Abstract
This study assesses the validity, reliability, and responsiveness of the Dutch version of the London Chest Activity of Daily Living scale (LCADL).
The English LCADL version was translated into Dutch and then back-translated to English to check if the translation was conceptually equivalent to the original LCADL.
Measurement properties were evaluated in191 patients with chronic obstructive pulmonary disease (COPD) (70 males; age 62 ± 9 years; FEV1 33 ± 10% pred). Construct validity was assessed using disease-specific health status, generic functional status, and functional and peak exercise capacity (Wmax). LCADL was completed twice to assess test–retest reliability. Responsiveness was assessed after 8 to 12 weeks inpatient pulmonary rehabilitation.
LCADL correlated significantly with the St. George Respiratory Questionnaire (r = 0.24 to 0.64), functional status (r = 0.45 to 0.82), walking distance (r = −0.3 to −0.58), and Wmax (−0.27 to −0.38) and Wmax % pred (−0.26 to −0.43). Test–retest reliability was high (ICC 0.87 to 0.98). The smallest detectable change for the LCADL total and domain score self-care, domestic, physical, and leisure was 4.5, 2.9, 3.3, 4.9, and 2.2, respectively. Improvement in LCADL after PR correlated significantly with improvement in Chronic Respiratory Questionnaire (−0.43; P < 0.001).
The Dutch LCADL is a reliable, valid, and responsive instrument to assess limitations in performing activities of daily living in patients with severe COPD.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a progressive pulmonary disease, which leads to reductions in pulmonary function and diminished exercise capacity.1 Resulting physical limitations and disruptions of daily life activities have serious implications for independence and quality of life. The primary goal of COPD rehabilitation is to achieve and maintain the individual's highest level of independence and functioning by focusing on alleviation or prevention of symptoms, ability to complete activities of daily living (ADL), improvement of functional status, and enhancing quality of life.2,3 Guidelines indicate that improvement in functional daily activities is an important goal in COPD treatment.2 Several instruments are available to measure different aspects of functional daily activities.4 The sensitivity of an instrument to assess a particular aspect of daily activities is of importance in order to detect clinically relevant changes after rehabilitative interventions. Dyspnea is a hallmark of exercise intolerance in patients with COPD. Physical exertion increases breathing difficulties and dyspnea is the symptom that limits activity in COPD patients most severely.5 Difficulties in executing daily activities due to disabling dyspnea are present in COPD in several domains of daily activity.6 The London Chest Activity of Daily living scale (LCADL) is a valid, reliable, and responsive instrument to assess the degree of dyspnea during ADL in patients with severe COPD.7,8 The advantage of a disease-specific questionnaire is that it is more likely to be sensitive to the specific problems associated with COPD. In addition, it may be more responsive to the effects of interventions like pulmonary rehabilitation (PR), compared to generic measures. In the UK the LCADL is recommended in NICE (National Institute for Health and Care Excellence) COPD guidelines.9
Several valid translations of the LCADL scale have been completed.10–12 There is a need for a valid Dutch version of the LCADL for use in both research and clinical practice. Translation into different languages will also allow the use of the LCADL in comparative international multi-center studies. Adequate psychometric properties of the Dutch LCADL scale are important to convince clinicians and investigators to use this instrument. The aim of the present study was to translate the LCADL in Dutch and to evaluate the cross-sectional validity and reliability, and responsiveness and longitudinal validity of the Dutch LCADL in patients with severe COPD.
METHODS
Patients and Procedures
Measurement properties of the Dutch version of the LCADL were evaluated in a multicenter observational study performed during PR. The following inclusion criteria were used: diagnosis of stage III/IV COPD based on the Global Initiative for Chronic Obstructive Lung Diseases (GOLD)13; forced expiratory volume in 1 second (FEV1) < 50% pred and FEV1/forced vital capacity (FVC) < 70% pred; no exacerbation for at least 4 weeks before entering the PR program. All questionnaires were self-administered and supervised by a test-assistant. Patients who had problems understanding the Dutch language were excluded. In a subset of clinically stable patients the LCADL was administered twice within 7 to 10 days. Under the Dutch Medical Research Involving Human Subjects Act (WMO), this study is exempt from ethical review. This was confirmed by the Medical Ethical Commission (METC), Academic Medical Center (Amsterdam, The Netherlands), protocol number 09/037. All patients provided written informed consent. In accordance with the original studies from Garrod et al,7,8 the St. George's Respiratory Questionnaire (SGRQ) was used for assessment of construct validity and the Chronic Respiratory Questionnaire (CRQ) for responsiveness of the LCADL.
