Abstract
Background:
Several randomized clinical trials (RCTs) conducted to evaluate the effect of triclosan-coated suture on surgical site infection (SSI) yield to controversial results. The primary purpose of this systematic review and meta-analysis was to analyze the available RCTs, comparing the effect of triclosan-coated suture with uncoated suture on the incidence of SSI after elective colorectal operations. As secondary endpoint of the analysis, we considered length of hospital stay after surgery.
Methods:
We performed a systematic literature review through Medline, Embase, Pubmed, Scopus, Ovid, ISI Web of Science, and the Cochrane Controlled Trials Register searching for RCTs published from 1990 to 2015. To conduct these meta-analyses, we followed the guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Study inclusion criteria were as follows: parallel-group RCTs in adult populations reporting the closure of the abdominal wall after elective colorectal operation with triclosan-coated suture or noncoated suture, and reporting the outcomes considered in the meta-analysis.
Results:
Six trials including 2168 patients (1102 treated and 1066 controls) provided data on SSIs. The overall rate was 11.7% (129/1102) in the triclosan group and 13.4% (143/1066) in the control group (odds ratio 0.81, 95% confidence interval [CI] 0.58–1.13, P = 0.220). Heterogeneity among studies was moderate (I2 = 44.9%). No evidence of publication bias was detectable. Five RCTs (1783 patients; 914 treated and 689 controls) described hospital length of stay with no significant effect (mean difference: −0.02, 95% CI −0.11 to −0.07, P = 0.668). The I2 test for heterogeneity was 0% (P = 0.836). Moderator analyses showed no significant differences were detected in analyses comparing the suture materials (polydioxanone vs polyglactin). In open-label trials, the odds ratio for SSI risk was 0.62 (95% CI 0.20–1.93, P = 0.413), 0.77 in single-blind (95% CI 0.31–1.95, P = 0.583) and 0.85 in double-blind trials (95% CI 0.46–1.54, P = 0.582).
Conclusions:
Our findings failed to demonstrate a significant protective effect of triclosan-coated sutures on the occurrence of SSI after elective colorectal resections. Further large RCTs are needed before introducing this technology into clinical practice.
Keywords: colorectal surgery, meta-analysis, surgical site infection, systematic review, triclosan
1. Introduction
Despite the continuous progress of awareness, protocol implementation, innovative surgical techniques, and preventive policies, infection of the surgical site remains an unsolved problem after elective colorectal resection.[1–3] It is well-recognized that the genesis of surgical site infections (SSIs) is multifactorial.[4,5] Among the potential causes of SSI, bacterial colonization of suture material with creation of a biofilm has been described.[6–8] Consequently, the use of sutures coated with broad-spectrum antiseptic agents such as triclosan has been introduced. The rational for assuming that the use of suture impregnated with triclosan may reduce the occurrence of SSI is well set by a series of robust data obtained by in vitro and in vivo experiments.[9–11] In fact, triclosan has been shown to have antiseptic property against a wide spectrum of bacteria responsible for SSIs. Moreover, coating provides a prolonged protection against colonization of sutures and surrounding tissues because its effect lasts for about 1 month.[12] This makes the use of triclosan-coated sutures even more attractive because of the considerable proportion of SSI presenting late after surgery.
Colorectal surgery may represent a favorable clinical model to evaluate the effect of sutures impregnated with triclosan because of the polymicrobial origin of SSI, allowing a true in vivo appraisal of the antimicrobial coverage of triclosan. To prove this hypothesis, several randomized clinical trials (RCTs)[13–18] were conducted to evaluate the effect of triclosan-coated suture on the incidence of postoperative SSI. Such trials produced controversial results. The potential reasons for disagreement among study results are the different designs, inclusion and exclusion criteria, sample size calculation, suture material used, blindness of patients and assessors, definition of SSI, evaluation of risk factors in the analysis, and several and potentially unrecorded behaviors of surgeons and sanitary personnel on the treatment of wounds and environmental parameters.
A meta-analytic approach may be useful to overcome the limitation of the limited sample size of single RCTs, allowing data pooling from multiple trials and thus providing a more complete estimation of the effect of a treatment, but it restricts only partially the confounding effect of the differences and heterogeneities among studies and populations. Five previous meta-analyses,[19–23] investigating the impact of triclosan on SSI rate, have been published. In all of them, the estimated risk of SSI was calculated by pooling trials with patients undergoing different types of operation and levels of contamination of the surgical field. Indeed, trials on brain, breast, appendix, colorectum, gynecological, vascular, cardiac, plastic, abdominal, and general surgery were analyzed together. Two of these meta-analyses reported subgroup analyses in patients undergoing colorectal surgery,[19,20] but they did not include the most recent large RCTs.[14,16] In this perspective, we choose to run a new meta-analysis to update the results and to select only RCTs designed for patients undergoing elective colorectal resection or RCTs including also several types of abdominal operation, but in which separate analysis on colorectal patients could be retrieved from the published data or by investigators who responded to our request of additional information. This enabled us to investigate the efficacy of triclosan in a homogeneous clinical setting and a more uniform population, and thus lower the heterogeneity of subgroup analyses. To our knowledge, the effect of triclosan on SSIs in such specific cohort has not been investigated yet.
