Abstract
This study examines whether the racial disparity in functional health grows unabated over the adult life course – the cumulative disadvantage hypothesis – or shrinks among the oldest old – the age-as-leveler hypothesis. Special emphasis is placed on the role of socioeconomic status (SES), which is highly associated with race. The analysis uses latent growth-curve modeling to examine differences in age trajectories of functional health between Black and White Americans and is based on nationally-representative panel data of 3,617 adults. Results cautiously support the age-as-leveler hypothesis. Net of functional health at baseline, Black adults experience a growing disadvantage in functional health over time until the oldest ages, when the gap in functional health begins to shrink. Results indicate that the potential leveling mechanisms of age may be specific to women. SES including financial assets explains the divergence in functional health across young and middle-aged Black and White adults, but not the later-life convergence. This study reveals the life course pattern of racial disparity in functional health and suggests that more theoretical development is needed in this field to explain how and why the age-as-leveler and cumulative disadvantage processes are outcome-specific.
Although many studies have verified that White adults have better functional health than Black adults in the US (Freedman, Martin, Schoeni, & Cornman, 2008; Hummer, 1996; Taylor, 2008; Thorpe, Kasper, Szanton, Frick, Fried, & Simonsick, 2008; Williams & Collins, 1995), few studies have examined whether and how the racial disparity in health varies over the adult life course (Ferraro, Thorpe, McCabe, Kelley-Moore, & Jiang, 2006; Shuey & Willson, 2008). Greater disparities at different life stages would have both important theoretical implications – such as providing clues to the underlying mechanisms involved in creating the health disparity – and social implications – such as the identification of life stages of particular disadvantage to Black adults that warrant intensified prevention and intervention efforts.
Previous studies of the life-course disparity patterns in health have focused on socioeconomic status (SES). Two distinct patterns of association between SES and health with age have been found in previous studies – one pattern of findings indicates a consistent diverging gap by SES in all adult life stages (Dupre, 2007; Kim & Durden, 2007; Ross & Wu, 1996; Willson & Shuey, 2007), and the other indicates a diverging gap until early-old age followed by convergence in later life (Herd, 2006; House, Lepkowski, Kinney, Mero, Kessler, & Herzog, 1994). These two patterns have been explained by two hypotheses, respectively the “cumulative advantage hypothesis” and the “age-as-leveler hypothesis” (Lynch, 2003).
According to Dannefer (2003), “cumulative advantage/disadvantage can be defined as the systemic tendency for interindividual divergence in a given characteristic (e.g., money, health, or status) with the passage of time” (S327). In SES and health research, the process of cumulative advantage implies that various resources related to SES accumulate with age to further advantage the health of people with higher SES (Ross & Wu, 1996). Although the age-as-leveler hypothesis has been contrasted with the cumulative advantage hypothesis in previous studies, the age-as-leveler hypothesis also posits increasing inequality with age until early old age (House et al., 1994). The hypothesis argues relatively weak or diminishing inequalities in older ages, while not denying the existence of inequality in older ages. For the age-related mechanisms to level the SES-based health disparity, House and colleagues suggested universal biological frailty in older ages and government assistance to the elderly such as Social Security and Medicare.
The present study examines the life-course disparity pattern between Black and White adults, testing the cumulative disadvantage hypothesis versus age-as-leveler hypothesis. These two hypotheses can be applied to the race-based health disparity pattern over adulthood because race is very closely related to SES in the US (Hummer, 1996; Williams & Collins, 1995). Black adults have lower SES than White adults, and the SES-based health pattern can be reflected in the race-based pattern over adulthood. White adults who have higher SES might gain a cumulative advantage in resources or health behaviors with age, generating health divergence between White and Black adults. This increasing racial disparity in health might be diminished in older adults because Social Security and Medicare may reduce the health disparity based on economic disparity, supporting the age-as-leveler hypothesis.
The present study intends to confirm the life-course pattern of health disparity between Black and White adults and examines the essential roles of SES factors including income, liquid assets, and educational attainment in the pattern. If the health divergence between Black and White adults disappears or substantially weakens with adjustment of certain SES factors, it would indicate that SES contributes to the divergence. If later-life convergence between Black and White adults disappears or substantially weakens with adjustment of certain SES factors, it would indicate that the SES contributes to generating the convergence.
Racial Disparity in Health over Adulthood
The few studies that have examined the racial disparity in health over the adult life course tend to support the cumulative disadvantage hypothesis. Focusing on the outcome of hospitalization, Ferraro et al. (2006) found amplified racial differences in the risk of death in the hospital in later life, supporting the cumulative disadvantage hypothesis. However, these results are of limited generalizability because they pertain only to people who had been hospitalized. Other analyses of the outcome of self-related health have reported similar findings. One study found a racial divergence over time of 18 years in an analysis that combined all adult age groups into one analysis pool, although this study did not focus on the oldest age groups and therefore did not test the age-as-leveler hypothesis (Shuey & Willson, 2008). A second study did consider differences in self-rated health by age group and found a consistent divergence throughout the adult life course (Ferraro & Farmer, 1996).
Analyses of the racial disparity in functional health in later life have produced mixed results. Functional health such as physical impartment is an important health outcome for racial health disparity research because it reflects the racial differences in quality of life and the risk of mortality (Verbrugge & Jette, 1994). Functional health is becoming more important in aging societies where life-expectancy is increasing but active life-expectancy is not guaranteed, especially for disadvantaged groups such as racial minorities (Verbrugge & Jette, 1994). Using longitudinal approaches, some studies indicate a growing disparity in functional health across older Black and White adults (Kelly-Moore & Ferraro, 2004), and other studies found a constant (Clark & Maddox, 1992) or diminishing gap over time among older adults (Clark, Maddox, & Steinhauser, 1993; Johnson, 2000).
These mixed results underscore the need for an analysis that both has the strengths of existing work and can avoid the limitations and potential biases that may have affected the substantive conclusions of previous analyses. Four characteristics are key to such a study. First, the data for analysis should be longitudinal because the “cumulative disadvantage” and “age-as-leveler” hypotheses refer to the aging process and it is difficult if not impossible to differentiate aging effects from cohort effects without panel data (Riley, 1987). Second, it is important for the data to include a wide range of adults from early to late adulthood for adequate consideration of the age-as-leveler hypothesis, which predicts widening disparities in young and middle adult ages and narrowing disparities among the oldest old.