Translation
A forward–backward method of translation was used to check if the translation was conceptually equivalent to the original LCADL. The English version of the LCADL scale developed by Garrod and colleagues7 was translated into Dutch separately by 2 researchers (HS and PK). The combined Dutch version was then back-translated into English by a native speaking healthcare professional (BP). Possible text-related problems were discussed and assessed until consensus was reached, leading to the final version.
London Chest Activity of Daily Living Scale
The LCADL consists of 15 questions designed to measure dyspnea during routine daily activities in patients with COPD.7 The LCADL consists of 4 components: self-care, domestic, physical, and leisure. Patients score from 0: “I wouldn’t do it anyway,” to 5: “someone else does this for me (or helps).” The total scores range from 0 to 75 with higher scores corresponding to greater limitation in ADL. We also interpreted a total LCADL score disregarding the questions in which the score was 0, as suggested previously, and presented it as a percentage of the total score.10
Construct Validity
Construct validity was assessed by correlating the LCADL scores with health status (SGRQ),7,14,15 the Groningen Activities Restriction Scale (GARS)16 severity scores, pulmonary functioning, maximal, and functional exercise capacity. The SGRQ is a valid and reliable measure of health status in patients with COPD.15 The SGRQ consists of 50 items divided into 3 domains component scores—symptoms, activity, and psychosocial impact and a total score (a score of 100 represents maximal disability). The GARS is a generic measure of subjective functional status (18 items) and is scored on a 4-point scale.16 Scores range from 18 (no disability) to 72 points (highly disabled). The total GARS score can be subdivided in a personal care domain (11 items) and an instrumental ADL domain (domestic activities; 7 items). Resting pulmonary function was determined, according to previously described recommendations and related to predicted normal values.17 Functional exercise capacity was assessed with the 6-minute walk distance (6MWD).18 Maximal workload (Wmax) was determined during an incremental symptom-limited cycle exercise test.19,20
Test–Retest Reliability
Test–retest reliability was assessed in a subgroup of 52 patients with a stable condition. Test retests were conducted 5 to 10 days apart. Stable clinical condition in the test–retest period was defined as21:
No visits to the pulmonologist for breathing problems.
A single item for self-perceived change in disease symptoms with a 5-point response scale (“How would you rate your health status at this moment: much improved—improved—the same—worse—much worse”).
Patients who visited the pulmonologist during the test–retest period and those who reported a change in self-perceived health status were omitted from test–retest reliability analysis.
Responsiveness
The sensitivity of the LCADL to detect change after an intervention was assessed in a separate group of patients who had undergone 8 to 12 weeks inpatient PR. Inpatient PR program consists of a comprehensive, multidisciplinary program which included among others: exercise training, functional daily activities training, dyspnea management, breathing retraining, and education regarding mechanisms of breathlessness, energy conserving techniques, medication management, and psychosocial support sessions relating to chronic disability.22
Changes in LCADL-total scores between the pre- and postrehabilitation were calculated and related to change in health status, assessed with the Dutch version of the CRQ self-reported.19,23 The CRQ has 4 domains (dyspnea, fatigue, emotion, mastery) each ranging from 1 (most severe impairment) to 7 (no impairment), and higher scores indicating better health status.23
Sample Size
Sample sizes were chosen according to the Consensus-based Standards for the selection of health Measurements Instruments (COSMIN).24 A minimum sample size of 100 patients is recommended for evaluation of validity and responsiveness. For reliability a minimum sample size of 50 patients is needed to obtain a confidence interval (CI) from 0.70 to 0.90 around an intraclass correlation coefficient (ICC) of 0.80.25
Data Analyses
Baseline characteristics of the patients were described using means with standard deviation.
Construct Validity
Pearson correlation coefficient (R) was used to determine relationships of the LCADL total and percentage of the total scores with other measures of functional and health status. We hypothesized no correlations for pulmonary function measures, weak to moderate correlations for maximal exercise capacity, moderate to strong correlations for 6 MWD, and strong correlations for generic functional status and health status.7 Strength of the correlations was interpreted using the criteria described by Guyatt and colleagues26: <0.2 as very weak, 0.2 to 0.35 as weak, 0.35 to 0.5 as moderate, and ≥0.5 as strong.
