Abstract
Alternative graft sources, [umbilical cord blood (UCB), matched unrelated donors (MUD), or mismatched unrelated donors (MMUD)] enable patients without a matched sibling donor to receive potentially curative hematopoietic cell transplantation (HCT). Retrospective studies demonstrate comparable outcomes among different graft sources. However, the risk and types of infections have not been compared among graft sources. Such information may influence the choice of a particular graft source. We compared the incidence of bacterial, viral, and fungal infections in 1,781 adults with acute leukemia who received alternative donor HCT (UCB = 568, MUD = 930, MMUD = 283) between 2008 and 2011. The incidence of bacterial infection at one year was 72%, 59%, and 65% (p<0.0001) for UCB, MUD and MMUD, respectively. Incidence of viral infection at one year was 68%, 45%, 53% (p<0.0001) for UCB, MUD, and MMUD respectively. In multivariable analysis, bacterial, fungal, and viral infections were more common after either UCB or MMUD than MUD, (p<0.0001). Bacterial and viral, but not fungal, infections were more common after UCB than MMUD (p=0.0009 and <0.0001, respectively). The presence of viral infection was not associated with an increased mortality. Overall survival (OS) was comparable among UCB and MMUD patients with Karnofsky performance status (KPS) ≥90%, but was inferior for UCB for patients with KPS < 90%. Bacterial and fungal infections were associated with poorer OS. Future strategies focusing on infection prevention and treatment are indicated to improve HCT outcomes.
Keywords: umbilical cord blood, leukemia, infection
INTRODUCTION
Umbilical cord blood (UCB) is an important hematopoietic cell source for patients without matched related (MRD) or matched unrelated donors (MUD). Several studies have shown comparable survival after hematopoietic cell transplantation (HCT) using either matched (MUD) or mismatched unrelated (MMUD) donors or UCB transplantation (UCBT)1,2,3. In general, engraftment is delayed in UCBT, but the incidence of chronic graft versus host disease (GvHD) is less. To overcome low cell dose in UCBT, multiple investigators have used double UCBT in adults following either myeloablative (MA) or reduced intensity conditioning (RIC) regimens4,5,6,7. Despite these advances, poor immune reconstitution remains a significant problem after UCBT8.
Several studies have reported a high rate of viral infection after UCBT. The incidence of human herpes virus (HHV)-6 infection ranges from 0–10% after MUD and 5 to 21% after UCBT9. High viral infection risk after UCBT is likely related to the delayed immune reconstitution post-transplant10, 11,12,13. Fungal infections remain an important cause of morbidity and mortality after allogeneic HCT, particularly alternative donor HCT. The incidence of invasive fungal infection (IFI) has been reported at 9% after allogeneic HCT; some studies show an increase in fungal infection with matched unrelated donor compared to matched related donor.14,15,16. In a study of 1400 patients in China, mortality was over 30% in patients with proven IFI15. Bacterial infections, especially after UCBT, are associated with high mortality. In 241 patients undergoing single UCBT, the incidence of bloodstream bacterial infection was 52% with a 12% mortality rate17.
The incidence, type, and risk factors for infection have not been formally compared in a large data set among MUD, MMUD, and UCBT. In this study, we seek to compare the incidence and type (fungal, viral, bacterial) of infections among transplant patients with acute leukemia that received UCB, MUD, and MMUD HCT. As infections are a significant cause of morbidity, mortality and resource utilization following HCT, data from this study may enable transplant physicians to better select the optimal donor source and to utilize more effective infection prevention and treatment strategies.
PATIENTS AND METHODS
Transplant registry
Data were obtained from the CIBMTR, a research affiliate of the International Bone Marrow Transplant Registry, Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program (NMDP) established in 2004. It comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute data on consecutive allogeneic and autologous HCT procedures to a statistical center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers report longitudinal data on all transplants and compliance is monitored by on-site audits. Transplant essential data, collected for consented patients participating in CIBMTR data collection, include demographic, disease type and stage, survival, relapse, graft type, the presence of GVHD, and cause of death data. A subset of CIBMTR participants are selected for comprehensive research level data collection by weighted randomization. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule. Studies conducted by the CIBMTR are performed under guidance and review of the Institutional Review Board of the National Marrow Donor Program.
Patients
The study population consists of patients ≥16 years with acute leukemia in first or second complete remission (CR) receiving transplant with a single or double unrelated UCBT, a MUD, or a single antigen/allele MMUD who were reported to the CIBMTR between 2008 and 2011. First HCTs, receiving either myeloablative (MA) or non-myeloablative/reduced intensity conditioning (NMA/RIC) regimens were included. Patients receiving ex-vivo T-cell depletion, CD34 selection, or post-transplant cyclophosphamide were excluded. Due to small sample size, patients receiving haploidentical HCT were excluded.
Infection data
Infections are reported to the CIBMTR using an organism code and site code. There are no data provided to assess infection prophylaxis, treatment, diagnostic criteria utilized by the center, or infection severity. Centers are instructed to report clinically significant infections with both on-line and in-person education regarding appropriate reporting. Data are reviewed by clinicians to assess appropriateness for inclusion in analyses. For yeast infections, sites were limited to lower respiratory infections, blood stream infections, and visceral organ involvement. Other fungal infections were included as reported by the center. Viral data excluded from analysis were suspected or “other virus” infection in the lips, nasopharynx/upper airway, feces, or skin. Bacterial data excluded were suspected bacterial infection in the oral cavity, lips, feces, nasopharynx/upper airway, and skin; H. pylori; vancomycin-resistant Enterococcus in the GI tract not specified, feces, genital area, skin not specified; E. coli or “other bacteria” in the genital tract; coagulase negative Staphylococcus in the oral cavity, nasopharynx/upper airway, GU tract not specified, or skin not specified; Enterococcus spp., Pseudomonas spp., or Streptococcus spp. in feces; and Streptococcus or Corynebacterium (non-diphtheroids) species on the skin.
Outcomes and study definitions
The primary objective of this study was to compare the incidence of bacterial, fungal, and viral infections at 100 days and one-year post transplantation, for alternative donor HCT. To account for multiple infectious episodes occurring in a single patient and adjusted for a period of time at risk, infection density was determined18. The infection density is: the infections per patient days of risk during the first year. A recurrent infection was standardly defined and incorporated once versus multiple times in the infection density calculation. The infection densities for gram-positive and gram-negative bacterial infections, cytomegalovirus (CMV), adenovirus (ADV), and Epstein-Barr virus (EBV) infections, and infection density of Aspergillus, non-aspergillus molds, and candida infections during the first year post-transplant were calculated. Infection as primary or secondary cause of death by day 100 and by 1 year was described. Secondary endpoints consisted of overall survival (OS) and relapse at 100 days and one year. All outcomes were assessed from the date of HCT. Death from any cause was considered an event. Surviving patients were censored at time of last follow-up.
