Supplemental Digital Content is available in the text
Abstract
Whether low-dose Bacillus Calmette-Guerin (BCG) treatment can reduce the side effects while maintaining efficacy for patients with nonmuscle invasive bladder cancer (NMIBC) is controversial.
To investigate whether low-dose BCG treatment can reduce the side effects while maintaining efficacy for patients with NMIBC when compared with standard-dose BCG treatment.
A comprehensive literature search of PubMed, EMBASE, CINAHL, LILACS, and CENTRAL databases was conducted to identify relevant randomized controlled trials (RCT) or quasi-randomized controlled trials (qRCT) that have assessed the efficacy of low- and standard-dose BCG therapy for patients with NMIBC. Systematic review and meta-analysis were performed according to Preferred Reporting Items for Systematic Reviews and Meta-analysis Criteria.
Six RCTs and 2 qRCTs were eligible for meta-analysis. Low-dose BCG instillation was not inferior to reduce the risk of bladder tumor recurrence (hazard ratio [HR], 1.15; 95% confidence interval [CI], 1.00–1.31; P = 0.05), meanwhile no difference was found regarding tumor progression (HR = 1.08; 95%CI, 0.83–1.42; P = 0.57). However, low-dose BCG provided a significantly lower incidence of overall side effects (RR = 0.75; 95%CI, 0.60–0.94; P = 0.01), systemic side effects (RR = 0.57; 95%CI, 0.34–0.97; P = 0.04), severe side effects (RR = 0.52; 95%CI, 0.36–0.74; P = 0.0003), and withdrawal due to BCG toxicity (RR = 0.49; 95%CI, 0.26–0.91; P = 0.02). In contrast, local side effects were comparable between low- and standard-dose arms (RR = 0.89; 95%CI, 0.73–1.08; P = 0.24).
Low-dose BCG instillation significantly reduces the incidence of overall side effects, especially severe and systemic symptoms in patients with NMIBC, while the oncological control efficacy of low-dose BCG is not inferior to standard-dose BCG. Further studies with stratification using different risk factors at randomization are required to assess whether the efficacy of low-dose BCG is comparable to standard dose BCG for different risk of patients.
PROSPERO registration No CRD42014014871 (www.crd.york.ac.uk/prospero/).
INTRODUCTION
Approximately 75% of patients with bladder cancer present with nonmuscle invasive bladder tumors (NMIBC).1 Although NMIBC could be completely eradicated by transurethral resection of the bladder tumor (TURBT), it is still characterized by a high risk of recurrence and to a lesser extent progression to muscle-invasive bladder cancer. Therefore, adjuvant therapy is necessary for most of NMIBC patients.2,3 Intravesical Bacillus Calmette-Guerin (BCG) immunotherapy has been confirmed as a more effective adjuvant treatment than TURBT alone or TURBT plus chemotherapy in preventing recurrence of intermediate or high-risk NMIBC.2
Side effects and cost are the major disadvantages of intravesical BCG treatment; consequently, urologists are reluctant to recommend BCG to their patients. It has been reported that only about 50% of patients with intermediate or high-risk NMIBC receive BCG therapy, and adverse effects related to BCG are one of the major obstacles.4 Therefore, many strategies have been explored to reduce the side effects of BCG, the most studied option being a decrease in dose.5 Two multicenter randomized clinical trials (RCT) conducted by the European Organization for Research and Treatment of Cancer, Genito-Urinary Cancer Group (EORTC-GU) and Club Urologico Espanol de Tratamiento Oncologico (CUETO) have demonstrated that the efficacy of low-dose BCG was not inferior to standard-dose BCG in preventing bladder tumor recurrence and progression. However, conflicting findings regarding the ability of low-dose BCG to reduce potential toxicity were reported in the 2 studies.3,6,7 It was reported by Martinez-Pineiro et al6 that dose reduction could significantly diminish the toxicity of BCG, while Oddens et al3 found no differences in toxicity between low- and standard-dose BCG.
To examine if low-dose BCG could reduce side effects while maintaining sufficient efficacy relative to standard-dose BCG, we performed a meta-analysis of RCT or quasi-randomized controlled trials (qRCT) that assessed the efficacy of low- and standard-dose BCG therapy for patients with NMIBC.
MATERIAL AND METHODS
Ethical Approval or Informed Consent Is Not Required for This Meta-Analysis Since Data Were Extracted From Previous Published Studies
Databases Search
A systematic literature search was performed in October 2015 using PubMed/MEDLINE, EMBASE, CINAHL, LILACS, Cochrane Central Register of Controlled Trials (CENTRAL), International Clinical Trials Register (ICTRP) Search Portal, and ClinicalTrials. gov databases. The exact search strategy for PubMed is listed in the Supplementary S1, and search strategies for other databases were similar. No limits were applied regarding language. Conference proceedings and reference lists of articles were also searched to obtain relevant literature.
Article Selection and Quality Assessment
Eligible RCT and qRCT studies had to meet the following criteria: Trials comparing different dose of BCG instillation for patients undergoing TURBT; patients included should have histologically confirmed NMIBC; and included studies must have been published in a peer-reviewed journal and adequate information provided or obtainable from the researchers. The qRCT studies were defined as RCTs in which allocations to treatment was obtained by predictable methods, for example, alteration, use of alternate medical records, or date of birth. We excluded trials that BCG was administered simultaneously with other adjuvant agents, such as interferon-alpha, for the patients. Trials with only conference abstracts available were not included because of a lack of detailed information. Eligibility assessment of published abstracts was performed independently by 2 reviewers (SXZ and ZSZ); disagreements between reviewers were further discussed with CLX. Full article text regarding the included abstracts was retrieved for further review (Figure 1). Pairs of reviewers (SXZ and ZSZ) worked independently to assess the quality of each included study; disagreements were discussed with CLX. The level of evidence of each study was assessed according to the criteria provided by the Oxford Centre for Evidence-based Medicine.8 The Cochrane Collaboration's tool for assessing risk of bias was used to assess the methodological quality of all eligible studies.9
FIGURE 1.