Reliability
Test–retest reliability of the Dutch-LCADL total scores was quantified using the ICC and smallest detectable change (SDC). ICC was calculated from analyses of variance (ANOVA). A 2-way random effects model was used, with a restricted maximum likelihood method. An ICC value >0.70 is considered acceptable.27
The standard error of measurement was calculated from the variance components test occasions (σo) and random error (σe) using the formula
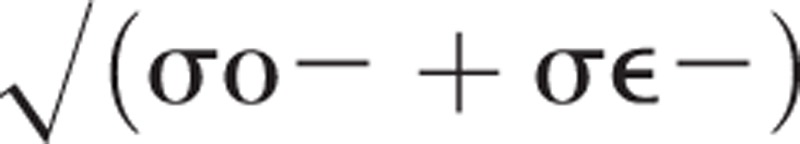 |
. Bland–Altman plots were constructed to visually inspect and rule out the presence of heteroscedasticity (ie, the SEM (standard error of measurement) not being independent of the mean).28 From the SEM, the smallest detectable change (SDC) was calculated which represents the minimum difference that can be considered a real change between measurements with 95% certainty. The SDC was calculated as 1.96∗SEM∗√2.
For all tests a level of significance of P ≤ 0.05 was used. Data were analyzed using SPSS version 20 (SPSS, Inc., Chicago, IL).
RESULTS
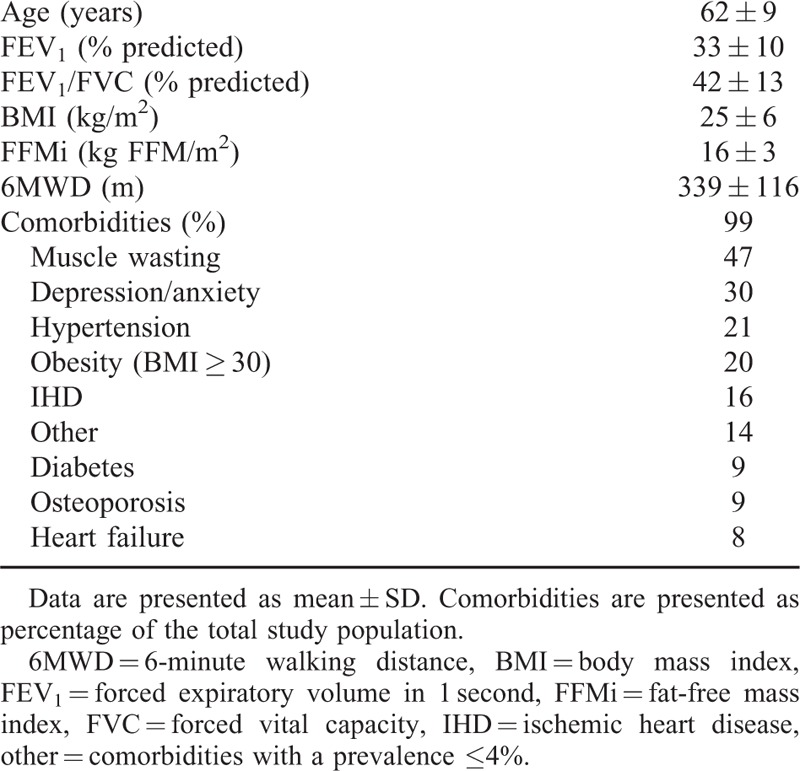
The baseline characteristics of all the patients (n = 191; 70 M, 121 F) included in the study are provided in Table 1. Patients had severe to very severe airway obstruction (GOLD III, 62%; GOLD IV, 38%) and a reduced functional exercise capacity. Almost all patients (97.4%) had 1 or more comorbidities.
TABLE 1.
