Abstract
Malignant ascites (MA) is most commonly observed in patients scheduled for epithelial ovarian cancer (EOC) surgery and is supposed as a major risk factor promoting perioperative hemodynamic deterioration. We aimed to assess the hemodynamic consequences of MA on systemic circulation in patients undergoing cytoreductive EOC surgery.
This study is a predefined post-hoc analysis of a randomized controlled pilot trial comparing intravenous solutions within a goal-directed algorithm to optimize hemodynamic therapy in patients undergoing cytoreductive EOC surgery. Ascites was used to stratify the EOC patients prior to randomization in the main study. We analyzed 2 groups according to the amount of ascites (NLAS: none or low ascites [<500 mL] vs HAS: high ascites group [>500 mL]). Differences in hemodynamic variables with respect to time were analyzed using nonparametric analysis for longitudinal data and multivariate generalized estimating equation adjusting the analysis for the randomized study groups of the main study.
A total of 31 patients in the NLAS and 16 patients in the HAS group were analyzed. Although cardiac output was not different between groups suggesting a similar circulatory blood flow, the HAS group revealed higher heart rates and lower stroke volumes during surgery. There were no differences in pressure-based hemodynamic variables. In the HAS group, fluid demands, reflected by the time to reindication of a fluid challenge after preload optimization, increased steadily, whereas stroke volume could not be maintained at baseline resulting in hemodynamic instability after 1.5 h of surgery. In contrast, in the NLAS group fluid demands were stable and stroke volume could be maintained during surgery. Clinically relevant associations of the type of fluid replacement with hemodynamic consequences were particularly observed in the HAS group, in which transfusion of fresh frozen plasma (FFP) was associated to an improved circulatory flow and reduced vasopressor and fluid demands, whereas the administration of artificial infusion solutions was related to opposite effects.
Malignant ascites >500 mL implies increased fluid demands and substantial alterations in circulatory blood flow during cancer surgery. Fresh frozen plasma transfusion promotes recovering hemodynamic stability in patients with malignant ascites >500 mL, in whom artificial infusion solutions could not prevent from hemodynamic deterioration.
INTRODUCTION
Epithelial ovarian cancer (EOC) is the most common cause of malignant ascites (MA).1 Malignant ascites is frequently observed in advanced stages of EOC and indicates tumor dissemination in the peritoneal cavity.2 The pathogenesis is complex, multifactorial determined, and primarily involves an increased production of peritoneal fluid due to altered vascular permeability and increased peritoneal neovascularization combined with an impaired lymphatic drainage from the peritoneal cavity due to obstructed lymphatic channels at a diaphragmatic level.3–5
As contemporary evidence on paracentesis prior to surgery is still limited and peritoneal fluid reaccumulates quickly,2 ∼50% of the patients scheduled for EOC surgery present with a substantial amount of MA on the day of surgery.6,7 Malignant ascites has recently been shown as a major determinant for the interdisciplinary clinical course in patients with EOC undergoing cytoreductive surgery with a significant impact on anesthesiological treatment and postoperative morbidity.6 Whereas major perioperative circulatory complications were of a moderate frequency in patients with none or low ascites (<500 mL), these were very frequently observed in patients with high amount of ascites (>500 mL).6
In this regard, MA >500 mL is supposed as a major risk factor promoting perioperative hemodynamic deterioration, but detailed data of hemodynamic consequences of MA on systemic circulation in patients undergoing cytoreductive EOC surgery are still limited. Furthermore, data on the appropriate type of intraoperative fluid replacement with respect to hemodynamic stability in patients with high amounts of MA are crucial but still not available.
Therefore, this study aimed to compare a none or low ascites group (<500 mL) with a high ascites group (>500 mL) regarding time courses of hemodynamic variables, time courses of variables of fluid, transfusion, and vasopressor demands; and to investigate the relation of administered artificial infusion solutions and fresh frozen plasma with hemodynamic variables as well as vasopressor and fluid demands in patients undergoing EOC surgery.
METHODS
This study is a predefined post-hoc analysis of a previously published randomized controlled pilot trial comparing a balanced crystalloid with a balanced colloid within a goal-directed hemodynamic algorithm in patients with advanced primary epithelial ovarian cancer undergoing cytoreductive surgery (BalaCriCo trial, ISRCTN 53154834).8 This subanalysis investigates the significance of malignant ascites on systemic circulation during surgery that was not reported in the previous publication of the main study.
Ethical approval was given by the Ethical Committee (No. EK 12 581/08) and the competent German authority (No. 4034705) and was internationally subscribed. The trial was conducted at Charité, University Medicine Berlin, Campus Virchow Klinikum, Berlin, Germany, from May 2009 to March 2011 and written informed consent was obtained from all patients.
Study Design and Patients
As the main study was a randomized controlled trial the patients were randomly assigned to receive either a balanced crystalloid or a balanced colloid solution for intraoperative hemodynamic optimization within a goal-directed algorithm.
On the day prior surgery, before randomization a consultant gynecologist performed an abdominal ultrasound examination including screening for ascites. If ascitic fluid was present, the amount was estimated by the formula: measure the maximal fluid depth or depth of the deepest pocket (AP diameter) (cm) × 100 = volume (mL). The result of the ultrasound examination in relation to ascites was the stratification criteria during the randomization process. According to the presence and amount of ascites we analyzed 2 groups in this subanalysis (NLAS: none or low ascites [<500 mL] vs HAS: high ascites group [>500 mL]).
The entire data for this subanalysis were obtained from the per-protocol groups of the BalaCriCo trial resulting in a subset of 48 patients. For this subanalysis, the patients of the per-protocol groups were merged and regrouped by ascites. One patient had to be excluded, as preoperative ultrasound screening was not performed. Consequently, a subset of 47 patients was eligible for statistical analysis (Flow Diagram Fig. 1).
FIGURE 1.
