Abstract
Conflicting results about the prognostic value of Glasgow Prognostic Score (GPS) in hepatocellular carcinoma (HCC) patients have been reported. We searched the available articles and performed the meta-analysis to clarify the predictive value of GPS in HCC patients’ outcome.
A systematic literature search was conducted using PubMed (Medline), Embase, Cochrane Library, Web of Science, ChinaInfo, and Chinese National Knowledge Infrastructure for all years up to September 2015. Studies analyzing the relationship of GPS and survival outcome were identified. Hazard ratio (HR) with 95% confidence interval (CI) was calculated to assess the risk.
A total of 10 studies were finally enrolled in the meta-analysis. The pooled estimates demonstrated a significant relationship between elevated GPS and inferior overall survival in patients with HCC (HR = 2.156, 95% CI: 1.696–2.740, P < 0.001). Patients with increased GPS had a tendency toward shorter progression-free survival (HR = 1.755, 95% CI: 0.943–3.265, P = 0.076). And elevated GPS was found to be significantly associated with advanced Child–Pugh class (odds ratio = 25.979, 95% CI: 6.159–109.573, P < 0.001). The publication bias analysis revealed that there was publication bias in the meta-analysis.
Glasgow Prognostic Score may be an independent prognostic factor in patients with HCC. More well-designed studies with adequate follow-up duration are warranted.
INTRODUCTION
Hepatocellular carcinoma (HCC) ranks as the sixth most common malignant cancer and the third most frequent cause of cancer-related mortalities worldwide.1 Despite the dramatic advancement in the surgical techniques, loco-regional treatment options and molecular target therapy, the prognosis of HCC is still dismal compared with other solid tumors. Currently, the clinical management toward malignant cancers chiefly depends on their clinical stage. Several clinical staging systems for HCC, including American Joint Committee on Cancer staging system,2 Barcelona Clinic Liver Cancer staging system,3 the Okuda classification,4 Cancer of the Liver Italian Program scoring system,5 and the Japan Integrated Staging score 6 have already been proposed. The accuracy of all the current ongoing staging systems in predicting the patients’ prognosis, however, is not satisfactory. Therefore, it is urgent to identify the prognostic index beyond the scope of current clinical staging systems to gain a rational classification of the patients according to their prognosis.
It has long been recognized that malignant cancer and chronic inflammation are closely interlinked.7 Tumor growth and invasion to adjacent tissue can induce inflammation. The cytokines produced by the inflammatory cells and the tumor cells themselves can drastically accelerate the process of cellular proliferation, invasion, epithelial–mesenchymal transition, and vascular genesis, and therefore prompt cancer progression.7,8 For HCC, the interrelationship is much easier to be understood as hepatitis B virus (HBV), hepatitis C virus (HCV), and ethanol are widely accepted as the etiologies of HCC. Thus, the incorporation of the inflammatory-related factors into the clinical decision making system may help us to stratify the patients at a more reasonable level. C-reactive protein (CRP), which is initially identified for its capacity to precipitate C-polysaccharide of Streptococcus pneumonia, is a highly sensitive and dynamic marker of systematic inflammation.9 The secretion of CRP by the hepatocytes is mainly stimulated by the oncogenic inflammatory cytokine as interleukin (IL)-6.10 In the meantime, hypoalbuminemia usually refers to body underperformance and poor nutrition status.11 Glasgow Prognostic Score [(GPS); detailed in Table 1], comprising of serum CRP and albumin, has been proved to be a prognostic indicator in patients with several kinds of malignant cancers including colorectal cancer,12 esophageal cancer,13 gastric cancer,14 and lung cancer.15 For patients with HCC, the prognostic value of GPS remains to be controversial. Horino et al16 suggested that GPS independently predicted poorer overall survival. Meanwhile, the study by Yamamura et al17 did not detect any significant relationship between GPS and patients’ outcome. In this setting, we searched the related articles and performed the current meta-analysis to gain a thorough understanding of the prognostic role of GPS in patients with HCC.
TABLE 1.
The Glasgow Prognostic Score

MATERIAL AND METHODS
Literature Research
Online databases, including PubMed (Medline), Embase, Cochrane Library, Web of Science, ChinaInfo, and Chinese National Knowledge Infrastructure were searched for all years up to September 2015. Terms used in our search included: “Glasgow Prognostic Score” (eg, “GPS”), “prognosis” (eg, “outcome,” “survival,” “mortality,” and “recurrence”), and “Hepatocellular carcinoma” (eg, “HCC,” “liver cancer,” “liver tumor,” and “liver neoplasm”). The articles should be written in English or Chinese. The reference lists of all reviewed articles were screened to identify additional related articles.
