Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is an important nosocomial pathogen in hospitals, and increases rapidly in the community, named as community-associated MRSA (CA-MRSA). We conducted a prospective/retrospective study to understand the epidemiology, antimicrobial susceptibility, and molecular characteristics of MRSA infections in adult patients in Taiwan.
From March to June, 2012, all clinical MRSA isolates were prospectively collected from adult patients in a tertiary hospital in northern Taiwan. Selective isolates were further characterized. We reviewed the detailed medical record of each case retrospectively.
A total of 857 clinical isolates were collected from 555 patients. A total of 749 isolates from 453 patients were classified as healthcare-associated (HA)-MRSA and 108 isolates from 102 patients as CA-MRSA by the epidemiologic criteria. Compared to HA-MRSA, CA-MRSA isolates were significantly more frequently identified from pus (78% vs 28%, P < 0.001) and less frequently from sputum (4.6% vs 43.8%, P < 0.001) and blood (3.7% vs 15%, P = 0.002). CA-MRSA isolates were more susceptible to all antibiotics tested. A total of 102 CA-MRSA and 101 HA-MRSA isolates were characterized, showing significantly different molecular characteristics between CA and HA isolates (P < 0.001). The clone of sequence type (ST) 59/t437 complex, with 2 pulsotypes, accounted for 70% of CA isolates. Three major clones were identified from HA-MRSA isolates, namely clonal complex (CC) 59 (32.7%), CC239 (29.7%), and CC5 (24.8%). Among HA isolates, a significant difference was also seen between community-onset and hospital-onset MRSA isolates in terms of the source of specimens, antibiotic susceptibility patterns, and molecular characteristics.
CA-MRSA isolates from adults in northern Taiwan were genetically significantly different from HA isolates. The community clones, CC59, spread into hospitals.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) has been an important nosocomial pathogen for a long time. Since mid-1990s, MRSA was not only identified in the hospital settings but also from persons without risk factors predisposing for the acquisition of MRSA. These isolates are classified as community-associated MRSA (CA-MRSA). The reports of CA-MRSA infections are increasing, and many studies indicate that CA-MRSA isolates have different clinical and molecular features from healthcare-associated MRSA (HA-MRSA) isolates. CA-MRSA infections occur more often in previously healthy young people, and mostly cause skin and soft tissue infections such as cellulitis and abscess. They possess smaller staphylococcal cassette chromosome (SCC)mec, predominately SCCmec IV or V, compared to SCCmec I, II, or III for HA-MRSA isolates. CA-MRSA isolates more commonly carry Panton–Valentine leukocidin (PVL) genes, and they are less resistant to non-β-lactam classes of antimicrobials.1 However, various MRSA clones have spread between community and hospitals, particularly CA-MRSA transmitted in hospital settings, making the distinctions between CA-MRSA and HA-MRSA blurred.2,3
CA-MRSA infections spread rapidly in the community and draw attention worldwide. So far, 5 major epidemic clones have been identified, namely multilocus sequence type 1 (USA400), ST8 (USA300) in North America, ST80 in Europe, ST59 in Asia-Pacific area, and ST30 worldwide.2,4 The prevalence of CA-MRSA varies markedly worldwide and was relatively high in Taiwan. The dominant clone of CA-MRSA in Taiwan was ST59 or its variants (namely, clonal complex [CC] 59).5 However, most studies regarding CA-MRSA from Taiwan were collected retrospectively, and limited in children. For few studies addressing CA-MRSA in adults all recruited the cases of blood stream infection only but not the full spectrum of clinical entities.6–8 Hence, we conduct a study to address the epidemiology and molecular characteristics of CA-MRSA in adults in Taiwan.