Baseline Characteristics of the Total Study Population
Construct Validity
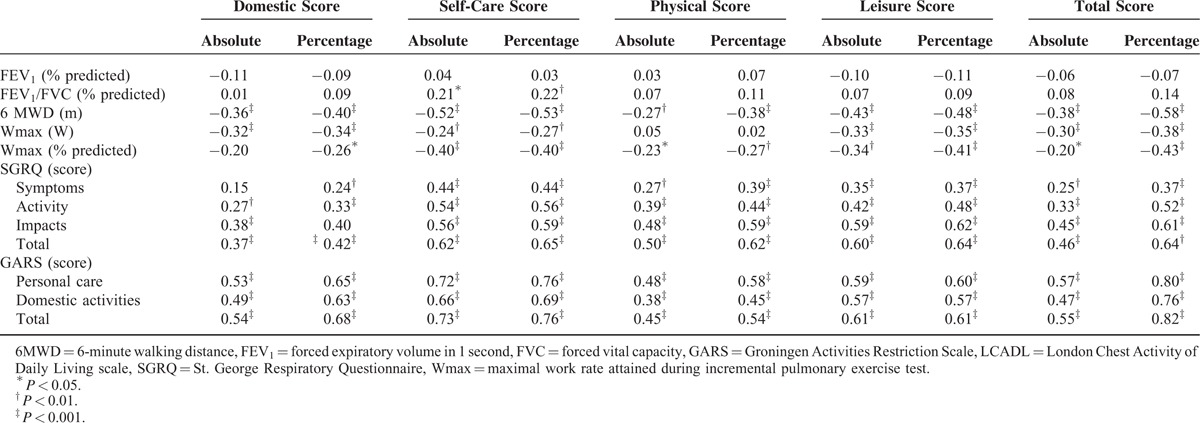
The Dutch LCADL showed good construct validity, as shown in Table 2. The percentage of the total score increased the number of confirmed hypothesis: pulmonary function 9 out of 10 hypothesis, peak exercise capacity 5 out of 10, 6MWD 5 out of 5, SGRQ and GARS 25 out of 35.
TABLE 2.
Construct Validity: Spearman Correlation Coefficients Between London Chest Activity of Daily Living Scale and Other Measures of Health-Status in Patients With Severe COPD (n = 137)
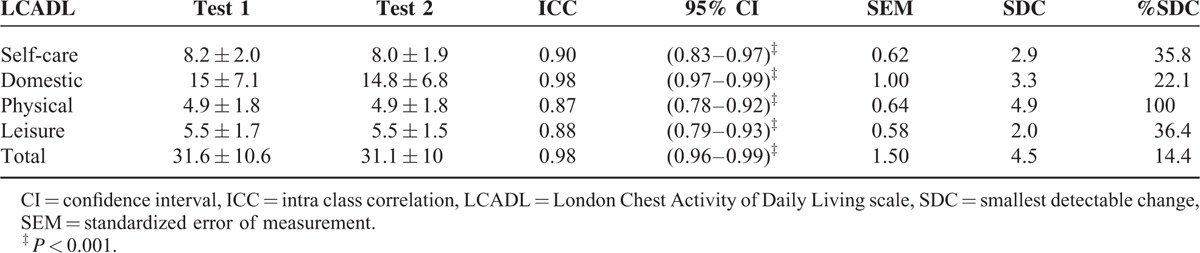
In 61 patients test–retest assessments were performed. However, 9 patients reported change (self-reported; 1 patient much worse, 5 patients worse, 3 patients much better). Therefore, 52 patients (63 ± 8 years, FEV1 34 ± 9% pred) were included in the analysis of reliability. As shown in Table 3, good test–retest reliability was found in all the domains of the LCADL scale.
TABLE 3.
London Chest Activity of Daily Living Scale Test–Retest Reliability (n = 52)
The sensitivity of the LCADL to detect change after 8 to 12 weeks inpatient PR was assessed in 60 patients (age 63 ± 9 years, FEV1% pred 34 ± 10).
As shown in Table 4, dyspnea during daily activities improved significantly after rehabilitation as demonstrated by a reduction in all components of the LCADL scale. In 55% of patients the improvement of LCADL total was larger than the SDC of the total score. For leisure, self-care, domestic, and physical, roughly 12%, 48%, 37%, and 2%, respectively, of individual improvements were above the SDC. The change in LCADL total score showed a moderate but significant correlation with the change in CRQ score, R = −0.47, P < 0.001 (see Fig. 1).
TABLE 4.
