Supplemental Digital Content is available in the text
Abstract
To evaluate prognostic significance of phosphoglycerate kinase 1 (PGK1) protein expression in patients with gallbladder cancer (GBC).
Ninety-five patients who underwent surgical resection for GBC between January 2004 and December 2010 were enrolled. Overall survival (OS) and disease-free survival (DFS) were evaluated over a 10-year follow-up. PGK1 expression was assessed by tissue microarray and immunohistochemistry. Prognostic significance was analyzed using multivariate Cox regression.
PGK1 was highly expressed in all gallbladder mucosa. Decreased PGK1 expression was detected in 54.7% (52/95) of patients with GBC. It was significantly down-regulated in GBC samples compared with that in gallbladder mucosa (P < 0.0001), and was associated with multiple clinicopathological factors. Multivariate survival analysis showed that low PGK1 expression was associated with shorter OS (median 12.8 vs 45.4 months; hazard ratio [HR] = 3.077; 95% confidence interval [CI], 1.373–6.897; P = 0.006) and DFS (median 8.3 vs 37.9 months; HR = 2.988; 95% CI, 1.315–6.790; P = 0.009), indicating that PGK1 expression was an independent prognostic factor in patients with GBC.
Low PGK1 expression was associated with progression in patients with GBC. PGK1 expression could be a useful prognostic biomarker for GBC.
INTRODUCTION
Gallbladder cancer (GBC) is a rare tumor that is characterized as highly aggressive and is associated with frequent metastases and an extremely poor prognosis, making GBC 1 of the most lethal malignancies of the hepatobiliary system.1 Patients with GBC have a mean overall survival (OS) of 6 months, and the 5-year survival rate is 5%.2 The reasons for the high mortality rate of GBC are complicated, and GBC is resistant to the majority of clinical anti-cancer regimens including chemotherapy and radiotherapy.3,4 Moreover, in comparison with other neoplasms, the pathogenesis and genetic profile of GBC have not been fully clarified.5
Metabolic reprogramming is considered a hallmark of cancer.6 Recent studies have indicated that cancer-related glucose metabolism changes mediate the development of drug resistance,7,8 evoking an interest in cancer cell metabolic pathway alterations.8,9 Phosphoglycerate kinase 1 (PGK1) is a key glycolytic pathway enzyme that transfers a phosphate group from 1,3-diphosphoglycerate to adenosine diphosphate to generate 3-phosphoglyceric acid and adenosine triphosphate.10 PGK1 has been associated with carcinogenesis and metastasis in various malignancies.11,12 This has prompted investigations into PGK1 as a potential oncogenic molecular biomarker and an indicator of poor prognosis in pancreatic cancer,13 gastric cancer,14 neuroblastoma,15 and hepatocellular carcinoma.16 A recent study revealed that PGK1 was a prognostic biomarker of chemoresistance to paclitaxel treatment in patients with breast cancer.17 PGK1 is believed to be highly expressed in malignant carcinoma to accommodate aerobic glycolysis through the well-known Warburg effect, and this upregulation was believed to meet the needs of tumor malignant transformation.18
In our preliminary proteomic study, PGK1 protein was significantly down-regulated in the highly metastatic GBC cell line.19 However, PGK1 expression levels have not been investigated in human GBC tissue. According to these findings, we hypothesized that PGK1 would be down-regulated in human GBC tissues. The aim of the present study was to investigate PGK1 expression in patient-derived GBC tissues compared with that in noncancerous gallbladder tissue and to explore its prognostic significance in relation to other established prognostic factors.
METHODS
Patient Selection and Follow-Up
Between January 2004 and December 2010, 95 consecutive patients who underwent surgical resection for primary GBC at Xinhua Hospital were included in this retrospective study. The patients with suspected GBC were ages 18 to 75 years old, had an estimated Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, and had been recruited prospectively to observe the surgical outcome of radical resection versus extended radical resection. All cases were eventually histopathologically confirmed as GBC by a gastrointestinal pathologist. Patients with severe concurrent illnesses and blood abnormalities that could affect surgical outcomes were excluded. None of the enrolled patients received additional chemotherapy or radiotherapy as such treatment had not been proved effective for treatment of GBC. GBC disease stage was defined using the American Joint Committee on cancer tumor, node, metastasis (TNM) classification system. For the present study, demographic and clinical patient information was extracted from medical records. Follow-up was conducted using direct interviews in outpatient clinics or telephone interviews with patients or their family when patients were lost to follow-up. Generally, follow-up was conducted every 3 months for the first 2 years and every 6 months for the next 3 years, then once a year for the following years. The survival time was calculated from the date of surgery to the deadline for follow-up, or to the date of death. Patient follow-up data were collected up to December 2014. The present study was approved by the Ethical Committee of Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from all patients. Additionally, Institutional Review Board approval was obtained for the molecular analysis of tumor blocks.