An analysis that would extend existing work and make unique contributions would have two additional characteristics. Such an analysis would consider the methodological issue of mortality selection bias, which can confound interpretation of aging effects. Mortality selection bias comes in two forms. “Sample selection bias” affects the baseline sample and stems from higher morality rates among the disadvantaged (Herd, 2006). People in the poorest health, disproportionately Black adults, are more likely to have died and are therefore unable to participate in a survey. This selection bias would artificially favor the age-as-leveler hypothesis because vulnerable Black adults tend to die before reaching old age and older Black survivors in the survey sample would be robust in comparison to their White counterparts (Gibson, 1994).
This issue is difficult to address methodologically, but can be addressed by group comparisons, specifically across sex. Because Black men have a higher mortality rate than Black women (Guralnik, Land, Blazer, Fillenbaum, & Branch, 1993; Hayward & Heron, 1999), it would be expected that insofar as sample selection bias influences the results, the later-life convergence should be stronger for men than it is for women. A finding that the convergence occurs only for women cannot be explained by sample selection bias.
The other type of mortality selection bias is specific to a longitudinal study. It can be called “sample attrition bias” due to mortality. If sample attrition is substantial in panel data, health changes among the disadvantaged may be underestimated due to their higher rates of mortality over time (Farmer & Ferraro, 2005; Lynch, 2003). Most previous longitudinal studies on racial disparities in health have not taken this possibility of sample attrition bias into account (for an exception see Kelly-Moore & Ferraro, 2004). If a longitudinal study on health does not have severe sample attrition, it is possible to solve the problem of this sample attrition bias. In this study we use panel data (the Americans’ Changing Lives survey, 1986-1994) based on a national probability sample of adults, and employ a latent growth-curve modeling approach that can reduce sample attrition bias with a missing data imputation method; we then examine the possibility that such bias may remain in our analyses.
A final characteristic for a study to extend this field into new territory is a more thorough consideration of the role of SES and a more complete measure of it. Several previous studies have examined the interaction between race and SES to explain the racial health disparity over the adult life course (Farmer & Ferraro, 2005; Shuey & Willson, 2008), but none have examined the mediating role of SES in the life-course disparity pattern. Many studies have found a substantial role of SES in racial disparities in health although they did not focus on the life-course pattern. Emphasizing the genetic differences between Black and White people in order to explain the health disparity incurs the risk of blaming the victims (Hummer, 1996). According to Hayward and colleagues (Hayward, Crimmins, Miles, & Yang, 2000), SES, not genetic differences, were the primary sources of racial disparity in chronic health. In particular, education as an early life-course resource played an important role in the racial disparity among adults. In other studies specific to functional health, the racial health disparity among the older adults has been explained by SES (Kelly-Moore & Ferraro, 2004; Liao, McGee, Cao, & Cooper, 1999). Although SES is an important structural mechanism to link race to health, it may not completely explain the racial disparity in health (Schoenbaum & Waidmann, 1997).
Ideally a better measure of SES in analyses of racial disparities in health over the life course would include analysis of educational attainment, income, and liquid assets, and not rely on single indicators. Although educational attainment and income have been examined as essential measures of SES in previous health studies, financial assets have been suggested as another potentially important indicator of SES (Williams & Collins, 1995). Financial assets may play a more important role in health in later life when the differences in assets between individuals become more pronounced. In addition, financial assets may play an important role in racial health disparity because racial disparity in financial assets is much greater than racial disparities in income or education (Conley, 1999). Our study takes into account liquid assets, education and income to examine the role of SES in the racial disparity in functional health through adulthood. Finally, changes or dynamics of financial resources may affect health changes over time (Willson & Shuey, 2007). However, existing longitudinal studies that examined the role of SES in the racial disparity have not taken into account the changes in financial resources over time.
The present study brings together all these four characteristics in one analysis to test the cumulative disadvantage vs. age-as-leveler hypotheses. In sum, this study examines the life-course disparity between Black and White adults in functional health, an important health outcome that has not yet been examined. To do so, this study (a) uses longitudinal data that includes adults from early to late adulthood, (b) takes into account mortality selection bias, and (c) considers the mediating role of SES, which is measured with multiple indicators.
Research Questions
In this study, we examine patterns of racial gaps in functional health across all adult life stages. We answer three specific questions: (1) Does functional health diverge over the follow-up period between Black and White adults for all age groups, supporting the hypothesis of cumulative disadvantage (e.g., an increasing racial disparity over time)? Or, does functional health diverge for young- and middle-aged adults but converge in later life, supporting the age-as-leveler hypothesis (e.g., a decreasing racial disparity over time in older ages)? (2) Does potential mortality selection bias substantially influence the results of this study regarding age variation in racial disparity in functional health? (3) Is the life-course disparity pattern (e.g., divergence or convergence) in functional health between Black and White adults explained by the racial differences in educational attainment, income, and liquid assets?
METHODS
Sample
Data are from the Americans’ Changing Lives (ACL) survey, a longitudinal data set
based on a nationally representative sample of non-institutionalized adults aged 24 to 96 in 1986. Sampling, interviewing, and coding for the surveys were conducted by the Survey Research Center of the University of Michigan. Information was obtained through face-to-face interviews with each respondent or a proxy respondent. The overall response rate at baseline was 68%. The initial multistage stratified area probability sample contained 3,617 adults with 100 percent oversamples of Black and those over the age of 60. As recommended by the ACL study team, all analyses are adjusted by the final centered post-stratification weight, which takes into account non-response as well as the sample design. The weighted sample maintains the original sample size and corresponds to the July 1986 Bureau of the Census population estimates by sex, age, and geographic region.
In this study, we restrict the sample to (self-identified non-Hispanic) Black and White adults (3,497 cases). The number of cases for other minorities is only 120 in the unweighted sample (Hispanic n=43, American Indian n=47, and Asian n=30) and seems too small to represent each minority group. This study uses three waves of data collected in 1986, 1989, and 1994. The three waves of ACL data can be classified into four exclusive groups by follow-up status. The first group is composed of the 2,285 respondents (65.3 percent) who participated in all three waves. The second group represents 495 respondents (14.2 percent) who participated in the first and the second surveys. The third group is composed of 206 respondents (5.9 percent) who participated in the first and the third surveys, and the fourth group represents 511 respondents (14.6 percent) who participated in the first wave only. Using a missing data imputation method, the latent growth model (LGM hereafter) estimated in this study contains all four groups of Black and White adults.