Change in London Chest Activity of Daily Living Scale After Pulmonary Rehabilitation (n = 60)
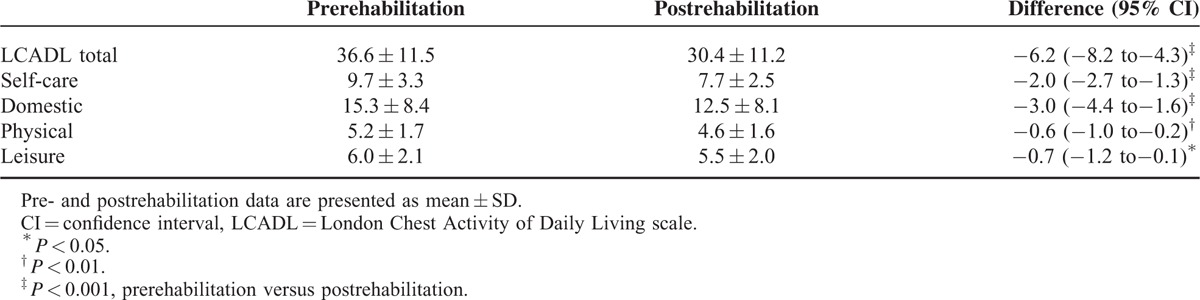
Figure 1.
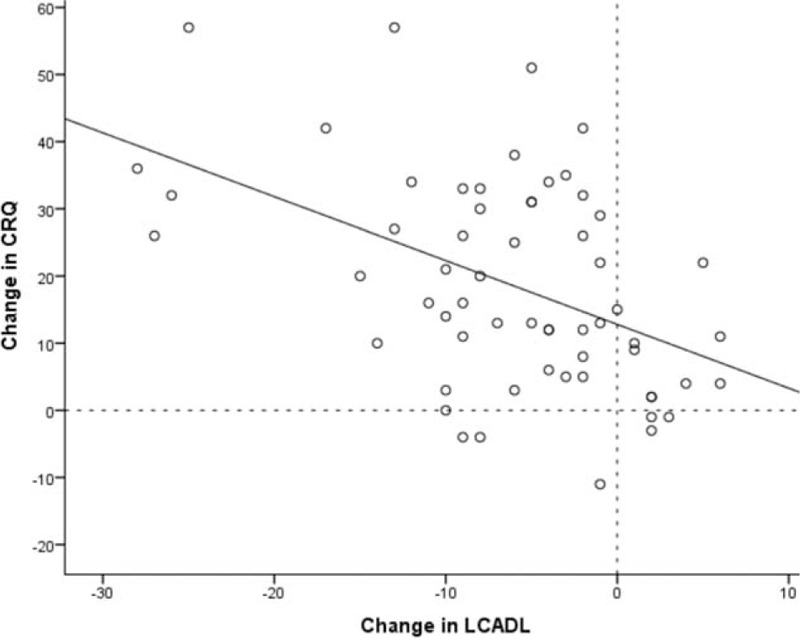
The relationship between the change in London Chest Activity of Daily Living (LCADL) and Chronic Respiratory Questionnaire (CRQ) after pulmonary rehabilitation. Improvement is indicated by positive CRQ-change scores and negative LCADL-change scores.
DISCUSSION
This study shows that the Dutch version of the LCADL is a reliable and valid instrument to measure dyspnea-related functional impairment of ADL in patients with severe COPD. Strong test–retest reliability was obtained for all 4 components and the total score. Construct validity was good, with moderate to strong correlations with health status, generic functional status and, maximal and functional exercise capacity. Furthermore, the LCADL was moderately sensitive to measure change after an intervention.
The test–retest reliability of the LCADL over repeated administration was considered good to very good, with ICC values ranging from 0.88 to 0.98. This result is in concordance with the original LCADL validation study and other cross-cultural validation studies which also showed high test–retest reliability.7,10–12 The minimal detectable change for LCADL total, self-care, domestic, physical, and leisure score found in the present study were slightly higher than those found in a recent study of Bisca et al.29 Our SDC values were higher which can possibly be explained by the smaller number of patients used in the Bisca et al29 study which results in larger measurement error. The SDC% for the LCADL total score was lower than the SDC% for the domain scores. This indicates that the total score is preferred in case of within-patient comparisons.