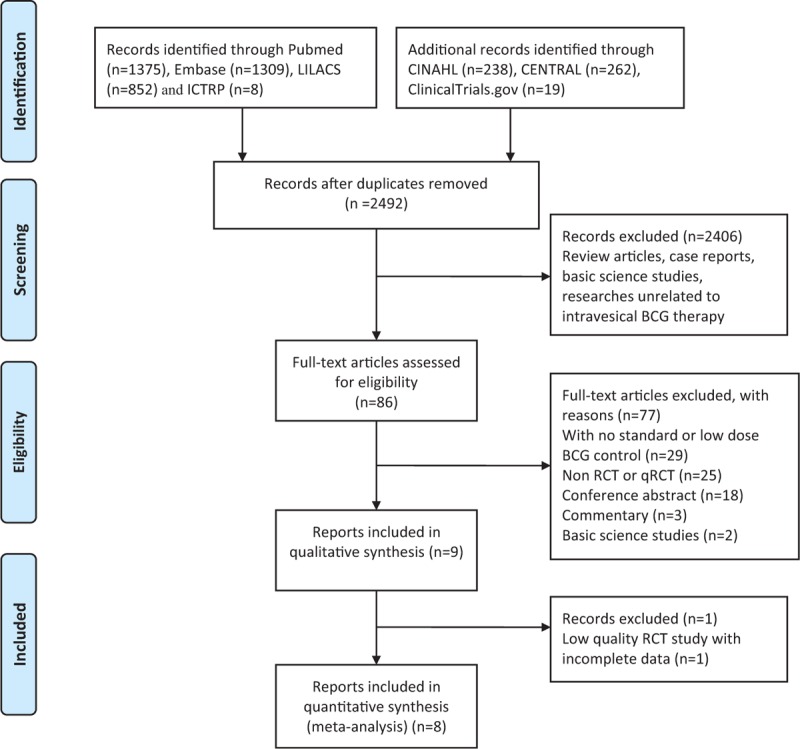
Preferred reporting items for systematic reviews and meta-analysis flow of study selection.
Study Outcomes and Definitions
The primary outcome was treatment efficacy of BCG for NMIBC, which was measured by: time to events (tumor recurrence, progression, and metastasis); frequency of events (tumor recurrence, progression, and metastasis). Tumor recurrence was defined as the first time recurrence after TURBT. Tumor progression was defined as upgrade of tumor grade and stage or evidence of metastasis. The secondary outcome was toxicity of BCG, which was measured by frequency of side effects. Side effects were classified by National Cancer Institute Common Toxicity Criteria (NCI-CTC), severe side effects were defined with grade 3 to 5.10
Data Extraction and Synthesis
Data from the included literature were extracted by 2 independent reviewers (SXZ and XWY); disagreements were resolved by discussion. Extracted data included: the characteristics of trial participants; time to bladder tumor recurrence, progression, and metastasis; the rate of recurrence, progression, and metastasis; and disease-specific survival and related side effects. Standard dose BCG was defined as the recommended dose of different BCG strains, while low-dose BCG could vary from 1/6 to 2/3 of the standard dose. We contacted 7 authors for further information regarding dose of BCG, randomization method, details of patients lost to follow-up, and criteria for side effects stratification,3,7,11–16 and 4 responded with accurate dosage of BCG,3 randomization method,14 criteria for side effects stratification,7 and details of conference abstract.11,16
Statistical Analysis
Time to event outcomes such as time to bladder tumor recurrence, progression, and metastasis were measured using the hazard ratio (HR) with a 95% confidence interval (CI). For studies with no published HRs, but with Kaplan–Meier curve or Wilcoxon P values, the method proposed by Tierney et al17 was employed to estimate the HR and 95% CI. The relative risk ratio (RR) was used for dichotomous variables, such as incidence of side effects. Multiarm trials would be included only with those interventions were relevant to the review and meet the inclusion criteria. Study with more than one arm meet the inclusion criteria would be combined by the method proposed by Hamling et al.18 This method ensured the data were included only once when pooling data and took into consideration the correlation between estimates.
Heterogeneity among the included studies was assessed using Cochran Q and I2 statistics.19 If the fixed-effect model (Mantel-Haenszel method) was used to pool estimates if no significant heterogeneity was detected (I2 < 50% and P > 0.1), otherwise, the random effect model (DerSimonian-Laird method) was used. The pooled effects were examined using the Z-test and α < 0.05 was considered statistically significant. Publication bias was examined using funnel plots.20 Sensitivity analysis was conducted as prespecified in the protocol, analysis was performed based on trial quality. Subgroup analyses were performed as indicated in the protocol, different BCG strains; BCG induction and BCG maintenance treatment; and different risks of bladder cancer patients. A meta-analysis was performed using Review Manager software (RevMan v 5.3, Cochrane Collaboration, Oxford, UK).
RESULTS
Search Results and Study Characteristics
Nine eligible reports were identified for qualitative evaluation (Figure 1), but one of them was assessed as a low-quality RCT with unclear randomization method and high risk of incomplete outcome data and selective reporting.15 Authors of this article were contacted for further information but we received no reply, this study was thus discarded. The baseline characteristics of the 8 studies included were presented in Tables 1 and 2. Two studies were 3-arm trials and the 2 different doses of low-dose BCG arms were combined into a single arm by the method described before.21,22 The 8 studies included 1260 cases in the low-dose and 1199 cases in the standard dose arms. Patient demographics in the included trials are detailed in Table 3.
TABLE 1.