Disease status was classified as CR1 or CR2 for leukemia patients.19,20,21. Preparative regimens were classified as MA or NMA/RIC according to standard definitions22,23.
Patient-, disease-, and transplant-related variables
The following patient-related variables were described: age, performance status, and pre-transplant fungal infection. Disease-related variables included disease: acute myeloid leukemia (AML) or acute lymphoid leukemia (ALL), disease status and time from diagnosis to transplant. Transplant-related variables were as follows: (a) HLA matching: 4/6 vs 5/6 vs 6/6 for single cord (n=106) and the combinations for both cord blood units (i.e., 4/6 + 4/6) for double cords (n=462); (b) Conditioning regimen intensity: (c) Total body radiation (TBI) in conditioning: yes versus no; (d) GvHD prophylaxis; (e) use and type of antithymocyte globulin (ATG): none versus horse versus rabbit; (f) Time to neutrophil and platelet engraftment; (g) Acute and chronic GvHD; (h) Use of intravenous gammaglobulin and growth factors; and (i) Donor/recipient CMV serostatus.
Statistical analysis
Patient-, disease- and transplant-related factors were compared between groups using the Chi-square test for categorical variables and the Wilcoxon two-sample test for continuous variables. Non-relapse mortality (NRM) was calculated using the cumulative incidence function with relapse as the competing event. The incidence of bacterial, fungal, and viral infection for the first infection event was determined using the cumulative incidence function with death and relapse as competing events. To account for multiple infections, the infection density was calculated for gram-positive bacteria, gram-negative bacteria, CMV, EBV, ADV, Aspergillus, non-Aspergillus molds, and Candida infections. The infection density was compared between groups using the Kruskal-Wallis test. Kaplan-Meier probabilities for OS were estimated.
Multivariable models for OS and leukemia free (LFS) survival, NRM, and the development of fungal, viral, and bacterial infections were calculated using the Cox model with examination of the proportional hazards assumption. If the proportional hazards assumption was violated, the variable was added as a time-dependent covariate. Variables analyzed (* denotes reference group) included donor source [MUD*, MMUD, UCB], age [16–30y*, 31 – 50y, >50y], KPS [≥90%*, <90%], disease [AML*, ALL], disease status [CR1*, CR2], time from diagnosis to transplant [<5m*, 6–11m, ≥12m], ATG [no*, yes], conditioning intensity [myeloablative*, reduced/non-myeloablative], donor/recipient CMV serostatus [neg/neg*, any positive], G-CSF use post-transplant [no*, yes], acute GvHD as a time dependent co-variate occurring prior to infection onset [grade 0/1*, grade 2–4], chronic GvHD as a time dependent co-variate occurring prior to infection onset [no*, yes], year of transplant [2008–2009*, 2010–2011], pre-transplant fungal infection [no*, yes], and time from fungal infection to transplant. The stepwise selection procedure was used to select significant covariates. An interaction between the main effect and time to infection and significant covariates was tested. In particular, time to infection was examined as a time-dependent covariate for OS, NRM, and relapse.
RESULTS
Patient, Disease, and Transplant Characteristics
Table 1 outlines the patient, disease, and transplant characteristics. Five hundred and sixty-eight (568) UCB from 109 centers, 930 MUD from 114 centers and 283 MMUD patients were included from 83 centers. Single and double UCB patients were combined for analysis, due to patient numbers and similar outcomes. 106 patients received a single cord, the majority receiving a unit with at least one mismatch [HLA match 6/6 = 17 (16%), 5/6 = 38 (36%), 4/6 = 51 (48%)]. Only 4% of the double UCBT patients received two 6/6 HLA matched units. The majority of patients in the MUD and MMUD groups received peripheral blood stem cells (MUD 82%, MMUD 81%). MUD and MMUD patients were more likely to be older, have a lower performance status, have AML, and be transplanted in CR1 than UCB patients. ATG was used most commonly in the MMUD patients. Table 2 describes the post-transplant variables of engraftment and development of acute and chronic GvHD. The median follow-up of survivors was 46 months, 58 months and 56 months for UCBT, MUD, and MMUD recipients, respectively.
Table 1.
Characteristics of patients (age>=16) with acute leukemia in remission receiving unrelated cord blood , 8/8 matched or 7/8 mismatched unrelated donor transplant, transplant reported to the CIBMTR between 2008 and 2011
| Variable | MUD N=930 N (%) |
MMUD N=283 N (%) |
UCB N=568 N (%) |
p-value | |
|---|---|---|---|---|---|
| Gender, male | 514(55%) | 141 (50%) | 267 (47%) | 0.006 | |
| Age, median (range), yrs | 48(16–78) | 47(16–73) | 43(16–72) | <0.001 | |
| Karnofsky score | 0.006 | ||||
| ≥90% | 599 (64%) | 186(66%) | 416(73%) | ||
| <90% | 309 (33%) | 93 (33%) | 145(26%) | ||
| Missing | 22 ( 2%) | 4( 1%) | 7( 1%) | ||
| Disease | <0.001 | ||||
| AML | 753(81%) | 226 (80%) | 395 (70%) | ||
| ALL | 177(19%) | 57 (20%) | 173(30%) | ||
| Disease status | 0.014 | ||||
| CR1 | 666 (72%) | 196(69%) | 366 (64%) | ||
| CR2 | 264 (28%) | 87(31%) | 202 (36%) | ||
| Conditioning intensity | 0.001 | ||||
| MAC | 690 (74%) | 202(71%) | 371 (65%) | ||
| NMA/RIC | 240 (26%) | 81 (29%) | 197(35%) | ||
| Conditioning Regimen | <0.001 | ||||
| Cy + TBI ± Other | 264 (28%) | 87(31%) | 424 (75%) | ||
| TBI ± Other | 113(12%) | 39(14%) | 51 ( 9%) | ||
| Bu + Cy ± Other | 175(19%) | 51 (18%) | 38 ( 7%) | ||
| Melphalan ± Other | 48 ( 5%) | 20 ( 7%) | 32 ( 6%) | ||
| Cy ± Other | 17 ( 2%) | 3( 1%) | 4(<1%) | ||
| Bu + Flu | 289(31%) | 78 (28%) | 15 ( 3%) | ||
| Other | 24 ( 3%) | 5 ( 2%) | 4(<1%) | ||
| GVHD prophylaxis | <0.001 | ||||
| CNI + MTX ± others | 622 (67%) | 174(61%) | 16 ( 3%) | ||
| CNI + MMF± others | 198(21%) | 74 (26%) | 472 (83%) | ||
| CNI ± others | 85 ( 9%) | 26 ( 9%) | 59(10%) | ||
| Other/UNK | 25 ( 3%) | 9 ( 3%) | 21 ( 4%) | ||
| ATG, yes | 325 (35%) | 143(50%) | 122(21%) | <0.001 | |
| Donor/Recipient CMV | <0.001 | ||||
| Pos/Pos | 224 (24%) | 86 (30%) | 0 | ||
| Pos/Neg | 99(11%) | 37(13%) | 0 | ||
| Neg/Pos | 323 (35%) | 90 (32%) | 381 (67%) | ||
| Neg/Neg | 273 (29%) | 69 (24%) | 183(32%) | ||
| Unknown | 11 ( 1%) | 1 (<1%) | 4(>1%) | ||
| G-CSF/GM-CSF, yes | 285(31%) | 91 (32%) | 488 (86%) | <0.001 | |
| Received IVIG, yes | 191 (21%) | 68 (24%) | 163(29%) | <0.001 | |
| Year of transplant | <0.001 | ||||
| 2008 | 337 (36%) | 126(45%) | 122(21%) | ||
| 2009 | 295 (32%) | 87(31%) | 152(27%) | ||
| 2010 | 180(20%) | 51 (18%) | 158(28%) | ||
| 2011 | 109(12%) | 19 ( 7%) | 136(24%) | ||
Table 2.
