Abstract
Whether patients with diverticular diseases exhibit a higher risk of developing pyogenic liver abscess (PLA) remains inconclusive.
From the inpatient claims in Taiwan's National Health Insurance Research Database, we identified 54,147 patients diagnosed with diverticulosis in the 1998 to 2010 period and 216,588 controls without the disorder. The 2 cohorts were matched by age, sex, and admission year, and were followed up until the end of 2010 to estimate the risk of PLA.
Overall, the incidence of PLA was 2.44-fold higher in the diverticular-disease group than in the controls (11.5 vs 4.65 per 10,000 person-year). The adjusted hazard ratio (aHR) of PLA was 2.11 (95% confidence interval [CI], 1.81–2.44) for the diverticular-disease group, according to a multivariate Cox proportional hazards regression model. The age-specific data showed that the aHR for the diverticular-disease group, compared with the controls, was the highest inpatients younger than 50 years old (aHR, 4.03; 95% CI, 2.77–5.85). Further analysis showed that the diverticular-disease group exhibited an elevated risk of PLA regardless of whether patients had diverticulitis.
The patients with diverticular diseases exhibited a higher risk of PLA.
INTRODUCTION
Pyogenic liver abscess (PLA) is a crucial clinical disease that can be fatal without prompt recognition and treatment.1 An increasing number of studies have demonstrated that PLA in Western and Asian countries differs in many aspects, including demographic characteristics, etiological factors, and clinical manifestations. For example, a significant proportion of PLA in Asia is associated with Klebsiella pneumoniae infection, whereas Escherichia coli is the predominant pathogen found in PLA in Western countries.2 Moreover, PLA in Asia is typically associated with diabetes or a cryptogenic etiology,3–6 compared with the biliary etiology observed in Western countries.
Colonic diverticular diseases are a relatively common health care problem and the most frequently reported pathology in routine colonoscopy.7 Autopsy studies have estimated the diverticular-disease prevalence at 5% to 52% in Western countries, and 1% to 19% in Asia.8 Approximately one-third of the population older than 45 years is estimated to have diverticular disease.9 Diverticular diseases can lead to disruption of the colonic mucosal barrier and therefore major clinical morbidities, including inflammation, obstruction, infection, hemorrhage, fistula formation, perforation, and peritonitis.10
Previous studies have demonstrated that the hematogenous spread of pathogens to the liver is a critical pathogenic factor for PLA, and colonic mucosal defects might be a route for bacterial invasion into the portal system.11 In a relatively small cohort study, colonoscopy revealed a colonic cause in 24.3% of patients with cryptogenic PLA.11 However, no study has been conducted in Asia to investigate the epidemiologic association between diverticular diseases and PLA by using a nationwide population-based dataset. We therefore hypothesized that patients with diverticular diseases may have a higher risk of PLA than patients without diverticular diseases and conducted this study by analyzing data from Taiwan's National Health Insurance Research Database (NHIRD), which is composed of deidentified medical claims from 99% of the 23 million residents of Taiwan.
METHODS
Data Source
The National Health Insurance (NHI) program was implemented in Taiwan on March 1, 1995, and provides insurance coverage for approximately 99% of the population of Taiwan (Ref1). The National Health Research Institutes (NHRI) maintains the NHIRD, comprising the administrative and health claims data of the NHI program, and makes it available for medical research. In this study, we used a subset of the NHIRD that includes files of inpatient claims from the Registry of Beneficiaries. The identification numbers of patients were scrambled before being released to users to protect the privacy of the insurant. Diseases are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH-104-REC2–115). The IRB also specifically waived the consent requirement.
Sample Patients
We identified patients with newly diagnosed diverticular diseases, including diverticulosis (ICD-9-CM codes 562.10, 562.12) and diverticulitis (ICD-9-CM codes 562.11, 562.13), from 2000 to 2011, from inpatient claims data. The initial date of hospitalization with a diagnosis of diverticular-disease was defined as the index date. Patients with history of PLA (ICD-9-CM code 572.0) or amebic liver abscess (ICD-9-CM code 006.3), missing data for sex or date of birth, or aged younger than 20 years were excluded from our study. NHI beneficiaries aged 20 years and older without diverticular were randomly selected for inclusion in a nondiverticulosis cohort and frequency matched with the patients in the diverticular cohort at a 4:1 ratio according to age (in 5-year bands), sex, history of diabetes mellitus (ICD-9-CM code 250), and index year, and the same exclusion criteria were applied.
