Abstract
Growing evidence showed that inflammation response plays an important role in cancer development and progression, and absolute lymphocyte count (ALC), absolute monocyte count (AMC), and lymphocyte to monocyte ratio (LMR) have been used as parameters of systemic inflammation in several tumors. In this study, we evaluated the prognostic significance of preoperative ALC, AMC and LMR in breast cancer and 2000 patients between January 2002 and December 2008 at Sun Yat-Sen University Cancer Center were enrolled. Patients were grouped by the cut-off value according to the receiver operating characteristics (ROC) curve analysis. Kaplan–Meier analysis showed that patients with elevated AMC levels (>0.48 × 109/L) had shorter overall survival (OS, P < 0.001). In multivariate analysis, preoperative AMC was identified as an independent prognostic parameter for OS in breast cancer patients (hazard ratio = 1.374, 95% confidence interval: 1.045–1.807). Subgroup analyses revealed that AMC was an unfavorable prognostic factor in stage II–III breast cancer patients and Luminal B, human epithelial growth factor receptor-2 overexpressing subtype, and triple-negative breast cancer (all P < 0.05). Additionally, the prognostic value of ALC and LMR could not be proven in the current study. Preoperative AMC may serve as an easily available and low-priced parameter to predict the outcomes of breast cancer.
INTRODUCTION
Breast cancer is by far the most common type of cancer in women and becoming a health concern globally. There were approximately 232,670 new cases and 40,000 deaths from breast cancer in the United States in 2014.1 The improvement in detection and various treatments, such as surgical resection, adjuvant chemotherapy, hormonal therapy, targeted therapy, and radiation therapy, are responsible for the improved survival and reduced risk of recurrence.2,3 Classified according to the tumor node metastasis (TNM) staging system and their molecular features,4,5 breast cancer patients received various treatment options and experienced different prognoses. However, recurrence and metastasis still remain big problems for cure, especially for patients in advanced stages.6
Currently, besides the tumor-related factors, the host-related factors are considered as important factors in determining cancer recurrence and survival. Systemic inflammatory response has long been associated with tumor development.7–9 Cancer-associated inflammation is potentially implicated in the process of proliferation and metastasis, promoting angiogenesis, restraining antitumor immunity, and inducing subsequent poor prognosis.10–12 Furthermore, cytokines produced by tumor cells, such as granulocyte colony stimulating factor (G-CSF) and interleukin (IL)-6, could stimulate the proliferation of leukocytes and the latter in turn link to the progression of cancer.13 Lymphocytes and monocytes are essential inflammatory cells in the systemic inflammatory response.14,15 By inducing cytotoxic cell death and inhibiting tumor cell proliferation, lymphocyte could serve as tumor suppressors.9,16 Conversely, monocyte could differentiate into tumor-associated macrophages (TAMs) at tumor sites and promote the invasive and metastatic ability of tumor cells by constructing tumor microenvironment.17,18 A low pretreatment lymphocyte to monocyte ratio (LMR), indicating a lower lymphocyte count and a higher monocyte count, is found to link with poor prognosis in colon cancer, lung cancer, and hematological malignancies.19–21
However, the link between peripheral LMR and breast cancer is not completely defined. Therefore, we performed a retrospective cohort study on breast cancer patients underwent surgical treatment and investigated the prognostic value of preoperative peripheral lymphocyte, monocyte, and LMR for breast cancer.
MATERIALS AND METHODS
Study Population
Patient histologically confirmed as primary breast cancer between January 2002 and December 2008 in Sun Yat-Sen University Cancer Center (SYSUCC) in Guangzhou, China were retrospectively reviewed. The inclusion criteria were as follows: received surgical treatment; female; and pathological diagnosed as invasive ductal carcinoma or invasive lobular carcinoma. Exclusion criteria included: received neoadjuvant chemotherapy or radiotherapy before surgery; had surgical treatment before admission; with previous or coexisting cancers other than breast cancer; confirmed metastasis; current or potential inflammation: neutrophilic granulocyte percentage >70% or C-reactive protein >10 mg/L; and not enough data can be extracted. All patients were followed up to December 31, 2014 or date of deaths from any causes.