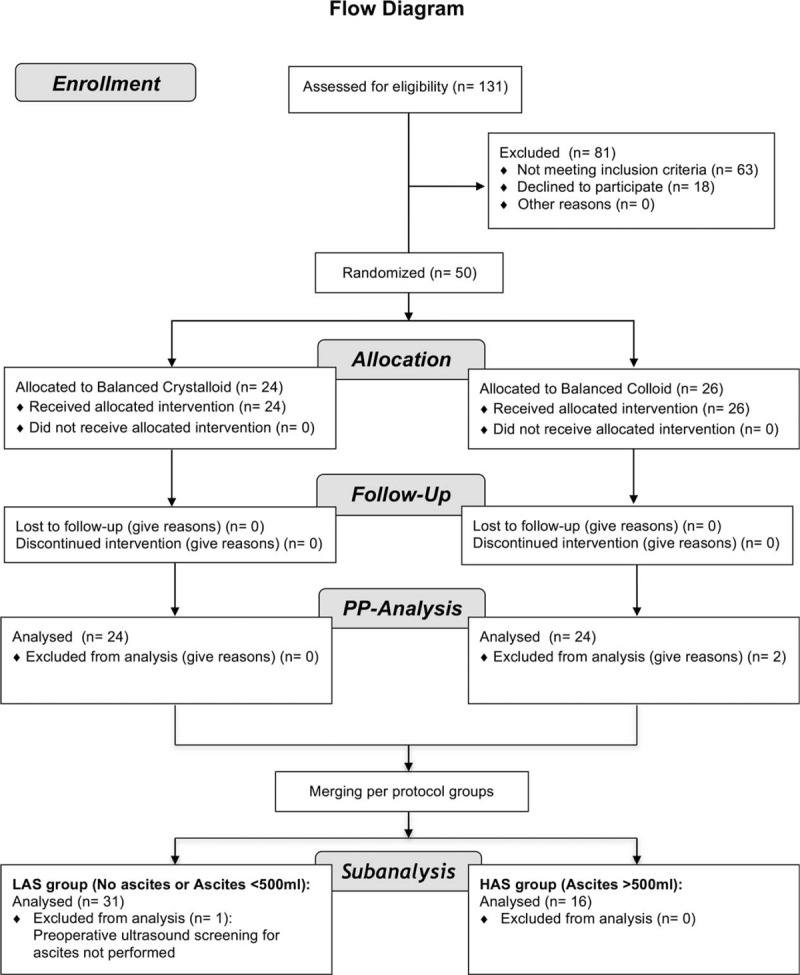
Advanced flow diagram of the study with respect to the subanalysis.
Intraoperative hemodynamic Management
The hemodynamic management was performed according to an outcome-based goal-directed hemodynamic algorithm guided by the oesophageal Doppler monitor (ODM, CardioQ-ODMTM, Deltex Medical, Chichester, UK).9
Briefly, after induction of anesthesia and establishing the hemodynamic monitoring hemodynamic optimization was started. First an initial fluid challenge of 200 mL of intravenous study fluid was given over 5 min. If stroke volume measured by ODM (SVODM) failed to rise by ≥10% no further fluid challenge was given. If SVODM increased of ≥10%, additional fluid challenges with an intravenous bolus of 200 mL were given until no further increase of SVODM of ≥10% could be measured. After a period of 15 min or clinically relevant hemodynamic changes of mean arterial pressure or heart rate SVODM was measured again and a decrease of >10% compared with SVODM after the last fluid challenge reindicated a further fluid challenge.
Fluid challenges were performed during the entire course of surgery as indicated by the goal-directed hemodynamic algorithm and conducted either with a balanced crystalloid (Jonosteril, Fresenius Kabi, Bad Homburg, Germany) or a balanced colloid solution (Volulyte, 6%, 130/0.4, Fresenius Kabi) according to the randomization. At the maximum dose of the study fluid (when 50 mL kg−1 body weight was reached), the fluid challenges were conducted by transfusion of fresh-frozen plasma (FFP). In addition, mean arterial pressure was maintained with bolus or continuous administration of norepinephrine and positive inotropic drugs were given if the cardiac index dropped <2.5 L min−1 m−2, while stroke volume could not be raised further by volume administration.
Endpoints
We compared 2 groups (NLAS group: none or low ascites [<500 mL] vs HAS group: high ascites group [>500 mL]) regarding: (1) time courses of hemodynamic variables including: heart rate (HR), stroke volume (SV), cardiac output (CO), corrected flow time (FTc), mean arterial pressure (MAP), and central venous pressure (CVP); (2) time courses of variables of fluid, transfusion, and vasopressor demands including: time to reindication of a fluid challenge (TTRI), total volume administered within the goal-directed therapy, transfusion of fresh frozen plasma (FFP), and norepinephrine (NE) requirements.
Time to reindication of a fluid challenge was introduced as a new approach to standardized characterize the patient's individual fluid demands at different time points during surgery to maintain stroke volume. Time to reindication of a fluid challenge was calculated as the time frame from preload optimization to reindication of a fluid challenge within the used goal-directed algorithm (Fig. 2).
FIGURE 2.