Sample Attrition Treatments
This study uses an effective correction method for sample attrition, multiple model-based imputation (MMBI hereafter) using Expectation Maximization (EM hereafter) algorithms (Little & Rubin, 2002). The MMBI-EM method, which is implemented in EQS version 6.1 and utilizes individual raw data (Bentler, 2003), assumes that the absence of values is the result of a combination of tendencies predictable from the observed values in the model as well as random chance (Collins, Schafer, & Kam, 2001).
Latent growth-curve modeling with the MMBI-EM method reduces potential sample attrition bias in two ways. First, the model includes the dropouts to predict their health trajectories not excluding them. If they were excluded in the model (i.e., to use only complete cases with listwise deletion method), the Black-White difference in health change might be underestimated because the health change among Black persons might appear better because of the attrition bias. Therefore, growth-curve modeling in which the dropouts are retained may reduce potential sample attrition bias to a certain degree. Second, the MMBI-EM method has the potential to additionally reduce sample attrition bias because it utilizes the tendencies predictable from the observed values to correct for attrition.
If the attrition rate is reasonably low as it is in this sample, then latent growth modeling with the MMBI-EM method can be effective to correct any attrition bias. Nevertheless, the possibility of sample attrition bias, which would result in underestimation of the diverging racial gaps in functional health, can not be completely ruled out (Lynch, 2003). To assess the influence of sample attrition due to mortality on the results, additional analyses were conducted. The ACL data include a variable that identifies the dropouts due to death. The indicator for mortality was added to the constant and change equations of LGM in this study. Controlling for attrition due to death did not make any substantial difference in the main results (analyses available on request).
Measures
Physical impairment measures
Two functional health measures are examined as indicators of physical impairment. The multi-indicator LGM uses each measure as a subscale for a latent factor of physical impairment to reduce measurement errors. The first subscale is functional impairment (F): (4) Most severe level = respondents who are currently confined to bed or a chair or who have a lot of difficulty bathing or cannot bathe, (3) Moderately severe = respondents who have a lot of difficulty climbing stairs or cannot climb stairs or have a lot of difficulty walking or cannot walk but are not in the previously defined level, (2) Least severe level = respondents who have a lot of difficulty doing heavy housework or cannot do heavy housework but who are not in the two previously defined levels, (1) No functional impairment = respondents answered no to all of the functional impairment questions. For the second subscale, activity limitation (L), respondents were asked to evaluate how much their daily activities are limited in any way by their health or health-related problems: (5) a great deal, (4) quite a bit, (3) some, (2) a little, or (1) not at all. Cronbach’s alpha for these two subscales is .798. The two subscales have skewed distributions. In the case of data that are not normally distributed, EQS 6.1 provides an option that corrects the chi-square and standard errors for nonnormality in its maximum likelihood estimation (Bentler, 2003). All latent-growth models in this study utilize this correction method.
Race, gender, and age variables
Black adults are coded 1 if the respondent self-identified as Black (non-Hispanic) adults and 0 for White (non-Hispanic) adults. Female is an indicator variable coded 1 for females and 0 for males. Age term is modeled as (Age-45)/10 and age-squared term is modeled as (Age2-45)/1000. Because the relationship between age and functional health may be curvilinear, age-squared terms will be assessed in relevant models. The centering method is conducted to reduce multicollinearity between the age terms and the interaction terms of age and race.
SES measures
Education is represented by the highest year of formal schooling completed through 1986. Family income, which is total household annual income before taxes in 1986. The ACL team imputed values for 311 missing cases and recommended using the imputed income variable at baseline for most purposes. To examine income dynamics, the change in income between baseline and wave 3 was calculated (income at wave 3 minus income at baseline). The income change variable is again coded into three dummy variables representing respondents in the highest third (income increased more than $16,555), the lowest third (income change is less than $2,225), respondents with missing values in income change (759 cases; 21.7%), with the middle third (income change is between $2,225 and $16,555) serving as the reference group. Liquid assets were measured with seven categories: (1) less than $10,000, (2) $10,000-$19,999, (3) $20,000-$49,999, (4) $50,000-$99,999, (5) $100,000-$199,999, (6) $200,000-$499,999, or (7) $500,000 or more. Respondents were asked, “Suppose you needed money quickly, and you cashed in all of your (and your spouse's) checking and savings accounts, and any stocks and bonds, and real estate (other than your principal home). If you added up what you got, about how much would this amount to?” This variable of liquid assets at baseline has 210 missing cases, and the MMBI-EM method is used for the missing cases. Taking into account the variable’s distribution, the asset variable is coded into two dummy variables representing respondents in categories 4, 5, 6, and 7 (high assets: 23.9%), categories 2 and 3 (middle assets: 29.1%), with category 1 (low assets: 47.0%) serving as the reference group. To examine asset dynamics, the change in assets between baseline and wave 3 was calculated. The asset change variable is again coded into three dummy variables representing respondents who experienced rising assets (37.9%), falling assets (10.1%), respondents with missing values in asset change (27.5%), with no change in assets (24.5%) serving as the reference group.
Analytic Model
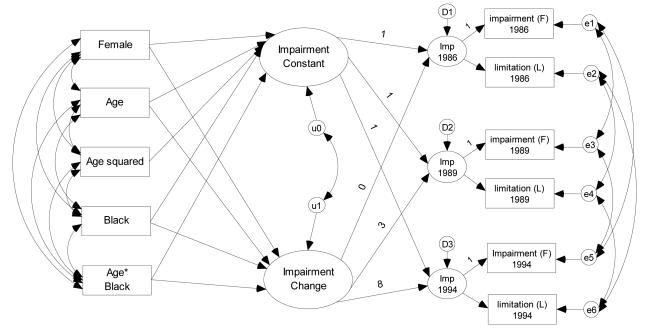
The LGM in this study consists of three sets of equations: a within-person equation, between-person structural equations, and measurement equations (Mirowsky & Ross, 2007). Figure 1 illustrates the model. The factor loading to the functional impairment (F) measure in each year is fixed to 1, which sets the metric of the latent factor to that of the functional impairment measure. Certain constraints were imposed to the model in order to generate a more parsimonious model. I set the factor loadings to activity limitation (L) and intercepts of activity limitation to have equal values over time and also set correlations of measurement errors to have equal values over time.
Figure 1.
Latent Growth-Curve Model for Physical Impairment over Eight Years by Race.
Note: This multi-indicator latent growth model allows correlation of measurement errors across time. The “Imp” in the latent factor of each year denotes a physical impairment level at each year. D1, D2, and D3 represent residuals of the latent factors. The “F” in the indicator box denotes the functional impairment measure, and the “L” in the indicator box denotes the activity limitation measure.