Study Inclusion/Exclusion Criteria
The inclusion criteria were as follows: the diagnosis of HCC was made based on pathologic examination or the current ongoing clinical guidelines; correlation of GPS with overall survival (OS)/progression-free survival (PFS) was presented in the article. The hazard ratios (HRs) with the respective 95% confidence interval (CI) were either directly reported or could be reconstructed by the relevant data18 or figures in the essay19; and for studies with overlapping study population, only the most informative one was included. Any divergences were addressed by discussion.
Exclusion criteria were defined as: abstracts, letters, editorials, expert opinions, reviews, case reports, case series less than 5 cases; articles without sufficient reported data for determining an estimate of HR [odds ratio (OR)] and a CI; and not human-based research.
Data Extraction
The extracted data included: first author's name, year of publication, country (region) of the population studied, patients’ age, sample size, gender, treatment, follow-up period, and clinicopathologic features; survival data including OS and PFS; cutoff value defining “elevated GPS” and number of high GPS expression. Overall survival was defined as the interval between the medical treatment and the death of patients or the last follow-up. Progression-free survival was calculated from the date of treatment to the detection of the recurrence tumor or death from any cause. In our analysis, surgical treatment was defined as surgical resection or liver transplantation. Nonsurgical treatment mainly referred to molecular target therapy, best supportive treatment, systematic chemotherapy, and loco-regional therapeutic options, such as percutaneous radiofrequency ablation, percutaneous ethanol injection, and transcatheter arterial interventional approaches.
Quality Assessment of Primary Studies
Newcastle–Ottawa Quality Assessment Scale was adopted as the appraising criteria of quality of the retrieved studies. Newcastle–Ottawa Quality Assessment Scale score ≥6 indicated high quality. Two reviewers (M-XL and X-YB) independently carried out the assessment. Consensus was finally reached through discussion when discrepancy occurred.
Statistical Analysis
The HRs and 95 % CIs were directly retrieved from the essays or were synthesized indirectly from available statistics and/or figure plots in the articles by methods reported by Parmar et al18 and Tierney et al.19 If several estimates were reported for the same value, the most persuading one was preferred (multivariate analysis was more advantageous than univariate analysis. And the latter one outweighed unadjusted Kaplan–Meier curve). Odds ratios and 95% CIs were used to assess the relationship between GPS and clinicopathologic parameters.
Interstudy heterogeneity among included studies was evaluated by the I2 statistics.20 If the I2 was larger than 50%, implying significant statistical heterogeneity between studies, the random-effects (DerSimonian–Laird method) models was adopted; in the presence of no observable interstudy heterogeneity (I2 < 50%), the fixed-effect model was applied. All P values were 2-sided and P < 0.05 were considered statistical significant. Evidence of publication bias was evaluated using the Begg test 21 and Egger test.22 “Trim and fill” analysis23 was additionally performed in case that publication bias was identified. All analyses were performed using STATA statistical software package version 12.0 (STATA Corp., College Station, TX).
RESULTS
Description of the Enrolled Studies
The initial literature search yielded a total of 38 studies. After reading the titles and abstracts, 16 articles were further assessed for eligibility. Six of them were subsequently excluded: 4 studies24–27 were removed because of their insufficient data to generate the estimate of HRs (ORs); Kinoshita et al28,29 and Ishizuka et al30,31 each published studies with overlapping study population. The latest ones with the most comprehensive information were admitted into the meta-analysis.29,31 Thus, 10 studies16,17,29,31–37 published between the year 2012 and 2015 with sample size ranging from 46 to 398 were finally enrolled into the meta-analysis. The flow chart of the literature selection was described in Figure 1.
FIGURE 1.