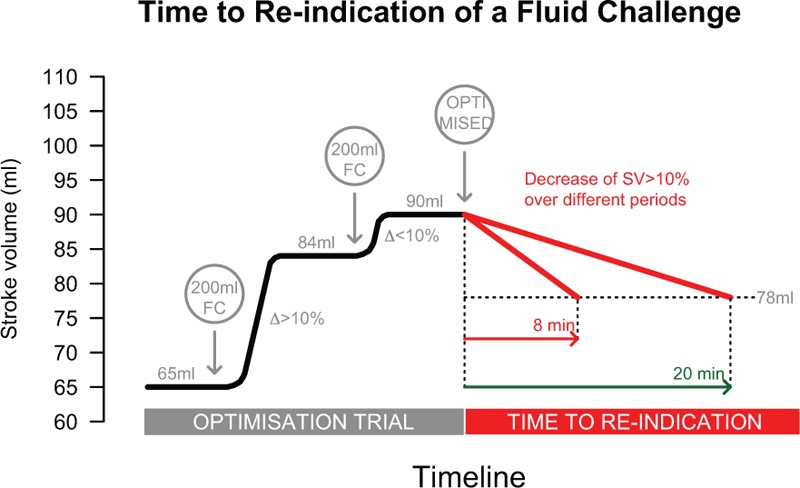
Determination of time to reindication (TTRI) of a fluid challenge within the goal-directed algorithm. During an optimization trial fluid challenges (FC) with 200 mL of balanced crystalloid or colloid or fresh frozen plasma were administered. If a fluid challenge results in an increase of stroke volume of >10%, further FC are given until no further increase of SV ≥10% could be measured. At this time the patient is considered as preload optimized. After a period of 15 min or clinically relevant hemodynamic changes of mean arterial pressure or heart rate SV is measured again and a decrease of >10% compared with SV after the last fluid challenge reindicated an optimization trial. The TTRI is calculated as the time frame from preload optimization to reindication of the next fluid challenge FC = fluid challenge, SV = stroke volume, TTRI = time to reindication.
Furthermore, we investigated the relation of time courses of SV, HR, NE, and TTRI with the time courses of administration of intravenous infusion solutions and fresh frozen plasma during surgery.
Statistical Analysis
To check the present data distributions, we used the graphic inspection by box plots, QQ plots, and histograms. Furthermore, we arithmetical examined the distributions by skewness. Because of limited sample sizes and non-normal distributions of the observations, data were expressed as median (25%, 75% quartiles), or frequencies (%), respectively. Therefore, differences between ascites groups in terms of continuous data were tested by using the Mann–Whitney U test for independent groups, whereas frequencies were tested by Fisher's exact test.
Due to the repeated measurements, differences in hemodynamic variables with respect to time between ascites groups were analyzed by using nonparametric analysis for longitudinal data (NparLD) as described by Brunner and colleagues.10 Therefore, all time points were simultaneously compared on the corresponding response curves. The following hypotheses have been tested with those analyses: differences between groups over time (group) and systematic changes in time separately for every group (time course). These analyses were complemented by adjusting for randomized study groups (crystalloid and colloid intervention group) of the main study (group (adjusted∗)) using the general estimating equation (GEE) as described by Liang and Zeger.11 To estimate the required sample size for multiple analysis with continuous outcomes, we followed the rule of Harrell for orientation.12 In this regard, the total sample size should be divided by 10 to 20 to obtain the number of predictors for a stable model. In our case, we included 48 patients for analysis resulting in a maximum of 2 to 3 predictors allowed. In this respect, the minimum requirements were fulfilled as we included 2 independent variables (ascites groups and randomized study groups) in the GEE model.