Clinical Data Collection
Baseline characteristics including age, menstrual status, pathological diagnosis, histologic grade, axillary lymph node status, hormonal receptor, and human epithelial growth factor receptor-2 (HER-2) status, date of last follow-up or death, and preoperative peripheral lymphocyte and monocyte count were collected. Blood sample was collected on an empty stomach and sent to the clinical laboratory. Complete blood count was performed as part of routine clinical evaluation prior to the surgery. The clinical stage was determined by the TNM staging system according to the American Joint Committee on Cancer. The intrinsic subtypes were as follow: Luminal A (estrogen receptor [ER]+, progesterone receptor [PR]+, HER-2−, and Ki-67 ≤ 14%), Luminal B (ER+ and HER-2+ or Ki-67 > 14%), HER-2 overexpressing (ER−, PR−, HER-2+) and triple-negativer breast cancer (ER−, PR−, HER-2−). HER-2 positive was defined as “3+” in immunohistochemical test or “positive” in HER-2 fluorescence in situ hybridization test. The follow-up of patients was performed through out-patient medical records, telephone, or letters by Department of Follow-Up and Medical Record Management.
Statistical Analyses
Preoperative peripheral lymphocyte and monocyte count are expressed as means (± standard deviation), and categorical data were described using numbers and percentages. The LMR was defined as the absolute lymphocyte count (ALC) divided by the absolute monocyte count (AMC). Young breast cancer patient was defined as <35 years old. The endpoints assessed were overall survival (OS), calculated from the time of pathological diagnosis to the date of death from any causes or last follow-up. The relationship between patients’ characteristics and the ALC, AMC, and LMR were assessed by unpaired t test or 1-way analysis of variance (ANOVA), and difference between categories was examined using the Chi-squared test. The clinical significance and the optimal cut-off value of ALC, AMC and LMR were determined by the receiver operating characteristic (ROC) curve analysis, and patients were stratified into 2 ranges according to the cut-off value. Kaplan–Meier method was performed for survival analyses and compared by log-rank test. Univariate analyses and multivariate analyses (Cox proportional hazards model) were performed to determine the influence of potential confounding factors on OS. Hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) estimated from the Cox analysis were regarded as relative risks, and a 2-tailed P value < 0.05 was considered significant. All statistical analyses were performed using SPSS 19.0 (SPSS, Inc., Chicago, IL) in the present study.
Ethics Statement
The study protocol was approved by the Independent Ethical Committee/Institutional Review Board of SYSUCC, and written informed consent was obtained from each participant prior to surgery. All patients were anonymous and de-identified prior to analysis.
RESULTS
Patient Characteristics
A total of 3156 consecutive patients with histopathologically confirmed breast cancer in SYSUCC were reviewed, and 2000 patients were finally enrolled after screening process (Figure 1) and no inflammatory breast cancer patient was included. Baseline clinicopathological characteristics are shown in Table 1. The median follow-up time was 75 months (range 3–144 months), and death occurred in 326 (16.3%) of the 2000 breast cancer patients. The median age of the enrolled patients was 49.4 years (range: 22–94 years), and 168 (8.4%) patients were under age 35. The mean ALC and AMC were 2.11 ± 0.62 (×109/L) and 0.47 ± 0.23 (×109/L), respectively, with a mean LMR of 5.10 ± 2.77.
FIGURE 1.
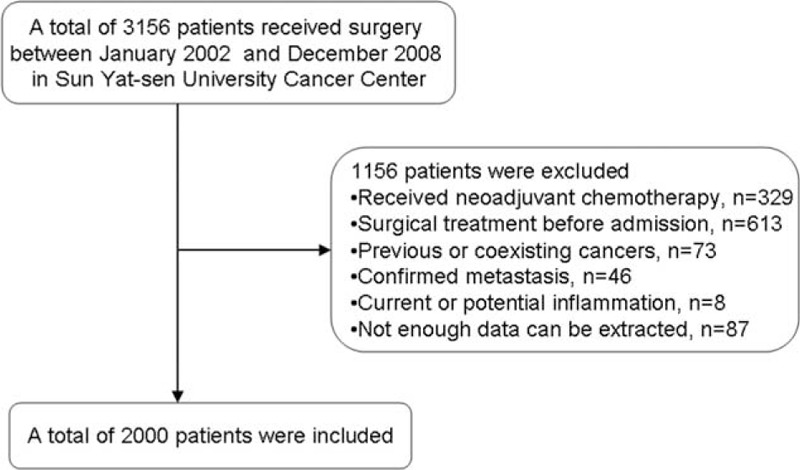
Flow chart of the patient selection.
TABLE 1.