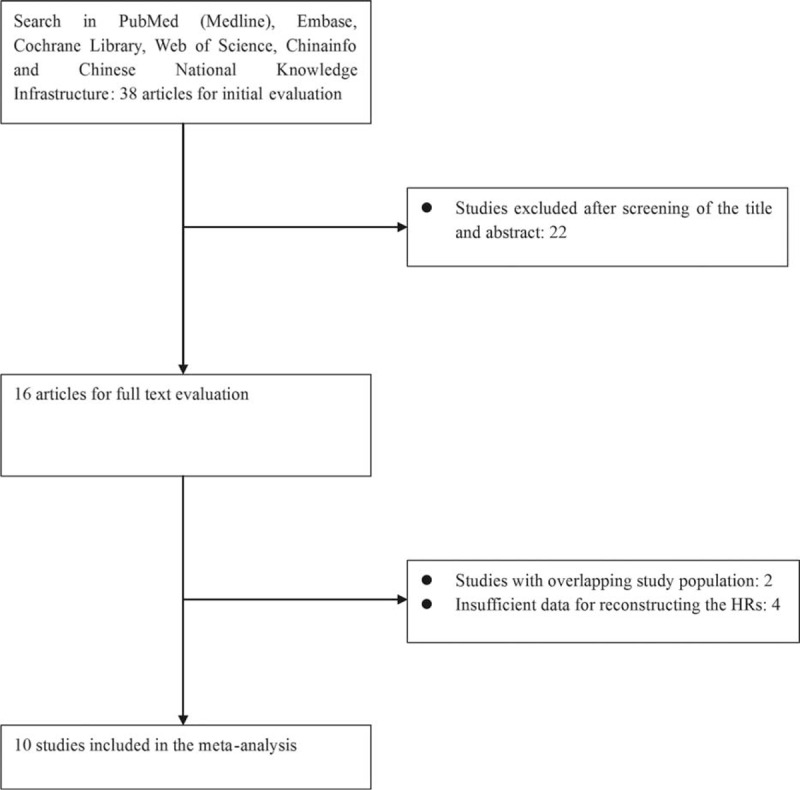
Flow chart describing the selection of eligible articles.
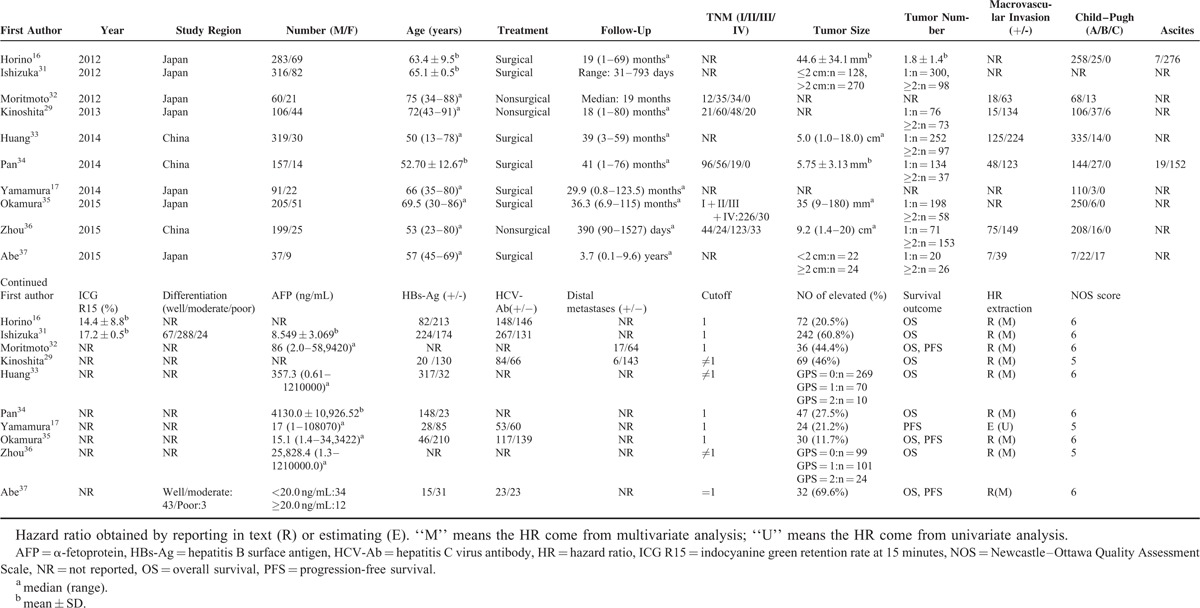
The characteristics of the included studies were summarized in Table 2. Of them, 7 were conducted in Japan and 3 were from China. Surgical treatment was the main treatment approach in 7 of the 10 included studies. Hazard ratio and 95% CI generated by the multivariate analysis were reported directly in 9 of the enrolled cohorts. Newcastle–Ottawa Quality Assessment Scale score was above 6 in 8 cohorts.