To study the association of time courses of administration of intravenous infusion solutions and transfusion of fresh frozen plasma with the time courses of SV, HR, NE, and TTRI during surgery, also the above methods for repeated measurements were used. Adjusted odds ratios (OR) with 95% confidence interval were calculated. Regarding the clinical interpretation, ORs of intravenous infusion solutions and fresh frozen plasma were scaled for the administration of 1 fluid challenge (200 mL) and 1 unit, respectively.
A 2-tailed P value of 0.05 was considered statistically significant. All numerical calculations were performed with IBM©SPSS© Statistics, Version 20,© Copyright 1989, 2010 SPSS Inc. and the free software from R project for Statistical Computing, Version 3.0.2 (R-packages used: “foreign” release 0.8–63, “gplots” release 2.16.0, “nparLD” release 2.1, “plotrix” release 3.4–8).
RESULTS
Patient Characteristics
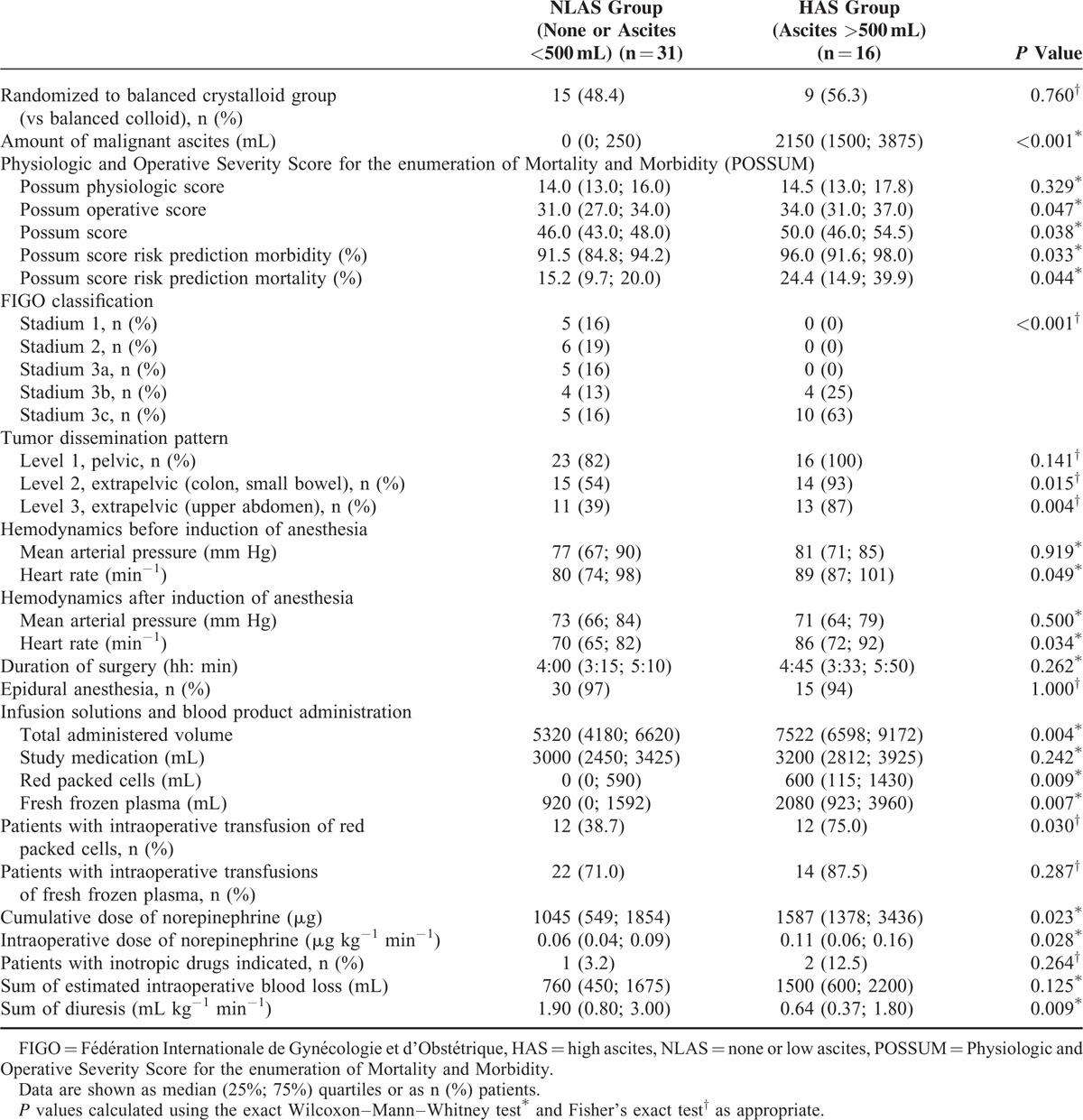
A total of 31 patients in the NLAS and 16 patients in the HAS group were analyzed. There were no differences in baseline characteristics except for lower preoperative albumin levels in the HAS group (Table 1). Patients randomized in the main study to receive balanced crystalloid or colloid were similarly distributed among NLAS and HAS groups (P = 0.760) (Table 2). The amount of malignant ascites in the NLAS and HAS groups was 0 (0; 250) and 2150 (1500; 3875) mL (P < 0.001), respectively (Table 2). Whereas scores assessing preoperative physiological condition and comorbidities were not different between groups, patients in the HAS group had a more progressed cancer disease and a higher risk prediction for perioperative morbidity and mortality. With respect to hemodynamics before and after induction of anesthesia, the HAS group had higher heart rates compared with the NLAS group, whereas mean arterial pressure was not different.
TABLE 1.
Baseline Characteristics of the Study Patients
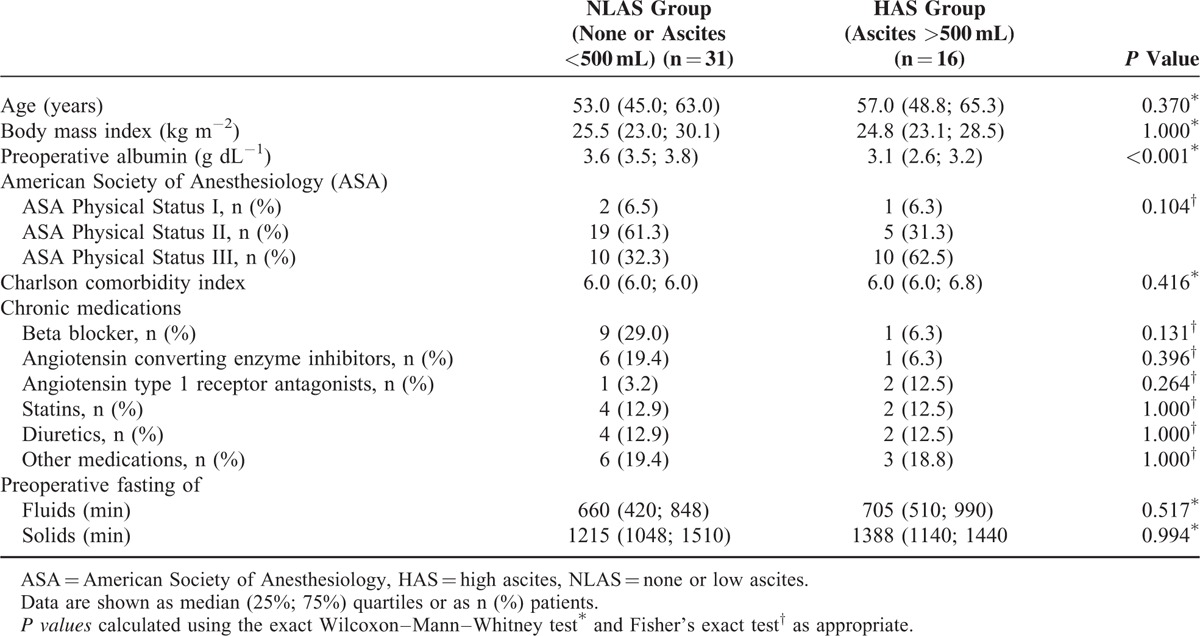
TABLE 2.
Intraoperative and Tumor Data of the Study Patients
Comparison of Time Courses of Hemodynamic Variables
While CO was not different between ascites groups suggesting a similar circulatory flow during the course of surgery, the HAS group revealed higher HR, lower SV, and lower FTc indicating that circulatory flow was not equivalent between groups (Fig. 3). There were no differences in pressure-based hemodynamic variables such as MAP and CVP.
FIGURE 3.