Description of pertinent post-transplant event variables included in the multivariable Cox model
| Variable | MUD N=930 |
MMUD N=283 |
UCB N=568 |
|
|---|---|---|---|---|
| Neutrophil engraftment, median (range), days |
||||
| MAC | 14 (1–73) | 14(3–35) | 23 (1 – 80) | |
| NMA/RIC | 14 (<1 −81) | 13(1 −26) | 15 (<1 −50) | |
| Acute GVHD | ||||
| Grade 0 −1 | 516(56%) | 141 (50%) | 288(51%) | |
| Grade 2 | 274 (29%) | 89(31%) | 194(34%) | |
| Grade 3–4 | 140(15%) | 53(19%) | 86(15%) | |
| Onset acute GVHD, Median (range), days |
28(7–176) | 26(8–119) | 29(7–178) | |
| Chronic GVHD, any severity, Yes |
508 (55%) | 149(53%) | 187(33%) | |
| Onset chronic GVHD, Median (range), months |
6 (2 – 75) | 6 (2 – 47) | 5 (2 – 53) | |
Effect of Donor Source on Infections and Post-Transplant Outcomes
We studied the effect of donor source on the incidence of bacterial, fungal, and viral infections, and on post-transplant outcomes, as shown in Table 3. Relapse was comparable among the donor sources. Non-relapse mortality (NRM) was comparable between UCB (33%) and MMUD (27%) patients and was higher than in MUD (14%) recipients, (p<0.0001).
Table 3.
Univariate outcomes and cumulative incidence of infection at 1 year by donor source.
| MUD % (95% CI) |
MMUD* % (95% CI) |
UCB* % (95% CI) |
p-value | |
|---|---|---|---|---|
| Transplant Outcomes | ||||
| OS | 69% (66 – 72%) |
60% (54 – 66%) |
51% (47 – 55%) |
<0.0001 |
| LFS | 56% (56 – 72%) |
49% (43 – 54%) |
44% (40 – 48%) |
<0.0001 |
| Relapse | 27% (24 – 30%) |
25% (20 – 30%) |
24% (21 – 28%) |
0.43 |
| NRM | 14% (12–16%) |
27% (22 – 32%) |
33% (29 – 36%) |
<0.0001 |
| Infection Incidence | ||||
| Bacterial | 59% (57 – 64%) |
65% (59 – 70%) |
72% (68 – 76%) |
<0.0001 |
| Viral | 45% (42 – 48%) |
53% (47 – 59%) |
68% (64 – 72%) |
<0.0001 |
| Fungal | 10% (8–12%) |
16% (12–20%) |
18% 15–21%) |
0.0001 |
The incidence at one year for any bacterial infection was 72% for UCB, 59% for MUD and 65% for MMUD (UCB vs MMUD p=0.04). Gram negative infections were also more frequent following UCB (UCB 28% vs MUD 21% vs MMUD 23%; p=0.013). Gram positive bacteremia represented approximately 70% of the gram positive infections for all the graft sources; gram negative bacteremia represented approximately 60% of the gram negative infections for all of the graft sources. Bacterial urine infections were approximately 16% of the bacterial infections for all of the graft sources. One-year incidence of fungal infections was 18% for UCBT, 10% for MUD, and 16% for MMUD (UCB vs MMUD, p = 0.42). Focusing on lung/lower respiratory tract, Aspergillus was reported in 48%, 46%, and 25% of the lung infections following UCB, MUD and MMUD respectively. Candida was reported in 24% of 32 pulmonary infections for UCB patients, 35% of 48 pulmonary infections in MUD, and 24% of 25 pulmonary infections in MMUD. Candidemia was seen in a similar percentage of candida infections across the graft sources. For viral infections, the incidence at one year was 68% for UCBT, 45% for MUD, and 53% for MMUD (UCB vs MMUD p<0.0001).
We investigated bacterial, viral, or fungal infection as the primary or secondary cause of death. For those patients (n= 264) who died, infection as the primary or secondary cause of death by Day 100 was similar across graft sources. By 1 year post-HCT 673 patients [UCB = 277 (49%), MUD = 284 (31%), MMUD = 112 (40%)] had died with infection reported as a primary or secondary cause of death greatest in UCB (UCB 39%, MUD 29%, MMUD 34%; p = 0.002).