Outcome and Comorbidities
The primary outcome was newly diagnosed PLA, obtained from hospitalization records. Person-years of follow-up were calculated for each patient until PLA diagnosis or censoring because of loss to follow-up, death, withdrawal from the insurance program, or the end of 2011. The baseline comorbidity history for each patient was determined from the inpatient claims data. The comorbidities comprised well-known risk factors for PLA, namely, alcoholic liver disease (ICD-9-CM codes 571.0, 571.1, 571.3), cholecystitis (ICD-9-CM code 575), cancer (ICD-9-CM codes 140-208), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 491, 492, 496), choledocholithiasis (ICD-9-CM code 574), cholangitis (ICD-9-CM code 576.1), diabetes mellitus (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), hypertension (ICD-9-CM codes 401-405), heart failure (ICD-9-CM code 428), liver cirrhosis (ICD-9-CM codes 571.2, 571.5, 571.6), and pancreatic diseases (ICD-9-CM code 577).
Statistical Analysis
Student t-tests and Chi-square tests were used to examine the differences in the demographic characteristics and comorbidities between the diverticular-disease and nondiverticular-disease cohorts. The incidence density rates (per 10,000 person-year) of PLA were estimated for both cohorts according to sex, age, and comorbidities. Univariate and multivariate Cox proportional hazards regression analysis was used to assess the risk of developing PLA associated with diverticular diseases, compared with the nondiverticular-disease cohort. The hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated in the Cox model. The multivariate models were simultaneously adjusted for age, sex, and comorbidities of diabetes mellitus, hypertension, hyperlipidemia, cancer, COPD, heart failure, choledocholithiasis, alcoholic liver disease, liver cirrhosis, cholangitis, cholecystitis, and pancreatic diseases. All data processing and statistical analyses were performed using SAS Version 9.4 (SAS Institute, Inc., Cary, NC); 2-tailed P < .05 was considered statistically significant.
RESULTS
Table 1 shows comparisons of the demographic characteristics and comorbidities of the diverticular-disease and nondiverticular-disease patients. The mean (±standard deviation [SD]) age of the diverticular-disease cohort was 57.7(±18.1) years and that of the nondiverticular-disease cohort was 57.2(±18.1) years, with 37.1% of the patients aged ≤49 years. Women were predominant (55.8% vs 44.2%). The principal diseases including hypertension, hyperlipidemia, cancer, COPD, heart failure, choledocholithiasis, alcoholic liver disease, liver cirrhosis, cholangitis, cholecystitis, pancreatic diseases, and percutaneous aspiration of the gallbladder and biliary tract in diverticular-disease patients were higher than those in the nondiverticular-disease cohort (Table 1). The mean follow-up periods were 4.79 ± 3.12 years for the diverticular cohort and 5.09 ± 3.06 years for the nondiverticular cohort.
TABLE 1.