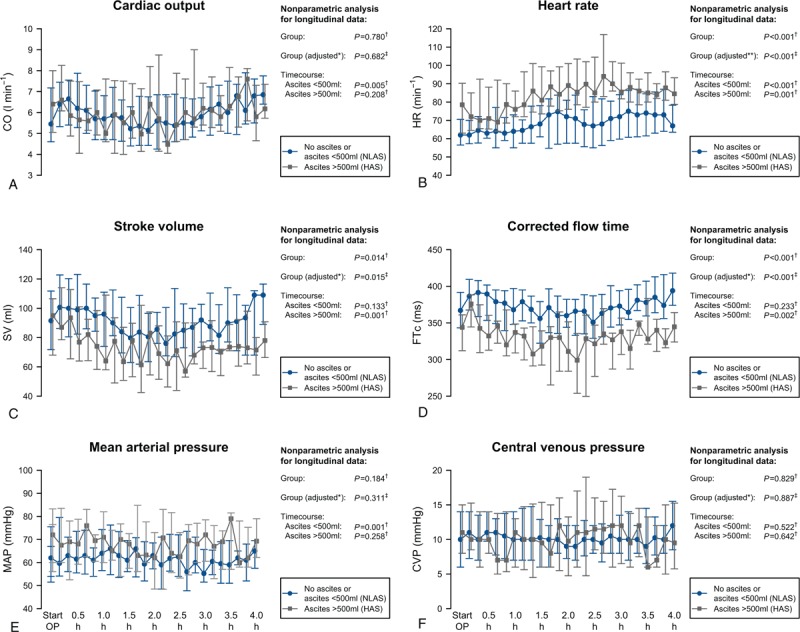
Intraoperative course of cardiac output (A), heart rate (B), stroke volume (C), corrected flow time (D), mean arterial pressure (E), and central venous pressure (F). Data are shown as median and (25%; 75%) quartiles. Results of the nonparametric analysis with respect to time courses are indicated. P Values calculated using NparLD† or GEE‡. ∗Adjusted for randomized study groups of the main study. ∗∗Adjusted for randomized study groups of the main study and beta blocker therapy. GEE = general estimating equation, NparLD = nonparametric analysis for longitudinal data.
In the HAS group, SV and FTc started to decrease steadily after beginning of surgery and could not be maintained at baseline reaching critically reduced values after 1.5 h of surgery. In contrast, in the NLAS group, SV and FTc could be mainly maintained and did not change significantly during the course of surgery. Although the HAS group had higher HR compared to the NLAS group, both groups showed a consecutive increase of HR during surgery. Generalized estimating equations regression analysis confirmed that the observed differences in time courses of HR, SV, and FTc between NLAS and HAS groups were independently associated to ascites groups after adjusting for the randomized study groups of the main study.
Comparison of Time Courses of Variables of Fluid, Transfusion, and Vasopressor Demands
During surgery, the time to reindication of a fluid challenge (TTRI) was significantly shorter in the HAS group. In the HAS group, after beginning of surgery TTRI decreased steadily reaching minimum values after 2 h and then showed an increase again reaching baseline values at the end of surgery (Fig. 4). In contrast, in the NLAS group the TTRI increased slightly and showed no substantial decrease during surgery. Patients in the HAS group received a higher amount of total volume within the GDT with more fresh frozen plasma (FFP) and red packed cells administered (Table 2, Fig. 4). Although total volume administration was similar between ascites groups during the first hour of surgery, the curves started to diverge 1 h after beginning of surgery. Furthermore, the HAS group required higher norepinephrine administrations during surgery, resulting in higher cumulative doses at the end of surgery (Table 2, Fig. 4). Similarly to total volume administration, there was a substantial increase in NE doses after 1.5 h in the HAS group. According to GEE regression analysis, the differences in TTRI, total volume administered, transfusion of FFP and NE administration during surgery were independently associated to ascites groups after adjusting for the randomized study groups of the main study.
FIGURE 4.
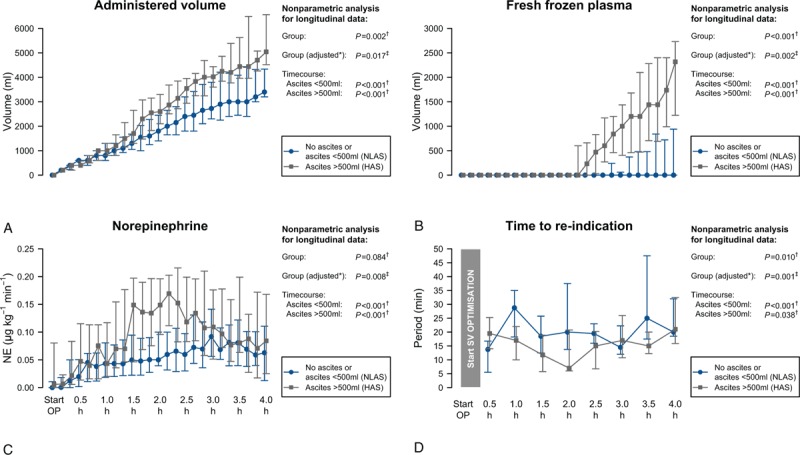
Intraoperative course of total administered volume (A), amount of transfused fresh frozen plasma (B), norepinephrine dose (C), and time to reindication of a fluid challenge (D). Data are shown as median and (25%; 75%) quartiles. Results of the nonparametric analysis with respect to time courses are indicated. P values calculated using NparLD† or GEE‡.∗Adjusted for randomized study groups of the main study. GEE = general estimating equation, NparLD = nonparametric analysis for longitudinal data.
Relation of the Type of Fluid Replacement with SV, HR, NE, and TTRI
Clinically relevant associations of the type of fluid replacement with hemodynamic consequences were particularly observed in the HAS group (Fig. 5). In the HAS group, administration of fresh frozen plasma was associated with increases of SV, prolongations of TTRI and decreases of HR and NE administration. In contrast, the transfusion of intravenous infusion solutions was associated with decreases of SV, shortenings of TTRI and increases of HR and NE administration. Although the relations were less considerable in the LAS group, the administration of intravenous infusion solutions was related to decreases of SV and increases of NE administration, whereas FFP transfusion was related to decreased NE administration.