Eligible Studies Compare Low- and Standard-Dose BCG Instillation for NMIBC
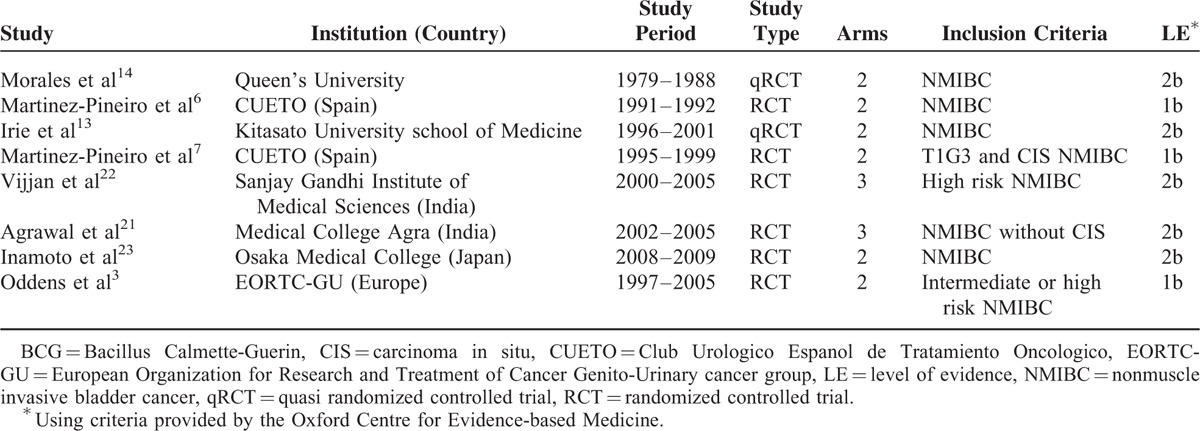
TABLE 2.
Patient Demographics of Included Trials
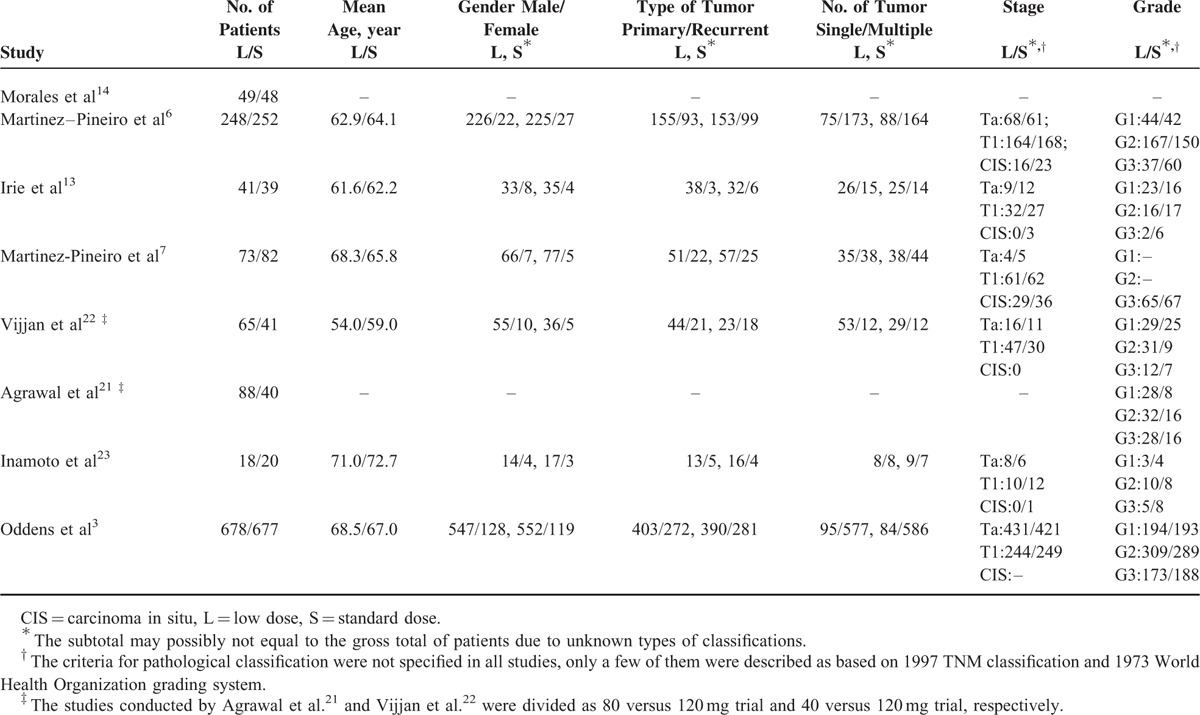
TABLE 3.
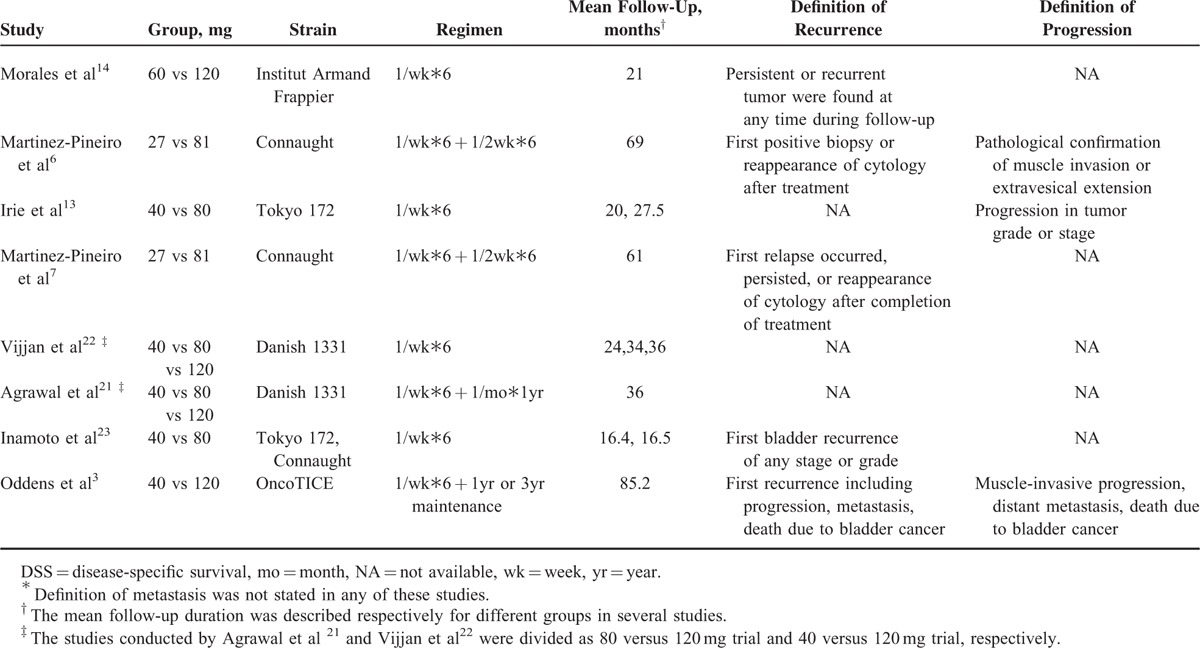
Treatment Characteristics and Definition of Primary Outcome of Studies∗
Assessment Risk of Bias
Eight eligible RCTs or qRCTs were eventually included to estimate the corresponding pooled estimates.3,6,7,13,14,21–23 Six studies were classified with proper randomization method,3,6,7,21–23 and 2 studies with patients sequentially allocated were categorized as qRCTs.13,14 Allocation concealment was not performed or unclear for all the included studies. Although none of the studies performed blinding of participants and personnel or masked the outcome assessors, the primary outcomes, for example, tumor recurrence and progression, were not likely to be influenced; therefore, corresponding domains were grades as “low risk.” Two studies were grades as high risk of attrition bias.13,21 The assessment of risk of bias outcome of included studies was summarized in Figure 2A, B.