TABLE 2.
Main Characteristics of All the Studies Included in the Meta-Analysis
Glasgow Prognostic Score and Overall Survival
Nine of the 10 included studies presented us with data regarding the relationship between GPS and overall survival. The pooled estimates demonstrated a significant relationship between elevated pretreatment GPS and inferior OS with heterogeneity (HR = 2.156, 95% CI: 1.696–2.740, P < 0.001, I2 = 56.2%, P value of Q test for heterogeneity test [(Ph) = 0.019, Figure 2; Table 3].
FIGURE 2.
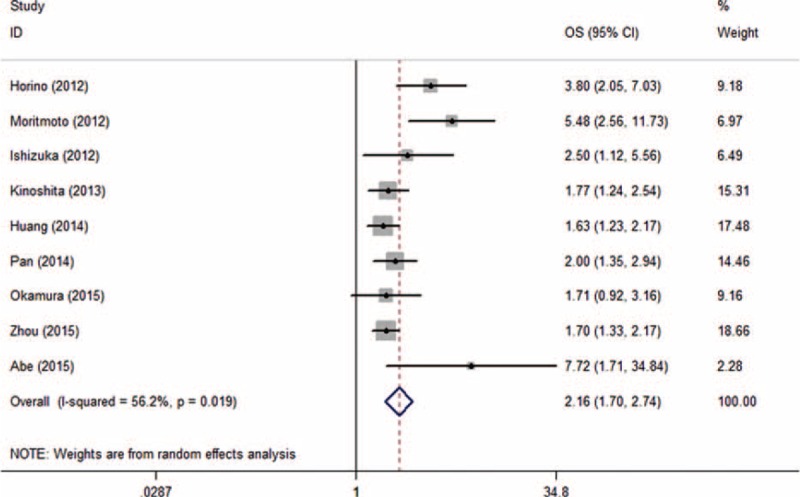
Forest plot of hazard ratio for the association between elevated Glasgow Prognostic Score and overall survival in patients with hepatocellular carcinoma with random effects model.
TABLE 3.
Summary of the Meta-Analysis Results
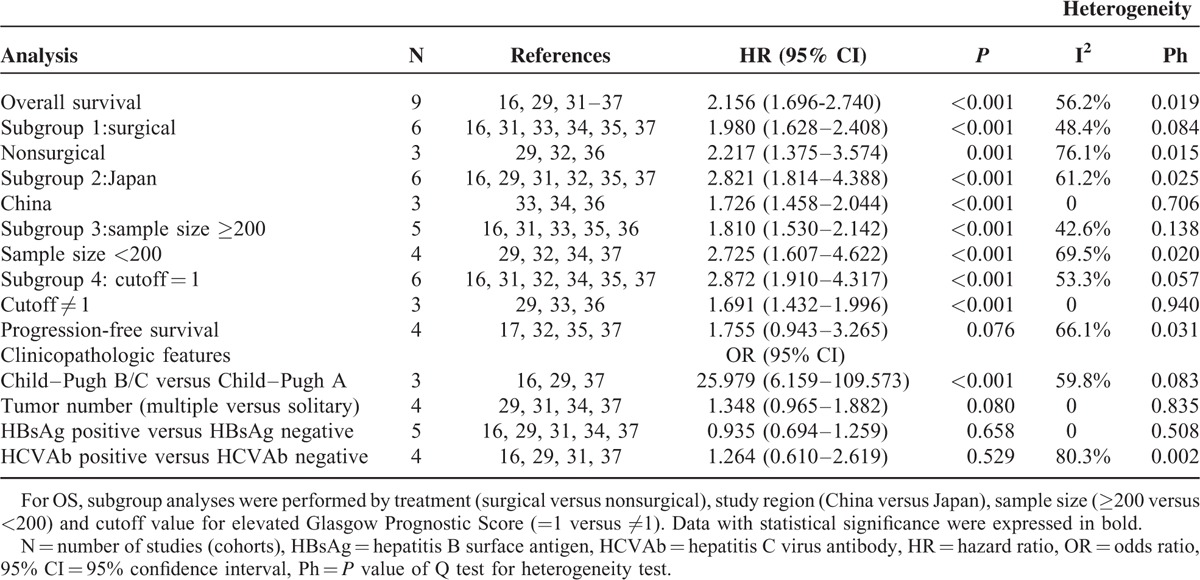
Subgroup analysis stratified by the main treatment (surgical versus nonsurgical), study region (China versus Japan), sample size (≥200 versus <200), and cutoff value (=1 versus ≠1) were performed. Significant relationship between increased GPS and inferior OS were detected in all the above subgroups (Table 3).