As illustrated in Figure 1, this model allows for a residual correlation between the change in health over time and the constant over time. It helps correct for floor or ceiling effects- that is, when high scores can decline more than low scores, and vice versa (Mirowsky & Kim, 2007). In order to assess whether the racial gap in health diverges over the follow-up period in all 1-year age groups at baseline, we focus on the effects of race on health change. For example, if the difference in change of physical impairment between White and Black adults has positive values in all age groups, it suggests health divergence over time in all age groups. In contrast, if the White/Black difference in change of physical impairment has negative values for certain age groups, it suggests health convergence over time for the age groups.
RESULTS
Racial Disparity in Physical Functioning and Mortality Selection Bias
Table 1 presents the differences in functional health at baseline and SES between racial groups for the weighted sample. Functional impairment and activity limitation in Black adults is higher than in White adults, but the differences are not statistically significant. As expected, White adults have the higher SES than Black adults. The Black-White difference in years of schooling is about 1.16. Family income of White adults is 1.42 times higher (about $8,690) than that of Black adults.
Table 1.
Descriptive Statistics at Baseline (1986) for Focal Variables across Race Groups
| Functional Impairment |
Activity Limitation |
Years of Schooling |
Family Income a |
Liquid Assets b |
|
|---|---|---|---|---|---|
| White Adults | 1.275 | 1.604 | 12.549*** | 2.961*** | 2.463*** |
| (n=2,323) | (.709) | (1.098) | (2.947) | (2.044) | (1.665) |
| Black Adults | 1.308 | 1.617 | 11.385 | 2.092 | 1.620 |
| (n=1,174) | (.757) | (1.156) | (3.436) | (1.753) | (1.134) |
| Total Sample | 1.279 | 1.605 | 12.414 | 2.860 | 2.366 |
| (N=3,497) | (.715) | (1.105) | (3.031) | (2.031) | (1.635) |
Note: Numbers in columns are means with standard deviations in parentheses (for the weighted sample); The provided number of cases (n) is for the unweighted sample.
Unit: $ 10,000.
This is an ordinal variable with 7 categories – the values ranged 1 to 7.
indicates that the means for Whites and Blacks are significantly different at p < .001.
Model 1 of Table 2 presents physical impairment trajectories (constant at baseline and change over time) predicted by race, age, and their interaction. In the prediction of health at baseline, Black adults have higher physical impairment than White adults for the group aged 45 at baseline (.049 in Column 1), and the gap is larger among participants at higher baseline ages (the gap in each age is .049+.034(Age-45)/10 in Column 1). Although this cross-sectional age pattern based on the constant equation appears to support the hypothesis of cumulative disadvantage, we cannot exclude the possibility that cohort effects are confounded with age effects, which could result if older cohorts had greater levels of racial disparities (Riley, 1987). According to Wilson (1978), racial inequality (e.g., in wage or occupational status) is generally greater in older cohorts. If this cohort attribution generated the greater health gap in older adults, it should be distinguished from cumulative disadvantage in aging.
Table 2.
Physical Impairment Constant and Change Regressed on Race and SES: Multi-Indicator Latent Growth Models with Missing Data Imputed by Expectation Maximization (Metric Coefficients with Robust Standard Errors for Non-Normal Data in Parentheses.)
| Model 1 a | Model 2 b | Model 3 c | Model 4 d | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | Constant | Change | Constant | Change | Constant | Change | Constant | Change |
| Female | .062*
(.025) |
−.001 (.003) |
.053*
(.024) |
−.002 (.003) |
.037 (.024) |
−.001 (.003) |
.038 (.024) |
−.002 (.003) |
| Age | .114***
(.009) |
.008***
(.001) |
.097***
(.009) |
.007***
(.001) |
.103***
(.009) |
.005***
(.001) |
.115***
(.010) |
.006***
(.001) |
| Age2 | .309***
(.045) |
.287***
(.044) |
.185***
(.046) |
.187***
(.046) |
||||
| Black Adults | .049*
(.023) |
.018***
(.003) |
.012 (.024) |
.016***
(.003) |
−.018 (.024) |
.014***
(.003) |
−.032 (.024) |
.013***
(.003) |
| Age × Black | .034*
(.015) |
−.004*
(.002) |
.016 (.014) |
−.005**
(.002) |
.019 (.014) |
−.004*
(.002) |
.017 (.014) |
−.005**
(.002) |
| Years of Schooling |
−.030***
(.004) |
−.002***
(.001) |
−.018***
(.005) |
−.001**
(.001) |
−.016***
(.005) |
−.001*
(.001) |
||
| Income e | −.043***
(.006) |
−.001 (.001) |
−.032***
(.006) |
.000 (.001) |
||||
| Income Rise f | −.000 (.004) |
.001 (.004) |
||||||
| Income Fall f | .009a
(.004) |
.007†
(.004) |
||||||
| Income | .046*** | .041*** | ||||||
| Missing f | (.003) | (.007) | ||||||
| Assets High g | −.106*
(.041) |
−.011*
(.005) |
||||||
| Assets Middle g | −.141***
(.028) |
.003 (.004) |
||||||
| Assets Rise h | −.006†
(.004) |
|||||||
| Assets Fall h | .013*
(.006) |
|||||||
| Change in Assets Missing h |
−.001 (.007) |
|||||||
| Intercept | 1.103***
(.024) |
.018***
(.003) |
1.499***
(.064) |
.045***
(.008) |
1.504***
(.062) |
.036***
(.008) |
1.508***
(.063) |
.036***
(.009) |
| Residual Variance |
.231***
(.016) |
.001**
(.000) |
.220***
(.016) |
.001**
(.000) |
.204***
(.015) |
.001**
(.000) |
.204***
(.015) |
.001**
(.000) |
| Residual Correlation |
−.275** | −.312*** | −.333*** | −.342*** | ||||
| R2 | .254 | .117 | .277 | .134 | .293 | .343 | .304 | .323 |
Note: Age is modeled as (Age-45)10−1, and Age2 is modeled as (Age-45)210−3; N= 3,497
Fit indexes: Yuan-Bentler scaled χ2=123.58, df=28, p < .001; CFI=.992, RMSEA=.024.
Fit indexes: Yuan-Bentler scaled χ2=134.40, df=32, p < .001; CFI=.992, RMSEA=.023.
Fit indexes: Yuan-Bentler scaled χ2=229.80, df=51, p < .001; CFI=.990, RMSEA=.032.