Clinicopathological Parameters of Breast Cancer Patients (n = 2000)
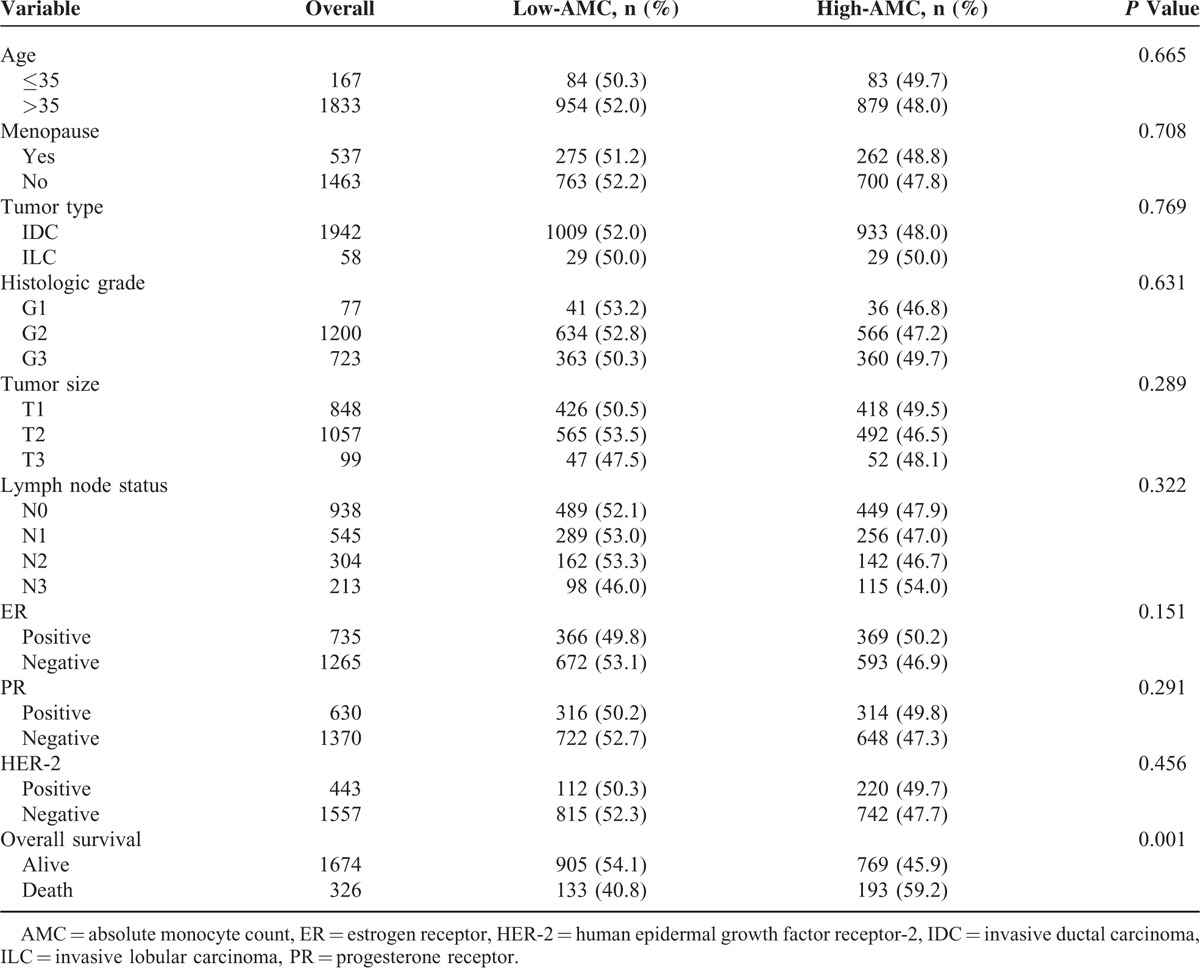
The Optimal Cut-Offs of ALC, AMC and LMR
ROC curve analysis was performed to determine the optimal cut-off values for ALC, AMC and LMR (Figure 2). The cut-off values of ALC, AMC and LMR were 2.20 × 109/L, 0.48 × 109/L, and 3.80, respectively, with highest Youden’ s index. Enrolled patients were stratified into 2 levels (low- and high-) according to cut-off points. One thousand one hundred fifty (57.7%) patients were categorized as low-ALC group and 850 (42.5%) patients were categorized as high-ALC group. Similarly, 1038 (51.9%) patients were categorized as low-AMC group while the remaining 962 (48.1%) patients as high-AMC group, and 590 (29.5%) patients were categorized as low-LMR group while the remaining 1410 (70.5%) patients as high-LMR group. No correlation was identified between AMC level and age, menopausal status, tumor types, histologic grades, tumor sizes, lymph node status, ER/PR status, and HER-2 status (all P > 0.05, Table 1). Patients who experienced poor outcome had significantly increased AMC compared with patients better prognosis (P < 0.001).