Characteristics of Patients Between Patients With Diverticular and Patients Without Diverticular
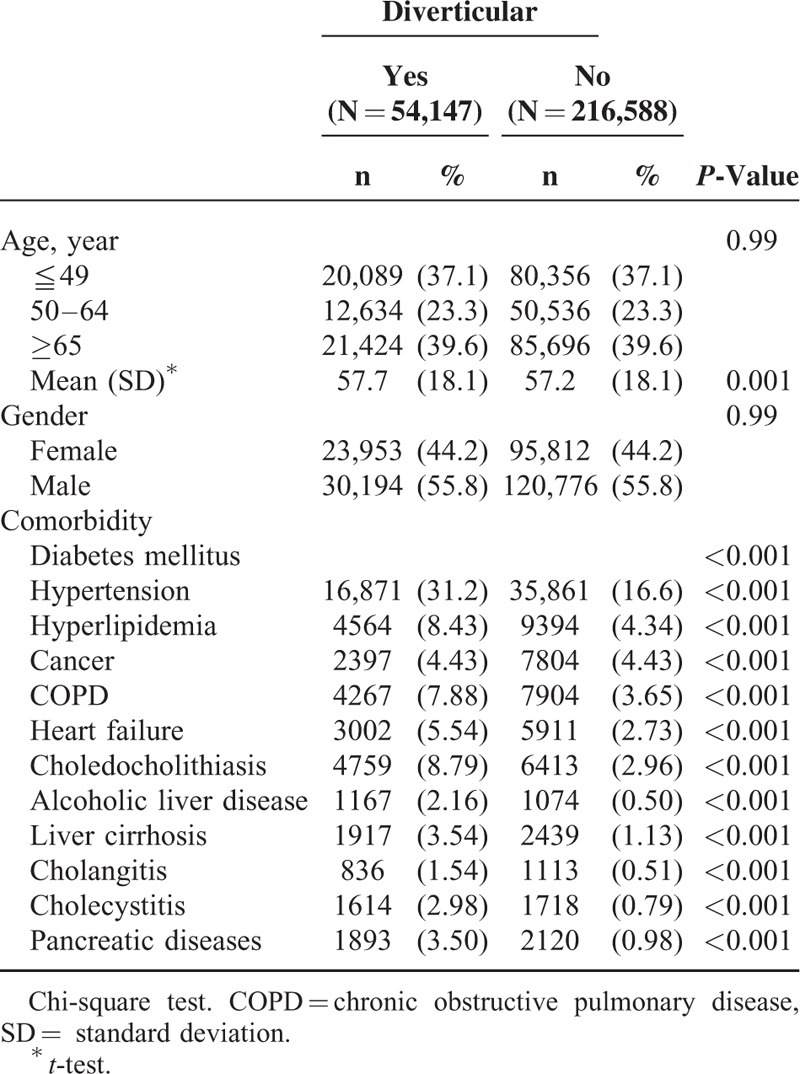
Then, we determined the incidence of PLA in both groups and found that diverticular diseases were associated with a significantly elevated risk of PLA (Table 2). The crude incidence of PLA was 2.44-fold higher in the diverticular-disease cohort than in the nondiverticular-disease cohort (11.5 vs 4.65 per 10,000 person-year) (Table 2). After adjustment for age, sex, and comorbidities, the adjusted HR (aHR) for developing PLA during follow-up was 2.10 (95% CI, 1.81–2.44) for the diverticular-disease cohort, as compared with the nondiverticular-disease cohort.
TABLE 2.
Incidence and Hazard Ratio of Pyogenic Liver Abscess Between Patients With Diverticular and Without Diverticular
Stratification analyses were performed to evaluate the risk of PLA in the subgroups stratified according to their sexes, ages, and comorbidities (Table 2). Our results showed that the elevated risk of PLA in patients with diverticular diseases could be observed in every stratification analysis. The risk of PLA in patients with diverticular diseases was significantly higher than in patients without diverticular disease, after stratification for sex (aHR, 1.83, 95% CI, 1.43–2.43 for women; aHR, 2.27, 95% CI, 1.88–2.75 for men), age (aHR, 4.03, 95% CI, 2.77–5.85 for patients aged ≤49 years; aHR, 2.17, 95% CI, 1.64–2.28 for patients aged 50–64 years; aHR, 1.65, 95% CI, 1.34–2.03 for the group aged ≥65 years), and comorbidities (aHR, 2.94, 95% CI, 2.32–3.73 for patients without comorbidities; aHR, 1.65, 95% CI, 1.37–1.98 for patients with comorbidities).