Glasgow Prognostic Score and Progression-Free Survival
There were 4 studies presenting the information with reference to GPS and PFS. Observable heterogeneity was detected (I2 = 66.1%, Ph = 0.031, Table 3). With marginal significance, patients with elevated GPS showed a tendency toward shorter PFS (HR = 1.755, 95% CI: 0.943–3.265, P = 0.076, Table 3; Figure 3).
FIGURE 3.
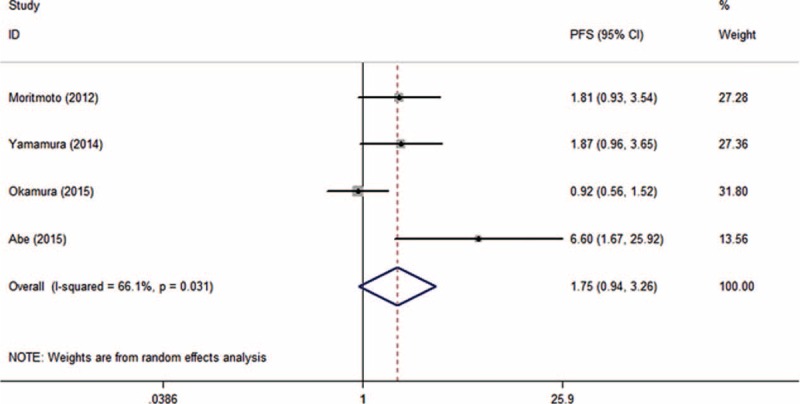
Forest plot of hazard ratio for the association between elevated Glasgow Prognostic Score and progression-free survival in patients with hepatocellular carcinoma with random effects model.
Glasgow Prognostic Score and Clinicopathologic Factors
Three of the included studies reported positive association between elevated GPS and advanced Child–Pugh class. The pooled OR of 25.979 displayed that patients with elevated GPS predisposed to be at advanced Child–Pugh class (OR: 25.979, 95% CI: 6.159–109.573, P < 0.001, I2 = 59.8%, Ph = 0.083, Table 3; Figure 4A). The relationship between GPS and tumor number had been reported in 4 studies. Without observable interstudy heterogeneity (I2 = 0%, Ph = 0.835, Table 3), the pooled estimates exhibited that patients with elevated GPS showed a tendency toward having multiple tumors (OR: 1.348, 95% CI: 0.965–1.882, P = 0.080, I2 = 0%, Ph = 0.835, Table 3; Figure 4B). But the relationship failed to gain statistical significance. And the pooled analyses showed no relationship between increased GPS and positive status of hepatitis B surface antigen (OR: 0.935, 95% CI: 0.694–1.259, P = 0.658, I2 = 0, Ph = 0.508, Table 3; Figure 4C) as well as positive status of hepatitis C virus antibodies (OR: 1.264, 95% CI: 0.610–2.619, P = 0.529, I2 = 80.3%, Ph = 0.002, Table 3; Figure 4D).
FIGURE 4.
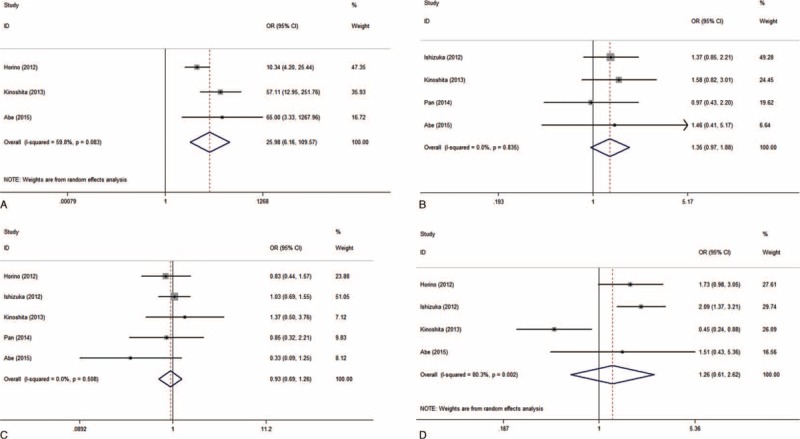
A, Forest plot of odds ratio for the association between elevated Glasgow Prognostic Score and Child–Pugh class (Child–Pugh class B and C versus Child–Pugh class A). B, Tumor number (multiple versus solitary). C, Status of hepatitis B surface antigen (positive versus negative). D, Status of hepatitis C virus antibody (positive versus negative) in patients with hepatocellular carcinoma with random effects model.