Infection Density
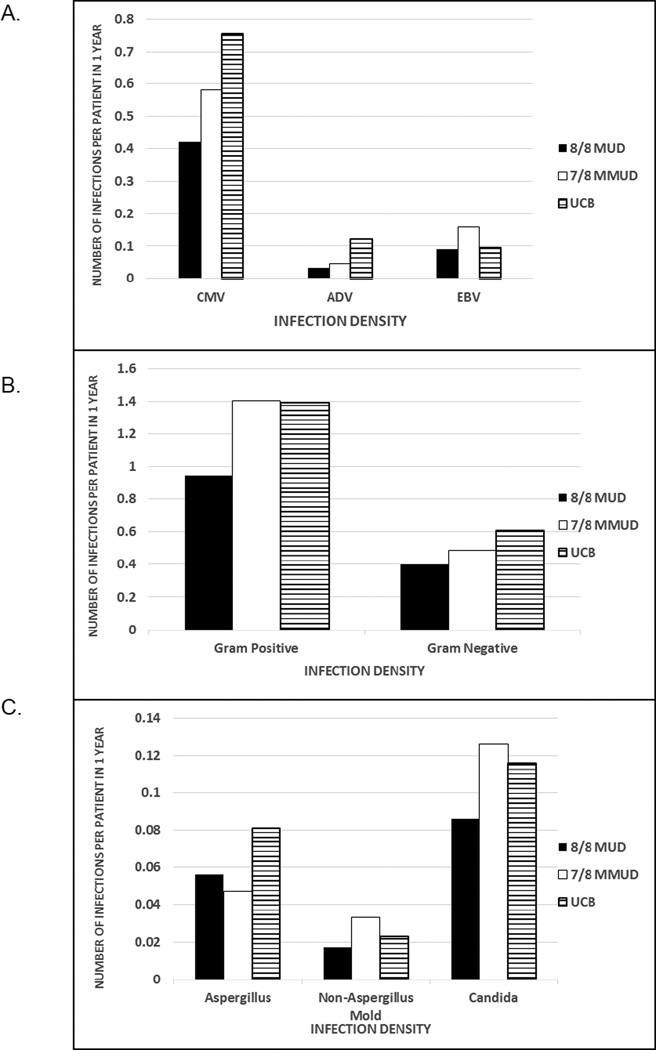
Infection density compares the normalized rate of infection of a specific type occurring over a specific interval and accounts for multiple infections. Patients receiving UCB had a higher rate of CMV and ADV infections (p>0.001); EBV infections occurred more frequently in MMUD recipients (p = 0.021) (Figure 1A). CMV disease represented about 5% of CMV infection in all of the graft sources. Adenovirus pneumonitis was rare and was reported in no UCBT patients, 2 patients with MUD, and 1 patient with MMUD. Gram-positive bacterial infections were more common in MMUD and UCB compared to MUD recipients (p <0.001). Infections with gram-negative bacteria were more frequent in the UCB recipients, compared to MUD and MMUD (p = 0.003) (Figure 1B). Mold infections with either Aspergillus spp or other molds occurred at similar rates and were uncommon regardless of graft source. Candida spp. infections were rare and also similar regardless of graft source (Figure 1C).
Figure 1.
Infection density for specific viral (A), bacterial (B), and fungal (C) infections by graft source
Multivariate Analysis of Infections among the Graft Sources
Table 4 provides the results of a multivariate analysis comparing infections among the different graft sources. Bacterial infections were 1.2-fold more common in MMUD (p = 0.029) and 1.6-fold more in UCB (<0.001) than MUD recipients. Similarly, MMUD recipients were almost 1.4-fold (p = 0.04) and UCB recipients 1.7-fold (p <0.0001) as likely to develop a fungal infection compared to MUD. However, there was no difference for the likelihood of fungal infection between MMUD and UCB recipients. Viral infections were notably higher in the UCB group with 2.3-fold higher risk compared to MUD (p<0.0001). MMUD recipients had a 1.3-fold higher risk compared to MUD (p = 0.0067) recipients but were 45% less likely than the UCB recipients to develop a viral infection (p<0.0001).
Table 4.
Results of multivariable analysis for risk of bacterial, fungal, and viral infections
| Variable | Bacterial Infections RR (95% CI) |
p-value | Fungal Infections RR (95% CI) |
p-value | Viral Infections RR (95% CI) |
p-value | |
|---|---|---|---|---|---|---|---|
| Main Effect | <0.0001 | 0.0002 | <0.0001 | ||||
| MUD | 1.00 | 1.00 | 1.00 | ||||
| MMUD | 1.196(1.018–1.406) | 0.0295 | 1.377(1.013–1.872) | 0.0413 | 1.283(1.072–1.535) | 0.0067 | |
| UCB* | 1.609(1.414–1.831) | <0.001 | 1.654(1.301 −2.102) | <0.0001 | 2.281 (1.989–2.615) | <0.0001 | |
| Acute GVHD, yes | 1.360(1.163–1.589) | 0.0001 | |||||
| Chronic GVHD, yes | 1.490(1.152–1.928) | 0.0024 | |||||
|
Conditioning regimen (< 3 mo) |
<0.0001 | ||||||
| MAC | 1.00 | ||||||
| NMA/RIC | 0.588 (0.505 – 0.683) | ||||||
|
Conditioning regimen (> 3 mo) |
0.9030 | ||||||
| MAC | 1.00 | ||||||
| NMA/RIC | 1.016(0.791 −1.304) | ||||||
| Year of Transplant | 0.0226 | ||||||
| 2008 – 2009 | 1.00 | ||||||
| 2010–2011 | 0.938(0.888–0.991) | ||||||
| ATG, yes | 1.604(1.400–1.838) | <0.0001 | |||||
| CMV D/R match | <0.0001 | ||||||
| Neg/Neg | 1.00 | ||||||
| Any positive | 1.629(1.413–1.878) | <0.001 | |||||
| Missing | 0.802(0.378–1.701) | 0.5647 | |||||
| Conditioning | 0.0002 | ||||||
| Regimen | 1.00 | ||||||
| MAC | 0.768 (0.667 – 0.883) | ||||||
| NMA/RIC | |||||||
| GVHD╪ | 0.0001 | ||||||
| aGVHD no, cGVHD no | 1.00 | ||||||
| aGVHD yes, cGVHD no | 1.863(1.601 −2.167) | <0.0001 | |||||
| aGVHD no, cGVHD yes | 2.331 (1.64–3.317) | <0.0001 | |||||
| aGVHD yes, cGVHD yes | 1.855(1.259–2.733) | 0.0018 | |||||
Contrast MMUD vs UCB: Bacterial, 0.74 (0.62–0.88), p<0.001; Fungal, 0.83 (0.61 – 1.14), p=0.26; Viral, 0.56 (0.47 – 0.68), p<0.0001
Contrast: aGVHD yes, cGVHD no vs aGVHD no, cGVHD yes, 0.799 (0.559 – 1.14), p = 0.218; aGVHD yes, cGVHD no vs aGVHD yes, 1.004 (0.680 – 1.481), p=0.984; aGVHD no, cGVHD yes vs. aGVHD yes, cGVHD yes, 1.256 (0.834 – 1.892), p=0.275
Additional risk factors identified to increase the risk of bacterial infections include development of acute or chronic GvHD and transplant between 2008 and 2009. In the first 3 months after transplant, patients receiving NMA/RIC were 42% less likely to have a bacterial infection. However, this benefit was lost after 3 months from transplant. Viral infections were also less likely following NMA/RIC regimens but this persisted throughout the post-transplant period. Use of ATG and donor or recipient positive CMV serostatus also increased the risk of viral infection. Development of acute or chronic GVHD also increased the risk of viral infection. No other factors were identified to increase the risk of fungal infections. Disease status (CR 1 vs CR 2) had no impact on the incidence of bacterial, viral, or fungal infection.