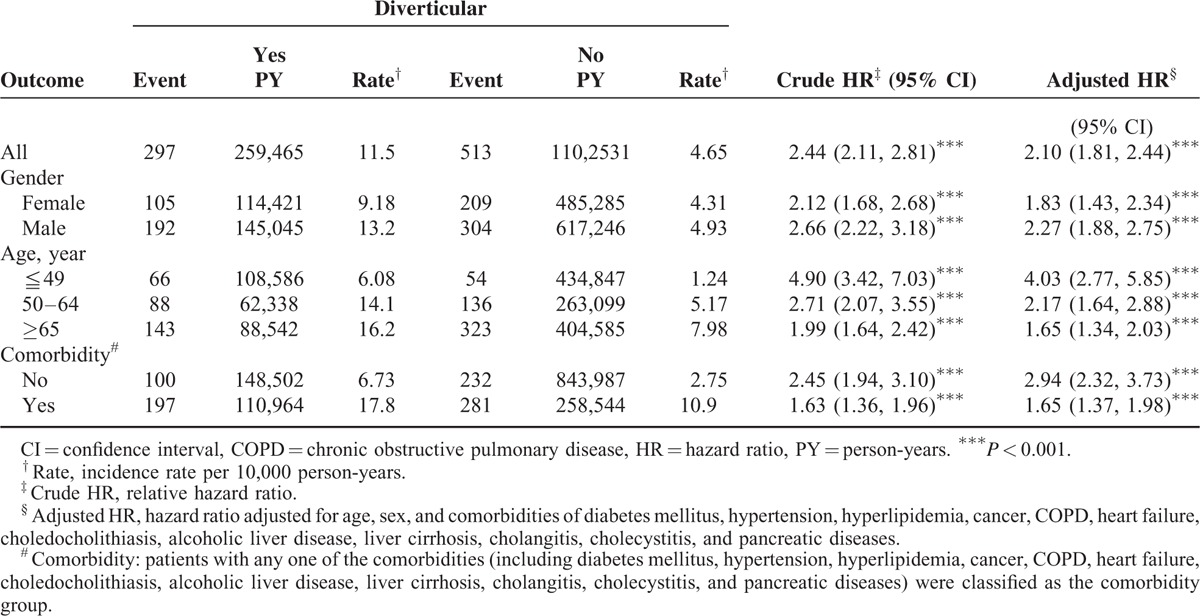
We then explored whether the inflammation of colonic diverticula was associated with PLA risks. The incidence and relative risks of PLA in patients with diverticulosis and diverticulitis are shown in Table 3. Compared with the nondiverticular-disease cohort, patients with diverticulosis had a significantly increased risk of PLA (aHR, 2.26, 95% CI, 1.86–2.75), followed by patients with diverticulitis (aHR, 1.98, 95% CI, 1.65–2.38).
TABLE 3.
Incidence and Hazard Ratio of Pyogenic Liver Abscess Between Different Entities Diverticular Diseases
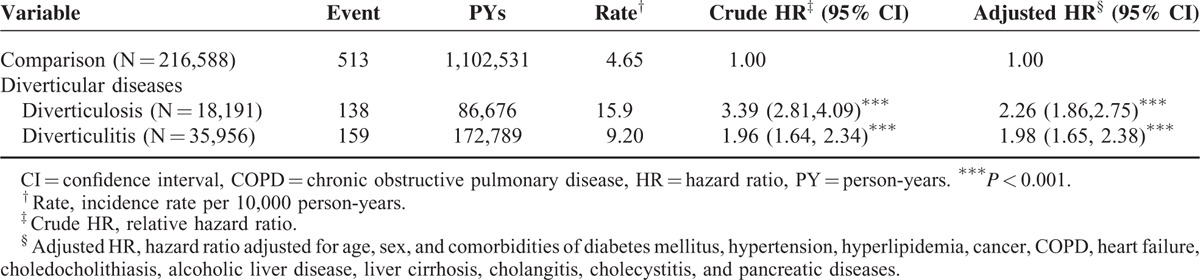
Finally, we would like to validate the accountability of our results by calculating the risk of PLA in the patients with some known risk factors of PLA. Theoretically, we should be able to demonstrate that patients with PLA risk factors, such as cholangitis, cholelithiasis, and diabetes mellitus, exhibited relatively high aHRs. On the other hand, patients with comorbidities not related to PLA should have lower aHRs of PLA. Compared with the patients without diverticular diseases and comorbidities, those with only cholangitis had a higher risk of PLA (aHR, 29.8, 95% CI, 7.39–120.1) followed by those with only diabetes mellitus (aHR, 3.35, 95% CI, 2.49–4.51), those with only choledocholithiasis (aHR, 2.50, 95% CI, 1.18–5.31), and those with only COPD (aHR, 2.33, 95% CI, 1.19–4.56) (Table 4). Moreover, compared with the diverticular-disease patients without these comorbidities, among the diverticular-disease patients, those with 5 or more comorbidities had a significantly increased risk of PLA (aHR, 8.08, 95% CI, 4.38–14.9), followed by those with 4 comorbidities (aHR, 5.30, 95% CI, 3.07–9.15), those with 3 comorbidities (aHR, 4.06, 95% CI, 2.69–6.14), those with any 2 comorbidities (aHR, 4.53, 95% CI, 3.36–6.60), and those with 1 comorbidity (aHR, 4.26, 95% CI, 3.32–5.47), regardless of comorbidity type.
