds ratio of 8.05 (P=5.65 × 10−5, Fisher's exact test). Allowing for a 1/100 probability of getting the disease, this would translate to a relative risk of 7.51. It is inevitable therefore, that MDS/AML driven by DDX41 LOF variants will sometimes appear as familial.
In summary, we report on novel germline heterozygous DDX41 variants exhibiting variable penetrance in families with MDS/AML and tendency to short telomeres. Our analysis suggests that rather than establishing a causal Mendelian link between DDX41 germline LOF variants and MDS/AML it is appropriate to consider these as genetic risk factors.
Acknowledgments
This work was supported by CNPq (The Brazilian National Council for Scientific and Technological Development), Bloodwise, Children with Cancer and MRC (Medical Research Council, UK).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Song W-J, Sullivan MG, Legare RD, Hutchings S, Tan X, Kufrin D et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet 1999; 23: 166–175. [DOI] [PubMed] [Google Scholar]
- Smith ML, Cavenagh JD, Lister TA, Fitzgibbon J. Mutation of CEBPA in familial acute myeloid leukaemia. N Engl J Med 2004; 351: 2403–2407. [DOI] [PubMed] [Google Scholar]
- Kirwan M, Vulliamy T, Marrone A, Walne AJ, Beswick R, Hillmen P et al. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukaemia. Hum Mutat 2009; 30: 1567–1573. [DOI] [PubMed] [Google Scholar]
- Hahn C N, Chong C-E, Carmichael CL, Wilkins EJ, Brautigan PJ, Li X-C et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukaemia. Nat Genet 2011; 43: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukaemia (Emberger syndrome). Nat Genet 2011; 43: 929–931. [DOI] [PubMed] [Google Scholar]
- Kirwan M, Walne AJ, Plagnol V, Velangi M, Ho A, Hossain U et al. Exome sequencing identifies autosomal-dominant SRP72 mutations associated with familial aplasia and myelodysplasia. Am J Hum Genet 2012; 90: 888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris P, Favier R, Alessi MC, Geddis AE, Kunishima S, Heller PG et al. ANKRD26-related thrombocytopenia and myeloid malignancies. Blood 2013; 122: 1987–1989. [DOI] [PubMed] [Google Scholar]
- Guo Y, Kartawinata M, Li J, Pickett HA, Teo J, Kilo T et al. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood 2014; 124: 2767–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MY, Churpek JE, Keel SB, Walsh T, Lee MK, Loeb KR et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat Genet 2015; 47: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polprasert C, Schulze I, Sekeres MA, Makishima H, Przychodzen B, Hosono N et al. Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell 2015; 27: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn M, Brown AL, Weinel LM, Phung C, Rafidi G, Lee MK et al. Novel germline DDX41 mutations define families with a lower age of MDS/AML onset, and lymphoid malignancies. Blood 2016; 127: 1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Pace FV. DEAD box RNA helicase functions in cancer. RNA Biol 2013; 10: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon R. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009; 37: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.