FIGURE 2.
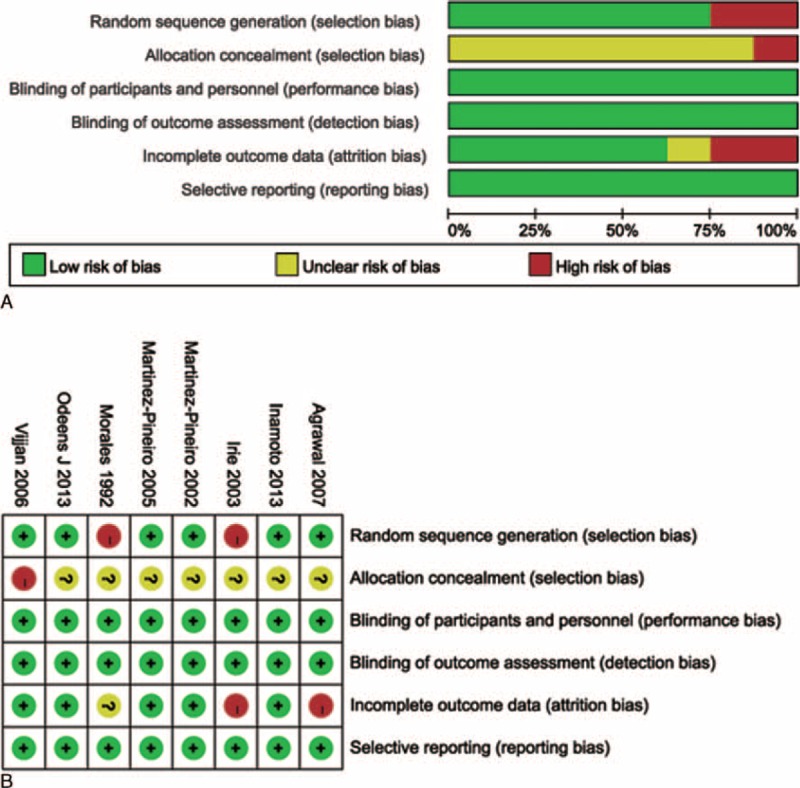
Treatment characteristics and definition of primary outcome of studies.
Primary Outcomes
Time to First Events Outcome
Oncological outcomes reported by Morales et al14 and Agrawal et al21 were shown as binary variables with a median follow-up of 21 and 36 months, respectively, and the individual patient data of these studies were not able to be obtained to estimate HR. So, the 2 studies were not included in the pooled estimates of HR. No significant difference was detected between the low- and standard-dose arms regarding bladder tumor recurrence (HR = 1.15; 95%CI, 1.00–1.31, P = 0.05; I2 = 0%) and tumor progression (HR = 1.08; 95%CI, 0.83–1.42; P = 0.57; I2 = 0%). No trial addressed comparison of time to metastasis. Heterogeneity among the above meta-analyses was not significant, so a fixed-effect model was used (Figure 3).
FIGURE 3.
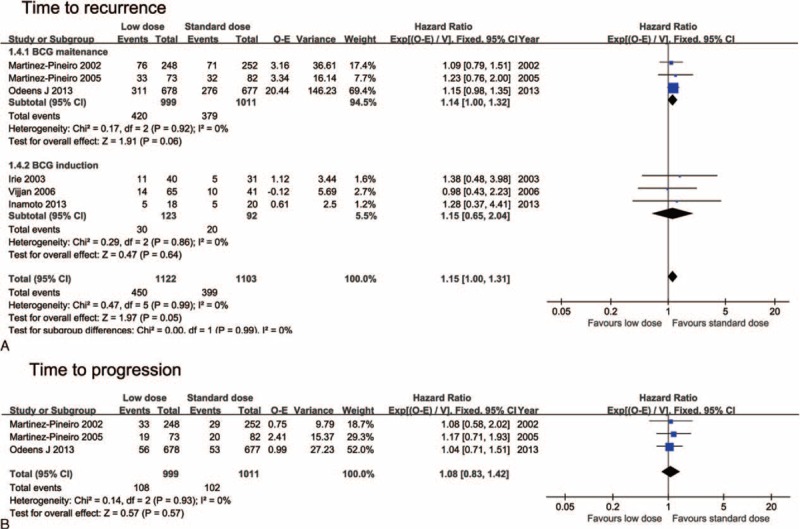
Forest plots for time to events analysis: (A) time to recurrence and subgroup analysis by different Bacillus Calmette-Guerin (BCG) instillation schedules, (B) time to progression.