MATERIALS AND METHODS
This study was conducted in Chang Gung Memorial Hospital (CGMH), which is a university-affiliated teaching hospital in northern Taiwan and provides a range of care, from primary to tertiary care, with 3700 beds. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital. Between 1 March and 30 June, 2012, all clinical MRSA isolates were collected prospectively from both inpatients and outpatients older than 18 years old. We evaluated the medical charts first and if needed, interviewed the patients or their caregivers for detailed medical history after a written consent was obtained. We then categorized the patients into CA-MRSA or HA-MRSA infections according to the definition proposed by Naimi et al.9 Briefly, MRSA identified after 48 hours of hospitalization or isolated from a lesion absent at admission was defined as hospital-onset (HO); conversely, community-onset (CO) was defined as MRSA isolated within 48 hours or from a lesion present at admission. Patients were classified as HA-MRSA infection if they had HO isolates, a permanent indwelling catheter or percutaneous medical device, history of hospitalization, surgery, and dialysis within previous 12 months, or lived in a long-term-care facility. Patients had none of the features were classified as CA-MRSA.
MRSA isolates from any site were collected during the study period. Specimens obtained for colonization surveillance and the patients without available medical records were excluded. From a single patient, only 1 isolate was selected for further characterization, usually the first isolate or the one from sterile sites. The demographics, clinical diagnosis, source of specimen, and antibiotic susceptibility were collected.
MRSA was identified according to Clinical and Laboratory Standards Institute (CLSI) guidelines.10 The susceptibility test was performed on Mueller–Hinton agar with disk-diffusion method following the protocol of CLSI and included oxacillin, teicoplanin, penicillin, trimethoprim/sulfamethoxazole (TMP/SMX), erythromycin, clindamycin, linezolid, fusidic acid, daptomycin, and tigecycline. For the determination of vancomycin susceptibility, if the clinical isolates were identified from blood stream specimens, minimal inhibition concentration (MIC) was determined for each isolate by E-test. Otherwise, a screening agar containing vancomycin 3 μg/mL was used first and then, if growth, E-test was performed to determine MICs of the isolates. Pulsed-field gel electrophoresis (PFGE) with SmaI digestion was used to fingerprint the isolates according to the procedure described previously.11,12 PFGE genotypes were designated in alphabetical order as in our previous studies,7,13 and those have less than 4-band differences from an existed genotype were defined as subtypes.14 Polymerase chain reaction (PCR) assays for SCCmec typing and PVL genes were performed according to the methods described previously.7,15–17 As our previous studies,18,19 some representative isolates from each PFGE pattern were sent for multilocus sequence typing and spa typing.
Chi-square test and Fisher exact test were used for categorical variable, and the independent t-test was used for continuous variables. Statistical significance was defined as P value <0.05 (2-sided). All analyses were performed with the software SPSS, version 17.0.
RESULTS
During the 4 months, a total of 881 MRSA isolates from adults were identified from the bacterial laboratory of Chang Gung Memorial Hospital. After excluding isolates from colonization surveillance (10 isolates), from patients with unavailable medical charts (13 isolates) or from patients refusing to participate in this study (1 isolate), a total of 857 MRSA isolates from 555 patients were included for analysis. Of the 857 isolates, 729 and 128 isolates were identified from the inpatients and the outpatients (including those visiting emergency department), respectively. A total of 749 and 108 were classified as HA-MRSA and CA-MRSA, respectively. Table 1 shows the distribution of specimens from which the 857 MRSA isolates were identified. Compared to HA-MRSA, CA-MRSA isolates were significantly more frequently isolated from pus (78.7% vs 28.0%, P < 0.001), and less frequently found from sputum (4.6% vs 43.8%, P < 0.001) and blood (3.7% vs 15.0%, P = 0.002). Among 749 HA-MRSA isolates, a statistically significant difference was also found between CO-MRSA and HO-MRSA isolates in terms of the sources from pus, blood, and sputum. Furthermore, CO-MRSA isolates were more significantly likely identified from the deep tissue specimen (P = 0.023).
TABLE 1.