Tissue Samples Process and Immunohistochemistry
Tissue blocks were collected from the surgically resected specimens of the 95 GBC patients, and from20 noncancerous gallbladder tissues obtained by cholecystectomy from patients with cholecystitis or gallstones. All tissues were formalin-fixed and paraffin-embedded. After hematoxylin and eosin-stained slides had been screened for optimal tumor content, tissue microarrays (TMAs) were constructed from the diagnosis-confirmed formalin-fixed paraffin-embedded tissues. Immunohistochemistry evaluation of PGK1 expression was then performed using the TMA. Additional details can be found in the Online Supplementary File.
Evaluation of PGK1 Expression
PGK1 proteins were stained yellowish-brown in the tumor cell nucleus, cytoplasm, and membrane. The intensity of PGK1 staining was scored by optical density using the semi-quantitative Image-Pro Plus software 6.0 (Media Cybernetics, Inc. Bethesda, MD) as described previously.21 In these cases, an optical density of <0.01 was regarded as low PGK1 expression and a score ≥0.01 was regarded as high PGK1 expression. The staining intensity and proportions were independently confirmed under a light microscope by 2 gastroenterology pathologists who were blinded to the clinical data.22,23 In cases of discrepancy, a consensus score was chosen for evaluation. The product of the scores for intensity and proportion were used to signify the protein expression level, and a cut-off value was determined based on a measure of heterogeneity using the log-rank test with respect to OS.
Statistical Analyses
All statistical analyses were performed using IBM SPSS 22 for Windows (SPSS, Chicago, IL). To assess the relationships between the PGK1 expression and the clinicopathological characteristics of patients with GBC, the χ2 test or Fisher exact test was used for categorical variables and Student t test or nonparametric Mann–Whitney U test for continuous variables. The cumulative OS and disease-free survival (DFS) rate were calculated using the life-table method, and patients with TNM IV were excluded in analyzing DFS. Significance differences between groups were analyzed using the Kaplan–Meier method with the log-rank test. Univariate survival