FIGURE 2.
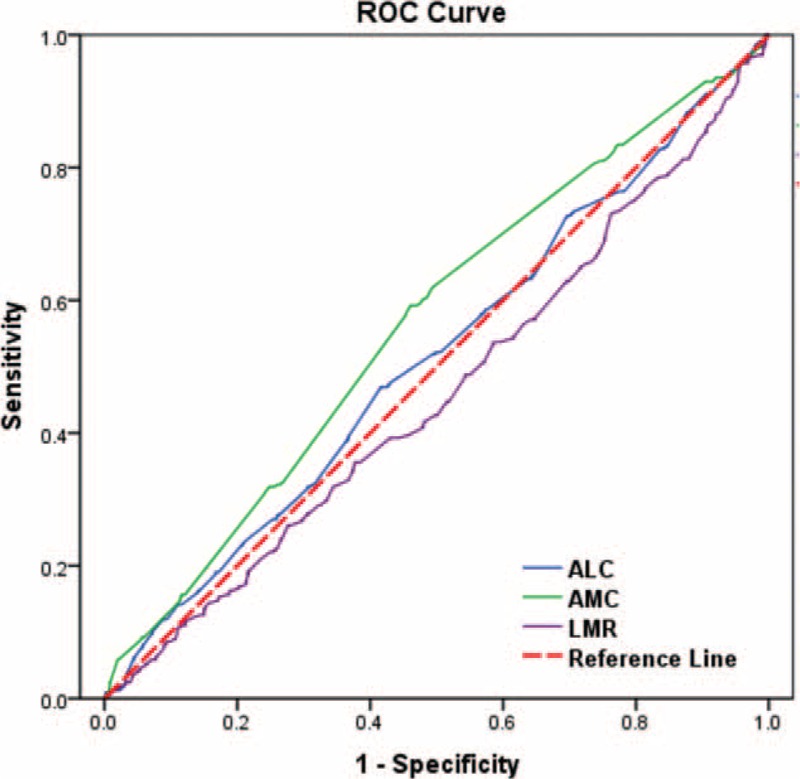
ROC curves assessing the cut-off of ALC, AMC, and LMR for predicting the overall survival in the cohort study. The AUCs for each parameter were 0.513 (P = 0.461), 0.562 (P < 0.001), and 0.459 (P = 0.02), respectively. ALC = absolute lymphocyte count, AMC = absolute monocyte count, LMR = lymphocyte to monocyte ratio, OS = overall survival, ROC = receiver operating characteristic.
Association of ALC, AMC and LMR With OS
The 10-year OS rate was 77.6% for all 2000 patients, and the mean survival time was 123.6 months (95% CI: 121.6–125.5). In the univariate analysis, AMC and LMR were both significantly associated with OS in breast cancer patients with the HR was 1.565 and 0.776, respectively (both P < 0.05). The HR for ALC was 1.166 (95% CI: 0.938–1.449, P = 0.167) and no prognostic significance was proven. Other identified prognostic factors for OS included menstrual status, tumor size, lymph node status, ER/PR status, and HER-2 status (Table 2).
TABLE 2.