Table 4.
Joint Effects for Acute Coronary Syndrome Between Diverticular and Acute Coronary Syndrome-Associated Risk Factor
DISCUSSION
This study is the first to address the long-term risk of PLA in Asian patients with diverticular diseases through analysis of a nationwide database with widespread coverage and complete follow-up evaluation. This population-based cohort study demonstrates that the diverticular-disease cohort exhibited a significantly elevated risk of PLA; after mortality was accounted for as a competing cause of risk and multiple known confounding factors were adjusted for. Additional clinical and basic studies are warranted for further investigation of the underlying causes of the observation in the present study.
Studies have reported that PLA can occur secondary to asymptomatic sigmoid diverticulitis12 and colon cancer.11,13,14 The present study, in analyzing a 13-year longitudinal database, further established a link between PLA and diverticular diseases. The large sample sizes collected in the present study enabled a highly reliable statistical analysis for assessing the PLA risk in patients with diverticular diseases and therefore avoided selection and recall biases.
Over the past few decades, the mortality associated with PLA has decreased gradually from more than 50% before the 1980s.15 However, recent studies have reported that PLA has continued to have a significant in-hospital mortality rate since 2000, ranging from 2.5% to 10%.2,3,16,17 One of the key factors contributing to fatality is delayed diagnosis. The presentation of PLA is often nonspecific, and its diagnosis requires a high degree of clinical suspicion. The median symptomatic duration prior to the diagnosis of PLA has been reported as approximately 1 week, whereas only 15% to 55% of PLA patients present with localized right upper quadrant tenderness.16,18 Therefore, identifying groups of patients who are at a risk of PLA is imperative. Based on the observations in this study, patients with diverticular diseases should undergo image screening for PLA when there is any clinical suspicion.
The mechanisms predisposing patients with diverticular diseases to PLA are multifactorial. First, patients with diverticular diseases tend to be immunocompromised. Diverticular diseases are associated with old age and diabetes,19 which was also found in the present study. Moreover, 10% to 25% of patients with diverticular diseases experience diverticulitis and its related complications,20–22 and therefore may undergo surgery at some point.23 Therefore, these unfavorable factors may compromise a patient's immunity and predispose the occurrence of PLA.24,25
As mentioned, a substantial proportion of patients with diverticular diseases encounter colonic inflammation, bleeding, and even perforation. Theoretically, mucosal disruption and underlying blood vessel exposure caused by diverticular inflammation may facilitate hematogenous spreading of colonic bacteria into portal circulation and the liver parenchyma. This hypothesis is further supported by a previous observation that nearly one-fourth of patients with cryptogenic PLA had a colonic cause when they underwent colonoscopic investigation.11
In addition to translocation via the mucosal defects, some intestinal bacteria, including K. pneumoniae, could also enter the bloodstream directly without breaking the mucosal integrity.26 In-vitro and in-vivo studies suggested that K. pneumoniae could translocate across the colonic epithelium via Rho GTPase-and phosphatidylinositol 3-kinase/Akt-dependent mechanisms.26 Other studies have reported that the expression of Rho GTPase27 and phosphatidylinositol 3-kinase/Akt activities28 were both enhanced within inflamed colonic segments. It will be of clinical interest to investigate whether direct bacterial translocation via intact intestinal mucosa contributes to the occurrence of PLA in patients with diverticular diseases.
