Abstract
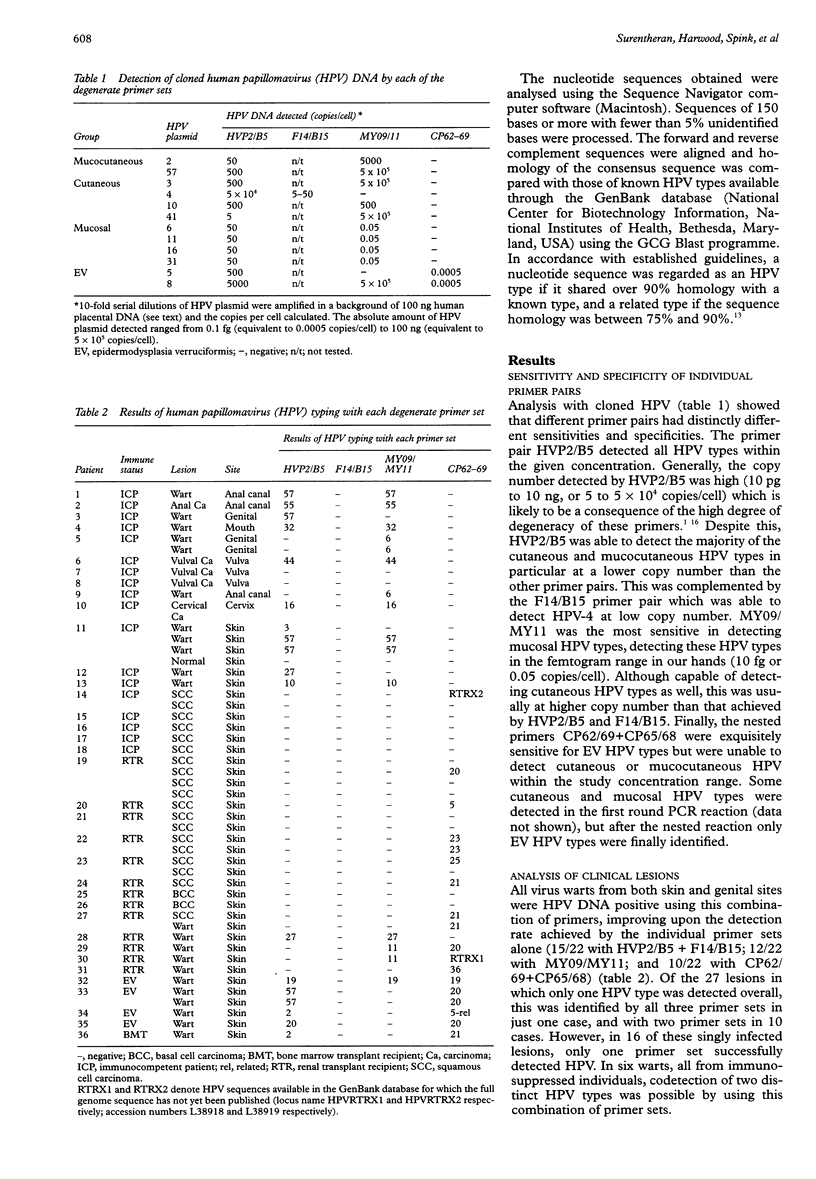
AIM: To develop a unified diagnostic approach for the detection of human papillomavirus (HPV) DNA in skin and mucosal biopsies from both immunocompetent and immunosuppressed individuals using a degenerate polymerase chain reaction (PCR) method. METHODS: The sensitivity and specificity of three published degenerate primer sets (HVP2/B5 and F14/B15; MY09/MY11; CP62/69 outer and CP65/68 nested primer pairs) were evaluated in PCR reactions with serial dilutions of 12 representative cloned HPV types. This combination of primers was then used to detect HPV DNA in 49 benign and malignant lesions of cutaneous and mucosal origin from immunosuppressed, immunocompetent, and epidermodysplasia verruciformis (EV) patients, and compared with detection rates using single primer sets alone. RESULTS: The observed sensitivity of MY09/MY11 and CP62/69 + CP65/68 was high for mucosal and EV HPV types, respectively. The sensitivity of all primer sets for cutaneous types was low, but nonetheless the use of this combination of primers allowed HPV DNA detection in all of the benign warts analysed. Several mixed infections were also identified. A high prevalence of HPV DNA was similarly detected in squamous cell carcinomas from immunocompromised patients; the HPV types found were exclusively EV related. CONCLUSIONS: The use of a combined degenerate primer PCR approach considerably improves HPV DNA detection over individual primer sets and allows detection of mixed infections. The findings may help explain the discrepancies in published reports relating to HPV DNA detection in benign and malignant skin lesions. Further modifications to this method are in progress which should significantly improve comprehensive HPV detection and typing for diagnostic purposes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkhout R. J., Tieben L. M., Smits H. L., Bavinck J. N., Vermeer B. J., ter Schegget J. Nested PCR approach for detection and typing of epidermodysplasia verruciformis-associated human papillomavirus types in cutaneous cancers from renal transplant recipients. J Clin Microbiol. 1995 Mar;33(3):690–695. doi: 10.1128/jcm.33.3.690-695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. Y., Delius H., Halpern A. L., Bernard H. U. Analysis of genomic sequences of 95 papillomavirus types: uniting typing, phylogeny, and taxonomy. J Virol. 1995 May;69(5):3074–3083. doi: 10.1128/jvi.69.5.3074-3083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith D., Trowell H., Dyall-Smith M. L. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991 Nov;30(11):785–789. doi: 10.1111/j.1365-4362.1991.tb04787.x. [DOI] [PubMed] [Google Scholar]
- Gassenmaier A., Fuchs P., Schell H., Pfister H. Papillomavirus DNA in warts of immunosuppressed renal allograft recipients. Arch Dermatol Res. 1986;278(3):219–223. doi: 10.1007/BF00412927. [DOI] [PubMed] [Google Scholar]
- Majewski S., Jabłońska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995 Nov;131(11):1312–1318. [PubMed] [Google Scholar]