Impact of Graft Sources and Infection on post-Transplant Outcomes
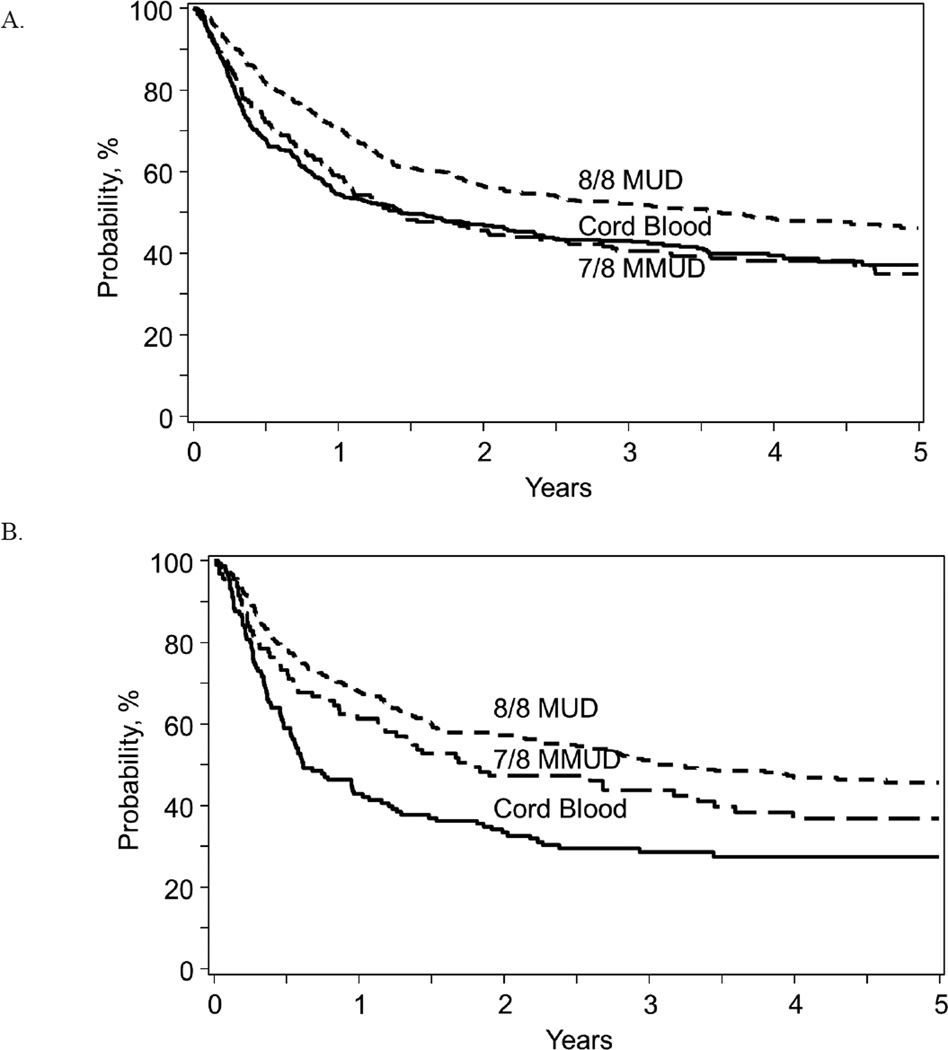
For OS and LFS, multivariable analysis found that graft source, bacterial and fungal infection, and age were significant factors (Table 5). Graft source affected OS and LFS for patients with a KPS < 90%. (Figure 2). For patients with KPS <90%, UCB recipients had inferior OS and LFS compared with either MUD [OS: p <0.0001; LFS: p <0.0001] or MMUD sources [OS: p=0.010; LFS: p=0.047]. LFS was comparable among UCB, MMUD, and MUD patients with a KPS ≥ 90%. OS was superior for MUD patients than MMUD or UCB for patients with KPS > 90%; OS was similar for UCB and MMUD for patients with KPS >90% (Figure 3). The development of a bacterial infection was associated with a 2.45-fold higher risk of death from any cause (p<0.0001). Fungal infections also negatively impacted OS and LFS [OS: RR 3.0, p<0.0001; LFS: RR 2.46, p<0.0001], while viral infections did not affect OS or LFS.
Table 5.