analyses were conducted using the Cox proportional hazards model, and in multivariate survival analyses we first adjusted for age and sex and then fully adjusted for other confounding factors. Receiver operating characteristic (ROC) curve analysis was used to determine the predictive value of the parameters. All P values were 2-sided, and a P value < 0.05 was considered statistically significant. Results are reported according to the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.24
RESULTS
Patients’ Characteristics
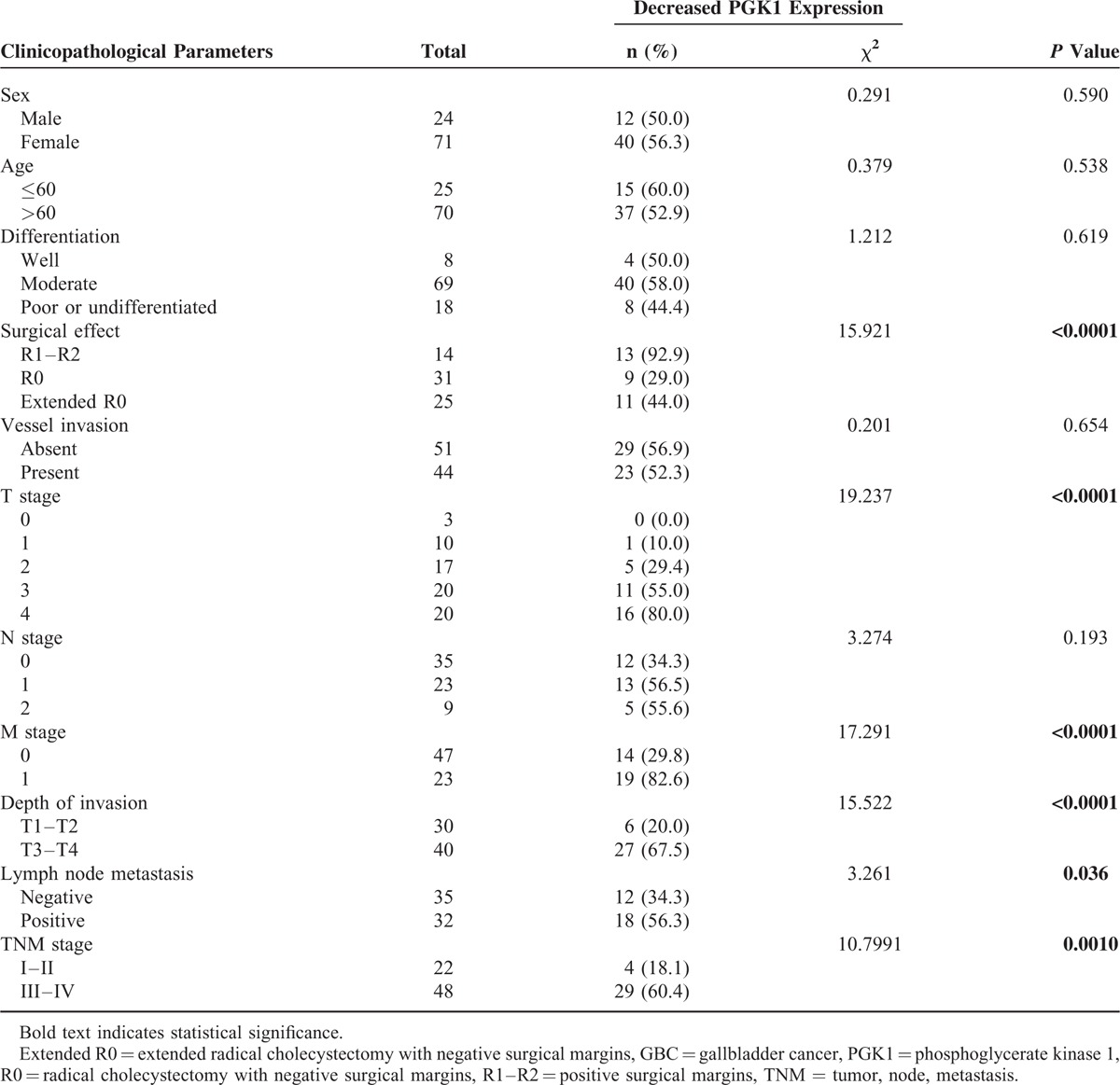
Patient characteristics and clinicopathological data, where available, are shown in Table 1. The median age at the time of surgery was 67 years (range: 39–75 years). All patients had follow-up records for 1 to 72 months, with a median of 17.5 (23.0 ± 19.0) months. The survival time was calculated from the date of surgery to the deadline for follow-up, or to the date of death. Thirteen patients had stage I GBC, 22 had stage II, 28 had stage III, and 32 had stage IV.
TABLE 1.
Patient Characteristics and Association With Decreased PGK1 Expression
PGK1 Expression and Association With Clinicopathological Parameters
PGK1 protein expression was significantly lower in patients with GBC, compared to that in the noncancerous gallbladder mucosa (see Supplementary Figure 1). The optical density score of PGK1 protein expression measured by Image-Pro Plus was 0.012 ± 0.015 in GBC tissue versus 0.021 ± 0.010 in none cancerous gallbladder mucosa (P < 0.0001, Fig. 1). The PGK1 expression in GBC was significantly associated with invasion depth (T3 and T4, P < 0.0001), TNM stage (stages III and IV, P = 0.001), the presence of lymph node metastasis (P = 0.036), and the presence of distant metastasis (P < 0.0001), but was not associated with sex, age, vessel invasion, or tumor differentiation status (Table 1).
FIGURE 1.
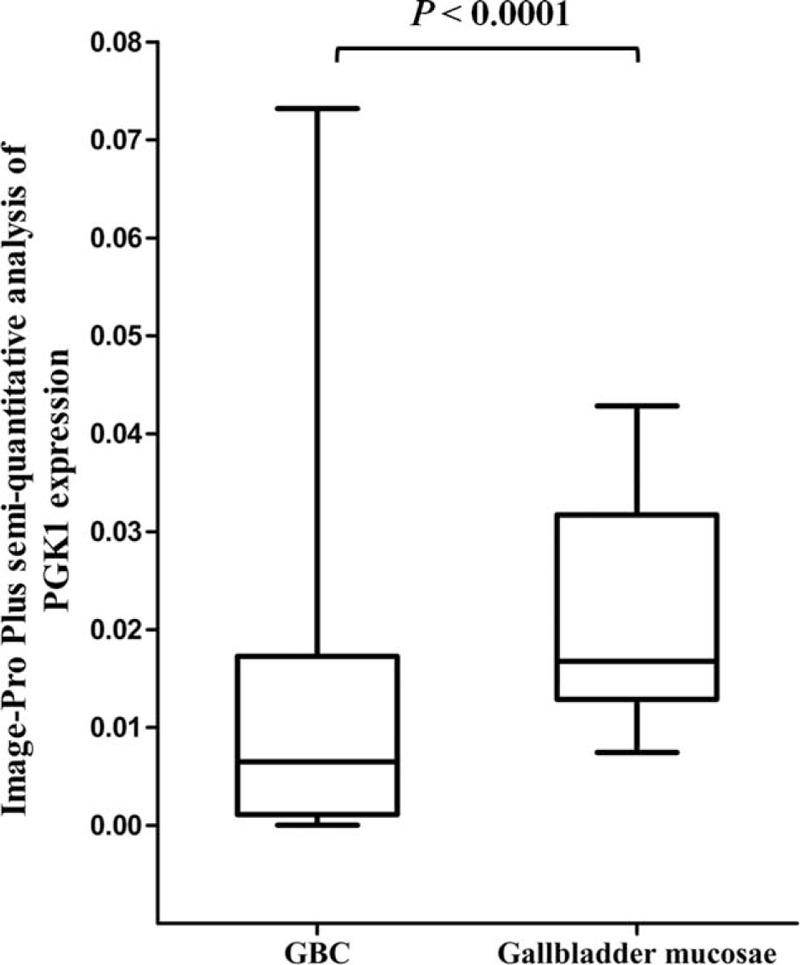
Optical density score of PGK1 protein expression in noncancerous gallbladder mucosa and GBC tissues measured using Image-Pro Plus. The PGK1 expression level in GBC tissues was significantly lower compared with that in noncancerous gallbladder mucosa. GBC = gallbladder cancer, PGK1 = phosphoglycerate kinase 1.