One possible confounding factor in our observation is that the diverticular-disease cohort may have had a higher risk of colorectal cancer, which is known as a risk factor for PLA.11,13,14 Whether colonic diverticular diseases are associated with an increased risk of colorectal cancer remains controversial. Diverticular diseases have been suggested to be a predisposing factor for left-side colorectal cancer.29 In a case–control study, sigmoid diverticulitis was associated with a significantly higher risk of left-side colorectal cancer, with an odds ratio of 4.2.30 Conversely, our previous study did not reveal any association between diverticular diseases and colorectal cancer.31 Because we adjusted for cancer as comorbidity, our results are unlikely to have been biased by this confounder.
There are some study limitations: the database has no detailed data about the smoking, alcohol, exercise, incomes, and diabetes mellitus control, which may the potentially confounding factors regarding the study biases; no detailed data in Eastern populations the to do further analyses about the prevalence and pattern of diverticular diseases, usually in the right-side colon.32 In addition, the pathogens of PLA may be different based on the location; we could not to confirm the diagnoses of PLA and diverticular diseases by checking the individual patient's medical record. However, these hospitalized patients whose diagnoses were strictly audited for reimbursement in this study; these patients were included from the database of inpatient admission, which is the potential selection bias. They might need more active diseases or greater access to hospital care. All of the above these study limitations might cause underestimated or overestimated the association between diverticular diseases and the risk of PLA.
In summary, we determined that Asian patients with diverticular diseases have a significantly increased risk of PLA. Based on our findings, further study on the rates of PLA for patients with diverticular diseases is warranted.
Acknowledgment
The authors thank Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan for the support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, PLA = pyogenic liver abscess, SD = standard deviation.
Y-TL and C-HK are contributed equally to this work.
Conception/Design: M-ST, C-HK; Provision of study materials: C-HK; Collection and/or assembly of data; Data analysis and interpretation; Manuscript writing; and Final approval of manuscript: All authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cerwenka H, Bacher H, Werkgartner G, et al. Treatment of patients with pyogenic liver abscess. Chemotherapy 2005; 51:366–369. [DOI] [PubMed] [Google Scholar]
- 2.Rahimian J, Wilson T, Oram V, et al. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659. [DOI] [PubMed] [Google Scholar]
- 3.Lee KT, Wong SR, Sheen PC. Pyogenic liver abscess: an audit of 10 years’ experience and analysis of risk factors. Dig Surg 2001; 18:459–465.discussion 465–456. [DOI] [PubMed] [Google Scholar]
- 4.Chen SC, Yen CH, Tsao SM, et al. Comparison of pyogenic liver abscesses of biliary and cryptogenic origin. An eight-year analysis in a University Hospital. Swiss Med Wkly 2005; 135:344–351. [DOI] [PubMed] [Google Scholar]
- 5.Chang FY, Chou MY. Comparison of pyogenic liver abscesses caused by Klebsiella pneumoniae and non-K. pneumoniae pathogens. J Formos Med Assoc 1995; 94:232–237. [PubMed] [Google Scholar]
- 6.Wang J, Yan Y, Xue X, et al. Comparison of pyogenic liver abscesses caused by hypermucoviscous Klebsiella pneumoniae and non-Klebsiella pneumoniae pathogens in Beijing: a retrospective analysis. J Int Med Res 2013; 41:1088–1097. [DOI] [PubMed] [Google Scholar]
- 7.Razik R, Nguyen GC. Diverticular disease: changing epidemiology and management. Drugs Aging 2015; 32:349–360. [DOI] [PubMed] [Google Scholar]
- 8.Korzenik JR. Case closed? Diverticulitis: epidemiology and fiber. J Clin Gastroenterol 2006; 40 Suppl 3:S112–S116. [DOI] [PubMed] [Google Scholar]
- 9.Tyau ES, Prystowsky JB, Joehl RJ, et al. Acute diverticulitis. A complicated problem in the immunocompromised patient. Arch Surg 1991; 126:855–858.discussion 858–859. [DOI] [PubMed] [Google Scholar]