Multivariable models for transplant outcomes
| Variable | Relative Risk (95% CI) |
p-value | Relative Risk (95% CI) | p-value | |
|---|---|---|---|---|---|
| Overall Survival | Leukemia Free Survival | ||||
| Graft source (KPS ≥ 90) | 0.143 | ||||
| MUD | 1.00 | 0.071 | 1.00 | ||
| MMUD | 1.269(1.026–1.571) | 0.028 | 1.221 (0.994–1.499) | 0.057 | |
| UCB* | 1.131 (0.955–1.341) | 0.155 | 1.101 (0.934–1.296) | 0.252 | |
| Graft source (KPS < 90) | <0.0001 | <0.001 | |||
| MUD | 1.00 | 1.00 | |||
| MMUD | 1.162(0.859–1.573) | 0.329 | 1.291 (0.969–1.719) | 0.081 | |
| UCB* | 1.785(1.392–2.88) | <0.0001 | 1.769(1.391 −2.251) | <0.0001 | |
| Age | <0.0001 | <0.0001 | |||
| 16–30 years | 1.00 | 1.00 | |||
| 31 – 50 years | 1.030(0.862–1.230) | 0.7483 | 0.982(0.831 −1.162) | 0.835 | |
| > 50 years╪ | 1.576(1.336–1.859) | <0.0001 | 1.374(1.174–1.608) | <0.0001 | |
| Bacterial infection, yes | 2.450(2.110–2.846) | <0.0001 | 1.724(1.512–1.965) | <0.0001 | |
| Fungal infection, yes | 3.004(2.584–3.491) | <0.0001 | 2.458(1.091 −2.888) | <0.0001 | |
| Viral Infection, yes | 1.043(0.903–1.205) | 0.5680 | |||
| Non-Relapse Mortality | Relapse | ||||
| Graft Source | 0.0006 | 0.8707 | |||
| MUD | 1.00 | 1.00 | |||
| MMUD | 1.370(1.078–1.740) | 0.0099 | 1.013(0.801 −1.282) | 0.9125 | |
| UCB* | 1.454(1.189–1.777) | 0.0003 | 1.051 (0.872–1.267) | 0.5999 | |
| Age | <0.0001 | ||||
| 16–30 years | 1.00 | ||||
| 31 – 50 years | 0.993(0.784–1.258) | 0.9557 | |||
| > 50 years* | 1.54 (1.220 – 1.979) | 0.0004 | |||
| Acute GVHD, yes | 1.257(1.052–1.501) | 0.0118 | |||
| Chronic GVHD, yes | 1.504(1.181 −1.915) | 0.0010 | |||
| Bacterial infection, yes | 3.170(2.524–3.982) | <0.0001 | |||
| Fungal infection, yes | 3.969(3.258–4.835) | <0.0001 | |||
| Viral infection, yes | 1.241 (1.024–1.505) | 0.0281 | |||
|
Conditioning regimen (≤ 3 months) |
0.0109 | ||||
| MAC | 1.00 | ||||
| NMA/RIC | 0.605(0.410–0.893) | ||||
|
Conditioning regimen (> 3 months) |
0.6844 | ||||
| MAC | 1.00 | ||||
| NMA/RIC | 0.947(0.730–1.22) | ||||
| Conditioning regimen | <0.0001 | ||||
| MAC | 1.00 | ||||
| NMA/RIC | 1.944 (1.645 – 2.296) | ||||
Contrast MMUD vs UCB: OS, KPS≥90% = 1.122 (0.899 – 1.400), p=0.307; OS, KPS <90% = 0.651 (0.470 – 0.903), p=0.010; LFS, KPS≥90% = 1.109 (0.896 – 1.374), p=0.341; LFS, KPS<90% = 0.729 (0.534 – 0.996), p=0.047; NRM = 0.942 (0.740 – 1.201), p=0.631; Relapse = 0.964 (0.747 – 1.244), p=0.777
Contrast 31–50 yrs vs >50 yrs: OS = 0.653 (0.566 – 0.754), p<0.0001; LFS = 0.715 (0.623 – 0.820), p<0.0001; NRM = 0.639 (0.516 – 0.792), p<0.0001
Figure 2.
Univariate curves for leukemia free survival by graft source for patients based on Karnofsky score ≥ 90% (A) and <90% (B).
Figure 3.
Univariate curves for Overall Survival by graft source for patients based on Karnofsky score ≥ 90% (A) and <90% (B).
Several factors, including graft source, influenced NRM. Specifically, recipients of MMUD had a 1.37-fold higher risk (p<0.001) and UCB a 1.45-fold higher risk (p<0.001) of death in remission compared with MUD. Notably, there was no difference between recipients of MMUD and UCB. In addition, the development of bacterial infection [RR: 3.17, p<0.0001], fungal infection [RR: 3.97, p<0.0001], and viral infection [RR: 1.24, p = 0.028] were all independent risk factors for higher NRM. The only independent predictor of relapse was the use of NMA/RIC conditioning.
DISCUSSION
In this study, one of the first of its kind, we compare bacterial, fungal, and viral infections among different donor graft sources, studying single and double UCBT, and 8/8 and 7/8 HLA-matched unrelated donor transplants. Matched sibling donor HCT was not included as the clinical decision is often choosing between UCBT and MUD or MMUD HCT. Since many retrospective studies have shown similar survival among these different graft sources, we focused on infections to help determine the appropriate graft source for each patient and to help design appropriate preventative strategies1,2,3, 24,25. The incidence of bacterial, fungal, and viral infections are high (68%, 45%, and 53% at one year for UCB, MUD, and MMUD respectively) after alternative donor transplant. Over 50% of patients developed bacterial infections at one year. In multivariable analysis, bacterial, fungal, and viral infections were more common after UCB than MUD (p <0.001, 0.001, and <0.001) respectively. Bacterial, fungal, and viral infections were more common after MMUD than MUD (p=0.0295, 0.041, and 0.0067 respectively). Bacterial and viral infections were more common after UCB than MMUD (p=0.0009 and <0.0001 respectively), perhaps due to slower engraftment and delayed immune recovery. However, there was no difference in fungal infections based on graft source. Bacterial and fungal infections were also associated with poorer survival. Surprisingly, the presence of a viral infection did not affect overall survival.
Bacterial infections have been studied in the early and late phases after HCT in 172 patients26. In this study, there were 100 episodes of blood-stream infections (BSI) in the pre-engraftment period and 89 episodes post engraftment. GvHD and steroids were predictors for late-onset bacteremia; graft source was not well studied. Kikuchi and colleagues reported on 122 UCB, 51 MRD, 26 haploidentical, 98 MUD, and 24 MMUD patients. Pre-engraftment BSI occurred in 39% of patients and post-engraftment BSI in 17% of patients; graft source did not affect this incidence27.
Strategies to prevent bacterial infection include prophylactic antibiotics such as quinolones. A meta-analysis of 17 trials and 1453 patients concluded that antibiotic prophylaxis reduced the incidence of fever and bacteremia, but did not impact all-cause or infection-related mortality28. C. difficile (C. Diff) was not a focus of our study, but other investigators have reported similar incidence and recovery from C. Diff infections after UCBT, MRD, or MUD29.
Viral infections have been analyzed extensively, especially after UCBT. Our study focused on CMV, ADV and EBV because these viral infections are significant causes of morbidity and mortality after allogeneic HCT. EBV-associated post-transplant lymphoproliferative disorder (PTLD) has been seen in up to 13% of patients receiving RIC and 3% for MA UCBT recipients30.