2. Methods
To conduct this research, we followed the guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses of studies evaluating healthcare interventions.[24]
Ethical approval was not necessary according to local legislation because of the type of study (meta-analysis).
2.1. Literature search
Three authors (MS, IM, and FU) independently performed a Medline, Embase, Pubmed, Scopus, Ovid, ISI Web of Science, and Cochrane Central Register of Controlled Trials and Cochrane Library database extended literature search of all studies published as original articles between March 1990 and June 2015. The following medical subject heading terms and words were used for the search, in all possible combinations: “triclosan,” “Vicryl plus,” “Monocryl Plus,” “PDS Plus,” “polyglactin,” “polydioxanone suture,” “coated suture,” “antiseptic,” “antimicrobial” AND “surgery,” “operation,” “procedure.”
The “related article” function was used to expand the search, and the reference lists of articles selected for full-text review were searched for additional articles. In the event of overlap of authors, institutions, or patients, the most recent article was chosen.
2.2. Study selection
Study inclusion criteria for eligibility were as follows: parallel-group RCTs in adult populations (age >18 years) reporting the closure of the abdominal wall after elective open or laparoscopic colorectal operation and reporting the primary outcome considered in the meta-analysis. No full-text available articles, opinion pieces, editorials, or non-English language papers were excluded.
2.3. Data extraction and quality assessment
An electronic database was created to collect all relevant trial data. The data were extracted independently by 2 investigators (MS, LN), and in case of disagreement, 2 impartial reviewers (LG, FU) cross-examined doubtful data and the decision was made after a consensus meeting. Agreement between authors was calculated to investigate the risk of bias (Cohen κ = 0.91).
Information extracted from the trials involved: first author, country of origin, year of publication, number of patients randomized, number of events (SSI) type of surgery, intention-to-treat reporting, double, single, or no blindness, calculated study sample size, and the different outcome measures. The manuscripts analyzing abdominal operations, but in which separate subgroup of patients undergoing colorectal patients was available from the published data or directly by the investigators who responded to our request for additional information, were also evaluated.
2.4. Risk of bias
Risk of bias and methodological quality of included studies were assessed using the Cochrane Collaboration tool for assessing risk of bias following the principles of the Cochrane Handbook for Systematic Reviews of Interventions[25] by 2 authors (LN and MS). A third author (LG) resolved the differences in opinions. Overall risk of bias was determined by the following areas: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; and incomplete outcome data. A risk-of-bias table was generated to summarize the results of the assessment.
2.5. Primary, secondary endpoints, and moderator analysis
The primary purpose of this systematic review and meta-analysis was to analyze the available RCTs, comparing the effect of triclosan-coated suture with uncoated suture on the incidence of SSI after elective colorectal operations. As secondary endpoint of the analysis, we considered length of stay (LOS) in hospital after surgery.
Moderator analyses were performed according to the following indicators: multicenter or monocenter study, type of suture materials (polydioxanone [PDS] or polyglactin [Vicryl]), and outcome masking (double or single-blind, or open-label method).
2.6. Statistical analysis
We performed a random-effects meta-analysis for each outcome of interest.[26] For SSI rate, the effect size was estimated by the odds ratio (OR), whereas for continuous outcome (LOS), the weighted mean difference (WMD) was used. Mean and standard deviation of LOS were calculated according to the method suggested by Hozo et al[27] for the studies where only median and range (or interquartile range) were reported. The weights assigned to each study were computed according to the inverse of the variance. OR and WMD values are reported with 95% confidence intervals (CIs), and P value <0.05 was considered statistically significant.
Heterogeneity was quantified using I-squared and tau-squared indexes and testing the null hypothesis that all studies share a common effect size. Moreover, we investigated the presence of publication bias by using the “trim and fill” method described by Duval and Tweedie.[28] Briefly, we constructed a plot of each study effect size against its precision (1/SE). These plots should be shaped like a funnel if no publication bias is present. However, since smaller or nonsignificant studies are less likely to be published, studies in the bottom left-hand corner of the plot are often omitted. For the meta-analyses, the right-most studies considered to be symmetrically unmatched are trimmed. The trimmed studies are then replaced and their missing counterparts imputed or “filled.” This then allows for the computation of an adjusted effect size and CI.[29]
All the analyses were performed using ProMeta software, version 2.1 (Internovi s.a.s. Cesena, Italy).
3. Results
Figure 1 depicts the flow diagram of the literature search and article selection. After duplicates removal, we identified 109 potentially relevant citations through the electronic searches. A total of 79 studies were excluded after title and abstract evaluation. Thirty manuscripts were fully analyzed for eligibility and 24 were further excluded for the following reasons: mixed operations (n = 21), unspecified abdominal surgery (n = 1), and 2 trials for not being or quasi randomized.
Figure 1.