Fit indexes: Yuan-Bentler scaled χ2=280.19, df=74, p < .001; CFI=.994, RMSEA=.023.
(family income)×10−4.
Compared to stable income change.
Compared to low assets.
Compared to no asset change.
p < .10;
p < .05;
p < .01;
p < .001 (2-tailed tests)
In order to confirm the cumulative disadvantage in aging, health change over time was predicted by race, age, and their interaction (Model 1). Black adults also have a worse change (i.e., within-person change rate per year or slope) in physical impairment than White adults for the group of age 45 at baseline (.018 in Column 2), and the difference in health change between Black and White adults is smaller among participants at higher baseline ages (the slope difference in each age is .018-.004(Age-45)/10 in Column 2 of Table 2). If the difference in health change has a positive value, it implies that health change of Black adults is worse (higher slope or steeper increase in physical impairment) than that of White adults. For the group of age 45 at baseline, Black adults have .018 higher slope in physical impairment than White adults, and the difference is significant at .05 level, implying a diverging Black-White gap over time for the age group. Net of physical impairment at baseline, Black adults experience additional disadvantage over the follow-up time for the group aged 45 at baseline.
However, the effect of age-race interaction on health change is also significant (−.004), implying that the magnitude of divergence or additional disadvantage diminishes among participants at higher baseline ages. The critical age at which the divergence disappears or the slope difference becomes zero is 90 (calculated from .018-.004(Age-45)/10 = 0). After age 90, Black adults exhibit a better health trajectory (i.e., lower within-person change in physical impairment) than White adults, and we call this pattern “convergence” in our study because the initial racial gap in health at baseline converges over the followup time for the oldest age groups. The health divergence between Black and White adults shifts to convergence for the age groups older than 90. If this convergence in later life does not result from mortality selection bias, the significant effect of the age-race interaction toward convergence supports the age-as-leveler hypothesis.
Two types of mortality selection bias need to be taken into account. As mentioned in the Method section, we found that “sample attrition bias” due to mortality is unlikely to distort the main findings such as later-life convergence in our study. For “sample selection bias” due to mortality, we examine the gender difference in the life-course pattern because Black men are known to have a higher mortality than Black women. If the sample selection bias generates the Black-White convergence in this study, the convergence should be more salient in men than in women. The same model with Model 1 of Table 2 was estimated in each gender group and the results are presented in Appendix A. The results demonstrate no significant difference in the constant of physical impairment between Black and White men and significantly higher constant of physical impairment in Black women than White women. It suggests the possibility of sample selection bias in this baseline sample because Black men are unexpectedly robust.
However, this study’s main interest is in the results of health change, not those of health constant. Among the men, Blacks have a consistently worse change in functional health than Whites for all age groups, and no convergence occurs in older age groups, as indicated by the significant coefficient of Black and the non-significant coefficient of Age×Black . Among the women, Blacks have worse change in functional health than Whites until early old age, but the diverging pattern switches to convergence in older age groups. Therefore, the health convergence in later life between Black and White adults can be attributed to the convergence between Black and White women, suggesting that the convergence is unlikely to be the result of sample selection bias. Therefore, the convergence in later life appears related to age rather than to the two types of mortality selection bias. The convergence between Black and White adults in older ages appears related to age-leveling mechanisms that are specific to women.
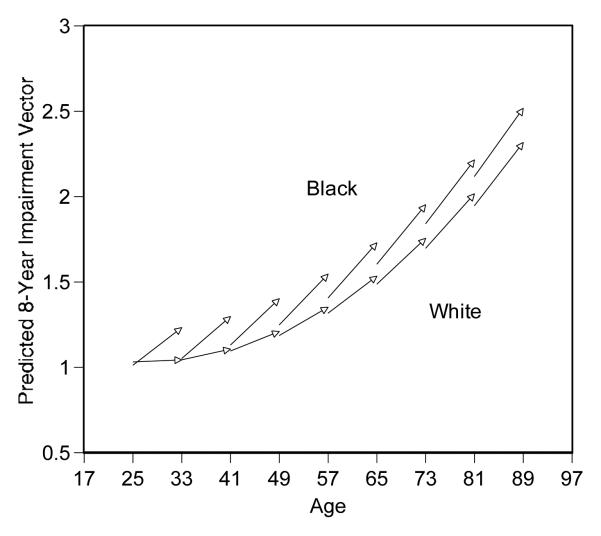
The graph in Figure 2 provides summaries of the Model 1 results presented in Table 2. Each arrow (vector) represents the predicted origin and change in physical impairment for a 1-year age group or birth cohort. The horizontal axis indicates the cohort’s age at the beginning and end of the survey period. The vertical axis indicates the cohort’s predicted health at the beginning and end of the survey period. The mean value (0.53) of the variable Female is used to generate predictions of the vectors, averaging the gender differences. If 1 is inserted in the Female variable, the predictions represent female’s pattern. If 0 is inserted in the Female variable, the predictions represent male’s pattern. If the mean value (0.53) is inserted in the Female variable, the predictions represent a pattern averaging the gender differences. To simplify the figure, vectors are shown for every eighth age group (Mirowsky and Kim 2007).
Figure 2.
Predicted 8-Year Aging Vectors of Physical Impairment by Race
Note: the vertical axis represents predicted physical impairment; the values range 1 to 4.
A series of vectors in the lower portion illustrates the health trajectories of White adults, and the vectors in the upper portion illustrate those of Black adults. Across most age groups or life stages, we can observe diverging health gaps by race over the 8-year follow-up period, but the diverging gap or the amount of additional disadvantage diminishes in older ages, providing cautious support for the age-as-leveler hypothesis.
The Roles of SES in the Racial Disparity in Functional Health
The racial disparity in functional health may be explained by racial differences in SES. As presented in Table 1, Black adults have lower levels of education, income, and liquid assets than White adults. The lower SES of Black adults might result in additional disadvantage in health over time net of health at baseline. Model 2 of Table 2 examines how much the effect of race on physical impairment is explained by differences in education at baseline. We add a variable, years of schooling, to Model 1 of Table 2. The effects of education on both baseline level and change of functional health are significant. As suggested in the comparison between the coefficients of Model 1 and Model 2, the Black-White difference in health at baseline is substantially reduced by adjusting for educational attainment. With adjustment for educational attainment, the Black-White difference in functional health at baseline becomes non-significant in young and middle adulthood. However, the Black-White difference in the rate of health change reduces only moderately after controlling education – the original Black-White slope difference at age 45 is .018 in the Model 1, and it changes into .016 in the Model 2. Net of health at baseline, additional disadvantage of Black adults in functional health over time is moderately explained by the Black-White difference in education.