- McGregor J. M., Farthing A., Crook T., Yu C. C., Dublin E. A., Levison D. A., MacDonald D. M. Posttransplant skin cancer: a possible role for p53 gene mutation but not for oncogenic human papillomaviruses. J Am Acad Dermatol. 1994 May;30(5 Pt 1):701–706. doi: 10.1016/s0190-9622(08)81498-3. [DOI] [PubMed] [Google Scholar]
- McGregor J. M., Proby C. M. The role of papillomaviruses in human non-melanoma skin cancer. Cancer Surv. 1996;26:219–236. [PubMed] [Google Scholar]
- Orth G. Epidermodysplasia verruciformis: a model for understanding the oncogenicity of human papillomaviruses. Ciba Found Symp. 1986;120:157–174. doi: 10.1002/9780470513309.ch11. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shamanin V., Delius H., de Villiers E. M. Development of a broad spectrum PCR assay for papillomaviruses and its application in screening lung cancer biopsies. J Gen Virol. 1994 May;75(Pt 5):1149–1156. doi: 10.1099/0022-1317-75-5-1149. [DOI] [PubMed] [Google Scholar]
- Shamanin V., Glover M., Rausch C., Proby C., Leigh I. M., zur Hausen H., de Villiers E. M. Specific types of human papillomavirus found in benign proliferations and carcinomas of the skin in immunosuppressed patients. Cancer Res. 1994 Sep 1;54(17):4610–4613. [PubMed] [Google Scholar]
- Shamanin V., zur Hausen H., Lavergne D., Proby C. M., Leigh I. M., Neumann C., Hamm H., Goos M., Haustein U. F., Jung E. G. Human papillomavirus infections in nonmelanoma skin cancers from renal transplant recipients and nonimmunosuppressed patients. J Natl Cancer Inst. 1996 Jun 19;88(12):802–811. doi: 10.1093/jnci/88.12.802. [DOI] [PubMed] [Google Scholar]
- Smith S. E., Davis I. C., Leshin B., Fleischer A. B., Jr, White W. L., Feldman S. R. Absence of human papillomavirus in squamous cell carcinomas of nongenital skin from immunocompromised renal transplant patients. Arch Dermatol. 1993 Dec;129(12):1585–1588. [PubMed] [Google Scholar]
- Tieben L. M., Berkhout R. J., Smits H. L., Bouwes Bavinck J. N., Vermeer B. J., Bruijn J. A., Van der Woude F. J., Ter Schegget J. Detection of epidermodysplasia verruciformis-like human papillomavirus types in malignant and premalignant skin lesions of renal transplant recipients. Br J Dermatol. 1994 Aug;131(2):226–230. doi: 10.1111/j.1365-2133.1994.tb08496.x. [DOI] [PubMed] [Google Scholar]
- Vousden K. H. Human papillomaviruses and cervical carcinoma. Cancer Cells. 1989 Oct;1(2):43–50. [PubMed] [Google Scholar]
- de Jong-Tieben L. M., Berkhout R. J., Smits H. L., Bouwes Bavinck J. N., Vermeer B. J., van der Woude F. J., ter Schegget J. High frequency of detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in biopsies from malignant and premalignant skin lesions from renal transplant recipients. J Invest Dermatol. 1995 Sep;105(3):367–371. doi: 10.1111/1523-1747.ep12320803. [DOI] [PubMed] [Google Scholar]
- de Villiers E. M. Human pathogenic papillomavirus types: an update. Curr Top Microbiol Immunol. 1994;186:1–12. doi: 10.1007/978-3-642-78487-3_1. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]