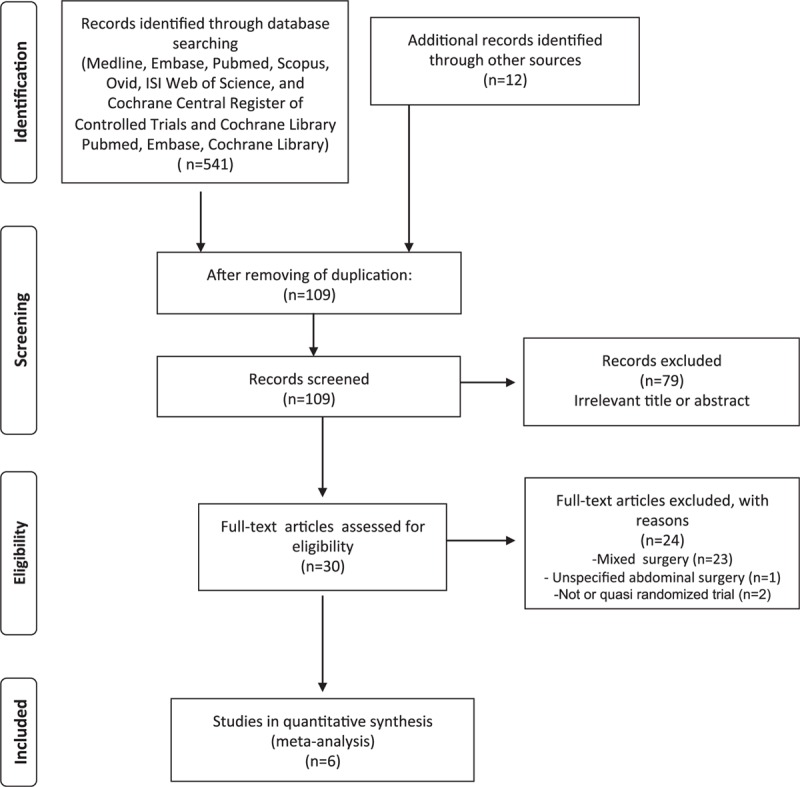
Flowchart of the literature search according the PRISMA statement. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.1. Study characteristics
Six RCTs were finally included in the meta-analysis, with a total of 2168 patients, 1102 (50.8%) receiving triclosan-coated material and 1066 (49.2%) uncoated sutures. The overall rate of SSI ranged from 6.8% to 16.8%. The mean number of patients/study was 361. The range of publication year was between 2011 and 2015. Three studies were multicenter, with a range of 4 to 24 hospitals and 3 single centers. Sample size was formally calculated in 4 out of 6 trials. SSI was declared as the primary endpoint of 5/6 of the studies, and SSI was defined according to the Centers for Disease Control and Prevention of Atlanta criteria[30] in 4 trials. In 2 publications, both patients and outcome assessors were blinded to treatment, whereas in 2 studies, only assessors and 2 trials were open-label. Wound suture material was PDS in 3 studies, Vicryl in 2, and both PDS and Vicryl in 1 study. Closure of the laparotomy was accomplished by a running single-layer mass techniques in 3 trials and miscellaneous techniques in the remaining 3 studies. Patients were followed up for late SSI appearance for 30 days after hospital discharge in 4 out of 6 trials. These results are summarized in Table 1.
Table 1.
Characteristics of the included randomized clinical trials.
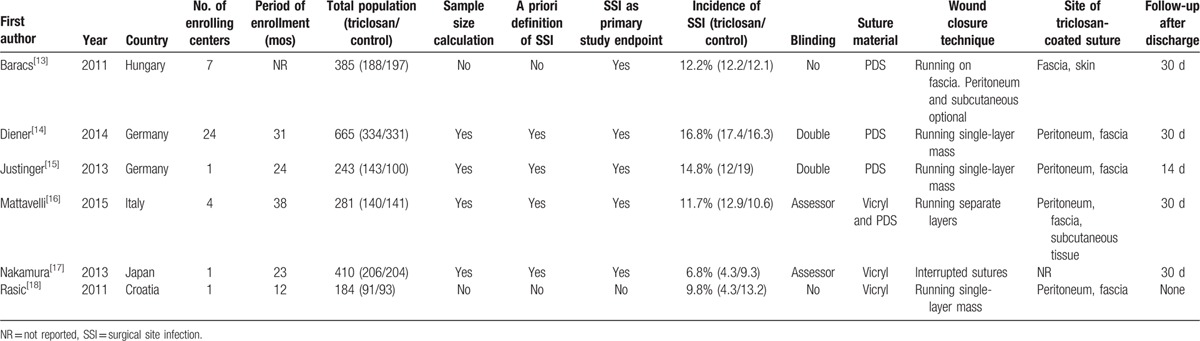
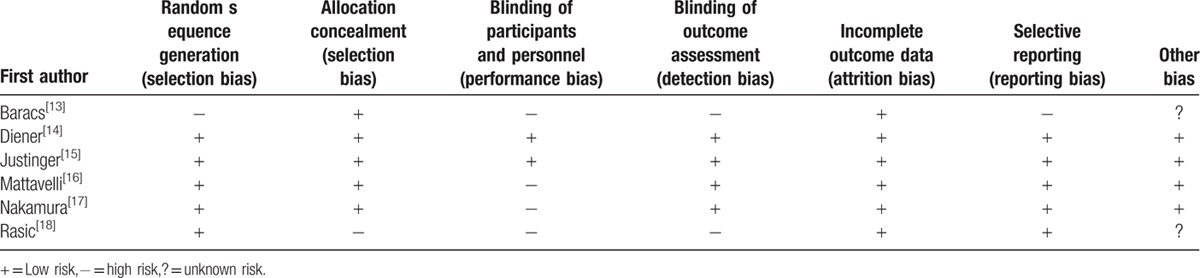
Table 2 describes the authors’ personal judgment of the publication bias. Overall, 2 RCTs[13,18] had a high risk of bias, whereas the remaining a low or moderate risk.