- 10.Kang JY, Melville D, Maxwell JD. Epidemiology and management of diverticular disease of the colon. Drugs Aging 2004; 21:211–228. [DOI] [PubMed] [Google Scholar]
- 11.Jeong SW, Jang JY, Lee TH, et al. Cryptogenic pyogenic liver abscess as the herald of colon cancer. J Gastroenterol Hepatol 2012; 27:248–255. [DOI] [PubMed] [Google Scholar]
- 12.Wallack MK, Brown AS, Austrian R, et al. Pyogenic liver abscess secondary to asymptomatic sigmoid diverticulitis. Ann Surg 1976; 184:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teitz S, Guidetti-Sharon A, Manor H, et al. Pyogenic liver abscess: warning indicator of silent colonic cancer. Dis Colon Rectum 1995; 38:1220–1223. [DOI] [PubMed] [Google Scholar]
- 14.Lonardo A, Grisendi A, Pulvirenti M, et al. Right colon adenocarcinoma presenting as Bacteroides fragilis liver abscesses. J Clin Gastroenterol 1992; 14:335–338. [DOI] [PubMed] [Google Scholar]
- 15.Johannsen EC, Sifri CD, Madoff LC. Pyogenic liver abscesses. Infect Dis Clin North Am 2000; 14:547–563.vii. [DOI] [PubMed] [Google Scholar]
- 16.Pang TC, Fung T, Samra J, et al. Pyogenic liver abscess: an audit of 10 years’ experience. World J Gastroenterol 2011; 17:1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol 2004; 2:1032–1038. [DOI] [PubMed] [Google Scholar]
- 18.Wong WM, Wong BC, Hui CK, et al. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol 2002; 17:1001–1007. [DOI] [PubMed] [Google Scholar]
- 19.Lee KM, Paik CN, Chung WC, et al. Clinical significance of colonic diverticulosis associated with bowel symptoms and colon polyp. J Korean Med Sci 2010; 25:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum 2014; 57:284–294. [DOI] [PubMed] [Google Scholar]
- 21.Boles RS, Jr, Jordan SM. The clinical significance of diverticulosis. Gastroenterology 1958; 35:579–582. [PubMed] [Google Scholar]
- 22.Wu CY, Huang HM, Cho DY. An acute bleeding metastatic spinal tumor from HCC causes an acute onset of cauda equina syndrome. Biomedicine (Taipei) 2015; 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman J, Davies M, Wolff B, et al. Complicated diverticulitis: is it time to rethink the rules? Ann Surg 2005; 242:581–583.576–581 discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branum GD, Tyson GS, Branum MA, et al. Hepatic abscess. Changes in etiology, diagnosis, and management. Ann Surg 1990; 212:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen WT, Yang CL. Yin MC. Protective effects from Houttuynia cordata aqueous extract against acetaminophen-induced liver injury. Biomedicine (Taipei) 2014; 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu CR, Pan YJ, Liu JY, et al. Klebsiella pneumoniae translocates across the intestinal epithelium via Rho GTPase-and Phosphatidylinositol 3-kinase/Akt-dependent cell invasion. Infect Immun 2015; 83:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connelly TM, Berg AS, Harris LR, et al. T-cell activation Rho GTPase-activating protein expression varies with inflammation location and severity in Crohn's disease. J Surg Res 2014; 190:457–464. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz PA, Haller D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-KB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J Nutr 2006; 136:664–671. [DOI] [PubMed] [Google Scholar]
- 29.Stefansson T, Ekbom A, Sparen P, et al. Increased risk of left sided colon cancer in patients with diverticular disease. Gut 1993; 34:499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefansson T, Ekbom A, Sparen P, et al. Association between sigmoid diverticulitis and left-sided colon cancer: a nested, population-based, case control study. Scand J Gastroenterol 2004; 39:743–747. [DOI] [PubMed] [Google Scholar]
- 31.Huang WY, Lin CC, Jen YM, et al. Association between colonic diverticular disease and colorectal cancer: a nationwide population-based study. Clin Gastroenterol Hepatol 2014; 12:1288–1294. [DOI] [PubMed] [Google Scholar]
- 32.Fischer MG, Farkas AM. Diverticulitis of the cecum and ascending colon. Dis Colon Rectum 1984; 27:454–458. [DOI] [PubMed] [Google Scholar]