Model 3 of Table 2 examines the role of family income in the racial disparity in functional health. When we add income at baseline and income change dummy variables to Model 3, the Black-White difference in functional health change is additionally reduced – the Black-White slope difference at age 45 is .016 in the Model 2, and it changes into .014 in the Model 3. The income change variables were modeled to predict only the health change not the health constant because it is causally illogical for the income change over the follow-up period to predict health at baseline. Among the income change variables, falling income variable is significant, implying that those who experienced falling income over the survey period have worse change in functional health than those who experienced relatively stable change in income. Although we cannot exclude the possibility of reverse causality between income change and health change, the income at baseline and income changes over time (particularly, falling income) may partially explain the Black-White difference in functional health change.
When we add baseline asset variables and asset change dummy variables to Model 4, the Black-White difference in functional health change is additionally reduced; for the group aged 45 at baseline, the Black-White slope difference substantially declined (from .016 to .013) with adjusting for income and liquid assets. Net of health at baseline and educational attainment, additional disadvantage of Black adults in functional health over time may be partially explained by the Black-White difference in economic status. In Model 4, a full model including all SES variables, several asset variables have significant effects. Particularly, net of asset change, those who had relatively high assets (more than $50,000) at baseline show better change in functional health (−.011) than those with low assets at baseline (less than $10,000). This result suggests the possibility of the existence of causal order from liquid assets to functional health because it satisfies the condition of temporal precedence. Among the asset change variables, falling assets has a significant effect on functional health change, indicating that those who lost assets over the survey period have more deterioration in functional health than those who experienced no substantial change in assets. Asset change appeared to be more important than income change in functional health change and in explaining the Black-White difference in functional health change, as demonstrated in the significance results of Model 4.
After controlling for education, income, and liquid assets, the Black-White difference in health change is substantially reduced – for the group aged 45 at baseline, the Black-White slope difference declined from .018 to .013. These results indicate that SES substantially explains the racial divergence. However, the additional disadvantage among Black adults still exists in young and middle adulthood net of education and economic status as demonstrated in the significant slope difference at age 45 (.013 in Model 4). After controlling for SES, the critical age at which the health divergence disappears changes to 71 (calculated from .013−.005(Age-45)/10 = 0) from 90 in the original model. The additional disadvantage of Black adults in functional health disappears after early-old ages with adjustment of SES, but additional disadvantage of Black adults still exists until early-old ages.
Finally SES factors do not explain the converging pattern between Black and White adults in older ages. In Model 2, 3, and 4, the negative age interaction on health change, which indicates the convergence, is still significant and does not disappear or weaken. These results indicate that SES does not explain the racial convergence.
DISCUSSION
The present study sets out to specify the life course pattern of racial disparity in functional health, taking into account all adult age groups. The focus of the analysis was whether growing racial differences in functional health reversed course with age and began to converge among the oldest old – the age-as-leveler hypothesis, or whether they diverged throughout the life course unabated – the cumulative disadvantage hypothesis. Strengths of this study include analysis of a representative, longitudinal panel study of adults who spanned a wide range of ages, a detailed consideration of potential mortality selection biases, and analysis of socioeconomic status.
Overall, the results support the age-as-leveler hypothesis. Net of physical impairment at baseline, the growing disadvantage that Black adults experienced slowed and then even reversed, resulting in convergence of functional health among the oldest old. These results run counter to the predominant support for the cumulative disadvantage hypothesis, which is based on analysis of other health outcomes such as hospitalization and self-rated health in several robust studies (Ferraro & Farmer, 1996; Ferraro et al., 2006). The study results therefore provide some evidence that age-as-leveler and cumulative disadvantage processes may vary by outcome. Further, they suggest the need for more theoretical development in this area to explain why these processes are outcome-specific.
Ruling out Methodological Artifacts
Support for the age-as-leveler hypothesis cannot readily be explained away as a methodological artifact. Cohort variation (i.e., a weak effect of race on health in older cohorts) is not a plausible explanation for the convergence because racial discrimination and inequalities in the labor market were generally greater in older cohorts (Wilson, 1978). Further, older cohorts have experienced racial discrimination and inequalities in economic opportunities for a longer time period than younger cohorts. To the extent that racial disparities result solely from racial discrimination they would be expected to grow unabated over the life span, a finding not supported in this analysis.
Most previous longitudinal studies that examined race-based disparity in functional health have focused on only older age groups and have not taken into account methodological issues such as two forms of mortality selection bias – sample attrition bias and sample selection bias. Support for the age-as-leveler hypothesis in this study is robust and remains strong after taking into account sample attrition bias due to mortality. Specifically, a higher mortality rate for disadvantaged groups during the course of the study may leave behind only the healthiest members, artificially lending support to the age-as-leveler hypothesis. To take this into account we used growth-curve modeling, and found that mortality attrition bias had little to no effect on the main results of this study.
Another factor that could affect the results in favor of the age-as-leveler hypothesis is sample selection bias. In brief, higher mortality rates among disadvantaged groups may have stacked the deck from the outset of the study in favor of the age-as-leveler hypothesis, if only the healthiest people in the disadvantaged group survived to participate in the baseline survey. While there is no elegant methodological way to address this bias, it is nevertheless still possible to evaluate it. Specifically, if this mortality selection bias is present, then its influence should be strongest among Black men, whose mortality rate is higher than that of Black women. However, the study results are opposite this expectation – the later-life convergence occurred only among women and not men – which makes the study findings difficult to explain as an artifact of sample selection bias.
The Role of Socioeconomic Status
SES provides only a partial explanation for the age-as-leveler pattern documented in this study. In brief, the results indicate that different levels of SES across racial groups explain in large part why functional health diverges across race in young and middle adult ages, but SES does not explain the convergence in later life. It was the uniquely detailed measurement and analysis of SES components in this study that allowed the analysis to specify a role for SES in the divergence of functional health across race in the earlier stages of adulthood. Specifically, levels and changes in liquid assets were important in the increasing disparity in functional health between Black and White adults. As the liquid assets of Black adults became increasingly disadvantaged in comparison to White adults, so too did their functional health.
Liquid assets and other SES components, however, fell short as an explanation for the study results in two ways. First, SES did not completely explain the worse change in the functional health of Black adults. Even after controlling a wide array of SES components, Black adults continued to have a worse change in health than White adults, suggesting that other factors are also at play.