Table 2.
Risk of bias.
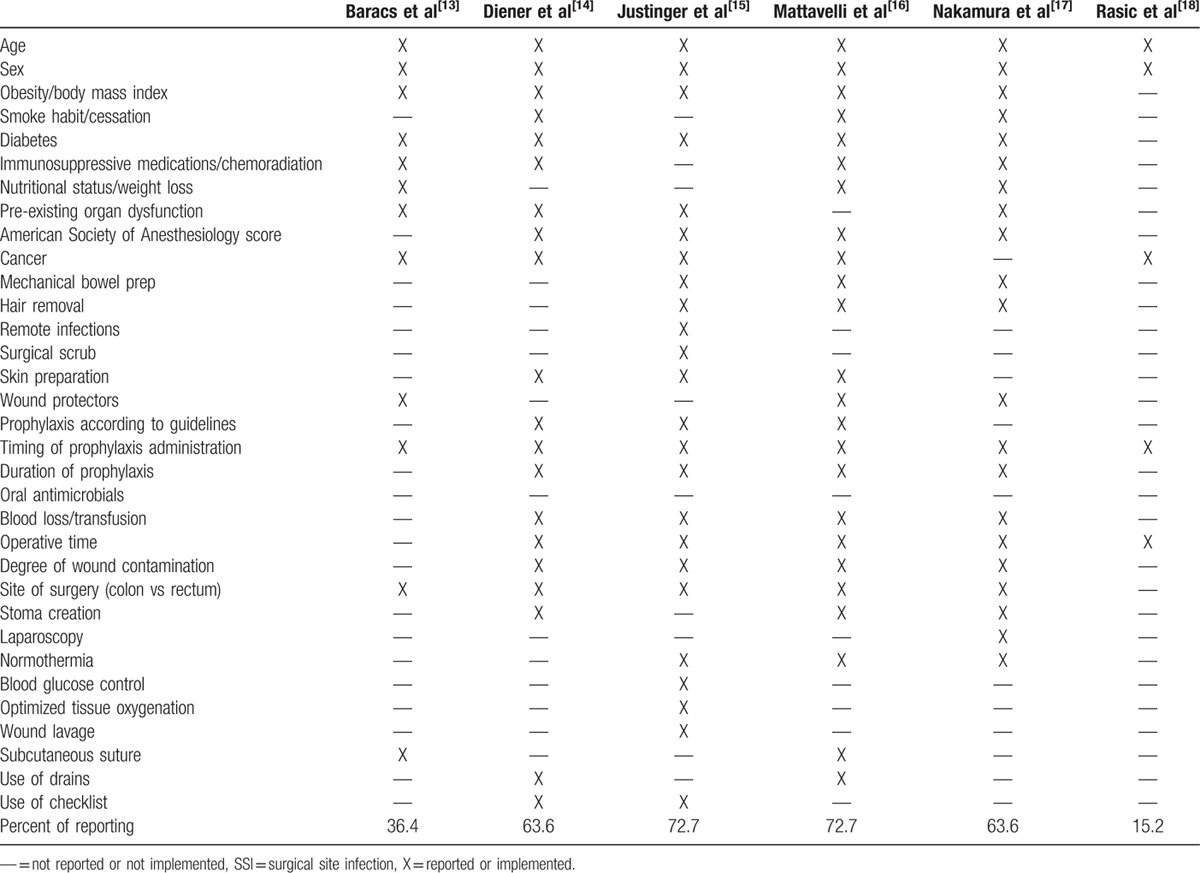
We described the potential risk factors for SSI and policies for prevention taken into account or implemented in the materials and methods of the RCTs (Table 3). The rate of reporting was between 15.2% and 72.7%, suggesting that in some studies, most of the recognized risk factors were not considered or calculated in the populations analyzed, and that significant strategies for prevention were not applied or reported.
Table 3.
Potential risk factors for SSI and strategies for prevention reported or implemented in the studies.
3.2. Primary endpoint
Six trials including 2168 patients (1102 treated and 1066 controls) provided data on SSIs. The overall rate was 11.7% (129/1102) in the triclosan group and 13.4% (143/1066) in control group. The OR was 0.81 (95% CI 0.58–1.13, P = 0.220) (Fig. 2). Heterogeneity among studies was moderate (I2 = 44.9%, Q = 9.1, P = 0.106). No evidence of publication bias was detectable (Fig. 3).
Figure 2.
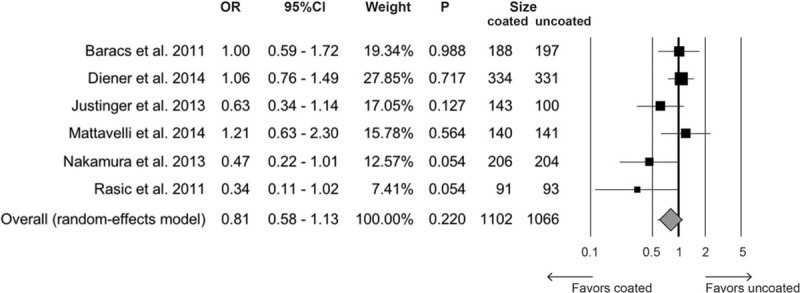
Forest plot for overall rate of surgical site infections comparing triclosan-coated suture versus uncoated. CI = confidence interval, OR = odds ratio.