Distribution of 857 Clinical Methicillin-Resistant Staphylococcus aureus Isolates, Stratified by Origin of Specimens
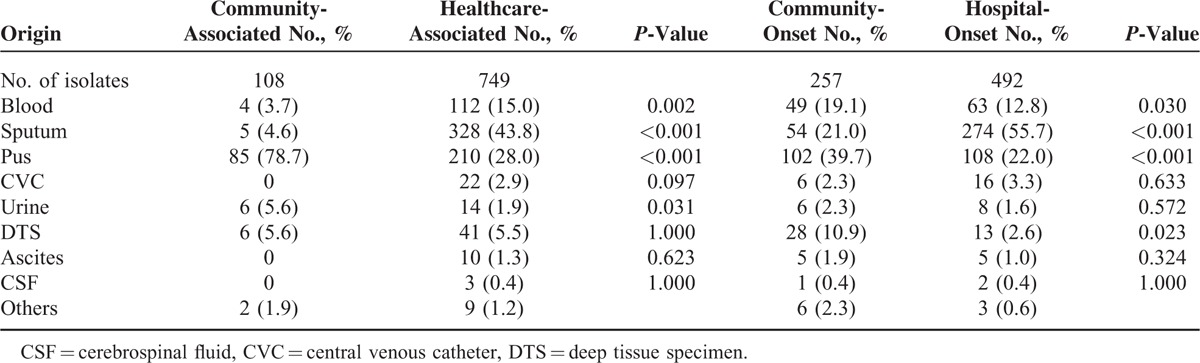
Of the 555 patients included, 122 patients were outpatients and 433 were inpatients. A total of 202 patients (18.4%) were identified as CA-MRSA infection. Among these 433 hospitalized patients with MRSA infection, only 43 (9.5%) had CA-MRSA infection. The mean age was 47 and 65 years for the patients with CA- and HA-MRSA infection, respectively (P < 0.001). In CA- and HA-MRSA group, 57.8% and 62.7% of patients were male. Of the 102 patients with CA-MRSA infection, 69 patients (67.6%) presented with skin and soft tissue infection (SSTI). Eight patients (7.8%) presented with bone and joint infection, and 3 patients (2.9%) presented with deep-seated soft-tissue infection, including necrotizing fasciitis for 2 cases and toe necrosis for 1 case. Seven patients (6.9%) had sinusitis or otitis media. Five patients (4.9%) presented with pneumonia, including necrotizing pneumonia for 1 case. There were 6 patients with MRSA isolated from urine, but all of these were not considered as pathogens. Two patients had eye infection. Two patients had bacteremia, 1 was secondary to wound infection and the other was due to prostatitis. All patients with CA-MRSA infection recovered uneventfully. The in-hospital case-fatality rate was 0% and 25.6% for CA- and HA-MRSA groups, respectively (P < 0.001).
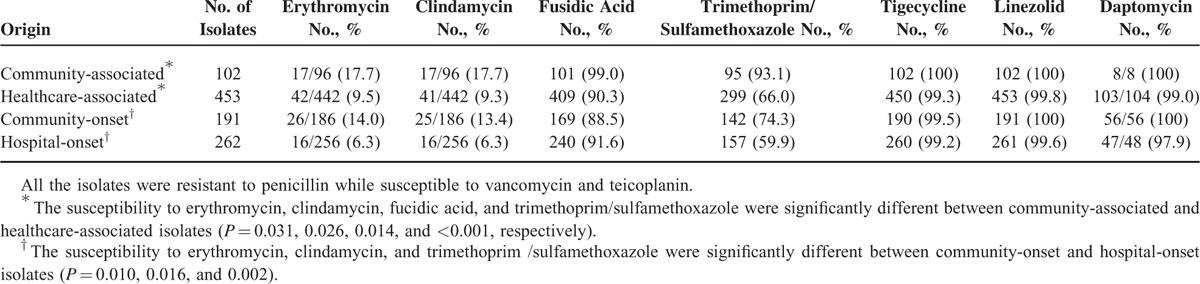
Table 2 illustrates the antibiotic susceptibility of the 555 isolates from the case patients. Nearly all the isolates were susceptible to vancomycin, teicoplanin, tigecycline, linezolid, and daptomycin. More than 80% of the isolates were resistant to erythromycin and clindamycin. However, CA-MRSA isolates were significantly more susceptible to erythromycin, clindamycin, fusidic acid, and TMP/SMX (P < 0.05 for all) than HA-MRSA isolates. Comparing to HO-MRSA, CO-MRSA isolates were significantly more susceptible to erythromycin, clindamycin, and TMP/SMX (P < 0.05).