One potentially important factor is the influence of racial discrimination on health independent of its effect on SES (Williams & Collins, 1995). For example, accessibility and quality of medical care is an important factor to affect physical impairment, and racial and ethnic minorities tended to receive a lower quality medical care even adjusting for income (Smedley, Stith, & Nelson, 2003),. Stereotyping and discrimination of healthcare providers contributes to the unequal treatment.
Second, the convergence of functional health across racial groups was not readily explained by SES, again suggesting that differential life-course pattern in health across racial groups is more than a reflection of differential SES. According to the results in this study, the leveling mechanisms of age may be specific to women because the later-life convergence between racial groups occurs only among women. Among men, the Black/White disparity increases over time with a constant rate for all the age groups and no convergence occurs in older age groups. We found this gender difference in the effect of race on change in functional health. We focus on this longitudinal pattern not the cross-sectional pattern in the gender difference because cohort effect and sample selection bias are confounded in the cross-sectional pattern. Universal biological frailty in older ages and Medicare, which previous studies have suggested as leveling mechanisms of age (House et al., 1994), may not explain the convergence between Black and White adults because the mechanisms are not gender-specific.
As a potential explanation for the health convergence between Black and White older adults, Gibson (1994) suggested that the effects of social relations and health behaviors on health might differ by age and race. If certain health behaviors or psychosocial factors have a more beneficial effect on functional health of Black female elders compared to White female elders, it may explain the health convergence between Black and White female elders, found in this study. In order to explain the gender difference, the effects of the health behaviors or psychosocial factors on functional health should be similar between Black and White male elders. However, few previous studies examined this possibility (Dupre, 2006).
Additional difficulty in exploring the potential factors to explain the convergence is that it may be specific to physical impairment not other health outcomes such as self-rated health or death in hospital. Therefore, it is particularly important to supplement the broad, general theories of cumulative disadvantage and age-as-leveler – which provide few expectations for specific outcomes and instead treat health as a monolithic concept – with insights on particular outcomes or types of outcomes. These insights could potentially come from many sources, including qualitative studies or the extension of theories and works in other areas to the specific topic of disparities over the life course. These insights will likely show that different health outcomes are strategic for analyses of different social processes. This theoretical development is key for future work in this area to be strategic and guided.
The study has limitations. One is that we cannot exclude the possibility of reverse causality – physical impairment might affect economic status through the loss of paid employment and medical costs. Second, although we have used more comprehensive measures of SES than prior studies, certain limitations may exist in measuring SES. One is that the respondents might not accurately report their liquid assets either intentionally or unintentionally, likely underestimating their assets. The other is that the same objective level of SES may have different meanings across racial/ethnic groups. For example, a high school education from a lousy, intercity school most likely means much less than a high school education from a prestigious, private school. Similarly, $100 buys you less in an inner-city supermarket than it does in a supermarket in a safe neighborhood. Thus, the fact that we find a health disparity even controlling for SES may result, in part, from the fact that the same measure of SES indicates fewer resources for people in disadvantaged racial/ethnic groups. If true, SES is still the main cause of the disparity but measurement issues prevent us from capturing it completely.
Third, we found the possibility that sample selection bias could be operating in our cross-sectional (i.e., health constant) results because Black men are unexpectedly robust in the baseline sample, as demonstrated in Appendix A. The potential bias likely generates the underestimation of the Black/White health disparity. As another limitation, we excluded other racial minorities because of their small numbers of cases in the data. The life-course pattern in functional health between White adults and adults in other racial/ethic populations such as Hispanics might be different, and needs to be examined in future research. Finally, we found a strong possibility of a gender interaction in the black/white life-course pattern in functional health but did not examine this issue completely in order to generate a more focused paper. Given that no previous studies examined the gender difference in the black/white life-course pattern in health, examining the gender interaction represents an important direction for future research in this topic.
Conclusion
Consistent with the age-as-leveler hypothesis, the disparity in functional health between Black and White adults increases until early old ages but then reverses, resulting in a convergence among the oldest old. The underlying processes behind this convergence remain to be identified, in large part because current theoretical work in the field cannot yet explain why cumulative disadvantage and age-as-leveler processes are outcome-specific. Such development is necessary to specify the influences at work and, ultimately, to inform policy aimed at the long-term reduction of health disparities.
Appendix A.
Physical Impairment Constant and Change Regressed on Race and its Interaction with Age in Gender Groups: Multi-Indicator Latent Growth Models with Missing Data Imputed by Expectation Maximization (Metric Coefficients with Robust Standard Errors for Non-Normal Data in Parentheses.)
| Model 1a (Male) | Model 2 b (Female) | |||
|---|---|---|---|---|
| Variables | Constant | Change | Constant | Change |
| Age | .114***
(.014) |
.007***
(.001) |
.112***
(.010) |
.008***
(.001) |
| Age2 | .213**
(.065) |
.361***
(.058) |
||
| Black | −.050†
(.030) |
.024***
(.004) |
.120***
(.034) |
.015**
(.005) |
| Age × Black | −.002 (.020) |
−.001 (.003) |
.052**
(.019) |
−.005* (.003) |
| Intercept | 1.131***
(.028) |
.017***
(.002) |
1.147***
(.025) |
.019***
(.002) |
| Residual Variance | .179***
(.020) |
.001* (.001) |
.262***
(.024) |
.001* (.001) |
| Residual Correlation | −.282* | −.233* | ||
| R2 | .232 | .110 | .264 | .122 |
Note: Age is modeled as (Age-45)10−1, and Age2 is modeled as (Age-45)210−3.
n=1,304; Fit indexes: Yuan-Bentler scaled χ2=55.01, df=24, p < .001; CFI=.995, RMSEA=.017.
n=2,194; Fit indexes: Yuan-Bentler scaled χ2=69.22, df=24, p < .001; CFI=.994, RMSEA=.022
p < .10;
p < .05;
p < .01;
p < .001 (2-tailed tests)
Contributor Information
JinYoung Kim, Department of Sociology, Korea University, Anamdong, Seongbuk-Gu, Seoul 136-701, Republic of Korea.
Richard Miech, Institute for Social Research, University of Michigan, 426 Thompson St., Ann Arbor, MI 48106.