Figure 3.
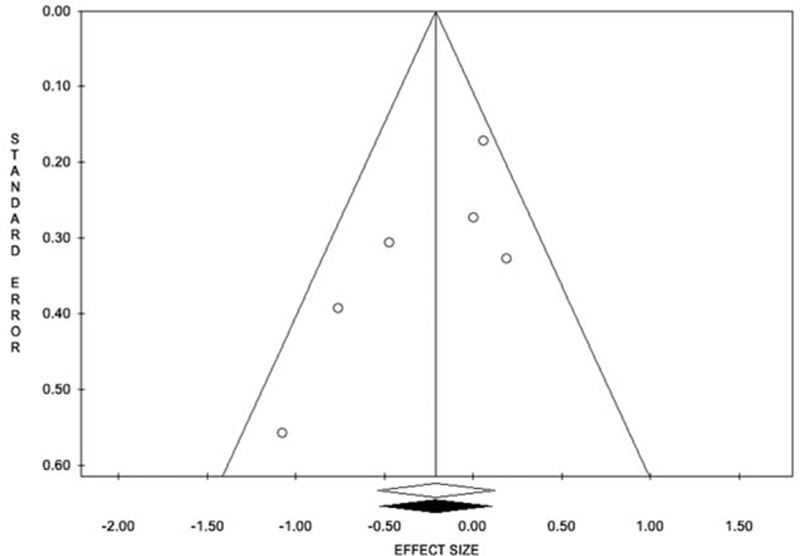
Funnel plot for overall the overall rate of surgical site infection. The estimated effect measure is plotted on the x-axis and its standard error on the y-axis.
3.3. Secondary endpoint
Five RCTs (1783 patients; 914 treated and 689 controls) described hospital LOS. WMD was −0.02 in favor of triclosan (95% CI −0.11 to −0.07, P = 0.668) (Fig. 4). The tau-squared test for heterogeneity among studies was 0% (Q = 1.45), with a P value of 0.836. Funnel plot suggested no evidence of publication bias (data not shown).
Figure 4.
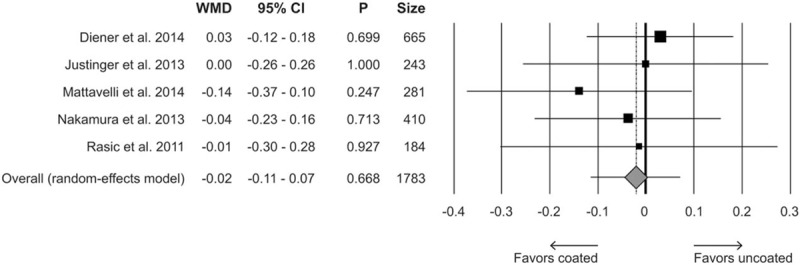
Forest plot for length of hospital stay comparing triclosan-coated versus uncoated suture. CI = confidence interval, WMD = weighted mean difference.
3.4. Moderator analyses
We performed different moderator analyses to evaluate possible effects of study design (multicenter or monocenter), type of suture materials (PDS or Vicryl), and outcome masking (double or single-blind, or open-label method) on the primary outcome measure.
Single-center studies were associated with a significant reduction of SSI in the group receiving triclosan-coated suture (OR 0.52, 95% CI 0.34–0.80, P = 0.003), whereas in trials conducted in multiple centers, this protective effect was not evident (OR 1.02, 95% CI 0.83–1.39, P = 0.602) (Fig. 5). Moreover, no significant differences were detected in analyses comparing the suture materials (PDS vs Vicryl) (Fig. 6) or level of marking. In fact, in open-label studies, the OR for SSI risk was 0.62 (95% CI 0.20–1.93, P = 0.413), 0.77 in single-blind (95% CI 0.31–1.95, P = 0.583) and 0.85 in double-blind trials (95% CI 0.46–1.54, P = 0.582).
Figure 5.
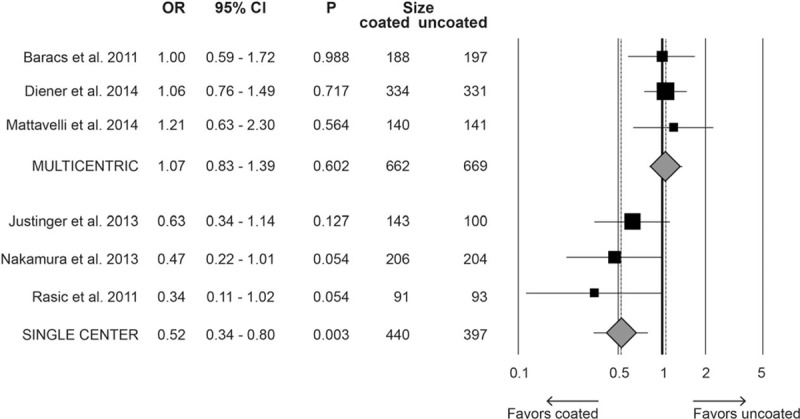
Forest plot for the moderator analysis evaluating the rate of surgical site infections in multicenter and monocenter studies comparing triclosan-coated versus uncoated suture. CI = confidence interval, OR = odds ratio.