Secondary Outcomes
Side Effects
The rate of patients suffering from either local or systemic side effects was significantly lower in the low-dose arm (RR = 0.75; 95%CI, 0.60–0.94; P = 0.01; I2 = 82%). Severe side effects (RR = 0.52; 95%CI, 0.36–0.74; P = 0.0003; I2 = 0%) and withdrawal due to BCG toxicity (RR = 0.49; 95%CI, 0.26–0.91; P = 0.02) were also significantly lower in the low-dose arm (Figure 4).
FIGURE 4.
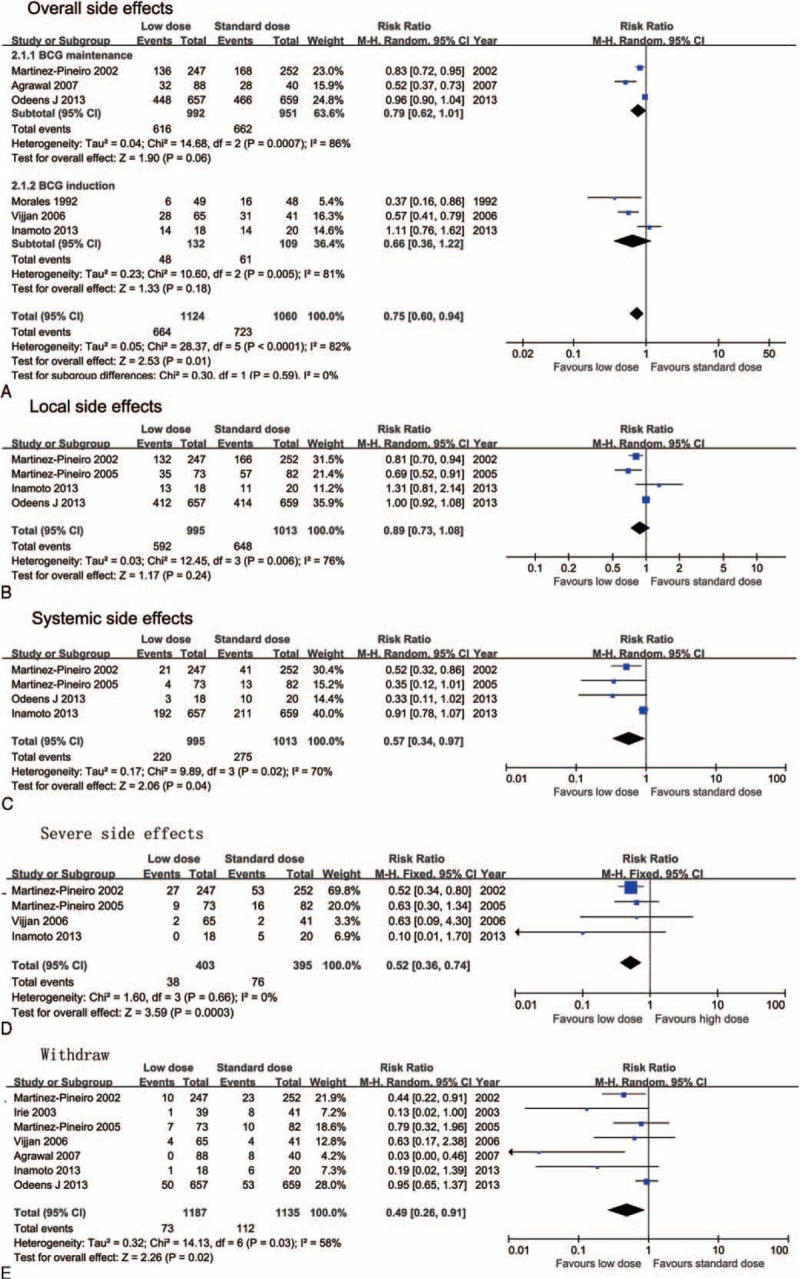
Forest plots for side effects: (A) overall side effects and subgroup analysis by different Bacillus Calmette-Guerin (BCG) instillation schedules, (B) local side effects, (C) systemic side effects, (D) severe side effects, and (E) withdraw rate.
Regarding local side effects, there was no significant difference in overall local side effects (RR = 0.74; 95%CI, 0.73–1.08; P = 0.24; I2 = 76%), hematuria (RR = 0.57; 95%CI, 0.28–1.15; P = 0.12; I2 = 87%), and cystitis (RR = 0.65; 95%CI, 0.27–1.59; P = 0.35; I2 = 96%), while the frequency was significantly lower in the low-dose arm (RR = 0.62; 95%CI, 0.41–0.95; P = 0.03; I2 = 83%) (Figure 4 and Supplementary Figure 1). Concerning systemic side effects, a significant difference was found in overall side effects (RR = 0.57; 95%CI, 0.34–0.97; P = 0.04; I2 = 70%), fever (RR = 0.47; 95%CI, 0.32–0.68; P < 0.0001; I2 = 48%), and malaise (RR = 0.63; 95%CI, 0.44–0.92; P = 0.02; I2 = 50%) (Figure 4 and Supplementary Figure 2). Heterogeneity was significant for all side effect meta-analyses except for severe side effects (P = 0.0003; I2 = 0%); the random effect model was thus used for pooled estimates.