Univariate and Multivariate Analyses of AMC and LMR for OS in Breast Cancer
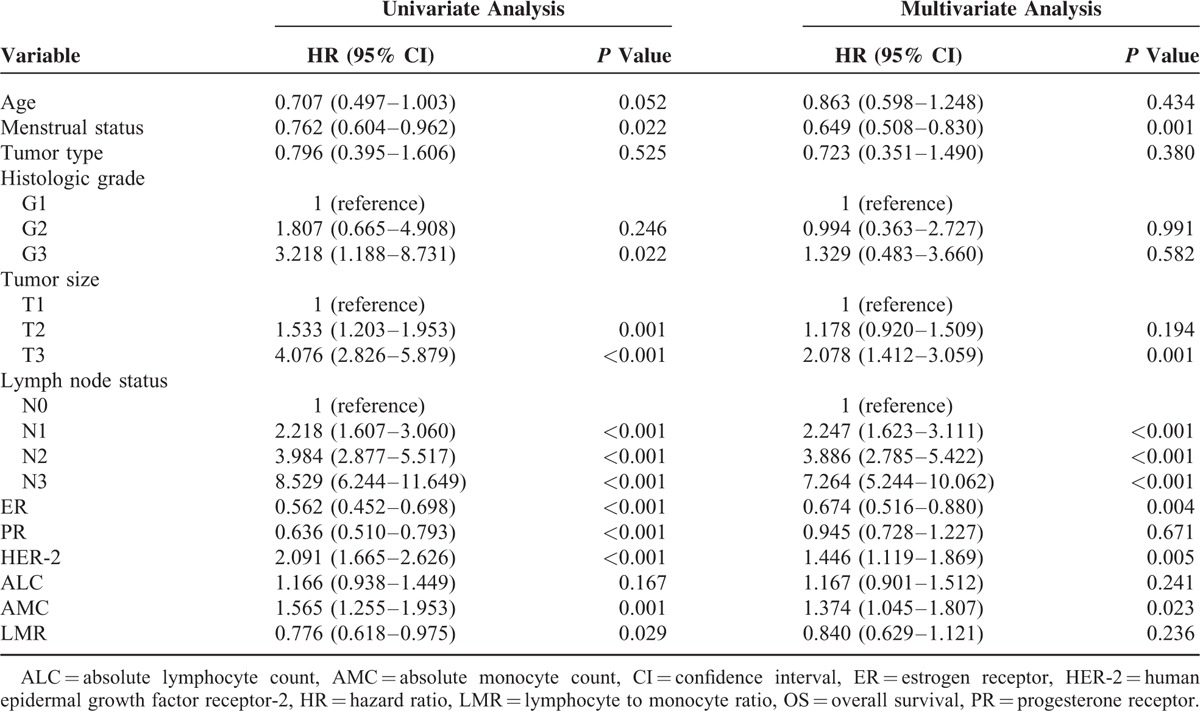
To identify independent prognostic factors for OS, multivariate analysis by the Cox proportional hazard model was performed and the AMC retained independent significance (HR = 1.374, 95% CI: 1.045–1.807, P = 0.023). Survival analysis showed that breast cancer patients with higher monocyte count (>0.48 × 109/L) had a significantly poorer survival than patients with lower monocyte count (116.0 vs 127.6 months, P < 0.001; Figure 3). No statistical significance of the prognostic effect of LMR was observed in the multivariate analysis (P = 0.236). Other prognostic factors included menstrual status, tumor size, lymph node status, ER status, and HER-2 status.
FIGURE 3.
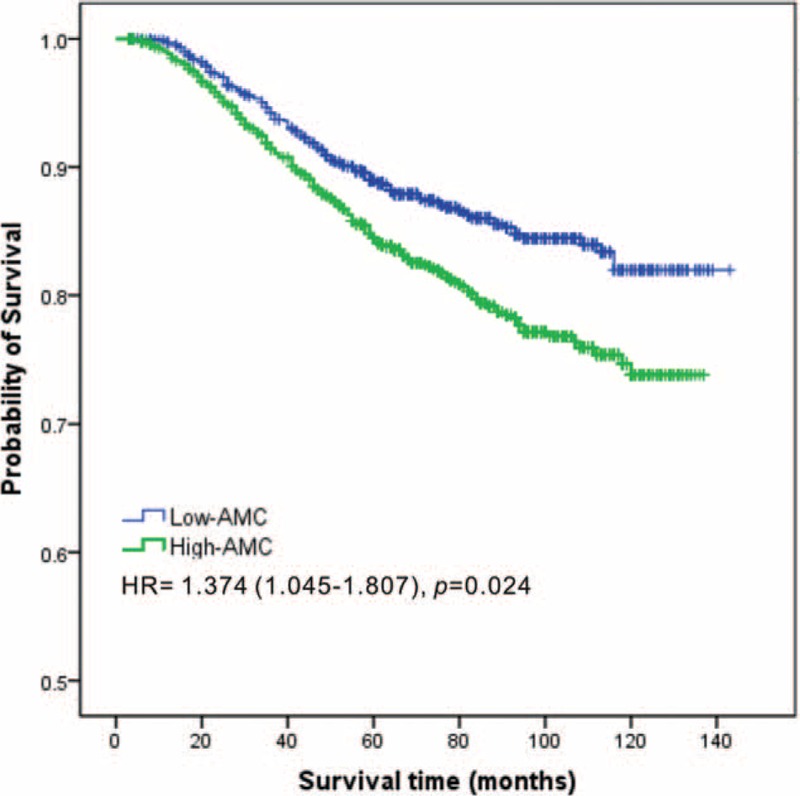
Kaplan–Meier estimates of the overall survival of breast cancer patients according to the AMC level in overall patients. AMC = absolute monocyte count.