Figure 6.
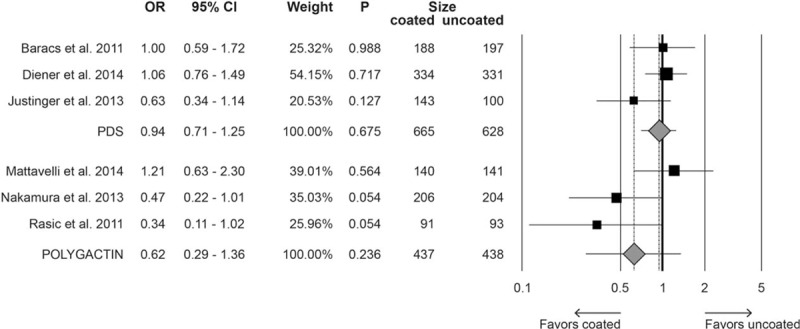
Forest plot for the moderator analysis evaluating the rate of surgical site infections by material used (PDS vs polyglactin) comparing triclosan-coated versus uncoated suture. CI = confidence interval, OR = odds ratio, PDS = polydioxanone.
4. Discussion
Several well-established and consolidated strategies and policies to prevent SSI have been recently reviewed,[2,31–33] although the quality of evidences and the strength of recommendations vary to a great extent among procedures, and the rate of their implementation in specific centers is largely unknown.
Surgical site infection still represents a severe burden for both patients and healthcare systems. SSIs not only lead to a significant increase in morbidity, intensive care unit admissions, and long-term surgical-site complications, but also result in an increased risk of death in patients having surgery.[34] Furthermore, SSIs challenge healthcare systems by requiring additional hospital bed occupancy, readmission, additional resource costs, and increased loss of working hours.[34–36] Thus, the implementation of any means of prevention should be of paramount significance in the patient management.
Because an infection of the surgical site results mainly from the balance between the amount of the microorganisms inoculated, and their virulence, and the ability of the immune system to clear them, is reasonable to utilize broad-spectrum agents to lessen the bacterial load at the wound site. The effect of surgical sutures embedded with triclosan has been tested in several RCTs enrolling subjects with fairly different patient-related and operation-related risk factors. Moreover, the trial quality and the potential confounding bias represent a limitation in the interpretation of the results and in drawing conclusive information on the true usefulness. Meta-analyses and systematic reviews may partially overcome these drawbacks if the study inclusion and exclusion criteria are set rigorously and the subgroup analyses are run with homogeneous cohorts. In this line of thought, we chose to perform a meta-analysis on the effect of triclosan on SSI, by selecting a specific population of patients undergoing elective colorectal resections to minimize heterogeneity of class of wound contamination[30] and type of operation.
The previously published meta-analyses did not or partially consider this specific cohort in subgroup analyses. Edmiston et al[21] and Chang et al[23] pooled all available RCTs without stratifying the risk for wound class contamination, type of operation, or organ/apparatus involved. Wang et al[22] reported that in a total of 1146 patients undergoing clean-contaminated surgery, which should correspond to elective colorectal resection, the relative risk of SSI was 0.69 (P = 0.026) in favor of the tricosan-treated group. In a further meta-analysis, Daoud et al[20] evaluated 1484 patients (enrolled in 6 studies) with class II wound contamination. The relative risk for SSI was 0.65 (P = 0.010), suggesting a significant protective effect of tricolsan. They also stratified the risk of operation by pooling 5 trials with 1322 patients who underwent colorectal resection or appendectomy, despite the considerable differences between these 2 procedures. The calculated risk ratio (0.687) approached statistical significance (P = 0.053). The latest meta-analysis by Apisarnthanarak et al[19] evaluated a subgroup analysis in patients undergoing colorectal resection without including the most recent RCTs,[14,16] and incorporated a duplicate publication by 2 different first authors.[13,37] Their results showed a nonsignificant effect of triclosan-coated sutures in this specific cohort (relative risk 0.73, 95% CI 0.46–1.17).
In the present meta-analysis, we included all the available trials that randomized only patients’ candidate to elective colorectal resection and the studies where it was possible to retrieve separate analysis on colorectal patients from the published data or by the investigators who responded to our request of additional information. This allowed us to increase the size of the sample to 2168 patients available for the final analysis. The results suggest that sutures impregnated with triclosan do not significantly affect the rate of SSI in patients undergoing elective colorectal resection. Nevertheless, even by considering the pooled results of potentially homogeneous trials for the level of wound contamination and type of operation, we observed a moderate heterogeneity among studies, whereas no evidence of publication bias with a symmetric distribution in the funnel plot was detected. Moreover, there was a wide range of incidence of SSI (6.8%–16.8%) and substantial differences in trial methodology and quality, suture materials, wound closure techniques, sites of tricolsan sutures application, and definition of SSI. Also, the reported details on the potentially confounding risk factors and the supplementary strategies implemented to prevent SSI were quite variable in the trials ranging from 15.2% to 72.7%. All these factors may represent a limitation on the interpretation of the present results.