Our study showed a higher incidence of viral infection with the use of either horse or rabbit ATG. However, single center studies have demonstrated a 30% incidence of viral infections even without ATG.31 CMV reactivation is quite common after UCBT, and clearance of CMV and survival is associated with de novo production of thymic derived T cells10. Due to the nature of the registry data, we were not able to evaluate the impact of strategies to reduce CMV reactivation, such as the use of IV ganciclovir during conditioning32. Delayed immune reconstitution after alternative donor HCT, particularly after UCBT, may explain the high risk of infection. A comparison of immune recovery after double UCBT and HLA-matched MUD revealed delay in recovery of naive (CD45RO-) and memory (CD45RO+) CD 4 T cells, CD8 T cells, and regulatory (CD4+CD25+) T cells, which correlates with the increased viral infection in the early post-transplant period8. A delayed time to recovery of thymopoiesis has been reported in recipients of UCBT compared to other graft sources11,33,34.
Fungal infections are an important cause of morbidity and mortality after HCT. The Gruppo Italiano Trapianto Midollo Osseo (GITMO) studied 1,858 patients undergoing allogeneic HCT35. Incidence of proven/probable invasive fungal disease was 8.8% at one year. Risk factors for developing invasive fungal disease were MUD or UCBT, active leukemia, prior fungal infection, and GvHD. In contrast, a study from Massachusetts General Hospital did not show a correlation between prior fungal infection and invasive fungal disease14. Parody and colleagues reported a higher incidence of invasive aspergillosis after UCBT but no difference in mortality compared to MUD36.
Although the primary endpoint was infection, LFS and OS were also analyzed in this study. OS was comparable among MUD, MMUD, and UCB for patients with a Karnofsky performance status (KPS) of 90% or greater; OS was inferior for UCB for patients with KPS < 90%. Therefore, when multiple graft sources are available, patients with poor performance status may have better outcomes with a MUD or MMUD HCT. Thus, these data may be used to help identify factors that may be modified, such as graft source, in the treatment approach. Many variables are used in the selection of the appropriate graft and information on infections and performance status may need to be considered along with other factors. Recently, the CIBMTR and Eurocord compared survivals for older patients with acute myeloid leukemia in CR 1 undergoing alternative donor transplant2. NRM was higher for patients receiving UCBT or 7/8 MUD HCT. Three-year OS was 43% in 8/8 matched MUD, 30% in UCBT (p=0.002) and 37% in 7/8 MUD (p=NS compared to UCB).
The study is limited by several factors. As with all registry studies, patients were treated in many centers with a variety of conditioning regimens, GvHD prophylactic treatments, and supportive care protocols. Furthermore, infections determined clinically significant are reported but there remains lack of data regarding diagnostic, prevention and treatment criteria. Therefore, this analysis is considered a high level overview and many important details regarding infection specifics and impact of immune reconstitution at the time of the infection cannot be addressed. Although parallel Phase II studies showed comparable OS between haploidentical HCT and UCBT, there were not enough haploidentical patients reported to the CIBMTR during the study period to include in this study37,38. Future studies will investigate infections after haploidentical transplant.
Clearly, better prevention and treatment of infection are needed to decrease the infection related mortality after alternative donor transplant. Strategies might include improved GVHD prophylaxis, new antifungal drugs or the use of ganciclovir before transplant as pioneered by the Seattle group32. Bacigalupo and colleagues have implemented the use of rituximab early after UCBT to decrease the incidence of EBV-related PTLD, even when high doses of ATG are given39. For patients that develop serious viral infection, an exciting new strategy is the use of either donor-derived or third party trivirus-directed cytotoxic T cells40. Use of these and other novel techniques may reduce the incidence of infection after UCBT or MMUD so that infection rates are comparable after all graft sources. The optimal donor source will need to be determined in a prospective trial. A randomized prospective trial comparing UCBT versus haploidentical is underway in the BMT Clinical Trials Network.
Highlights.
Bacterial and viral, but not fungal, infections are more common after UCBT than MMUD.
Bacterial and fungal, but not viral, infections were associated with poorer survival.
Overall survival is comparable among graft sources if performance status is ≥90%.
Acknowledgments
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Be the Match Foundation; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Mesoblast; *Millennium: The Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; Oxford Immunotec; Perkin Elmer, Inc.; Pharmacyclics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Telomere Diagnostics, Inc.; TerumoBCT; Therakos, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP
Karen Ballen, Kwang Woo Ahn, Min Chen, and Marcie Riches designed the study, analyzed data, wrote the manuscript, and reviewed the final manuscript.
Hisham Abel-Azim, Ibrahim Ahmed, Mahmoud Aljurf, Joseph Antin, Ami Bhatt, Mic Boeckh, George Chen, Christopher Dandoy, Biju George, Mary Laughlin, Hillard Lazarus, Margaret MacMillan, David Margolis, David Marks, Maxim Norkin, Joseph Rosenthal, Ayman Saad, Bipin Savani, Harry Schouten, Jan Storek, Paul Szabolcs Celatettin Ustun, Michael Verneris, Edmund Waller, Daniel Weisdorf, Kirsten Willia John Wingard, Baldeep Wirk, Tom Wolfs, Jo-Anne Young, Jeffrey Auletta, Krishna Komanduri, and Caroline Lindemans designed the study, wrote the manuscript, an reviewed the final manuscript.