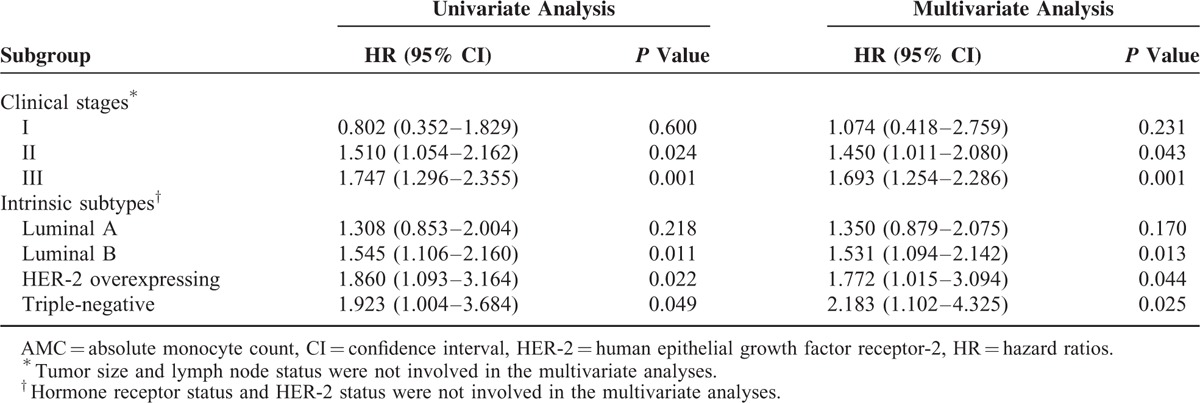
In multivariate analysis stratified by clinical stages, elevated AMC was significantly related to worse OS in stage II–III breast cancer patients (HR = 1.450 and 1.693, respectively, both P < 0.05; Table 3), while no statistical significance was proven in the stage I patients (P = 0.231). Meanwhile, the AMC was indicated as risk factor for breast cancer patients of Luminal B, HER-2 overexpressing subtype, and triple-negative breast cancer subtypes (all P < 0.05; Table 3). The clinical outcomes of Luminal A breast cancer patients with high monocyte counts were not significantly different with patients with low monocyte counts (P = 0.170).
TABLE 3.
Prognostic Impact of AMC in Various Clinical Stages and Intrinsic Subtypes
DISCUSSION
Growing evidence has suggested an important role of inflammation in cancer development, tumor angiogenesis, and metastasis. Chronic oxidative stress and the oxygen free radicals caused by the inflammatory response could lead to cancer initiation and promotion.22,23 As a marker of systemic inflammation, preoperative leukocyte levels are considered to be in association with prognosis in various carcinoma.24,25 The leukocytic cell ratios, including neutrophil to lymphocyte ratio, platelets to lymphocyte ratio, and LMR, are routinely available markers of the systemic inflammatory response and identified as prognostic parameters in several solid tumors and leukemia.26–29
In the present study, we retrospectively analyzed 2008 consecutive breast cancer patients who received surgeries as primary treatments and found that: only AMC was an independent prognostic factor for patients with breast cancer, the prognostic value of ALC and LMR could not be confirmed (P = 0.241 and P = 0.236); high AMC levels (>0.48 × 109/L) were associated with poor clinical outcome in more advanced stages (II–III) and Luminal B, HER-2 overexpressing, and triple-negative breast cancer subtypes. Previous studies have shown a link between monocyte and cancer progression. The prognostic value of monocyte was reported not only in lymphomas, but also in nonhematologic malignancies.30,31 Increase of circulating monocyte was associated with poor progression-free survival and OS in nonsmall cell lung cancer, gynecological cancer, and gastrointestinal cancer.32–34
The possible mechanism of the independent association between circulating monocyte and cancer prognosis may be related to the activation of innate immunity during the tumor progression.34 Act as chemotactic factor, the monocyte chemoattractant protein 1 is up regulated in tumors and can induce the increase of monocyte lineage cells in the tumor site directly, which usually correlate with a more advanced stage of cancer.35 Furthermore, the inflammatory monocytes recruited into tumor sites by the CCL2/CCR2 chemokine axis can differentiate into TAMs17 and become a major component of the tumor microenvironment. Positive correlation between the TAM count and circulating monocytes is demonstrated in previous study.36 By producing a great diversity of cytokines, such as VEGFA, MMP-9 and PDGF, TAMs can promote the invasion and metastasis of cancer cells.37 Coculture of cancer cells with macrophages can enhance cancer cell matrix-degrading activity, promote the invasive potential of cancer cells, which correlate with poor prognosis in cancers.18
In the subgroup analysis based on different clinical stages and intrinsic subtypes, the predictive value of AMC was significant in patients with stage II–III (both P < 0.05), while the stage I patients showed no statistical significance (P = 0.231). It is in line with the idea that the monocyte mainly plays roles in the cancer invasion and metastasis, not in the proliferation of the tumor cell.37,38 That may be the possible reason for no prognostic effect of AMC in the early stage breast cancer patients.