REFERENCES
- Bentler PM. EQS 6 for Windows program manual. Multivariate Software; Los Angeles, CA: 2003. [Google Scholar]
- Clark DO, Maddox GL, Steinhauser K. Race, aging, and functional health. Journal of Aging and Health. 1993;5(4):536–553. [Google Scholar]
- Clark DO, Maddox GL. Racial and social correlates of age-related changes in functioning. Journal of Gerontology: Social Sciences. 1992;47:S222–S232. doi: 10.1093/geronj/47.5.s222. [DOI] [PubMed] [Google Scholar]
- Collins LM, Schafer JL, Kam C-M. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods. 2001;6(4):330–351. [PubMed] [Google Scholar]
- Conley D. Being black, living in the red: Race, wealth, and social policy in America. University of California Press; Berkeley: 1999. [Google Scholar]
- Dannefer D. Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. Journal of Gerontology: Social Sciences. 2003;58B(6):S327–S337. doi: 10.1093/geronb/58.6.s327. [DOI] [PubMed] [Google Scholar]
- Dupre ME, Franzese AT, Parrado EA. Religious attendance and mortality: Implications for the black-white mortality crossover. Demography. 2006;43(1):141–164. doi: 10.1353/dem.2006.0004. [DOI] [PubMed] [Google Scholar]
- Dupre ME. Educational differences in age-related patterns of disease: Reconsidering the cumulative advantage and age-as-leveler hypotheses. Journal of Health and Social Behavior. 2007;48:1–15. doi: 10.1177/002214650704800101. [DOI] [PubMed] [Google Scholar]
- Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status. Social Science & Medicine. 2005;60(1):191–204. doi: 10.1016/j.socscimed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Ferraro KF, Thorpe RJ, Jr., McCabe GP, Kelley-Moore JA, Jiang Z. The color of hospitalization over the adult life course: Cumulative disadvantage in black and white? Journal of Gerontology: Social Sciences. 2006;61B(6):S299–S306. doi: 10.1093/geronb/61.6.s299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro KF, Farmer MM. Double jeopardy to health hypothesis for African Americans: Analysis and critique. Journal of Health and Social Behavior. 1996;37(1):27–43. [PubMed] [Google Scholar]
- Freedman VA, Martin LG, Schoeni RF, Cornman JC. Declines in late-life disability: The role of early- and mid-life factors. Social Science & Medicine. 2008;66:1588–1602. doi: 10.1016/j.socscimed.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson RC. The age-by-race gap in health and mortality in the older population: A social science research agenda. The Gerontologist. 1994;34:454–462. doi: 10.1093/geront/34.4.454. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Land KC, Blazer D, Fillenbaum GG, Branch LG. Educational status and active life expectancy among older Blacks and Whites. The New England Journal of Medicine. 1993;329(2):110–116. doi: 10.1056/NEJM199307083290208. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Crimmins EM, Miles TP, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review. 2000;65(6):910–930. [Google Scholar]
- Hayward MD, Heron M. Racial inequality in active life among adult Americans. Demography. 1999;36(1):77–91. [PubMed] [Google Scholar]
- Herd P. Do functional health inequalities decrease in old age?: educational status and functional decline among the 1931-1941 birth cohort. Research on Aging. 2006;28(3):375–392. [Google Scholar]
- House JS, Lepkowski M, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. Journal of Health and Social Behavior. 1994;35:213–234. [PubMed] [Google Scholar]
- Hummer RA. Black-White differences in health and mortality. Sociological Quarterly. 1996;37:105–125. [Google Scholar]
- Johnson NE. The racial crossover in comorbidity, disability, and mortality. Demography. 2000;37(3):267–283. [PubMed] [Google Scholar]
- Kelley-Moore JA, Ferraro KF. The Black/White disability gap: Persistent inequality in later life? Journal of Gerontology: Social Sciences. 2004;59B(1):S34–S43. doi: 10.1093/geronb/59.1.s34. [DOI] [PubMed] [Google Scholar]
- Kim J, Durden E. Socioeconomic status and age trajectories of health. Social Science & Medicine. 2007;65(12):2489–2502. doi: 10.1016/j.socscimed.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Liao Y, McGee DL, Cao G, Cooper RS. Black-White differences in disability and morbidity in the last years of life. American Journal of Epidemiology. 1999;149(12):1097–1103. doi: 10.1093/oxfordjournals.aje.a009763. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. 2 John Wiley and Sons; New York: 2002. [Google Scholar]
- Lynch SM. Cohort and life-course patterns in the relationship between education and health: A hierarchical approach. Demography. 2003;40(2):309–331. doi: 10.1353/dem.2003.0016. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE. Life course trajectories of perceived control and their relationship to education. American Journal of Sociology. 2007;112(5):1339–1382. [Google Scholar]
- Mirowsky J, Kim J. Graphing age trajectories: Vector graphs, synthetic and virtual cohort projections, and cross-sectional profiles of depression. Sociological Method and Research. 2007;35(4):497–541. [Google Scholar]
- Riley MW. On the significance of age in Sociology. American Sociological Association, 1986 presidential address. American Sociological Review. 1987;52(1):1–14. [Google Scholar]
- Ross CE, Wu C-L. Education, age, and cumulative advantage in health. Journal of Health and Social Behavior. 1996;37:104–120. [PubMed] [Google Scholar]
- Schoenbaum M, Waidmann T. Race, socioeconomic status, and health: Accounting for race differences in health. Journal of Gerontology. 1997;52B:61–73. doi: 10.1093/geronb/52b.special_issue.61. Special Issue. [DOI] [PubMed] [Google Scholar]
- Shuey KM, Willson AE. Cumulative disadvantage and Black-White Disparities in life-course health trajectories. Research on Aging. 2008;30(2):200–225. [Google Scholar]
- Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. National Academies Press; Washington DC: 2003. [PubMed] [Google Scholar]
- Taylor MG. Timing, accumulation, and Black/White disability gap in later life. Research on Aging. 2008;30(2):226–250. [Google Scholar]
- Thorpe RJ, Kasper JD, Szanton SL, Frick KD, Fried LP, Simonsick EM. Relationship of race and poverty to lower extremity function and decline: Findings from the women's health and aging study. Social Science & Medicine. 2008;66:811–821. doi: 10.1016/j.socscimed.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Williams DR, Collins C. U.S. socioeconomic and racial differences in health: Patterns and explanations. Annual Review of Sociology. 1995;21:349–386. [Google Scholar]
- Willson AE, Shuey KM. Cumulative advantage processes as mechanisms of inequality in life course health. American Journal of Sociology. 2007;112(6):1886–1924. [Google Scholar]
- Wilson WJ. The declining significance of race: Black adults and changing American institutions. University of Chicago Press; 1978. [Google Scholar]