Association of PGK1 Expression With Prognosis
Kaplan–Meier survival analysis revealed that high PGK1 expression was significantly associated with longer OS and DFS (all P < 0.0001, Fig. 2). Stratification by TNM stage yielded similar results (P = 0.007 for early stage and P = 0.031 for advanced stage).
FIGURE 2.
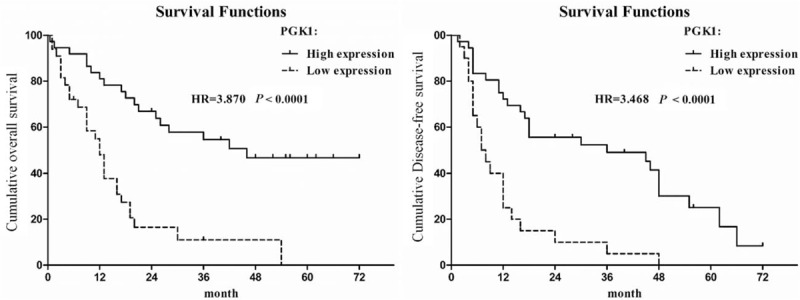
Kaplan–Meier survival curves of GBC patients with differential PGK1 expression. The overall survival in GBC patients with high PGK1 expression versus low PGK1 expression. The disease-free survival in GBC patients with high PGK1 expression versus low PGK1 expression. GBC = gallbladder cancer, PGK1 = phosphoglycerate kinase 1.
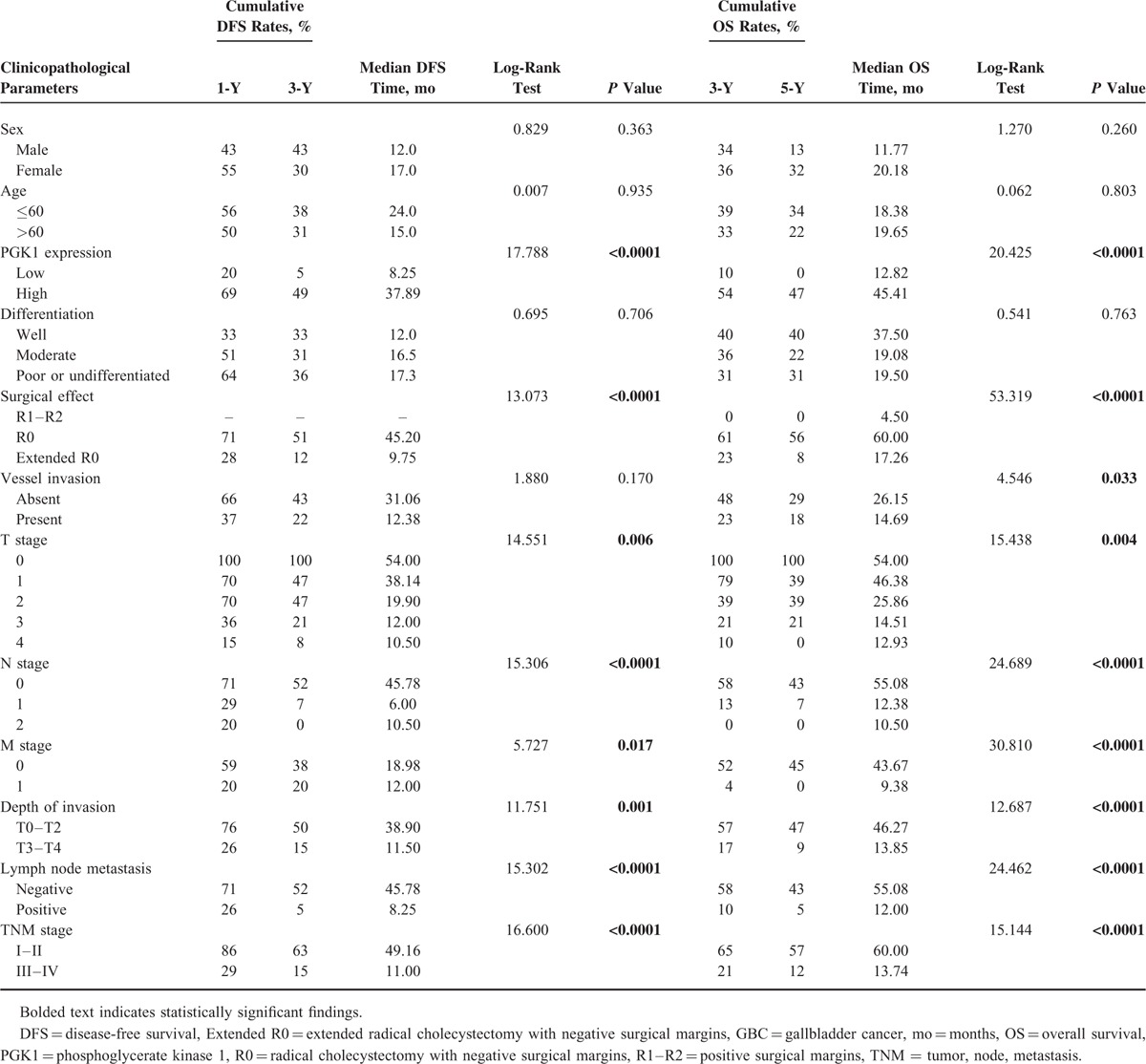
Table 2 shows the relationships between PGK1 expression and survival outcomes according to univariate analysis. In patients with GBC, low PGK1 expression was associated with significantly shorter DFS and OS (P < 0.0001). Invasion depth, TNM stage, differentiation grade, the presence of lymph node or distant metastasis, and surgical outcomes were significantly associated with DFS and OS. Sex, age, vessel invasion, and histological type were not associated with prognosis (Table 2).