Subgroup and Sensitivity Analysis
Maintenance intravesical BCG instillation was performed in 3 studies,3,6,7,21 while patients only received BCG induction treatment in the other trials.13,14,22,23 The tumor recurrence risk and overall side effects were not significantly different between the low- and standard-dose arms when trials were divided into different subgroups (Figures 3A and 4A). Subgroup analysis of different BCG strains was also predetermined in the protocol. However, 5 different strains were used in the 8 studies; thus, this subgroup analysis was not performed. Subgroup analysis of different risks of patients was not performed since stratified analysis regarding different risks of bladder cancer was only performed by Oddens et al.3
The primary outcomes of the meta-analysis were unchanged after qRCT trials were omitted in the sensitivity analysis (HR = 1.14, 95%CI, 0.54–1.31, P = 0.06, figure not shown), when the studies only with level of evidence Ia were included, the tumor recurrence HR was 1.14 (95%CI, 1.00–1.32, P = 0.06) (Figure 2A). No significant evidence of publication bias for primary outcome was detected based on funnel plots (Supplementary Figure 3).
DISCUSSION
Intravesical instillation of BCG has been confirmed as the most effective strategy for the prophylaxis of NMIBC recurrence after complete TURBT. However, BCG side effects present a serious therapeutic conundrum for urologists.2 Numerous investigations have been conducted regarding reduction of adverse effects while maintaining or improving tumor control, for example, antibiotic prophylaxis, concurrent use of interferon, and dose reduction.3,24,25 Dose reduction is one of the most investigated methods for reducing the side effects of BCG instillation. Most studies have suggested that dose reduction could significantly reduce the incidence of side effects without compromising treatment efficacy.6,7,21,22 However, the largest RCT conducted by EORTC-GU found no significant difference between low- and standard-dose BCG instillation.3,5 Thus, the present analysis was conducted to systematically identify and critically evaluate high-quality trials, comparing low- and standard-dose BCG instillation. The important finding of our meta-analysis was that the oncological efficacy of low-dose BCG was not inferior to standard-dose; however, low-dose BCG could significantly reduce side effects, especially systemic side effects and severe side effects.
The Role of Low-Dose BCG in Tumor Control
The pooled estimate of the primary outcome was consistent with the majority of results from previous studies. Two convincing studies conducted by CUETO6 and EORTC-GU3 found low-dose BCG was not inferior to the standard dose, with an HR of 1.09 (95%CI, 0.79–1.51; P = 0.045) and 1.15 (95%CI, 0.98–1.35; P = 0.5864), respectively. The study conducted by EORTC-GU was designed as a noninferiority study that needed α < 0.0025 to ascertain significance. Therefore, the pooled results of RCT or qRCT studies with larger sample sizes might be crucial in confirming these nonsignificant differences. Furthermore, there was also no significant difference for tumor progression regarding different BCG doses, which agreed with previous clinical trials.
Although the length and frequency of BCG instillation are still controversial, it was recommended by the European Association of Urology that at least 1 year of BCG instillation was required to maintain its efficacy.2 One of the most widely used schedules is based on the Southwest Oncology Group regimen, which is initiated with 6-weekly BCG instillations followed by 3-weekly instillations at 3 and 6 months, and subsequently every 6 months for 3 years.5,26 However, BCG was administered weekly for 6 weeks without maintenance among half of the included studies. Intravesical BCG instillation was only maintained for 1 or 3 years in the study conducted by the EORTC-GU; nevertheless, no significantly improved efficacy was found between 3 and 1-year maintenance therapy.3 When studies were divided into different groups based on whether BCG instillation schedules, no significant difference was found in subgroup analysis for recurrence; the HR was 1.14 (95%CI, 1.00–1.32; P = 0.06) and 1.15 (95%CI, 0.65–2.04; P = 0.64) for the BCG maintenance and BCG induction subgroups, respectively. Low-dose BCG maintenance regimens with a reduced number of instillations have been investigated in 2 RCT studies; it was found in the reduced instillation group that treatment was effective with no impact on tumor recurrence or progression.27,28
In our meta-analysis, subgroup analysis concerning different risks of bladder cancer was not performed since stratified analysis regarding different risks of bladder cancer was only performed by Oddens et al.3 It was suggested by Oddens et al3 that standard dose BCG was more likely to reduce recurrences in high-risk patients relative to low-dose BCG; however, the difference was not significant. Meanwhile, it was also observed by Martínez-Piñeiro et al6 that disease-free survival tended to be longer in patients with T1G3 and carcinoma in situ in the standard arm. However, the efficacy of low-dose BCG instillation for prophylaxis of high-risk bladder cancer is unclear. Further RCT studies stratified using different risks for bladder cancer are required to elucidate the efficacy of low-dose BCG for patients with high-risk bladder cancer.
The impact of different strains on the efficacy of BCG instillation was predetermined for subgroup analysis in the present meta-analysis protocol. However, 5 different strains of BCG were used in the 10 trials; thus, it was not appropriate to perform further subgroup analysis. To the present, no study has investigated the potential impact of different strains of low-dose BCG on the efficacy of tumor control. So, there is no conclusive evidence that differences exist regarding the clinical efficacy of various BCG strains.2
In the included trials, low-dose BCG was defined as doses ranging from 1/3 to 2/3 of the standard dose (1/3 in 5 trials,3,6,7,21,22 1/2 in 3 trials, 13,14,23 and 2/3 in 2 trials).21,22 The efficacy of 1/6 and 1/3 of the standard dose was compared by the CUETO group; it was shown that 1/3 of the standard dose seemed to be the minimum effective dose as adjuvant treatment.29 However, Morales et al14 suggested that the ideal BCG dose might not be universal and should be tailored based on the different characteristics of bladder tumors and populations. Indeed, the immune response to BCG for populations in different geographical locations may vary greatly. It was considered by Martinez-Pineiro et al7 that the different results concerning low-dose BCG instillation between Morales et al14 and other studies may be the result of less prior BCG immunization and lower tuberculosis exposure for Europeans.7 To the best of our knowledge, the impact of different human nationalities and geographical location on the efficacy of BCG for bladder cancer has not yet been fully studied.