Sensitivity Analyses
A single study involved in the meta-analysis was deleted each time to unveil the influence of the individual data to the wholesome result. No significant deviation from the overall results was detected.
Publication Bias
Substantial publication bias was detected in the Begg test (Pr > |z| = 0.029, Figure 5) and Egger test (P > |t| = 0.007) in the pooled estimates for OS. We further performed the “trim and fill” analysis. The results showed at least 1 relevant study was unpublished. The filled meta-analysis concerning OS (HR = 2.104, 95% CI: 1.645–2.692, P < 0.001) upheld the strength of our pooled results.
FIGURE 5.
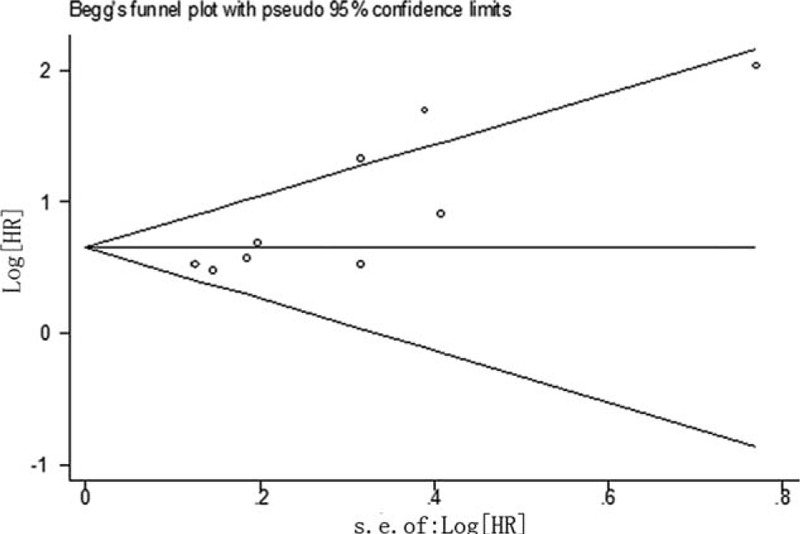
Funnel plot for elevated Glasgow Prognostic Score and overall survival in patients with hepatocellular carcinoma.
DISCUSSION
The current meta-analysis, to our knowledge, is the first meta-analysis evaluating the prognostic value of GPS in patients with HCC. The pooled estimates of 10 studies involving 2094 patients indicated that patients with elevated GPS predisposed to have inferior survival outcome. The significant relationship between GPS and OS was detected in all the subgroup analyses stratified by the main treatment (surgical versus nonsurgical) study region (China versus Japan), sample size (≥200 versus <200), and cutoff value (=1 versus ≠1), which suggested that our results were stout. And patients with elevated GPS tended to have impaired liver function. Being based on only 2 conventional laboratory data without additional imaging techniques or histologic examinations, GPS can be a practical index for stratification of the HCC patients according to their prognosis.
The underlying biologic mechanism explaining the prognostic role of GPS in patients with HCC has not been well established. C- reactive protein secretion are usually triggered by the inflammatory cytokines, such as IL-6, IL-8, or tumor necrosis factor-α, which can in turn boost the development of HCC through facilitating cancer growth, invasion, metastasis, angiogenesis, and immunosuppression to tumor cells.38 Increased CRP levels can act as a measure of tumor progression activity. Through a meta-analysis, Zheng et al39 had proved that CRP was a significant predictor of poorer survival outcome in patients with HCC. The serum albumin level, which partially reflects the body function and nutrition status, has long been recognized as a prognostic factor for HCC.40 And it has already been incorporated into several staging system such as Child–Pugh classification 41 and Cancer of the Liver Italian Program system .5 Combining these, we can reckon that the increased GPS, incorporating elevated CRP and declined serum albumin levels, denotes enhanced neoplastic reaction and weakened body performance.