TABLE 2.
Univariate Analysis of the Correlation Between Decreased PGK1 Expression With DFS and OS in Patients With GBC
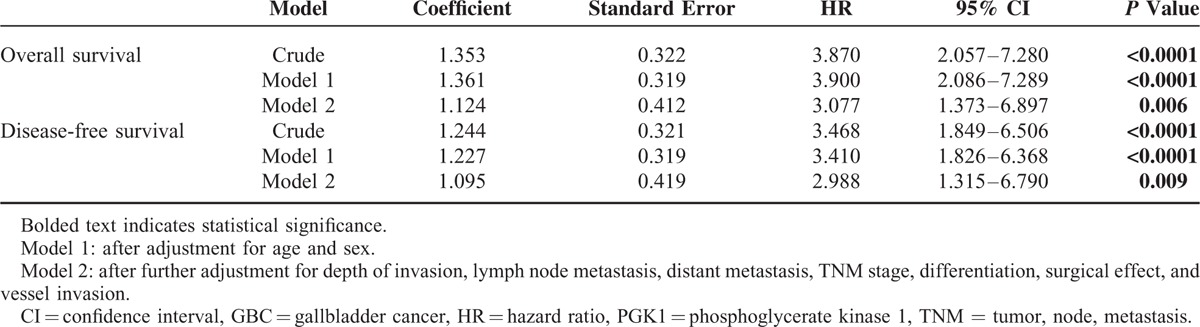
Multivariate Cox regression analysis revealed that decreased PGK1 expression was a significant and independent predictor of poor OS and DFS (hazard ratio [HR] = 3.077, 95% confidence interval [CI]: 1.373–6.897, P = 0.006 and HR = 2.988, 95% CI: 1.315–6.790, P = 0.009, respectively; Table 3).
TABLE 3.
Multivariate Analysis of the Association Between Decreased PGK1 Expression and Survival In-Patients With GBC
ROC Analysis of a Combination of PGK1 Expression and TNM Staging for Prognosis
ROC analysis was used to evaluate the efficacy. The area under curve (AUC) was 0.680 using TNM stage as predictive model (95% CI: 0.558–0.787; P = 0.013) and 0.691 using PGK1 expression (95% CI: 0.569–0.796; P = 0.008). To generate a more sensitive predictive model for patient outcome, we combined PGK1 expression and TNM stage to create a prognostic scoring system. The combination improved prognostic value; the AUC was 0.746 (95% CI: 0.628–0.842; P = 0.001), which was larger when compared with TNM stage (95% CI for difference, 0.009–0.122; P = 0.023) or PGK1 expression (95% CI for difference, −0.033 to 0.142; P = 0.221) alone (Fig. 3). The C-index was 0.6571 when assessed by TNM stage alone, and increased to 0.7571 when PGK1 expression was added.
FIGURE 3.