Side Effects of Low-Dose BCG
The main purpose of adopting low-dose BCG instillation is to decrease BCG toxicity. Most investigations have demonstrated the efficacy of low-dose BCG in reducing side effects.6,7,13,14,21–23 In contrast, the largest RCT trial conducted by the EORTC-GU group found no significant differences in side effects between low- and standard-dose arms.3,5 However, our meta-analysis suggested that low-dose BCG had significant efficacy in decreasing the incidence of overall side effects (RR = 0.75; 95%CI, 0.60–0.94; P = 0.01). In clinical practice, severe side effects associated with BCG instillation are of the utmost concern. The current meta-analysis demonstrated that low-dose BCG had a 48% lower risk of severe side effects than the standard dose (RR = 0.52; 95%CI, 0.36–0.74; P = 0.0003). Patients were more compliant to accept low-dose BCG instillation and this was reflected by a lower toxicity resulted withdrawal rate in the low-dose arm (RR = 0.49; 95%CI, 0.26–0.91; P = 0.02). The main benefit of low-dose BCG, in terms of side effects, was a decrease in systemic side effects. It was found in our meta-analysis that the incidence of systemic side effects, especially fever and malaise, was significantly reduced; while local side effects (eg, hematuria and cystitis) were comparable in the low and standard dose arms (with the exception of frequency).
The result of pooled estimate of adverse effect should be considered with caution. It should be noted that the heterogeneity of all the pooled estimates of side effects was significant except for severe side effects (P = 0.66; I2 = 0%). This heterogeneity might be the result of different standards of reporting regarding the side effects of BCG treatment and schedules of BCG instillation. The pooled data regarding severe side effects with grade 3 to 4 toxicity were based on the reports using the NCI-CTC grading of severity. Therefore, no significant heterogeneity was detected in this situation. Different standards and definitions of adverse effects reporting were likely to play a critical role in heterogeneity. This reflects the importance of adopting a unified standard to classify side effects, which would make the results of different studies considerably more compatible. To date, however, no standard guidelines or criteria exist for reporting the side effects of adjuvant prophylaxis treatment in the field of urology.2,30
Study Limitations
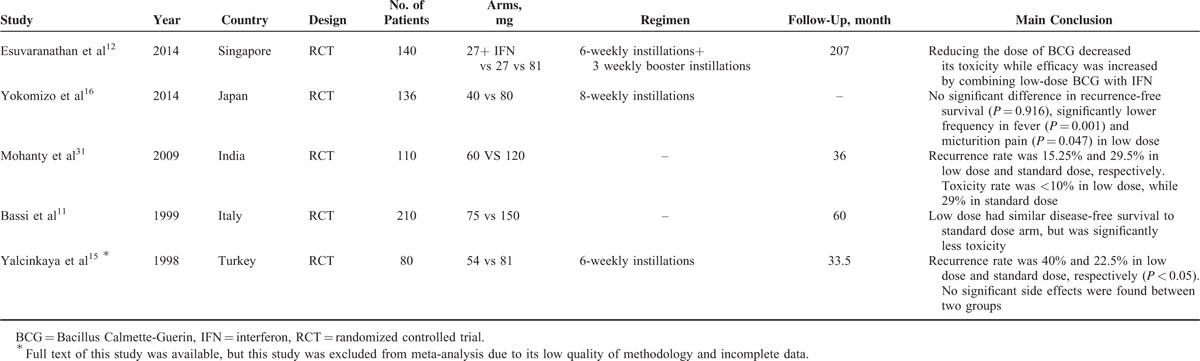
The present analysis was conducted based on 8 studies involving 8 studies. Six of the 8 studies were RCTs with low (1 study) to medium (5 studies) methodological quality, and 2 studies were qRCTs with patients sequentially allocated to each arm. Two studies did not adhere to the intention-to-treat analysis principle.13,14 The blinded method was not used in any of the 8 studies. However, the primary outcome of these studies was oncological outcome, for example, recurrence and progression, which was less likely to be influenced by the unblinded method. Several RCTs involving only conference abstracts were not included in the meta-analysis because the methodological quality was not assessable, and the data were incomplete for extraction; the results of these studies with only abstracts available are listed in Table 4.11,12,15,16,31 Last but not least, the clinical relevancy of bladder tumor recurrence changes dramatically with the risk category; however, no study was randomized with risk factor stratifications. Therefore, it was not possible to infer whether the efficacy of low-dose BCG was comparable to both intermediate and high risk patients.
TABLE 4.
Results of RCT Studies Comparing Low- and Standard-Dose BCG With Only Abstracts Available or Incomplete Data
Implication for Future Studies
There is a lack of evidence regarding comparison of the efficacy of low-dose BCG with standard BCG for different risks concerning bladder tumors. Patients need to be stratified using different risk factors at randomization in future studies of low-dose BCG. The current multicenter studies involving BCG are usually conducted in Europe; the results of these studies may not represent the immune response to BCG on other continents. In developing countries with higher tuberculosis exposure and poor sanitary conditions, the efficacy of BCG may vary and requires proving in large multicenter RCT studies. Finally, the heterogeneity of side effects suggests that unified criteria and definitions for their recording are strongly expected to be established in future clinical trials.
In conclusion, this meta-analysis of 8 RCTs or qRCTs comparing low- and standard-dose BCG suggests that low-dose BCG instillation significantly reduces the incidence of overall side effects, especially severe and systemic symptoms in patients with NMIBC, while the oncological control efficacy of low-dose BCG is not inferior to standard dose BCG. Low-dose BCG for patients with high-risk factors should be recommended with meticulous discussion, further studies with stratification using different risk factors at randomization are required to assess whether the efficacy of low-dose BCG is comparable to standard-dose BCG for the different risk of patients.
Supplementary Material
Supplementary Material
Acknowledgments
to the authors thank Professor Chunbo Li, the director of the Chinese Satellite of Cochrane Schizophrenia Group, who kindly gives us advice to perform this systematic review and meta-analysis.
Footnotes
Abbreviations: BCG = Bacillus Calmette-Guerin, CI = confidence interval, CUETO = Club Urologico Espanol de Tratamiento Oncologico, EORTC-GU = European Organization for Research and Treatment of Cancer, Genito-Urinary Cancer Group, HR = hazard ratio, NMIBC = nonmuscle invasive bladder cancer, qRCT = quasi-randomized controlled trials, RCT = randomized controlled trials, RR = risk ratio, TURBT = transurethral resection of the bladder tumor.
