Abstract
Few studies have evaluated the risk factors for in-hospital mortality in critically ill surgical patients who have undergone emergency gastrointestinal (GI) surgery. The aim of this study was to identify the risk factors associated with in-hospital mortality in critically ill surgical patients after emergency GI surgery.
The medical records of 362 critically ill surgical patients who underwent emergency GI surgery, admitted to intensive care unit between January 2007 and December 2011, were reviewed retrospectively. Perioperative biochemical and clinical parameters of survivors and nonsurvivors were compared. Logistic regression multivariate analysis was performed to identify the independent risk factors of mortality.
The in-hospital mortality rate was 15.2% (55 patients). Multivariate analyses revealed cancer-related perforation (odds ratio [OR] 16.671, 95% confidence interval [CI] 2.629–105.721, P = 0.003), preoperative anemia (hemoglobin <10 g/dL; OR 6.976, 95% CI 1.376–35.360, P = 0.019), and preoperative hypoalbuminemia (albumin <2.7 g/dL; OR 9.954, 95% CI 1.603–61.811, P = 0.014) were independent risk factors of in-hospital mortality after emergency GI surgery.
The findings of this study suggest that in critically ill patients undergoing emergency GI surgery, cancer-related peritonitis, preoperative anemia, and preoperative hypoalbuminemia are associated with in-hospital mortality. Recognizing risk factors at an early stage could aid risk stratification and the provision of optimal perioperative care.
Keywords: critically ill, emergency surgery, mortality
1. Introduction
Peritonitis is one of the most common surgical emergencies,[1] and has relatively high morbidity and mortality rates.[2] However, mortality rates remain high despite advances in surgical techniques, antimicrobial therapy, and intensive care support.[2,3] The 2009 updated guidelines for managing patients with intra-abdominal infection recommend rapid fluid resuscitation, early initiation of antibiotics, and appropriate source-control procedures.[4]
Numerous studies have tried to identify prognostic biomarkers in critically ill patients. Of those identified, lactates or lactate clearance,[5,6] base excess,[7] and serum pro-calcitonin (PCT)[8] are used most often in clinical practice. In addition, many scoring systems, such as the Acute Physiology and Chronic Health Evaluation II (APACHE II) score,[9] the Simplified Acute Physiology Score II (SAPS II),[10] the Sequential Organ Failure Assessment (SOFA),[11] and the Mannheim Peritonitis Index (MPI)[12] systems, have been introduced to estimate disease severity and prognosis in critically ill patients.[13]
However, with the exception of the MPI system, none of the scoring systems mentioned above is specific for peritonitis. Furthermore, these scoring systems are difficult to implement and little help during decision-making in alone. Few studies have evaluated risk factors in critically ill surgical patients with peritonitis. It is important that these risk factors be identified to stratify risks and optimize perioperative care. Accordingly, we performed this study to identify perioperative risk factors for in-hospital mortality in critically ill patients after emergency gastrointestinal (GI) surgery.
2. Materials and method
2.1. Setting and population
This study was performed using a retrospective cohort design. The analysis was conducted using the records of a surgical intensive care unit (ICU) at a single tertiary referral hospital in Seoul, Korea. The data of 500 patients who underwent emergency GI surgery for peritonitis between January 2007 and December 2011 were reviewed. One hundred thirty-eight patients admitted to a general ward postoperatively or who were below 20 years of age were excluded. Finally, 362 patients were enrolled. Approval for this study was obtained beforehand from our institutional review board (IRB No. 4–2015–0424).
2.2. Variables and definition
Demographic data, such as age, sex, underlying disease, American Society of Anesthesiologist (ASA) score, APACHE II score, and cause of peritonitis, were collected. Lengths of hospital stays, lengths of ICU stays, durations of mechanical ventilation, and information on re-operations were collected and analyzed. Perioperative biochemical parameters, such as hemoglobin (Hb) levels, renal function test results, arterial blood gas analysis (ABGA) findings, serum albumin levels, and the presence of septic shock, were analyzed. Blood culture and peritoneal fluid culture results and times of antibiotic administration were included among perioperative variables. Operative variables were evaluated by surgical approach, operation type, and anatomic origin.
Preoperative biochemical parameters were measured immediately after emergency admission, or for in-patients, were the poorest recorded during the 24 hours before surgery. Postoperative biochemical parameters were measured immediately after surgery.