TABLE 2.
Antibiotic Susceptibility of 555 Clinical Methicillin-Resistant Staphylococcus aureus Isolates From Case Patients, Stratified According to the Origin of Acquisition
Further molecular analyses were performed for a total of 203 MRSA isolates, including 102 CA-MRSA and 101 HA-MRSA isolates (Table 3). All of the 102 CA-MRSA isolates were included while 50 isolates from CO-MRSA patients and 51 from HO-MRSA patients (1 per 4 and 1 per 5 consecutive isolates, respectively) were selected for molecular characterization. Twelve PFGE patterns were identified for CA-MRSA and HA-MRSA isolates, respectively. Nine pulsotypes were singletons. Among the CA-MRSA isolates, pulsotype D and C were the most common patterns, accounting for 56.9% and 19.6%, respectively. Six SCCmec types were found with the predominant types of VT (57.8%) and IV (33.3%). Among the 101 HA-MRSA isolates, pulsotype F (22.8%), A (21.8%), and D (20.8%) were the most common types. Except 4 untypable isolates, 6 SCCmec types were identified. Type II and III accounted for 23.8%, respectively. PVL genes were detected in fewer HA-MRSA isolates than CA-MRSA ones (19.8% vs 65.7%). A total of 23 CA-MRSA isolates and 21 HA-MRSA isolates were selected for MLST, and 10 and 8 sequence types were identified, respectively. A total of 56 isolates were selected for spa typing and 17 spa types were identified.
TABLE 3.
Molecular Characteristics of 203 Selective Clinical Methicillin-Resistant Staphylococcus aureus Isolates From Case Patients, Stratified by the Origin of Acquisition and Pulsed-Field Gel Electrophoresis Patterns
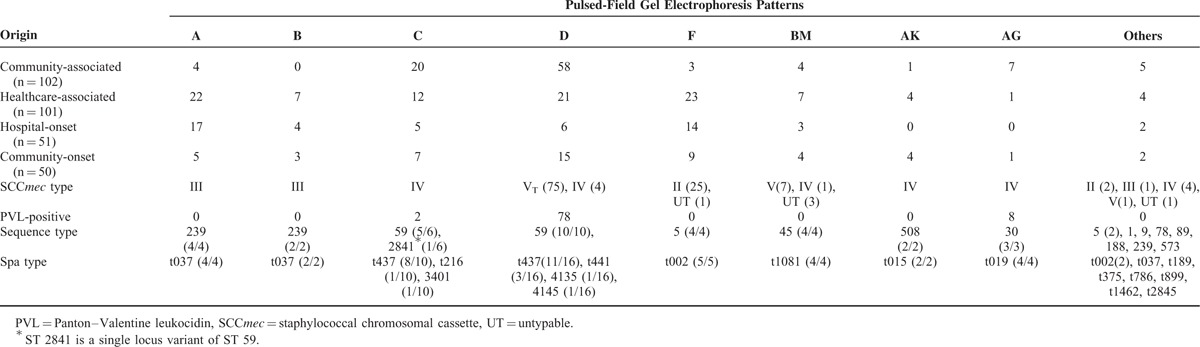
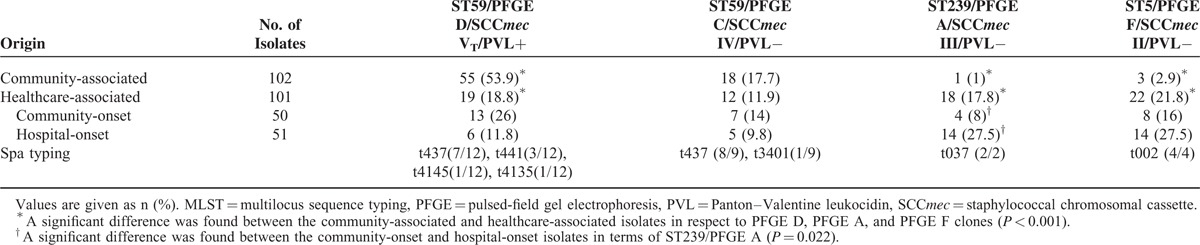
Table 4 shows the comparison of 4 major clones identified among the CA- and HA-MRSA isolates. ST59/PFGE type D/SCCmec VT/PVL(+)/t437 was significantly more commonly seen in CA-MRSA isolates (53.9%) than in HA-MRSA isolates (18.8%); ST5/PFGE type F/SCCmec II /PVL(−)/t002 (21.8%) and ST239/PFGE type A/SCCmec III/PVL(−)/t037 (17.8%) were significantly more detected in HA-MRSA isolates than in CA-MRSA isolates (2.9% and 1.0%, respectively) (all P < 0.001). Likewise, ST239/PFGE type A was significantly more commonly seen in HO-MRSA isolates (27.5%) than in CO-MRSA isolates (8.0%) (P = 0.022).