SZ, XY, and CM contributed equally to this work.
This study was supported by the foundation of Excellent Academic Leaders of Shanghai (XBR2013076), authors declared that they have no relevant financial interests.
REFERENCES
- 1.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013; 63:234–241. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013; 64:639–653. [DOI] [PubMed] [Google Scholar]
- 3.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013; 63:462–472. [DOI] [PubMed] [Google Scholar]
- 4.Witjes JA, Palou J, Soloway M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): results of an international individual patient data survey (IPDS). BJU Int 2013; 112:742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brausi M, Oddens J, Sylvester R, et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol 2014; 65:69–76. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Piñeiro JA, Flores N, Isorna S, et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guérin with a reduced dose of 27 mg in superficial bladder cancer. BJU Int 2002; 89:671–680. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Pineiro JA, Martinez-Pineiro L, Solsona E, et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol 2005; 174:1242–1247. [DOI] [PubMed] [Google Scholar]
- 8.Phillips B, Ball C, Sackett D, et al. Oxford Centre for Evidence-based Medicine–levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. Available from http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. [Google Scholar]
- 9.Higgins J, Altman D, Sterne J. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011; Available from http://handbook.cochrane.org/. [Google Scholar]
- 10.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13:176–181. [DOI] [PubMed] [Google Scholar]
- 11.Bassi P, Nicola P, Spinatdin R, et al. Low dose vs standard dose BCG therapy of superficial bladder cancer: final results of a phase 3 randomized trial. Eur Urol 1999; 35:152.Abstract. [Google Scholar]
- 12.Esuvaranathan K, Tham SM, Ravuru M, et al. Long term results of a double-blind randomized controlled trial of interferon alpha-2b and low dose bcg in patients with high risk non-muscleinvasive bladder cancer. J Urol 2014; 191:e571. [Google Scholar]
- 13.Irie A, Uchida T, Yamashita H, et al. Sufficient prophylactic efficacy with minor adverse effects by intravesical instillation of low-dose bacillus Calmette-Guerin for superficial bladder cancer recurrence. Int J Urol 2003; 10:183–189. [DOI] [PubMed] [Google Scholar]
- 14.Morales A, Nickel JC, Wilson JWL. Dose-response of bacillus Calmette-Guerin in the treatment of superficial bladder cancer. J Urol 1992; 147:1256–1258. [DOI] [PubMed] [Google Scholar]
- 15.Yalcinkaya F, Kamis L, Ozteke O, et al. Prospective randomized comparison of intravesical BCG therapy with standard dose versus low doses in superficial bladder cancer. Int Urol Nephrol 1998; 30:41–44. [DOI] [PubMed] [Google Scholar]
- 16.Yokomizo A, Akaza H, Ozono S, et al. A randomized control study to evaluate the efficacy, safety and QOL in low-dose bacillus calmette-guerin instillation therapy for non-muscle invasive bladder cancer. Urology 2014; 84:S46. [Google Scholar]
- 17.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008; 27:954–970. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal MS, Agrawal M, Bansal S, et al. The safety and efficacy of different doses of bacillus Calmette Guerin in superficial bladder transitional cell carcinoma. Urology 2007; 70:1075–1078. [DOI] [PubMed] [Google Scholar]
- 22.Vijjan V, Mandhani A, Kapoor R, et al. A randomized trial comparing low dose (40 or 80 mg) with standard dose (120 mg) of bacillus calmette-guerin for superficial bladder cancer. Indian J Urol 2006; 4:317–321. [Google Scholar]
- 23.Inamoto T, Ubai T, Nishida T, et al. Comparable effect with minimal morbidity of low-dose Tokyo 172 strain compared with regular dose Connaught strain as an intravesical bacillus Calmette-Guerin prophylaxis in nonmuscle invasive bladder cancer: Results of a randomized prospective comparison. Urol Ann 2013; 5:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemdan T, Johansson R, Jahnson S, et al. 5-Year outcome of a randomized prospective study comparing bacillus Calmette-Guerin with epirubicin and interferon-alpha2b in patients with T1 bladder cancer. J Urol 2014; 191:1244–1249. [DOI] [PubMed] [Google Scholar]
- 25.Vegt PD, van der Meijden AP, Sylvester R, et al. Does isoniazid reduce side effects of intravesical bacillus Calmette-Guerin therapy in superficial bladder cancer? Interim results of European Organization for Research and Treatment of Cancer Protocol 30911. J Urol 1997; 157:1246–1249. [PubMed] [Google Scholar]
- 26.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000; 163:1124–1129. [PubMed] [Google Scholar]
- 27.Galliot I, Gall S, Rigaud J, et al. Bacillus Calmette-Guerin maintenance treatment in non-invasive bladder tumors: 1 year follow-up results of multicenter URO-BCG-4 trial. [French]. Prog Urol 2013; 5:336–346. [DOI] [PubMed] [Google Scholar]
- 28.Pfister C, Kerkeni W, Rigaud J, et al. Efficacy and tolerance of one-third full dose bacillus Calmette-Guerin maintenance therapy every 3 months or 6 months: two-year results of URO-BCG-4 multicenter study. Int J Urol 2015; 22:53–60. [DOI] [PubMed] [Google Scholar]
- 29.Ojea A, Nogueira JL, Solsona E, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol 2007; 52:1398–1406. [DOI] [PubMed] [Google Scholar]
- 30.Mitropoulos D, Artibani W, Graefen M, et al. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol 2012; 61:341–349. [DOI] [PubMed] [Google Scholar]
- 31.Mohanty N, Arora RP, Nayak RL, et al. Can in vitro tumour cell culture and cyto immuno assay individualise intra vesical therapy for TCC of urinary bladder – an innovative idea. Eur Urol, Suppl 2009; 8:188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