The prognostic value of AMC was statistically significant in Luminal B, HER-2 overexpressing subtype, and triple negative breast cancer subtypes, not in Luminal A subtype. Since the OS was distinctly different among various subtypes, and patients of Luminal A subtype experience best OS. HER-2 is considered as an oncogene of the MAP-Kinase and PI3K/AKT/mTOR signaling pathways leading to cell growth and proliferation.39,40 Previous studies indicate that patients with ER/PR-negative experienced higher risk of cancer relapse than those with ER/PR-positive.41 Thus monocyte may contact with tumor cells and assist the metastasis in the more invasive subtypes.
In our study cohort, the ALC was not identified as an independent favorable prognostic factor in breast cancer. The reason may be that different lymphocyte subsets manifest various effects in tumor development and metastasis. In breast cancer and lung cancer, CD8+ T lymphocytes were considered as antitumor lymphocyte and have favorable effect on cancer patients.42,43 Conversely, CD4+CD25+Foxp3+ regulatory T lymphocytes (Tregs) can inhibit cytotoxic responses and associate with poor clinical outcome.44 Thus the ALC could not accurately reflect the prognostic effect of lymphocytes in cancer patients.
AMC provides an easily available and low-priced biomarker for prognostic evaluation. However, several limitations existed in the present study. Firstly, because of the retrospective nature of the study, selection bias could not be excluded even though consecutive patients were enrolled. Secondly, although we excluded patients with potential inflammation by neutrophilic granulocyte percentage and CRP levels, potential confounding factors, such as autoimmune disease and hepatic dysfunction, still may still existed and affected the circulating monocyte counts. Finally, specific quality control analysis was performed by different quality inspectors since the white blood cell count was obtained as a routine clinical test before surgery.
In conclusion, our study suggested that high pretreatment count of circulating monocyte is independently associated with poor prognosis in breast cancer patients. And large, prospective studies are needed to validate the prognostic effect of circulating monocyte in the future.
Footnotes
Abbreviations: ALC = absolute lymphocyte count, AMC = absolute monocyte count, ANOVA = 1-way analysis of variance, ER = estrogen receptor, HER-2 = human epithelial growth factor receptor-2, HR = hazard ratios, LMR = lymphocyte to monocyte ratio, PR = progesterone receptor, ROC = receiver operating characteristic, TAM = tumor-associated macrophages.
Supplemental Digital Content is available for this article.
This work was supported by funds from the National Natural Science Foundation of China (81472575, 8127251, and 81372133), the Key Programme of the National Natural Science Foundation of China (31030061), the Science and Technology Planning Project of Guangdong and Guangzhou (2013B060300009 and 2014J4100169).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Fedele P, Orlando L, Schiavone P, et al. Recent advances in the treatment of hormone receptor positive HER2 negative metastatic breast cancer. Crit Rev Oncol Hematol 2015; 94:291–301. [DOI] [PubMed] [Google Scholar]
- 3.Moran MS. Radiation therapy in the locoregional treatment of triple-negative breast cancer. Lancet Oncol 2015; 16:e113–e122. [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295:2492–2502. [DOI] [PubMed] [Google Scholar]
- 5.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol 2002; 20:3628–3636. [DOI] [PubMed] [Google Scholar]
- 6.Carlson RW, Allred DC, Anderson BO, et al. Metastatic breast cancer, version 1.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2012; 10:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12:223–226. [DOI] [PubMed] [Google Scholar]
- 8.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010; 6:149–163. [DOI] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book 2013; 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009; 30:1073–1081. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674. [DOI] [PubMed] [Google Scholar]
- 13.Kasuga I, Makino S, Kiyokawa H, et al. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer Am Cancer Soc 2001; 92:2399–2405. [DOI] [PubMed] [Google Scholar]
- 14.Delogu G, Famularo G, Tellan G, et al. Lymphocyte apoptosis, caspase activation and inflammatory response in septic shock. Infection 2008; 36:485–487. [DOI] [PubMed] [Google Scholar]
- 15.Gillette DD, Tridandapani S, Butchar JP. Monocyte/macrophage inflammatory response pathways to combat Francisella infection: possible therapeutic targets? Front Cell Infect Microbiol 2014; 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008; 454:436–444. [DOI] [PubMed] [Google Scholar]