FIGURE 5.
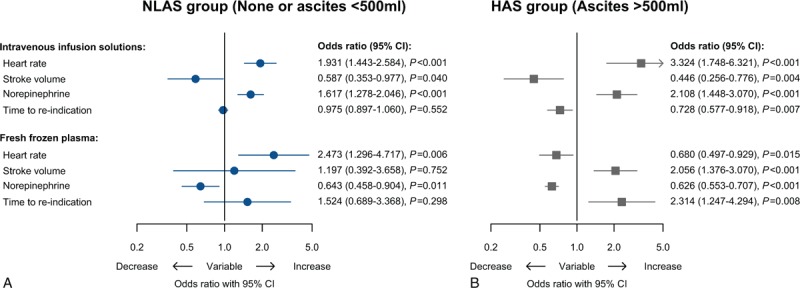
Forest plot visualizing the association of administered infusion solutions and fresh frozen plasma with heart rate and stroke volume, norepinephrine dose, and time to reindication by presenting adjusted odds ratios (OR) obtained from GEE regression analysis for longitudinal data with adjustment considering multiple measurements per patient. Odds ratios are drawn on a logarithmic scale. Odds ratios of heart rate, stroke volume, norepinephrine, and time to reindication are scaled for changes of min−1, mL, 0.01 μg kg−1 min−1 and min, respectively. GEE = general estimating equation, OR = odds ratios.
DISCUSSION
Our findings address important issues regarding hemodynamic consequences within a goal-directed algorithm in patients with malignant ascites undergoing EOC surgery.
The main findings of the study are (1) that although cardiac output was not different, higher heart rates, lower stroke volumes, and lower corrected flow times in the high ascites group indicate that circulatory flow was not equivalent between groups; (2) that in the high ascites group after start of surgery fluid demands increased steadily whereas stroke volume decreased continuously resulting in hemodynamic instability after 1.5 h of surgery with massive fluid and vasopressor requirements; (3) that clinically relevant associations of the type of fluid replacement were particularly observed in the high ascites group, in which transfusion of FFP was associated to an improved circulatory flow, hemodynamic stability, and reduced fluid demands, whereas the administration of artificial infusion solutions was related to opposite effects.
Although cardiac output was similar in NLAS and HAS groups, stroke volume (SV) and heart rate (HR) as determinants of circulating blood flow showed substantial differences during the course of surgery. Whereas a maintained SV during surgery has been widely shown to improve outcome13 and intraoperative tachycardia was related to increased mortality,14 little is known about consequences of an elevated HR below tachycardic threshold. However, recent data indicate that lower HR is related to improved microvascular blood flow and blood flow heterogeneity suggesting that HR is not only a multiplier of SV to generate cardiac output.15 In this respect, despite a similar cardiac output, the combination of higher HR and lower SV suggests alterations of circulatory blood flow in patients with high amount of ascites.
In the HAS group, the fluid demands increased steadily during the first 1.5 h after beginning of surgery reflected by a continuously decreasing time to reindication of fluid optimization (TTRI), whereas simultaneously stroke volume could not be maintained at baseline values resulting in hemodynamic instability after 1.5 h of surgery. From a rational view, detrimental effects of MA on the circulation are due to the impact on fluid homeostasis prior and during surgery while considering consequences of loss of MA following laparotomy. In patients with MA, the exudative loss is predominantly caused by a massive increase of vascular permeability and neovascularization of small peritoneal blood vessels.16 Vascular endothelial growth factor released by tumor cells is a major determinant altering vascular permeability and promoting uncontrolled vascular growth.17 The combination of substantial neoangionesis of nonmatured peritoneal blood vessels with massive hyperpermeability results in a substantial fluid shift from the intravascular space to the peritoneal cavity and is considered as a major mechanism for ongoing fluid loss during surgery and might explain the substantial fluid demands in the high ascites group. As patients with MA commonly present with elevated intraabdominal pressures,18 the rapid pressure decrease following laparotomy is hypothesized to augment the fluid shift due to an increase of intravascular to peritoneal cavity hydrostatic pressure gradient and to cause changes in splanchnic circulation. A rapid decrease of external pressure on the splanchnic vasculature is supposed to be followed by a reactive release of vasodilative mediators increasing splanchnic arterial blood flow. In this regard, an increased arterial splanchnic inflow results not only in an elevated hydrostatic pressure in the peritoneal blood vessels but causes a blood pooling within the splanchnic veins due to the flow–pressure–volume relation in the splanchnic circulation.19,20 Blood pooling decreases mean systemic filling pressure and results in a reduced venous return pressure gradient.19,21 In this respect, an impaired venous return might explain the rapid onset of a decreasing stroke volume after start of surgery in the high ascites group whereas there were no signs for a compensation by release of external pressure of the large intra-abdominal veins. In addition, the low albumin concentration in patients with high amounts of ascites might affect circulatory blood flow. As blood albumin contributes up to 75% to 80% to maintain the colloid osmotic pressure, a reduced albumin concentration prior surgery is supposed to affect intravascular and tissue fluid distribution, which might contribute to a decreased intravascular volume at the time of surgery.22
Knowledge on the appropriate type of fluid replacement in patients with high amounts of MA undergoing cytoreductive surgery is crucial but still limited.23 Our findings show that clinically relevant associations of the type of fluid replacement with hemodynamic consequences were particularly observed in the high ascites group, in which administering artificial infusion solutions was associated to an impaired circulatory flow and impaired hemodynamic stability, whereas the administration of FFP was related with recovering hemodynamic stability reflected by increases of SV, prolongations of TTRI, and decreases of HR and NE administration. To date, FFP is used frequently during surgery, but its efficacy is controversial whereas its use involves several serious risks.24,25 Currently, there is an ongoing debate about the effect of transfusion-related immune modulation of different blood products but to date, there is no conclusive evidence that transfusion of FFP in patients undergoing cancer surgery promotes cancer recurrence and decreases overall survival.26–28 With respect to the perioperative period, the latest guideline published by the American Association of Blood Banks (AABB) has recommended using FFP only during massive transfusion after trauma and in patients with warfarin therapy-related intracranial hemorrhage.29 However, they could not recommend for or against transfusion of plasma for patients undergoing surgery in the absence of massive transfusion owing to the evidence addressing this practice was of very low quality.29 In this regard, our findings suggest that FFP transfusion could help recovering hemodynamic stability in patients with high amount of malignant ascites undergoing cancer surgery in whom intravenous infusion solutions could not prevent from hemodynamic deterioration. In this context, the link between FFP transfusion and reconstitution of hemodynamic stability is hypothesized to be multifactorial related. In contrast to any intravenous infusion solution, FFP contains significant content of proteins while the percentage of albumin of ∼60% is comparable to normal serum.30 In addition, FFP has a secondary hyperosmolal effect due a rapid metabolism of the preservation solutions’ citrate to hydrogen carbonate ions in the liver with a 1:3 ratio.31 Furthermore, whereas FFP contains adequate levels of hemostatic factors,32 recent in vitro and in vivo studies indicate that FFP transfusion is related to a reduced vascular endothelial cell permeability and to a decreased expression of endothelial adhesion markers.33–35 These findings were recently confirmed in critical ill patients indicating the role of FFP transfusion to preserve endothelial integrity.36 In summary, to date, the reconstitution of hemodynamic stability observed after FFP administration in patients with MA sustaining substantial exudative losses during surgery is supposed to be attributed by replacing albumin content in circulating blood volume, administering a secondary hyperosmolal solution, and restoring and maintaining endothelial integrity.