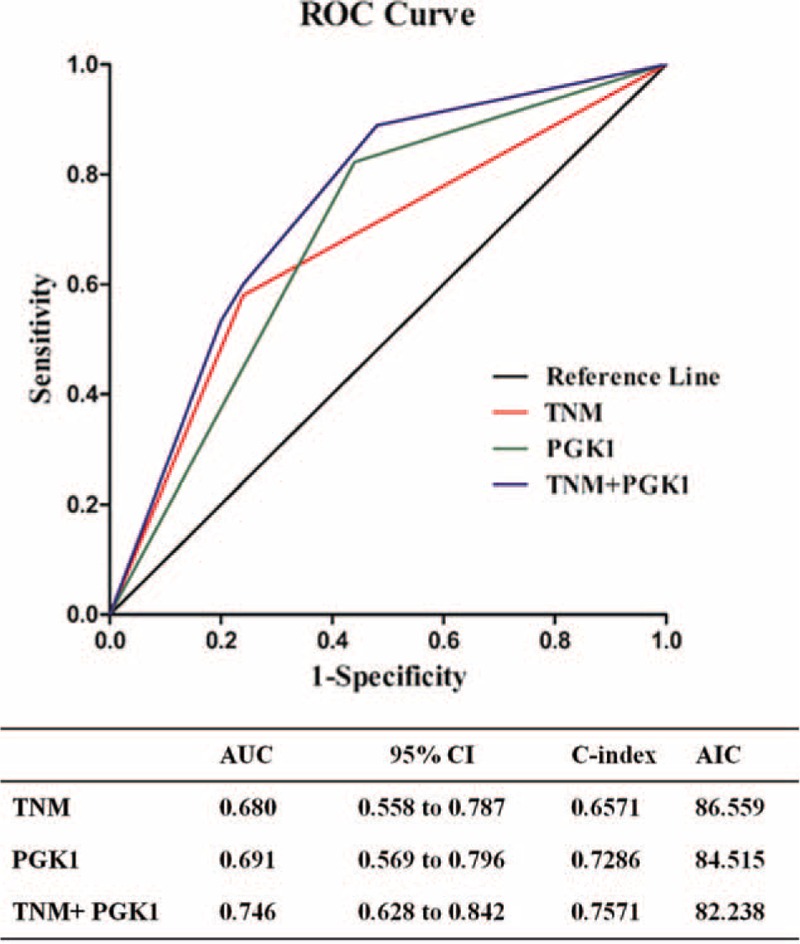
ROC analysis for the predictive value of PGK1 expression in patients with GBC. ROC analysis of the sensitivity and specificity for the predictive value of combined PGK1 and TNM stratification model (P = 0.001), TNM model (P = 0.013), and PGK1 model (P = 0.008). The AUC of the combined PGK1 and TNM stratification model was larger than each individual model (compared with the PGK1 model, P = 0.221; compared with the TNM model, P = 0.023). AIC = Akaike information criterion, AUC = area under curve, ROC = receiver operating characteristic, GBC = gallbladder cancer, PGK1 = phosphoglycerate kinase 1, TNM = tumor, node, metastasis.
DISCUSSION
Aberrant PGK1 expression has been noted in several carcinomas, with the majority reports declaring that overexpression of PGK1 was associated with poor prognosis.11–16 However, the functional role of PGK1 expression in carcinogenesis remains unclear. From western blot and qRT-PCR, we noticed that PGK1 was down-regulated in the high metastasis GBC cell line in comparison with the low metastasis homologous cell line.19 In the present study, we evaluated PGK1 expression in patients with GBC and its prognostic implications. PGK1 expression was lower in GBC tissues compared with that in normal gallbladder mucosa. Low PGK1 expression was an independent indicator of poor OS and DFS. Moreover, combining the PGK1 expression score and TNM stage had a better predictive value for clinical outcomes compared with either score alone.
The PGK1 expression in biliary duct system has not been reported to our knowledge. PGK1 was once regarded as a common reference gene for quantitative real-time polymerase chain reaction (qRT-PCR)25 until a qRT-PCR-based study revealed that PGK1 expression was elevated in squamous-cell carcinoma samples. There were accumulated reports of PGK1 expression in relation with malignancy, recently. In a study including 10 peritoneal carcinomatosis and 10 primary gastric cancers, PGK1 expression was upregulated in metastatic lesions indicating that PKG1 overexpression might be associated with disease progression.26 Another study that utilized human neuroblastoma tissues revealed that overexpression of PKG1 was significantly and positively correlated with tumor dissemination to the bone marrow and had an adverse impact on survival.15 In pancreatic ductal carcinoma, elevated serum PGK1 correlated with poor prognosis.13 However, the findings in the present study in patients with GBC were paradoxical to previous findings in other cancers, with higher PGK1 expression conferring improved survival outcomes. We presume that this is most likely due to a tissue-specific role for PGK1. However, to our knowledge, studies comparing of PGK1 expression levels in human tissues have not been conducted. We examined PGK1 expression in the human protein ATLAS database (http://www.proteinatlas.org/ENSG00000102144-PGK1/tissue) and found high expression in the gallbladder, kidney, testis, breast, cervix, tonsil, thyroid gland, and parathyroid gland. In contrast, PGK1 expression in the liver, pancreas, oral mucosa, esophagus, small intestine, ovaries, and cardiac muscle was low (Supplemental Figure 2). The high PGK1 expression in gallbladder mucosa reported in the ATLAS database is consistent with our findings in normal mucosa derived from the cholecystectomy specimens. However, there are no data addressing GBC in the ATLAS database.