TABLE 4.
Comparison of Major Clones From Community-Associated and Healthcare-Associated Methicillin-Resistant Staphylococcus aureus Isolates
DISCUSSION
To our knowledge, this is the first study regarding molecularly characterizing all clinical MRSA isolates collected from any specimen sites in adult patients in Taiwan. The results showed a significant difference between CA- and HA-MRSA isolates as well as between CO- and HO-MRSA isolates in terms of the source of specimens, antibiotic susceptibility patterns, and molecular characteristics, a scenario similar to that seen in children in Taiwan. Clinically, the patients with CA-MRSA infection were younger and had better outcomes than those with HA-MRSA infection. These again suggest that CA-MRSA was a distinct pathogen from HA-MRSA in Taiwan. In the present study, CA-MRSA isolates accounted for 12.6% of all MRSA isolates, 18.4% of patients with MRSA infection were classified as CA-MRSA infection, and only 10% of MRSA isolates from inpatients were categorized as CA isolates. The prevalence of CA-MRSA among adult MRSA-infected patients in Taiwan may be underestimated, particularly inpatients, because this is a hospital-based study and performed in a tertiary hospital.
Clinically, the sources of clinical specimens obtained may reflect the disease spectrum. Similar to our previous study in children with same design,7 the most common source of specimens for CA-MRSA isolates in the present study was pus. However, the percentage of SSTI (70%) in adults was lower than that in children (92%), the ratio of bone and joint infection was higher in adults than in children (7.8% vs 1.9%, respectively). In 2000s in Taiwanese children, SSTI accounted for 72% to 86% of CA-MRSA infections and bone and joint infection accounted for 2% to 12%.5 In USA, 87% to 95.6% of CA-MRSA infections presented as SSTIs, and bone and joint infections accounted for up to 3% only.20–23 In addition, 12.7% of patients with CA-MRSA infections in the present study had invasive diseases, including osteomyelitis, necrotizing fasciitis, and necrotizing pneumonia, a rate higher than that reported from US (6%).20 However, none of the cases in the present study died. In contrast, HA-MRSA isolates were more commonly found in respiratory tract infection and bacteremia than CA-MRSA isolates, and thus contributed to a higher mortality, as shown in the present study.