The study has some limitations. First, post-hoc analyses of randomized controlled trials often have to handle heterogeneous data and the interventions usually constitute the most important confounding factors. In our case, the main study revealed significant differences in stroke volume, corrected flow time, and fluid administration between the crystalloid and colloid intervention groups. However, using advanced statistical methods allowed us to adjust the analyses of time courses of all investigated endpoints for the intervention groups of the main study providing valid results for this post hoc analysis and minimizing the potential to undermine the conclusions. Second, implications regarding the beneficial effects of FFP with respect to hemodynamic stability have to be drawn carefully as this study was not designed to compare FFP with intravenous infusion solutions and consequently FFP transfusion was started when maximum dose of the study fluid was reached whereas the findings are based on regression analysis not allowing simply stating causation. However, when revealing interesting associations a potential causal relationship has to be considered, discussed, further investigated, and eventually confirmed or rejected.
In summary, malignant ascites >500 mL is related to increased fluid demands and substantial alterations in circulatory blood flow during cancer surgery resulting in hemodynamic instability after 1.5 h of surgery. Fresh frozen plasma administration could help recovering hemodynamic stability in patients with malignant ascites >500 mL, in whom intravenous infusion solutions could not prevent from hemodynamic deterioration.
ACKNOWLEDGMENTS
The authors would like to thank the BalaCriCo study group members for their support in conduction the study, Ansgar Jones, MD, and Olga Müller, MD, for participating recruiting patients and Jean-Philipp Zallet, MD, Heike Sieglitz, MD, Kathrin Solzbach, MD, David Liehre, MD, and Julienne Köhler, MD, for the data acquisition.
Department to which the work should be attributed: Department of Anaesthesiology and Intensive Care Medicine, Department of Gynaecology, Campus Charité Mitte and Campus Virchow, Klinikum, Charité- University Medicine Berlin, Berlin, Germany.
Footnotes
Abbreviations: ASA = American Society of Anesthesiology, CO = cardiac output, CVP = central venous pressure, EOC = epithelial ovarian cancer, FFP = fresh frozen plasma, FIGO = Fédération Internationale de Gynécologie et d’Obstétrique, FTc = corrected flow time, GDT = goal-directed therapy, GEE = generalized estimating equations, HAS = high ascites, HR = heart rate, MA = malignant ascites, MAP = mean arterial pressure, NE = norepinephrine, NparLD = nonparametric analysis for longitudinal data, NLAS = none or low ascites, ODM = oesophageal Doppler monitor, OR = odds ratio, POSSUM = Physiologic and Operative Severity Score for the enumeration of Mortality and Morbidity, SV = stroke volume, TTRI = time to reindication.
∗ Presented in part at the European Society of Anaesthesiology Euroanaesthesia 2015 Annual Meeting in Berlin, May 2015.
OH and CF equally contributed to this study.
Authors’ contributions—study concept, design of the study: AF, CS. Acquisition of data: OH, CF, KP, MK, JS, AF. Interpretation of data: OH, CF, KP, CS, AF. Statistical analysis: AK, OH, CS, AF. Drafting of the manuscript: OH, CF, CS, AF. Critical revision of the manuscript for important intellectual content: All authors. Final revision of manuscript: AF. Obtained funding: CS. Study supervision: AF, CS.
Funding: this research was an investigator-initiated study. It was supported by an unrestricted grant by Fresenius Kabi, Bad Homburg, Germany. The implementation of the ODM technology in the department was supported by Deltex Medical by an unrestricted grant unrelated to this study. The funders had no input into, or control over, study design, data collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Ethical approval: Ethical approval was given by the Ethical Committee of Charité - University Medicine Berlin (No. EK 12 581/08) and the competent German authority (No. 4034705).