CONFLICT OF INTEREST DISCLOSURES
Conflict of Interest: The authors report no conflict of interest
References
- 1.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor hematopoietic stem cell transplantation in adults with acute leukemia; a retrospective analysis. Lancet Oncol. 2010;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisdorf D, Eapen M, Ruggeri A, Zhang MJ, Zhong X, Brunstein C, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a Center for International Blood and Marrow Transplant Research-Eurocord Analysis. Biol Blood Marrow Transplant. 2014;20(6):816–822. doi: 10.1016/j.bbmt.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancies; relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YB, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biology Blood Marrow Transplant. 2012;18:805–812. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Peffault de Latour R, Brunstein CG, Porcher R, Chevallier P, Robin M, Warlick E, et al. Similar overall survival using sibilng, unrelated donor, and cord blood grafts after reduced intensity conditioning for older patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2013;19(9):1355–1360. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson CA, Turki A, McDonough SM, Stevenson KE, Kim HT, Kao G, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation:comparison wtih unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogata M, Fukuda J, Teshima T. Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation. 2015;50(8):1030–1036. doi: 10.1038/bmt.2015.76. [DOI] [PubMed] [Google Scholar]
- 10.Brown JA, Stevenson K, Kim HT, Cutler C, Ballen K, McDonough S, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstittution of thymopoiesis. Blood. 2010;115:4111–4119. doi: 10.1182/blood-2009-09-244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danby R, Rocha V. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Frontiers in Immunology. 2014;5:1–19. doi: 10.3389/fimmu.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshrine BR, Li Y, Teachey DT, Heimall J, Barrett DM, Bunin N. Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2013;19:1581–1589. doi: 10.1016/j.bbmt.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks DI, Woo KA, Zhong X, Appelbaum FR, Bachanova V, Barker JN, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2014;99(2):322–328. doi: 10.3324/haematol.2013.094193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omer AK, Ziakas PD, Anagnostou T, Coughlin E, Kourkoumpetis T, McAfee SL, et al. Risk Factors for invasive fungal diseases after allogeneic hematopoietic stem cell transplantation: a single center experience. Biol Blood Marrow Transplant. 2013;19(8):1190–1196. doi: 10.1016/j.bbmt.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Meng F, Han M, Zhang X, Yu L, Huang H, et al. Epidemiology, management, and outcome of invasive fungal disease in patients undergoing hematopoietic stem cell transplantation in China. Biol Blood Marrow Transplant. 2015;21(6):1117–1126. doi: 10.1016/j.bbmt.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Norkin M, Wingard JR. Diagnostic strategies for invasive fungal infection in patients with hematologic malignancies and in hematopoietic stem cell recipients. J Natl Comp Canc Netw. 2013;11(8):941–949. doi: 10.6004/jnccn.2013.0115. [DOI] [PubMed] [Google Scholar]
- 17.Sanz J, Cano I, Gonzalez-Barbera EM, Arango M, Reyes J, Montesinos P, et al. Bloodstream infections in adult patients undergoing cord blood transplantation from unrelated donors after myeloablative conditioning regimen. Biol Blood Marrow Transplant. 2015;21(4):755–760. doi: 10.1016/j.bbmt.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Tomblyn M, Young JA, Haagenson MD, Klein JP, Trachtenberg EA, Storek J, et al. Decreased infections in recipients of unrelated donor hematopoietic cell transplantation from diseases with an activating KIR genotype. Biol Blood Marrow Transplant. 2010;16(8):1155–1161. doi: 10.1016/j.bbmt.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serna DS, Lee SJ, Zhang MJ, Baker KS, Eapen M, Horowits MM, et al. Trends in survival rates after allogeneic hematopoietic stem cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. J Clin Oncol. 2003;21(20):3756–3760. doi: 10.1200/JCO.2003.03.133. [DOI] [PubMed] [Google Scholar]
- 20.Baker KS, Davies SM, Majhail NS, Hassebroek A, Klein JP, Bigelow CL, et al. Race and socieconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1543–1554. doi: 10.1016/j.bbmt.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballen KK, Klein JP, Pedersen TL, Bhatla D, Duerst R, Kurtzberg J, et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18(6):903–912. doi: 10.1016/j.bbmt.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning workshop: defining the dose spectrum Report of a workshop convened by the Center for International Bone Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacigalupo A, Ballen K, RIzzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(2):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eapen M, Rubinstein P, Zhang MJ, et al. Comparison of outcomes after transplantation of unrelated donor umbilical cord blood and bone marrow in children with leukemia. Lancet. 2007;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 25.Warlick ED, Peffault de Latour R, Shanley R, Robin M, Bejanyan N, Xhaard A, et al. Allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia: similar outcomes regardless of donor type. Biol Blood Marrow Transplant. 2015;21(2):357–363. doi: 10.1016/j.bbmt.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gudiol C, Garcia-Vidal C, Arnan M, Sanchez-Ortega I, Patino B, Duarte R, et al. Etiology, clinical features, and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant. 2014;49(6):824–830. doi: 10.1038/bmt.2014.37. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi M, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, et al. Risk factors for pre- and post-engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transplant Infect Dis. 2015;17:56–65. doi: 10.1111/tid.12345. [DOI] [PubMed] [Google Scholar]
- 28.Kimura S, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, et al. Antibiotic prophylaxis in hematopoietic stem cell transplantation. A meta-analysis of randomized controlled trials. J Infection. 2014;69(1):13–25. doi: 10.1016/j.jinf.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Hosokawa K, Takami A, Tsuji M, Araoka H, Ishiwata K, Takagi S, et al. Relative incidences and outcomes of Clostridium difficile infection following transplantation of unrelated cord blood, unrelated bone marrow and related peripheral blood in adult patients:a single institute study. Transplant Infect Dis. 2014;16(3):412–420. doi: 10.1111/tid.12224. [DOI] [PubMed] [Google Scholar]
- 30.Sanz J, Arango M, Senent L, Jarque I, Montesinos P, Sempere A, et al. EBV-associated post transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant. 2014;49:397–402. doi: 10.1038/bmt.2013.190. [DOI] [PubMed] [Google Scholar]
- 31.Sauter C, Abboud M, Jia X, Hller G, Gonzales AM, Lubin M, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant. 2011;17:1460–1471. doi: 10.1016/j.bbmt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milano F, Pergam SA, Xie H, Leisenring WM, Gutman JA, Riffkin I, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011;118(20):5689–5693. doi: 10.1182/blood-2011-06-361618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood. 2014;124:3201–3211. doi: 10.1182/blood-2014-07-589176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenbosch K, Ovetchkine P, Champagne MA, Haddad E, Alexandrov L, Duval M. Varicella-zoster disease is more frequent after cord blood than after bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14:867–871. doi: 10.1016/j.bbmt.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Girmenia C, Raiola AM, Piciocchi A, Algarotti A, Stanzani M, Cudillo L, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation:a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) Biol Blood Marrow Transplant. 2015;50(2):282–288. doi: 10.1016/j.bbmt.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Parody R, Martino R, de la Camara R, Garcia-Noblejas A, Esquirol A, Garcia-Cadenas I, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant. 2015;50:274–281. doi: 10.1038/bmt.2014.229. [DOI] [PubMed] [Google Scholar]
- 37.Brunstein CG, Fuchs EJ, Carter SJ, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partiall HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eapen M, O’Donnell P, Brunstein CG, Wu J, Barowski K, Mendizabal A, et al. Mismatched related and unrelated donors for allogeneic hematopoietic cell transplantation for adults with hematologic malignancies. Biology Blood and Marrow Transplant. 2014;20:1485–1492. doi: 10.1016/j.bbmt.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominietto A, Tedore E, Saracco M, et al. In-vivo B-cell depletion with Rituximab after allogeneic donor hematopoietic stem cell transplantation. Bone Marrow Transplant. 2012;47(1):101–106. doi: 10.1038/bmt.2011.28. [DOI] [PubMed] [Google Scholar]
- 40.Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Liu H, Martinez CA, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21(11):2113–2112. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]