To test construct validity, the LCADL total score should, consistent with the underlying theoretical construct, correlate with related domains of functioning. No significant correlations were found for LCADL score with pulmonary function measures. This is in accordance with previous reports that performance of ADL and disease severity are influenced by factors other than airflow limitation solely.1,30,31 This highlights the multifactorial nature of intolerance to daily activities seen in many patients with COPD.32 The LCADL is intended for people with severe COPD as with increasing disease severity dyspnea begin to have an impact on ADL.33 Functional and maximal exercise tests represent7,10–12 different constructs of exercise capacity.34 Therefore, associations with both 6MWD and peak exercise capacity were explored. Significant weak to moderate, negative correlations were found for maximal and functional exercise capacity with LCADL total score. Some activities are, however, never performed by some patients, which let them to give a score of 0, thereby reducing the total score. Carpes et al10 showed an improvement in the correlation between LCADL and 6MWD when using the percentage of the total LCADL score. By analyzing the LCADL as a percentage of the total score the association with maximal exercise capacity increased from weak to moderate and with functional exercise capacity (distance walked in 6 minutes) from moderate (−0.38) to strong (−0.58). Garrod et al7 showed an identical association between LCADL and the incremental shuttle walk test (−0.58). The negative correlations imply that patients who have a lower physical fitness are more hindered in performing functional activities. These results support the validity of the LCADL. Successful performance of physical activities requires a complex interaction of cardiorespiratory and musculoskeletal systems. Obviously, a change in physical fitness will have an influence on activities in daily life. However, this must not detract from the fact that performance of activities in daily life and exercise performance are considered to be distinct constructs.1,35 The benefits of exercise training are particularly specific and more likely restricted to the activities used during training than other forms of activity.36 The relationship between traditional performance tests and actual performance in specific ADL tasks in patients’ home setting warrants further study. To further test construct validity, LCADL total score was correlated with health status (SGRQ) and a generic measure of subjective functional status (GARS). Weak to moderate correlations were found with the SGRQ and moderate to strong correlations with the GARS. When analyzed as percentage of the total score, the association of the LCADL with SGRQ total, activity and impacts increased to strong. The correlation with SGRQ activity increased as well but remained moderate. This result is comparable with the original validation study of Garrod and colleagues and the Portuguese LCADL.7,11 The relationship with the GARS became even stronger, raising all correlations to 0.76 to 0.82. Reardon et al37 showed that walking up stairs, house work, walking on level ground, bathing and showering and making a bed are the activities most often selected as causing the most dyspnea. All of these activities are represented in the LCADL which is also a strong confirmation of the construct validity.
In the majority of the study population one or more comorbidities were present. Several comorbid conditions are associated with dyspnea (eg, anxiety/depression, cardiac conditions).38 These concurrent morbidities might influence the LCADL outcome. During our PR program comorbidities are treated according to local current guidelines. PR has been shown to reduce dyspnea, anxiety, and depression.38 Moreover, comorbidities do not seem to have an important influence on obtaining clinical and significant improvements following PR.39
Strengths of the study are that whilst number of patients are small they are significantly greater than in other validation studies of the LCADL10,11,40 and are sufficient according to the COSMIN criteria.24 Moreover, construct validity and test–retest reliability was assessed in groups of outpatients and inpatients. A limitation of our study was that responsiveness was only assessed following inpatient PR which raises the question of generalizability.
CONCLUSION
Multicomponent assessment and a combination of tests may be necessary to capture all aspects relevant to patients’ limitation of meaningful activities in daily life.4,41 The LCADL scale is a user-friendly and feasible clinical instrument, for assessing dyspnea-related functional impairment in patients with severe COPD. The instrument is moderately sensitive to measure change after an intervention with improvements exceeding the recently suggested minimal detectable change.29 The SEM values can be used to assess the relevance of observed changes in individual patients.42 In addition, since %SDC was lowest for LCADL total, we suggest to use the LCADL total score for individual patients. The Dutch LCADL measures a relevant aspect in the assessment of ADL intolerance. In order to develop tailored treatment programs, the authors propose that the Dutch LCADL should be part of a comprehensive assessment of ADL in patients with severe COPD.
Acknowledgments
The authors would like to thank the patients for their participation in this study. They are also grateful to the department of data management and function testing.
Footnotes
Abbreviations: 6MWD = 6-minute walking distance, ADL = activities of daily living, ANOVA = analyses of variance, BMI = body mass index, CI = confidence interval, COPD = chronic obstructive pulmonary disease, COSMIN = Consensus-based Standards for the selection of health Measurements Instruments, CRQ = Chronic Respiratory Questionnaire, FEV1 = forced expiratory volume in 1second, FFMi = fat-free mass index, FVC = forced vital capacity, GARS = Groningen Activities Restriction scale, GOLD = Global Initiative for Chronic Obstructive Lung Diseases, ICC = intraclass correlation coefficient, IHD = ischemic heart disease, LCADL = London Chest Activity of Daily Living scale, METC = Medical Ethical Commission, NICE = National Institute for Health and Care Excellence, PR = pulmonary rehabilitation, SDC = smallest detectable change, SEM = standard error of measurement, SGRQ = St. George Respiratory Questionnaire, Wmax = maximal work rate attained during incremental pulmonary exercise test, WMO = Medical Research Involving Human Subjects Act.