The original study was internationally subscribed: BalaCriCo, ISRCTN 53154834 at the ISRCTN Registry (registered 27/08/2009, www.isrctn.com/).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol 2007; 18:945–949. [DOI] [PubMed] [Google Scholar]
- 2.Woopen H, Sehouli J. Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res 2009; 29:3353–3359. [PubMed] [Google Scholar]
- 3.Cavazzoni E, Bugiantella W, Graziosi L, et al. Malignant ascites: pathophysiology and treatment. Int J Clin Oncol 2013; 18:1–9. [DOI] [PubMed] [Google Scholar]
- 4.Sangisetty SL, Miner TJ. Malignant ascites: a review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg 2012; 4:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamsma JT, Keizer HJ, Meinders AE. Pathogenesis of malignant ascites: Starling's law of capillary hemodynamics revisited. Ann Oncol 2001; 12:1353–1357. [DOI] [PubMed] [Google Scholar]
- 6.Feldheiser A, Braicu EI, Bonomo T, et al. Impact of ascites on the perioperative course of patients with advanced ovarian cancer undergoing extensive cytoreduction: results of a study on 119 patients. Int J Gynecol Cancer 2014; 24:478–487. [DOI] [PubMed] [Google Scholar]
- 7.Sehouli J, Senyuva F, Fotopoulou C, et al. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J Surg Oncol 2009; 99:424–427. [DOI] [PubMed] [Google Scholar]
- 8.Feldheiser A, Pavlova V, Bonomo T, et al. Balanced crystalloid compared with balanced colloid solution using a goal-directed haemodynamic algorithm. Br J Anaesth 2013; 110:231–240. [DOI] [PubMed] [Google Scholar]
- 9.Feldheiser A, Conroy P, Bonomo T, et al. Development and feasibility study of an algorithm for intraoperative goaldirected haemodynamic management in noncardiac surgery. J Int Med Res 2012; 40:1227–1241. [DOI] [PubMed] [Google Scholar]
- 10.John Wiley & Sons, Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. 2002. [Google Scholar]
- 11.Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika 1986; 73:13–22. [Google Scholar]
- 12.Springer, Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. 2001. [Google Scholar]
- 13.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg 2011; 112:1392–1402. [DOI] [PubMed] [Google Scholar]
- 14.Reich DL, Bodian CA, Krol M, et al. Intraoperative hemodynamic predictors of mortality, stroke, and myocardial infarction after coronary artery bypass surgery. Anesth Analg 1999; 89:814–822. [DOI] [PubMed] [Google Scholar]
- 15.Morelli A, Donati A, Ertmer C, et al. Microvascular effects of heart rate control with esmolol in patients with septic shock: a pilot study. Crit Care Med 2013; 41:2162–2168. [DOI] [PubMed] [Google Scholar]
- 16.Nagy JA, Masse EM, Herzberg KT, et al. Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res 1995; 55:360–368. [PubMed] [Google Scholar]
- 17.Cheng D, Liang B, Kong H. Clinical significance of vascular endothelial growth factor and endostatin levels in the differential diagnosis of malignant and benign ascites. Med Oncol 2012; 29:1397–1402. [DOI] [PubMed] [Google Scholar]
- 18.Gotlieb WH, Feldman B, Feldman-Moran O, et al. Intraperitoneal pressures and clinical parameters of total paracentesis for palliation of symptomatic ascites in ovarian cancer. Gynecol Oncol 1998; 71:381–385. [DOI] [PubMed] [Google Scholar]
- 19.Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology 2008; 108:735–748. [DOI] [PubMed] [Google Scholar]
- 20.Gelman S, Mushlin PS. Catecholamine-induced changes in the splanchnic circulation affecting systemic hemodynamics. Anesthesiology 2004; 100:434–439. [DOI] [PubMed] [Google Scholar]
- 21.Magder S. Bench-to-bedside review: an approach to hemodynamic monitoring—Guyton at the bedside. Crit Care 2012; 16:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth 2000; 85:599–610. [DOI] [PubMed] [Google Scholar]
- 23.Vorgias G, Iavazzo C, Mavromatis J, et al. Determination of the necessary total protein substitution requirements in patients with advanced stage ovarian cancer and ascites, undergoing debulking surgery. Correlation with plasma proteins. Ann Ssurg Oncol 2007; 14:1919–1923. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Stanworth S, Hopewell S, et al. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion 2012; 52:1673–1686.quiz 1673. [DOI] [PubMed] [Google Scholar]
- 25.Puetz J. Fresh frozen plasma: the most commonly prescribed hemostatic agent. J Thrombosis Haemost 2013; 11:1794–1799. [DOI] [PubMed] [Google Scholar]
- 26.Shiba H, Ishida Y, Haruki K, et al. Negative impact of fresh-frozen plasma transfusion on prognosis after hepatic resection for liver metastases from colorectal cancer. Anticancer Res 2013; 33:2723–2728. [PubMed] [Google Scholar]
- 27.Tomimaru Y, Wada H, Marubashi S, et al. Fresh frozen plasma transfusion does not affect outcomes following hepatic resection for hepatocellular carcinoma. World J Gastroenterol 2010; 16:5603–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cata JP, Wang H, Gottumukkala V, et al. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth 2013; 110:690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion 2010; 50:1227–1239. [DOI] [PubMed] [Google Scholar]
- 30.Ewalenko P, Deloof T, Peeters J. Composition of fresh frozen plasma. Crit Care Med 1986; 14:145–146. [DOI] [PubMed] [Google Scholar]
- 31.Zander R. [Physiology and clinical aspects of the extracellular bicarbonate pool: plea for cognizant use of HCO3-]. Infusionstherapie Transfusionsmedizin 1993; 20:217–235. [PubMed] [Google Scholar]
- 32.Theusinger OM, Baulig W, Seifert B, et al. Relative concentrations of haemostatic factors and cytokines in solvent/detergent-treated and fresh-frozen plasma. Br J Anaesth 2011; 106:505–511. [DOI] [PubMed] [Google Scholar]
- 33.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg 2011; 112:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pati S, Matijevic N, Doursout MF, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma 2010; 69 Suppl 1:S55–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Z, Pati S, Potter D, et al. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock 2013; 40:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straat M, Muller MC, Meijers JC, et al. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care 2015; 19:163. [DOI] [PMC free article] [PubMed] [Google Scholar]