As known, there was significant difference in antibiotic susceptibility between CA- and HA-MRSA isolates. Unlike a high susceptibility rate to erythromycin and clindamyin for CA-MRSA isolates from the USA,9 the susceptibility rate was only 17.7% for CA isolates in this study. Similar to previous reports from Taiwan,5 the largest susceptibility difference between CA- and HA-MRSA isolates24 was noted for TMP/SMX, to which 93% of CA isolates were susceptible, significantly higher than HA-MRSA isolates (66%). This scenario was also seen between CO- and HO-MRSA isolates in this study. TMP/SMX was suggested to use for simple SSTIs.24,25 However, the evidence of TMP/SMX to treat invasive or severe MRSA infections was still inadequate.24
The molecular characteristics of CA-MRSA isolates from adults in this study were similar to those from Taiwanese children.5 ST59/pulsotype D/SCCmec VT/PVL(+)/t437, named as Taiwan clone, was the most common clone and accounted for more than half of the clinical CA-MRSA isolates from adult patients, as in pediatric patients. Nearly 20% of the isolates were ST59/pulsotype C/SCCmec IV/PVL(−), which was also a major community clone in Taiwan but more frequently identified from colonized subjects in Taiwan.5,26–29 The clone of ST30/SCCmec IV/PVL(+)/t019, named as southwest Pacific clone and rarely reported from Taiwan previously, accounted for 7 (6.9%) CA-MRSA isolates and deserved further observation. Interestingly, 1 ST9/t899 isolate, a livestock-associated MRSA in Taiwan,30 was found in pus from a pig farmer hospitalized for skin and soft-tissue infection and subsequent osteomyelitis.
For molecular characteristic of HA-MRSA isolates in the present study, there were 3 major clones identified, namely CC 59 (32.7%), CC239 (29.7%), and CC5 (24.8%), which were consistent with an islandwide study in 2010.31 ST239/pulsotype A/SCCmec III (Hungarian or Brazilian clone), the previously most epidemic HA clone in Taiwan, decreased year by year since 2000 (from 73% in 2000 to 26% in 2010),31 and accounted for less than 20% in the current study. Its dominance was lost by the clone of ST5 and the community clone of ST59, both of which emerged in late 1990s, increased gradually and were among the major clones of bloodstream isolates up to 2010.3,31,32 In our previous study in children,7 ST 59 also accounted for 30.7% of HA-MRSA isolates and 40% of CO-MRSA. All of these findings indicated that the community clone had spread into hospital settings in Taiwan.
There are several limitations in this study. First, the clinical cases as well as the clinical isolates of MRSA were not collected all year round but for only 4 months, which limited the full epidemiologic features, since the case number was too huge to be handled. Second, not every HA-MRSA isolate, but selected isolates, were molecularly characterized. Third, clinical outcomes were not evaluated and correlated with HA- or CA-MRSA infections as well as genotypes of MRSA infections.
In conclusion, the characteristics of CA- and HA-MRSA isolates from Taiwanese adults were significantly different in terms of clinical disease spectrum, antibiotic susceptibility, and molecular characteristics. The major CA-MRSA clones were CC59/t437 and included ST59/pulsotype D/SCCmec VT/PVL(+) and ST59/pulsotype C/SCCmec IV/PVL(−). The major HA-MRSA clones were CC59/t437, CC239, and CC5. Further longitudinal molecular surveillance for MRSA and population-based studies are needed to provide more clinical information.
Acknowledgments
The authors thank Chang Gung Memorial Hospital (BMRP 236) for the support.
Footnotes
Abbreviations: CA = community-associated, CC = clonal complex, CO = community-onset, HA = healthcare-associated, HO = hospital-onset, MRSA = methicillin-resistant Staphylococcus aureus, PFGE = pulsed-field gel electrophoresis, PVL = Panton–Valentine leukocidin, SCC = staphylococcal cassette chromosome.
Y-JC and K-LL contributed equally to this work.