Author contribution: PK, ML, AK, and HS contributed to the study concept, data interpretation, and/or writing of the manuscript. PK and ML were responsible for data collection. AB, RG, MB, BP, and AS contributed to interpretation of results and writing of the manuscript. PK, HS, and BP contributed to the translation process of the LCADL. PK, ML, and HS were responsible for the integrity and the accuracy of the data analysis. All authors have read and approved the final manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188:e13–e64. [DOI] [PubMed] [Google Scholar]
- 2.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007; 131 (5 Suppl.):4S–42S. [DOI] [PubMed] [Google Scholar]
- 3.Bourbeau J. Activities of life: the COPD patient. COPD 2009; 6:192–200. [DOI] [PubMed] [Google Scholar]
- 4.Janaudis-Ferreira T, Beauchamp MK, Robles PG, et al. Measurement of activities of daily living in patients with COPD: a systematic review. Chest 2014; 145:253–271. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD 2007; 4:225–236. [DOI] [PubMed] [Google Scholar]
- 6.Leidy NK, Haase JE. Functional performance in people with chronic obstructive pulmonary disease: a qualitative analysis. ANS Adv Nurs Sci 1996; 18:77–89. [DOI] [PubMed] [Google Scholar]
- 7.Garrod R, Bestall JC, Paul EA, et al. Development and validation of a standardized measure of activity of daily living in patients with severe COPD: the London Chest Activity of Daily Living scale (LCADL). Respir Med 2000; 94:589–596. [DOI] [PubMed] [Google Scholar]
- 8.Garrod R, Paul EA, Wedzicha JA. An evaluation of the reliability and sensitivity of the London Chest Activity of Daily Living Scale (LCADL). Respir Med 2002; 96:725–730. [DOI] [PubMed] [Google Scholar]
- 9.National Clinical Guideline Centre. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. 2010; London: National Clinical Guideline Centre, Assessed 20-10-2015. [Google Scholar]
- 10.Carpes MF, Mayer AF, Simon KM, et al. The Brazilian Portuguese version of the London Chest Activity of Daily Living scale for use in patients with chronic obstructive pulmonary disease. J Bras Pneumol 2008; 34:143–151. [DOI] [PubMed] [Google Scholar]
- 11.Pitta F, Probst VS, Kovelis D, et al. [Validation of the Portuguese version of the London Chest Activity of Daily Living Scale (LCADL) in chronic obstructive pulmonary disease patients]. Rev Port Pneumol 2008; 14:27–47. [PubMed] [Google Scholar]
- 12.Vilaro J, Gimeno E, Sanchez FN, et al. [Daily living activity in chronic obstructive pulmonary disease: validation of the Spanish version and comparative analysis of 2 questionnaires]. Med Clin (Barc) 2007; 129:326–332. [DOI] [PubMed] [Google Scholar]
- 13.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555. [DOI] [PubMed] [Google Scholar]
- 14.Royal Dutch Society for Physical Therapy. Clinical Practice Guideline for physical therapy in patients with COPD. Dutch J Phys Ther 1998; 108 Suppl 5:1–44. [Google Scholar]
- 15.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145:1321–1327. [DOI] [PubMed] [Google Scholar]
- 16.Kempen GI, Miedema I, Ormel J, et al. The assessment of disability with the Groningen Activity Restriction Scale. Conceptual framework and psychometric properties. Soc Sci Med 1996; 43:1601–1610. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16:5–40. [PubMed] [Google Scholar]
- 18.ATS. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117. [DOI] [PubMed] [Google Scholar]
- 19.Palange P, Ward SA, Carlsen KH, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J 2007; 29:185–209. [DOI] [PubMed] [Google Scholar]
- 20.Jones NL, Killian KJ. Exercise limitation in health and disease. N Engl J Med 2000; 343:632–641. [DOI] [PubMed] [Google Scholar]
- 21.van Stel HF, Maille AR, Colland VT, et al. Interpretation of change and longitudinal validity of the quality of life for respiratory illness questionnaire (QoLRIQ) in inpatient pulmonary rehabilitation. Qual Life Res 2003; 12:133–145. [DOI] [PubMed] [Google Scholar]