Publication bias is one of the intrinsic limitations of meta-analysis. As researches with negative results predisposed to be unpublished, the results of meta-analysis may thus be somewhat overvalued. We surmised that the publication bias may partly attribute to the statistical instability secondary to limited number of included studies. We further performed the “trim and fill” analysis23; the results of “filled” analysis did not change the results substantially. Regarding this, our results may also be robust.
Subgroup analysis in terms of the study region (China versus Japan) did not alter the overall results materially. As HBV and HCV are the predominant etiologies of HCC in China and Japan, respectively, our results may have implications in both HBV and HCV dominant areas to some extent. Of note, all the 10 included studies were conducted in Asian medical institutions. Although it may partially be ascribed to high burden of HCC among Asian populations,42 doubts toward the extrapolation to Caucasian populations were also raised. It ought to be minded that the ethnic background and life styles may contribute to the variations in the patients’ prognosis. Thus, the results of our meta-analysis should still be interpreted with caution when comes to the Non-Asian population. Providing these, the findings of our meta-analysis emphasize further researches studying patients from regions other than Asia to gain a comprehensive understanding of the prognostic value of GPS.
There were several other limitations of the meta-analysis. First of all, the treatment details, the baseline characteristics of the study population, and the follow-up information varied from institution to institution. These confounding factors might lead to heterogeneity. And a remarkable portion of the included studies were retrospectively performed, which was susceptible to some biases. Secondly, the HRs and 95% CIs of the study by Yamamura et al17 were retrieved indirectly from figure plots, which was to some extent less accurate than those generated by the directly reported multivariate analyses. Thirdly, tumor recurrence and progression is a major concern in the prognosis of patients with HCC. The meta-analysis, which only took 4 studies included, found that relationship between elevated GPS and PFS only gained marginal statistical significance. It is quite possible that as more and more relevant studies publish in the future, the propensity of increased GPS toward shortened PFS may gain statistical significance. In addition, interstudy heterogeneity was observed in the meta-analysis. As metaregression analysis is best applicable for meta-analysis including more than 10 individual studies, we did not perform the metaregression analysis to figure out the source of heterogeneity. Subgroup analysis revealed that the study region (China versus Japan) and the cutoff value (=1 versus ≠1) may explain the source of heterogeneity to some extent. The differences in the dominant etiologies along with the genetic backgrounds in China and Japan may partially explain the source of interstudy heterogeneity. No uniform cutoff value defining elevated GPS has been erected. Seven of the included studies adopted 1 as the cutoff value whereas the remaining 3 studies used cutoff value other than 1. These all could be the source of bias.
Collectively, GPS, an easily obtained and reproducible inflammatory index, is a promising prognostic factor in patients with HCC. More strictly designed studies focusing on this theme are required before GPS can move forward into routine clinical practice as a complementary prognostic factor to the current staging systems.
Footnotes
Abbreviations: AFP = a-fetoprotein, CI = confidence interval, CRP = C-reactive protein, GPS = Glasgow Prognostic Score, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HCVAb = hepatitis C virus antibody, HR = hazard ratio, ICG R15 (%) = indocyanine green retention rate at 15 minutes, IL = interleukin, NOS = Newcastle–Ottawa Quality Assessment Scale, OR = odds ratio, OS = overall survival, PFS = progression-free survival, Ph = P value of Q test for heterogeneity test.