It is also noteworthy that despite the overall effect of triclosan was not significant, the use of coated sutures yield to a reduction of 19% in the rate of SSI. This figure is not negligible because such scenario may potentially result in a substantial financial saving even if the cost of embedded material is higher that the standard one. At the moment, there are no well-designed cost-benefit studies that may prove that this new technology is a dominant strategy, even though some data might suggest it. Nakamura et al[17] estimated that if 0.5% of SSIs are prevented by the use of triclosan in closing the abdominal wound after colorectal surgery, then this policy may be cost-effective, given the mean cost of SSI more than 2000 US dollars.
Our moderator analyses suggested that neither the level of blinding nor the material used was effective in reducing the rate of SSI after colorectal surgery. These findings are of some interest because they seem to confirm that braided sutures are less susceptible, with respect to monofilament, to be colonized by bacteria,[38] and that double-blinding technique is not superior to a true blinded assessor of complications.
The present findings showed that in single-center, but not in multicenter RCTs, triclosan-embedded suture was superior to uncoated one in reducing SSI. This observation is puzzling because it might imply that when triclosan-coated suture is introduced as a single variable in a consolidated, homogenous, and uniform background and surgical behavior of a center, the effect is more apparent. On the contrary, multicenter trials mimic much better the everyday reality, with the numerous variables affecting results, and if a new treatment is truly effective, it should stand the challenge of multifactorial events. One possible interpretation of the discrepancies of results among single and muticenter trials is the composition of the colonic microbiota that differs substantially in different populations mainly according to the alimentary habits and the environmental conditions.[39–41] Because the main source of SSI after colorectal resection is the intestinal flora, different bacterial strains harboring the gut and their susceptibility to triclosan antimicrobial properties may account for the variability of results among trials conducted in different regions.
Our analysis may have several methodological limitations. The true incidence of SSI in both groups should have been based and analyzed by weighting single risk factors extrapolated by data of individual patients and this was not done. Despite the definition of SSI was well-described and established in most of the trials, the classification of this complication partially remains on subjective judgment. This may introduce clinical heterogeneity so that all the potential biases cannot be completely ruled out. The reliability of the results of a meta-analysis is determined by the quality of the included trials, which in this case was not always outstanding.
In conclusion, our findings failed to demonstrate a significant protective effect of triclosan-coated sutures on the occurrence of SSI after elective colorectal resections. Since the present meta-analysis did not completely rule out and solve the conflicting results in the literature on the benefit of impregnated materials on wound infection, further large RCTs that take into account all risk factors and the supplementary preventive strategies are needed before introducing it in a routine clinical use, unless well-performed health technology assessment evaluation will prove a dominant cost-effectiveness ratio.
Acknowledgments
The authors are truly thankful to Professor Markus K. Diener and Professor Markus W. Būchler for providing additional information and data on their work.[14]
Footnotes
Abbreviations: CI = confidential interval, LOS = length of stay, OR = odds ratio, PDS = polydioxanone, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized clinical trial, SSI = surgical site infection, WMD = weighted mean difference.
Author contributions: MS and IM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: LG, MS, and IM; acquisition of data: MS, LN, and FU; analysis and interpretation of data: LG, MS, LN, IM, and FU; drafting of the manuscript: MS, LG, and IM; critical revision of the manuscript for important intellectual content and final approval: MS, IM, FU, LN, and LG; statistical analysis: MSandini; study supervision: LG.
The authors have no conflicts of interest to disclose.
References
- 1.Leaper DJ. Surgical-site infection. Br J Surg 2010; 97:1601–1602. [DOI] [PubMed] [Google Scholar]
- 2.Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann Surg 2004; 239:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hrandjec T, Sweason R, Sawyer RG. Surgical site infection prevention: how we do it. Surg Infect 2010; 11:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fry DE. Surgical site infections and the surgical care improvement project (SCIP): evaluation of national quality measures. Surg Infect 2008; 9:579–584. [DOI] [PubMed] [Google Scholar]
- 5.Tang R, Chen HH, Wang YL, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg 2001; 234:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander JW, Kaplan JZ, Altemeier WA. Role of suture materials in the development of wound infection. Ann Surg 1967; 165:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz S, Izhar M, Mirelman D. Bacterial adherence to surgical sutures. A possible factor in suture induced infection. Ann Surg 1981; 194:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrit K, Hitchins VM, Neale VR. Tissue colonization from implantable biomaterials with low numbers of bacterial. J Biomed Material Res 1999; 44:261–265. [DOI] [PubMed] [Google Scholar]
- 9.Barbolt TA. Chemistry and safety of triclosan, and its use as an antimicrobial coating on coated Vicryl Plus antibacterial suture (coated polyglactin 910 suture with triclosan). Surg Infect 2002; 3 suppl 1:S45–S53. [DOI] [PubMed] [Google Scholar]