- 17.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011; 475:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JJ, Lin YC, Yao PL, et al. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol 2005; 23:953–964. [DOI] [PubMed] [Google Scholar]
- 19.Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer 2014; 110:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz E, Goldstein J, Benacquista T, et al. Diagnosis related group “all payor” hospital payment and medical diseases. Financial risk and hospital cost in medical noncomplicating condition-stratified diagnosis related groups. Arch Intern Med 1989; 149:417–420. [DOI] [PubMed] [Google Scholar]
- 21.Wei X, Huang F, Wei Y, et al. Low lymphocyte-to-monocyte ratio predicts unfavorable prognosis in non-germinal center type diffuse large B-cell lymphoma. Leuk Res 2014; 38:694–698. [DOI] [PubMed] [Google Scholar]
- 22.Salim AS. The permissive role of oxygen-derived free radicals in the development of colonic cancer in the rat. A new theory for carcinogenesis. Int J Cancer 1993; 53:1031–1035. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SP, Aguilar F, Amstad P, et al. Oxy-radical induced mutagenesis of hotspot codons 248 and 249 of the human p53 gene. Oncogene 1994; 9:2277–2281. [PubMed] [Google Scholar]
- 24.Tibaldi C, Vasile E, Bernardini I, et al. Baseline elevated leukocyte count in peripheral blood is associated with poor survival in patients with advanced non-small cell lung cancer: a prognostic model. J Cancer Res Clin Oncol 2008; 134:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer 2005; 93:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013; 88:218–230. [DOI] [PubMed] [Google Scholar]
- 27.Cihan YB, Ozturk A, Mutlu H. Relationship between prognosis and neutrophil: lymphocyte and platelet: lymphocyte ratios in patients with malignant pleural mesotheliomas. Asian Pac J Cancer Prev 2014; 15:2061–2067. [DOI] [PubMed] [Google Scholar]
- 28.Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005; 91:181–184. [DOI] [PubMed] [Google Scholar]
- 29.Li ZM, Huang JJ, Xia Y, et al. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS ONE 2012; 7:e41658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai YD, Wang CP, Chen CY, et al. Pretreatment circulating monocyte count associated with poor prognosis in patients with oral cavity cancer. Head Neck 2014; 36:947–953. [DOI] [PubMed] [Google Scholar]
- 31.Rochet NM, Kottschade LA, Grotz TE, et al. The prognostic role of the preoperative absolute lymphocyte count and absolute monocyte count in patients with resected advanced melanoma. Am J Clin Oncol 2015; 38:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botta C, Barbieri V, Ciliberto D, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther 2013; 14:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YY, Choi CH, Sung CO, et al. Prognostic value of pre-treatment circulating monocyte count in patients with cervical cancer: comparison with SCC-Ag level. Gynecol Oncol 2012; 124:92–97. [DOI] [PubMed] [Google Scholar]
- 34.Bishara S, Griffin M, Cargill A, et al. Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur J Obstet Gynecol Reprod Biol 2008; 138:71–75. [DOI] [PubMed] [Google Scholar]
- 35.Marcus B, Arenberg D, Lee J, et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer Am Cancer Soc 2004; 101:2779–2787. [DOI] [PubMed] [Google Scholar]
- 36.Koh YW, Kang HJ, Park C, et al. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin's lymphoma: correlation with tumor-associated macrophages. Oncologist 2012; 17:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Zhang J, Chen S, et al. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer 2011; 74:188–196. [DOI] [PubMed] [Google Scholar]
- 38.Park KY, Li G, Platt MO. Monocyte-derived macrophage assisted breast cancer cell invasion as a personalized, predictive metric to score metastatic risk. Sci Rep 2015; 5:13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127–137. [DOI] [PubMed] [Google Scholar]
- 40.Yakes FM, Chinratanalab W, Ritter CA, et al. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 2002; 62:4132–4141. [PubMed] [Google Scholar]
- 41.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res 2012; 14:R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer Am Cancer Soc 2008; 113:1387–1395. [DOI] [PubMed] [Google Scholar]
- 43.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949–1955. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y, Xu X, Guo S, et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS ONE 2014; 9:e91551. [DOI] [PMC free article] [PubMed] [Google Scholar]