Initially broad-spectrum antibiotics were administered empirically, and these were changed to specific antibiotics after obtaining blood culture or peritoneal fluid culture results. First results available after hospital arrival were viewed as preoperative biochemical parameters.
Septic shock was defined by a systolic arterial pressure <90 mm Hg, a mean arterial pressure <60 mm Hg, or in case of using vasopressors.[14]
Patients were divided into 2 groups: survivors (n = 307) and nonsurvivors (n = 55). These 2 groups were compared with respect to perioperative biochemical and clinical variables.
2.3. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 20.0 (IBM Co. Armonk, NY). Categorical data were compared using the chi-square or Fisher exact test, and continuous variables were compared using the independent t test or Mann–Whiney U test. The continuous variables were converted into categorical variables for the analysis. Factors found to be significantly associated with mortality (P < 0.05) by univariate analysis were included in the multivariate analysis, which was performed using the logistic regression model using the maximum likelihood method and backward stepwise selection. Goodness of fit was assessed using the Hosmer–Lemeshow test.
3. Results
3.1. Baseline characteristics and clinical outcomes
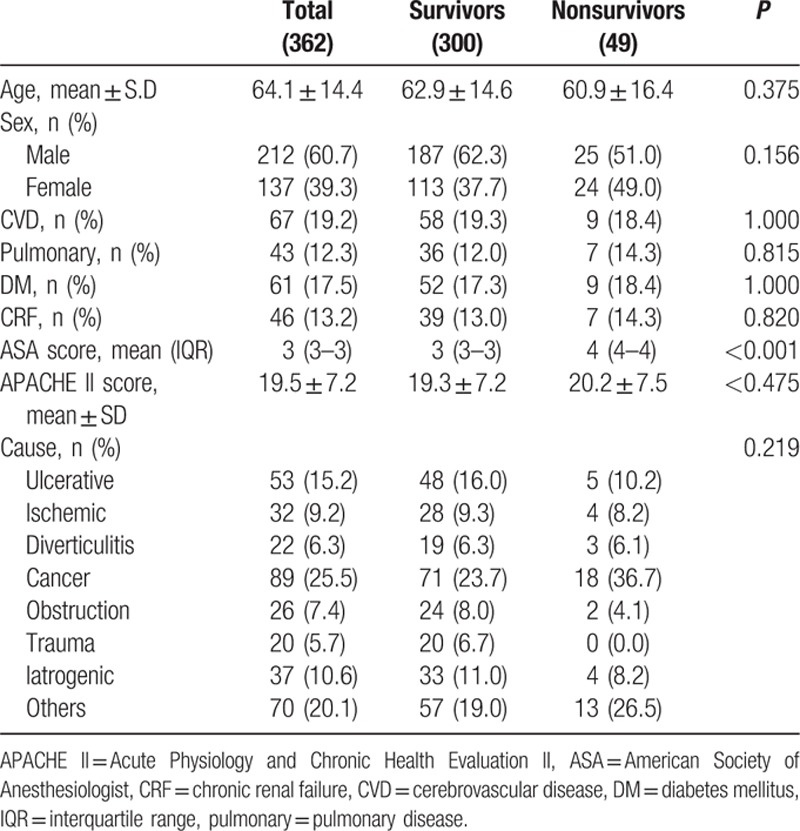