This work was supported by The State Key Project on Infection Diseases of China (Grant No. 2012ZX10002016).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010; 30:61–74. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985; 56:918–928. [DOI] [PubMed] [Google Scholar]
- 5.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998; 28:751–755. [DOI] [PubMed] [Google Scholar]
- 6.Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000; 32:1224–1229. [DOI] [PubMed] [Google Scholar]
- 7.Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013; 13:759–771. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008; 454:436–444. [DOI] [PubMed] [Google Scholar]
- 9.Hurlimann J, Thorbecke GJ, Hochwald GM. The liver as the site of C-reactive protein formation. J Exp Med 1966; 123:365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castell JV, Gomez-Lechon MJ, David M, et al. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology 1990; 12:1179–1186. [DOI] [PubMed] [Google Scholar]
- 11.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013; 39:534–540. [DOI] [PubMed] [Google Scholar]
- 12.Dreanic J, Maillet M, Dhooge M, et al. Prognostic value of the Glasgow Prognostic Score in metastatic colorectal cancer in the era of anti-EGFR therapies. Med Oncol 2013; 30:656. [DOI] [PubMed] [Google Scholar]
- 13.Vashist YK, Loos J, Dedow J, et al. Glasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol 2011; 18:1130–1138. [DOI] [PubMed] [Google Scholar]
- 14.Li QQ, Lu ZH, Yang L, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev 2014; 15:945–950. [DOI] [PubMed] [Google Scholar]
- 15.Mimatsu K, Oida T, Fukino N, et al. Glasgow prognostic score is a useful predictive factor of outcome after palliative gastrectomy for stage IV gastric cancer. Anticancer Res 2014; 34:3131–3136. [PubMed] [Google Scholar]
- 16.Horino K, Beppu T, Kuroki H, et al. Glasgow Prognostic Score as a useful prognostic factor after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol 2013; 18:829–838. [DOI] [PubMed] [Google Scholar]
- 17.Yamamura K, Sugimoto H, Kanda M, et al. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci 2014; 21:682–688. [DOI] [PubMed] [Google Scholar]
- 18.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 19.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015; 22:803–810. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara Y, Shiba H, Furukawa K, et al. Glasgow prognostic score is related to blood transfusion requirements and post-operative complications in hepatic resection for hepatocellular carcinoma. Anticancer Res 2010; 30:5129–5136. [PubMed] [Google Scholar]
- 26.Ni XC, Yi Y, Fu YP, et al. Prognostic value of the Modified Glasgow Prognostic Score in patients undergoing radical surgery for hepatocellular carcinoma. Medicine 2015; 94:e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YF, Ke Z, Zhou Y, et al. Primary discussion on prognostic value of Glasgow prognostic score in patients with primary hepatic carcinoma. J Clin Surg 2015; 22:568–570. [Google Scholar]
- 28.Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer 2012; 107:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinoshita A, Onoda H, Imai N, et al. The Glasgow Prognostic Score, an inflammation based prognostic score, predicts survival in patients with hepatocellular carcinoma. BMC Cancer 2013; 13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishizuka M, Kubota K, Kita J, et al. Usefulness of a modified inflammation-based prognostic system for predicting postoperative mortality of patients undergoing surgery for primary hepatocellular carcinoma. J Surg Oncol 2011; 103:801–806. [DOI] [PubMed] [Google Scholar]
- 31.Ishizuka M, Kubota K, Kita J, et al. Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patients. Am J Surg 2012; 203:101–106. [DOI] [PubMed] [Google Scholar]
- 32.Morimoto M, Numata K, Moriya S, et al. Inflammation-based prognostic score for hepatocellular carcinoma patients on sorafenib treatment. Anticancer Res 2012; 32:619–623. [PubMed] [Google Scholar]
- 33.Huang J, Xu L, Luo Y, et al. The inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomy. Med Oncol 2014; 31:883. [DOI] [PubMed] [Google Scholar]
- 34.Pan QX, Zhang JH, Su ZJ, et al. The Glasgow Prognostic Score is an independent prognostic predictor of hepatocellular carcinoma following radical resection. Oncol Res Treat 2014; 37:192–197. [DOI] [PubMed] [Google Scholar]
- 35.Okamura Y, Ashida R, Ito T, et al. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg 2015; 39:1501–1509. [DOI] [PubMed] [Google Scholar]
- 36.Zhou DS, Xu L, Luo YL, et al. Inflammation scores predict survival for hepatitis B virus-related hepatocellular carcinoma patients after transarterial chemoembolization. World J Gastroenterol 2015; 21:5582–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe T, Tashiro H, Hattori M, et al. Prediction of long-term survival by using the Glasgow Prognostic Score in patients with hepatocellular carcinoma after liver transplantation. Hepatol Res 2015; doi: 10.1111/hepr.12597. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009; 30:1073–1081. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Z, Zhou L, Gao S, et al. Prognostic role of C-reactive protein in hepatocellular carcinoma: a systematic review and meta-analysis. Int J Med Sci 2013; 10:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh S, Shirabe K, Matsumoto Y, et al. Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann Surg Oncol 2014; 21:3063–3068. [DOI] [PubMed] [Google Scholar]
- 41.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. [DOI] [PubMed] [Google Scholar]
- 42.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA: Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]