This study was supported by a grant from Chang Gung Memorial Hospital (BMRP 236).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010; 23:616–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLeo FR, Otto M, Kreiswirth BN, et al. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010; 375:1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CJ, Hsueh PR, Su LH, et al. Change in the molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream infections in Taiwan. Diagn Microbiol Infect Dis 2009; 65:199–201. [DOI] [PubMed] [Google Scholar]
- 4.Chuang YY, Huang YC. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Asia. Lancet Infect Dis 2013; 13:698–708. [DOI] [PubMed] [Google Scholar]
- 5.Huang YC, Chen CJ. Community-associated meticillin-resistant Staphylococcus aureus in children in Taiwan, 2000s. Int J Antimicrob Agents 2011; 38:2–8. [DOI] [PubMed] [Google Scholar]
- 6.Wu KC, Chiu HH, Wang JH, et al. Characteristics of community-acquired methicillin-resistant Staphylococcus aureus in infants and children without known risk factors. J Microbiol Immunol Infect 2002; 35:53–56. [PubMed] [Google Scholar]
- 7.Huang YC, Ho CF, Chen CJ, et al. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin Microbiol Infect 2008; 14:1167–1172. [DOI] [PubMed] [Google Scholar]
- 8.Wang JL, Chen SY, Wang JT, et al. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin Infect Dis 2008; 46:799–806. [DOI] [PubMed] [Google Scholar]
- 9.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003; 290:2976–2984. [DOI] [PubMed] [Google Scholar]
- 10.Institute CaLS. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. M100-s21. Clinical and Laboratories Standards Institute. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. M100-s21, 2011. [Google Scholar]
- 11.Huang YC, Su LH, Lin TY. Nasal carriage of methicillin-resistant Staphylococcus aureus among pediatricians in Taiwan. PLoS One 2013; 8:e82472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YC, Su LH, Wu TL, et al. Molecular epidemiology of clinical isolates of methicillin-resistant Staphylococcus aureus in Taiwan. J Clin Microbiol 2004; 42:307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YC, Lien RI, Su LH, et al. Successful control of methicillin-resistant Staphylococcus aureus in endemic neonatal intensive care units – a 7-year campaign. PLoS One 2011; 6:e23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995; 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo Y, Ito T, Ma XX, et al. Combination of multiplex pcrs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother 2007; 51:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira DC, de Lencastre H. Multiplex pcr strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2002; 46:2155–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of panton-valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 1999; 29:1128–1132. [DOI] [PubMed] [Google Scholar]
- 18.Enright MC, Day NP, Davies CE, et al. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 2000; 38:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmsen D, Claus H, Witte W, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 2003; 41:5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005; 352:1436–1444. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis 2005; 40:1785–1791. [DOI] [PubMed] [Google Scholar]
- 22.Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at driscoll children's hospital. Arch Pediatr Adolesc Med 2005; 159:980–985. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in Sanfrancisco, 2004–2005. Clin Infect Dis 2008; 46:1637–1646. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52:285–292. [DOI] [PubMed] [Google Scholar]
- 25.Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med 2014; 370:1039–1047. [DOI] [PubMed] [Google Scholar]
- 26.Chen CJ, Huang YC, Chiu CH, et al. Clinical features and genotyping analysis of community-acquired methicillin-resistant Staphylococcus aureus infections in Taiwanese children. Pediatr Infect Dis J 2005; 24:40–45. [DOI] [PubMed] [Google Scholar]
- 27.Huang YC, Su LH, Chen CJ, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without identifiable risk factors in northern Taiwan. Pediatr Infect Dis J 2005; 24:276–278. [DOI] [PubMed] [Google Scholar]
- 28.Huang YC, Hwang KP, Chen PY, et al. Prevalence of methicillin-resistant Staphylococcus aureus nasal colonization among Taiwanese children in 2005 and 2006. J Clin Microbiol 2007; 45:3992–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YC, Chao AS, Chang SD, et al. Association of Staphylococcus aureus colonization in parturient mothers and their babies. Pediatr Infect Dis J 2009; 28:742–744. [DOI] [PubMed] [Google Scholar]
- 30.Fang HW, Chiang PH, Huang YC. Livestock-associated methicillin-resistant Staphylococcus aureus in pigs and related personnel in Taiwan. PLoS One 2014; 9:e88826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CJ, Huang YC, Su LH, et al. Molecular epidemiology and antimicrobial resistance of methicillin-resistant Staphylococcus aureus bloodstream isolates in Taiwan, 2010. PLoS One 2014; 9:e101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YC, Su LH, Wu TL, et al. Changing molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates from a teaching hospital in northern Taiwan. J Clin Microbiol 2006; 44:2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]