- 10.Edmiston CE, Seabrook GR, Goheen MP, et al. Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination? J Am Coll Surg 2006; 203:481–489. [DOI] [PubMed] [Google Scholar]
- 11.Rothenburger S, Spangler D, Bhende S. In vitro antimicrobial evaluation of coated Vicryl Plus antibacterial suture (coated polyglactin 910 with triclosan) using zone of inhibition assays. Surg Infect 2002; 3 suppl 1:539–587. [DOI] [PubMed] [Google Scholar]
- 12.Leaper D, Assadian O, Hubner NO, et al. Antimicrobial sutures and prevention of surgical site infection: assessment of the safety of the antiseptic triclosan. Int Wound J 2011; 8:556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baracs J, Huszár O, Sajjadi SG, et al. Surgical site infections after abdominal closure in colorectal surgery using triclosan-coated absorbable suture (PDS Plus) vs. uncoated sutures (PDS II): a randomized multicenter study. Surg Infect (Larchmt) 2011; 12:483–489. [DOI] [PubMed] [Google Scholar]
- 14.Diener MK, Knebel P, Kieser M, et al. Effectiveness of triclosan-coated PDS Plus versus uncoated PDS II sutures for prevention of surgical site infection after abdominal wall closure: the randomised controlled PROUD trial. Lancet 2014; 384:142–152. [DOI] [PubMed] [Google Scholar]
- 15.Justinger C, Slotta JE, Ningel S, et al. Surgical-site infection after abdominal wall closure with triclosan-impregnated polydioxanone sutures: results of a randomized clinical pathway facilitated trial (NCT00998907). Surgery 2013; 154:589–595. [DOI] [PubMed] [Google Scholar]
- 16.Mattavelli I, Rebora P, Doglietto G, et al. Multicentre, randomized, controlled trial on the effect of triclosan-coated suture on surgical site infection after colorectal surgery. Surg Infect 2015; 16:226–235. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Kashimura N, Noji T, et al. Triclosan-coated sutures reduce the incidence of wound infections and the costs after colorectal surgery: a randomized controlled trial. Surgery 2013; 153:576–583. [DOI] [PubMed] [Google Scholar]
- 18.Rasić Z, Schwarz D, Adam VN, et al. Efficacy of antimicrobial triclosan-coated polyglactin 910 (Vicryl Plus) suture for closure of the abdominal wall after colorectal surgery. Coll Antropol 2011; 35:439–443. [PubMed] [Google Scholar]
- 19.Apisarnthanarak A, Singh N, Bandong AN, et al. Triclosan-coated sutures reduce the risk of surgical site infections: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015; 36:169–179. [DOI] [PubMed] [Google Scholar]
- 20.Daoud FC, Edmiston CE, Jr, Leaper D. Meta-analysis of prevention of surgical site infections following incision closure with triclosan-coated sutures: robustness to new evidence. Surg Infect (Larchmt) 2014; 15:165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edmiston CE, Jr, Daoud FC, Leaper D. Is there an evidence-based argument for embracing an antimicrobial (triclosan)-coated suture technology to reduce the risk for surgical-site infections? A meta-analysis. Surgery 2013; 154:89–100. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZX, Jiang CP, Cao Y, et al. Systematic review and meta-analysis of triclosan-coated sutures for the prevention of surgical-site infection. Br J Surg 2013; 100:465–473. [DOI] [PubMed] [Google Scholar]
- 23.Chang WK, Srinivasa S, Morton R, et al. Triclosan-impregnated sutures to decrease surgical site infections: systematic review and meta-analysis of randomized trials. Ann Surg 2012; 255:854–859. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Available at: http://www.cochrane-handbook.org. Accessed May 30, 2015. [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 27.Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 29.Gilbody SM, Song F, Eastwood AJ, et al. The causes, consequences and detection of publication bias in psychiatry. Acta Psychiatr Scand 2000; 102:241–249. [DOI] [PubMed] [Google Scholar]
- 30.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999: Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999; 20:250–278. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JD, Podgorny K, Berríos-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol 2014; 35:605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murry BW, Hueeta S, Dineen S, et al. Surgical site infection in colorectal surgery: a review of the nonpharmacologic tolls of prevention. J Am Coll Surg 2010; 211:812–822. [DOI] [PubMed] [Google Scholar]
- 33.Alexander JW, Solomkin JS, Edwards MJ. Updated recommendations for control of surgical site infections. Ann Surg 2011; 253:1082–1093. [DOI] [PubMed] [Google Scholar]
- 34.Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 1999; 20:725–730. [DOI] [PubMed] [Google Scholar]
- 35.Braga M, Gianotti L, Vignali A, et al. Hospital resources consumed for surgical morbidity: effect of preoperative arginine and omega-3 fatty acids supplementation on costs. Nutrition 2005; 21:1078–1086. [DOI] [PubMed] [Google Scholar]
- 36.Leaper DJ, van Goor H, Reilly J, et al. Surgical site infection: a European perspective of incidence and economic burden. Int Wound J 2004; 1:247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huszár O, Baracs J, Tóth M, et al. Comparison of wound infection rates after colon and rectal surgeries using triclosan-coated or bare sutures: a multi-center, randomized clinical study. Magy Seb 2012; 65:83–91. [DOI] [PubMed] [Google Scholar]
- 38.Edmiston CE, Krepel CJ, Marks RM, et al. Microbiology of explanted suture segments from infected and noninfected surgical cases. J Clin Microbiol 2013; 51:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Human Microbiome Project. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power SE, O’Toole PW, Stanton C, et al. Intestinal microbiota, diet and health. Br J Nutr 2014; 111:387–402. [DOI] [PubMed] [Google Scholar]
- 41.Hold GL. Western lifestyle: a ‘master’ manipulator of the intestinal microbiota? Gut 2014; 63:5–6. [DOI] [PubMed] [Google Scholar]