The functional role of PGK1 overexpression in carcinogenesis has been suggested as the regulation of the Warburg effect,18 which describes increased aerobic glycolysis in tumor cells despite the presence of oxygen. Besides its catalytic role in the glycolytic pathway, PGK1 has been shown to play multiple cellular roles that link PGK1 expression to tumor biology, although such functions have not been fully elucidated. PGK1 has disulfide reductase activity during tumor angiogenesis.26 Moreover, via a positive interaction with CXCR4, PGK1 can increase gastric cancer cell invasion dramatically, resulting in peritoneal dissemination.27 However, the functional consequences of changes in PGK1 expression in cancer are controversial. For instance, high PGK1 expression can inhibit tumor angiogenesis by promoting the extracellular formation of angiostatin from plasmin.28 In addition, PGK1 has been suggested to be a critical downstream target of CXCL12 and an important negative regulator of angiogenesis.29 Moreover, in lung cancer cells, PGK1 overexpression reduced cyclooxygenase 2 expression, and decreased tumor growth and promoted anti-tumor immunity in vivo.30,31 PGK1 can bind DNA, influencing DNA replication and repair in mammalian cell nuclei.32,33 A proteomic analysis of glycolysis in lung cancer tissues revealed that poorly differentiated tumors had significantly lower PGK1 levels compared with moderate or well-differentiated tumors, indicating that PGK1 was down-regulated during lung cancer carcinogenesis.34 This finding might explain why PGK1 elevations have been associated with survival in patients with stage I lung cancer.35 A recent study demonstrated that PGK1 expression was blocked by MVIH, a long noncoding RNA, and this inhibition was associated with poor postoperative prognosis in patients with hepatocellular carcinoma,36 further indicating PGK1's tumor suppressor role in certain malignancy. Therefore, the decreased expression of PGK1 with poor prognosis of GBC seemed not a causal relationship. The current results indicated that down-regulation could be 1 of the oncogenic step in GBC carcinogenesis.
Although our study provided evidence for the prognostic value of PGK1 in patients with GBC, there remain some limitations. This is a single center retrospective study, so our results might not be generalized to other studies. The number of patients enrolled is relatively small due to relative lower incidence of the disease. And we cannot rule out the possibilities of type 1 error, which might lead to a false positive finding. More larger, multicentered, prospective studies are needed to validate these results.
In summary, we found that low PGK1 expression was an independent prognostic biomarker for poor OS and DFS. PGK1 may serve as an objective and effective biomarker to help identify patients with a high risk for tumor invasion and metastasis, thereby guiding the clinical decision making. Furthermore, because GBC is refractory to chemotherapy, modulation of PGK1 expression might be a promising target to improve survival outcomes and treatment efficacies, which might provide additional curative clinical strategies for patients with GBC.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AIC = Akaike information criterion, AUC = area under curve, CI = confidence interval, DFS = disease-free survival, ECOG = estimated Eastern Cooperative Oncology Group, GBC = gallbladder cancer, HR = hazard ratio, OS = overall survival, PGK1 = phosphoglycerate kinase 1, STROBE = STrengthening the Reporting of Observational studies in Epidemiology, TMAs = tissue microarrays, TNM = tumor, node, metastasis.
WL, JG, and JY have contributed equally to this work.
This work was supported by China National High Technology Research and Development Program (863 Program) (No. 2012AA022606) to YL; National Natural Science Foundation of China (No. 91440203, 81172026, 81272402, 81301816, and 81172029) to YL; China Postdoctoral Science Foundation (No. 2014M561487) and Interdisciplinary Program of Shanghai Jiao Tong University (No. 14JCRY05) to WL. JY's research was supported by NIH/NIA R01AG036042 and the Illinois Department of Public Health.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Dwivedi AN, Jain S, Dixit R. Gall bladder carcinoma: aggressive malignancy with protean loco-regional and distant spread. World J Clin Cases 2015; 3:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014; 6:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Aretxabala X, Roa I, Berrios M, et al. Chemoradiotherapy in gallbladder cancer. J Surg Oncol 2006; 93:699–704. [DOI] [PubMed] [Google Scholar]
- 4.Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012; 30:1934–1940. [DOI] [PubMed] [Google Scholar]
- 5.Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol 2015; 33:1845–1848. [DOI] [PubMed] [Google Scholar]
- 6.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 2012; 21:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todor IN, Lukyanova NY, Shvets YV, et al. Metabolic changes during development of Walker-256 carcinosarcoma resistance to doxorubicin. Exp Oncol 2015; 37:19–22. [PubMed] [Google Scholar]