- 22.Klijn P, van Keimpema A, Legemaat M, et al. Nonlinear exercise training in advanced chronic obstructive pulmonary disease is superior to traditional exercise training. A randomized trial. Am J Respir Crit Care Med 2013; 188:193–200. [DOI] [PubMed] [Google Scholar]
- 23.Gosselink HAAM, Wagenaar RC, Keimpema van ARJ, et al. The effect of a rehabilitation program in patients with COPD and asthma. Ned Tijdschr Fysioth 1990; 100:193–199. [Google Scholar]
- 24.Mokkink LB, Terwee CB, Patrick DL, et al. COSMIN Checklist Manual. 2012; http://www.cosmin.nl/images/upload/File/COSMINchecklistmanualv9.pdf [Google Scholar]
- 25.Giraudeau B, Mary JY. Planning a reproducibility study: how many subjects and how many replicates per subject for an expected width of the 95 per cent confidence interval of the intraclass correlation coefficient. Stat Med 2001; 20:3205–3214. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, King DR, Feeny DH, et al. Generic and specific measurement of health-related quality of life in a clinical trial of respiratory rehabilitation. J Clin Epidemiol 1999; 52:187–192. [DOI] [PubMed] [Google Scholar]
- 27.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60:34–42. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–310. [PubMed] [Google Scholar]
- 29.Bisca GW, Proenca M, Salomao A, et al. Minimal detectable change of the London Chest Activity of Daily Living Scale in patients with COPD. J Cardiopulm Rehabil Prev 2014; 34:213–216. [DOI] [PubMed] [Google Scholar]
- 30.Annegarn J, Meijer K, Passos VL, et al. Problematic activities of daily life are weakly associated with clinical characteristics in COPD. J Am Med Dir Assoc 2012; 13:284–290. [DOI] [PubMed] [Google Scholar]
- 31.Williams SJ, Bury MR. ‘Breathtaking’: the consequences of chronic respiratory disorder. Int Disabil Stud 1989; 11:114–120. [DOI] [PubMed] [Google Scholar]
- 32.Debigare R, Maltais F. The major limitation to exercise performance in COPD is lower limb muscle dysfunction. J Appl Physiol 2008; 105:751–753. [DOI] [PubMed] [Google Scholar]
- 33.Reardon JZ, Lareau SC, Zuwallack R. Functional status and quality of life in chronic obstructive pulmonary disease. Am J Med 2006; 119 (10 Suppl. 1):32–37. [DOI] [PubMed] [Google Scholar]
- 34.Lacasse Y, Martin S, Lasserson TJ, et al. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A Cochrane systematic review. Eura Medicophys 2007; 43:475–485. [PubMed] [Google Scholar]
- 35.Leidy NK. Functional status and the forward progress of merry-go-rounds: toward a coherent analytical framework. Nurs Res 1994; 43:196–202. [PubMed] [Google Scholar]
- 36.Hickson RC, Hidaka K, Foster C. Skeletal muscle fiber type, resistance training, and strength-related performance. Med Sci Sports Exerc 1994; 26:593–598. [PubMed] [Google Scholar]
- 37.Reardon J, Patel K, Zuwallack R. Improvement in quality of life is unrelated to improvement in exercise endurance after outpatient pulmonary rehabilitation. J Cardiopulm Rehabil 1993; 13:51–54. [Google Scholar]
- 38.Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis 2014; 9:871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesquita R, Vanfleteren LE, Franssen FM, et al. Objectively identified comorbidities in COPD: impact on pulmonary rehabilitation outcomes. Eur Respir J 2015; 46:545–548. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho VO, Garrod R, Bocchi EA, et al. Validation of the London Chest Activity of Daily Living scale in patients with heart failure. J Rehabil Med 2010; 42:715–718. [DOI] [PubMed] [Google Scholar]
- 41.Ortega F, Montemayor T, Sanchez A, et al. Role of cardiopulmonary exercise testing and the criteria used to determine disability in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994; 150:747–751. [DOI] [PubMed] [Google Scholar]
- 42.Wyrwich KW, Wolinsky FD. Identifying meaningful intra-individual change standards for health-related quality of life measures. J Eval Clin Pract 2000; 6:39–49. [DOI] [PubMed] [Google Scholar]