- 8.Tung JC, Barnes JM, Desai SR, et al. Tumor mechanics and metabolic dysfunction. Free Rad Biol Med 2015; 79:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciacovelli M, Gaude E, Hilvo M, et al. The metabolic alterations of cancer cells. Methods Enzymol 2014; 542:1–23. [DOI] [PubMed] [Google Scholar]
- 10.Mikawa T, LLeonart ME, Takaori-Kondo A, et al. Dysregulated glycolysis as an oncogenic event. Cell Mol Life Sci 2015; 72:1881–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad SS, Glatzle J, Bajaeifer K, et al. Phosphoglycerate kinase 1 as a promoter of metastasis in colon cancer. Int J Oncol 2013; 43:586–590. [DOI] [PubMed] [Google Scholar]
- 12.Zieker D, Konigsrainer I, Weinreich J, et al. Phosphoglycerate kinase 1 promoting tumor progression and metastasis in gastric cancer—detected in a tumor mouse model using positron emission tomography/magnetic resonance imaging. Cell Physiol Biochem 2010; 26:147–154. [DOI] [PubMed] [Google Scholar]
- 13.Hwang TL, Liang Y, Chien KY, et al. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics 2006; 6:2259–2272. [DOI] [PubMed] [Google Scholar]
- 14.Zieker D, Konigsrainer I, Traub F, et al. PGK1 a potential marker for peritoneal dissemination in gastric cancer. Cell Physiol Biochem 2008; 21:429–436. [DOI] [PubMed] [Google Scholar]
- 15.Ameis HM, Drenckhan A, von Loga K, et al. PGK1 as predictor of CXCR4 expression, bone marrow metastases and survival in neuroblastoma. PLoS ONE 2013; 8:e83701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ai J, Huang H, Lv X, et al. FLNA and PGK1 are two potential markers for progression in hepatocellular carcinoma. Cell Physiol Biochem 2011; 27:207–216. [DOI] [PubMed] [Google Scholar]
- 17.Sun S, Liang X, Zhang X, et al. Phosphoglycerate kinase-1 is a predictor of poor survival and a novel prognostic biomarker of chemoresistance to paclitaxel treatment in breast cancer. Br J Cancer 2015; 112:1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qing G, Skuli N, Mayes PA, et al. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1alpha. Cancer Res 2010; 70:10351–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JW, Peng SY, Li JT, et al. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett 2009; 281:71–81. [DOI] [PubMed] [Google Scholar]
- 20.Zhan C, Zhang Y, Ma J, et al. Identification of reference genes for qRT-PCR in human lung squamous-cell carcinoma by RNA-Seq. Acta Biochim Biophys Sin 2014; 46:330–337. [DOI] [PubMed] [Google Scholar]
- 21.Prasad K, Prabhu GK. Image analysis tools for evaluation of microscopic views of immunohistochemically stained specimen in medical research—a review. J Med Syst 2012; 36:2621–2631. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein NS, Hewitt SM, Taylor CR, et al. Members of Ad-Hoc Committee On Immunohistochemistry S. Recommendations for improved standardization of immunohistochemistry. Appl Immunohistochem Mol Morphol 2007; 15:124–133. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Zhang S, Wang Z, et al. Prognostic significance of nemo-like kinase (NLK) expression in patients with gallbladder cancer. Tumour Biol 2013; 34:3995–4000. [DOI] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 25.Shimamoto Y, Kitamura H, Niimi K, et al. Selection of suitable reference genes for mRNA quantification studies using common marmoset tissues. Mol Biol Rep 2013; 40:6747–6755. [DOI] [PubMed] [Google Scholar]
- 26.Lay AJ, Jiang XM, Kisker O, et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature 2000; 408:869–873. [DOI] [PubMed] [Google Scholar]
- 27.Zieker D, Konigsrainer I, Tritschler I, et al. Phosphoglycerate kinase 1 a promoting enzyme for peritoneal dissemination in gastric cancer. Int J Cancer 2010; 126:1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26:225–239. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Wang J, Dai J, et al. A glycolytic mechanism regulating an angiogenic switch in prostate cancer. Cancer Res 2007; 67:149–159. [DOI] [PubMed] [Google Scholar]
- 30.Tang SJ, Ho MY, Cho HC, et al. Phosphoglycerate kinase 1-overexpressing lung cancer cells reduce cyclooxygenase 2 expression and promote anti-tumor immunity in vivo. Int J Cancer 2008; 123:2840–2848. [DOI] [PubMed] [Google Scholar]
- 31.Ho MY, Tang SJ, Ng WV, et al. Nucleotide-binding domain of phosphoglycerate kinase 1 reduces tumor growth by suppressing COX-2 expression. Cancer Sci 2010; 101:2411–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vishwanatha JK, Jindal HK, Davis RG. The role of primer recognition proteins in DNA replication: association with nuclear matrix in HeLa cells. J Cell Sci 1992; 101:25–34. [DOI] [PubMed] [Google Scholar]
- 33.Popanda O, Fox G, Thielmann HW. Modulation of DNA polymerases alpha, delta and epsilon by lactate dehydrogenase and 3-phosphoglycerate kinase. Biochim Biophys Acta 1998; 1397:102–117. [DOI] [PubMed] [Google Scholar]
- 34.Murphy JP, Pinto DM. Targeted proteomic analysis of glycolysis in cancer cells. J Proteome Res 2011; 10:604–613. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Gharib TG, Wang H, et al. Protein profiles associated with survival in lung adenocarcinoma. Proc Natl Acad Sci USA 2003; 100:13537–13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan SX, Yang F, Yang Y, et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology 2012; 56:2231–2241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