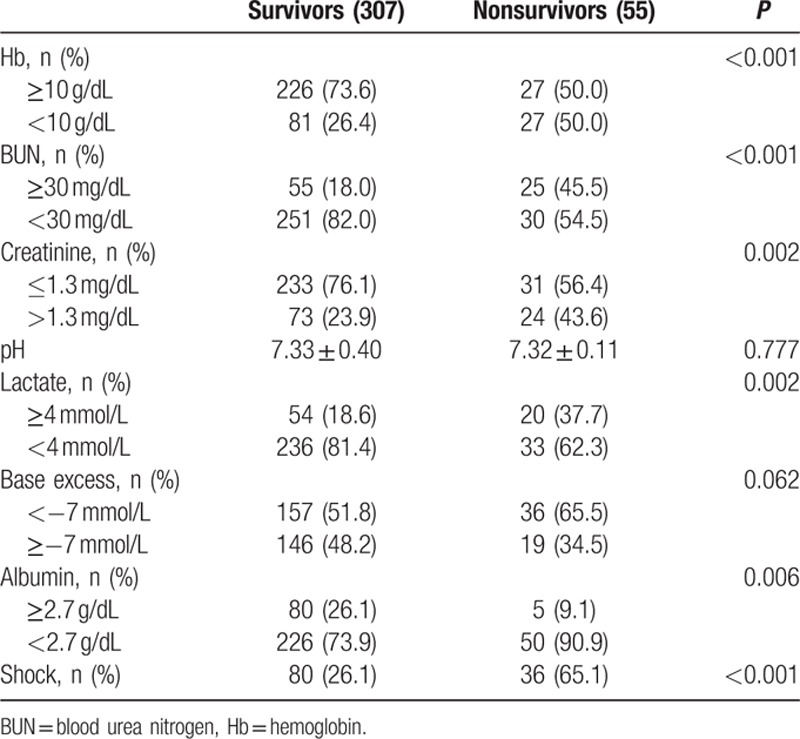
Of the 362 study subjects, 307 (84.8%) survived and 55 (15.2%) patients died. Overall mean subject age was 62.4 ± 15.0 years. Mean age, sex, and underlying diseases were nonsignificantly different in the survivor and nonsurvivor groups. Mean APACHE II scores in the 2 groups were 18.3 ± 6.1 and 22.9 ± 6.8, respectively (P < 0.001). Malignancy of the GI tract was the most common cause of bowel perforation (Table 1).
Table 1.
Baseline characteristics of patients.
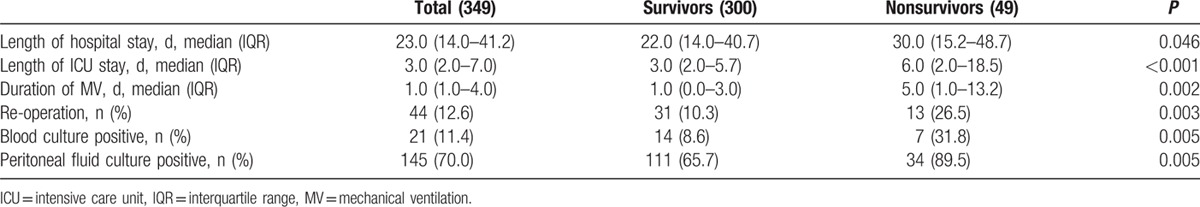
Nonsurvivors stayed in the ICU longer, and more frequently required mechanical ventilation (Table 2).
Table 2.
Baseline characteristics of patients.
3.2. Analysis of perioperative biochemical parameters
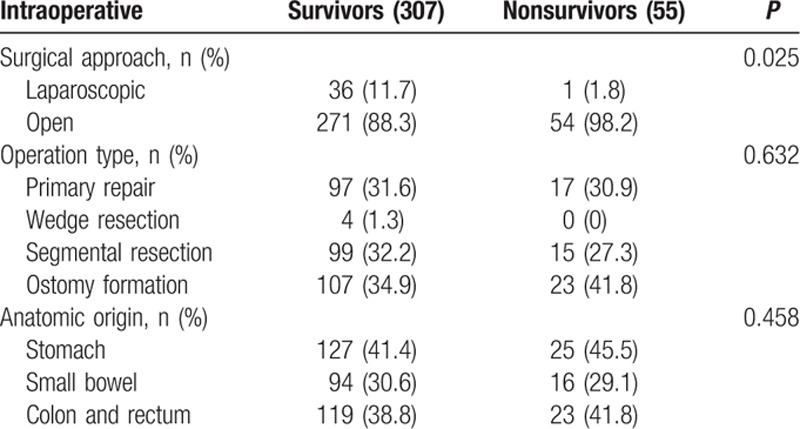
Preoperative anemia (Hb <10 g/dL) was detected in 37 patients, and was more common in nonsurvivors (39.3%) than in survivors (11.8%). Blood urea nitrogen (BUN) was significantly higher in nonsurvivors (P = 0.008), whereas serum creatinine levels were no different in the 2 groups. Initial hyperlactatemia (>4 mmol/L) was more common in nonsurvivors (37.7% vs 19.8 %; P = 0.073), and hypoalbuminemia (<2.7 g/dL) was significantly higher in nonsurvivors (P < 0.001) (Table 3). Laparoscopic surgery was more frequently performed in survivors (Table 4).
Table 3.
Preoperative parameters by univariate analysis.
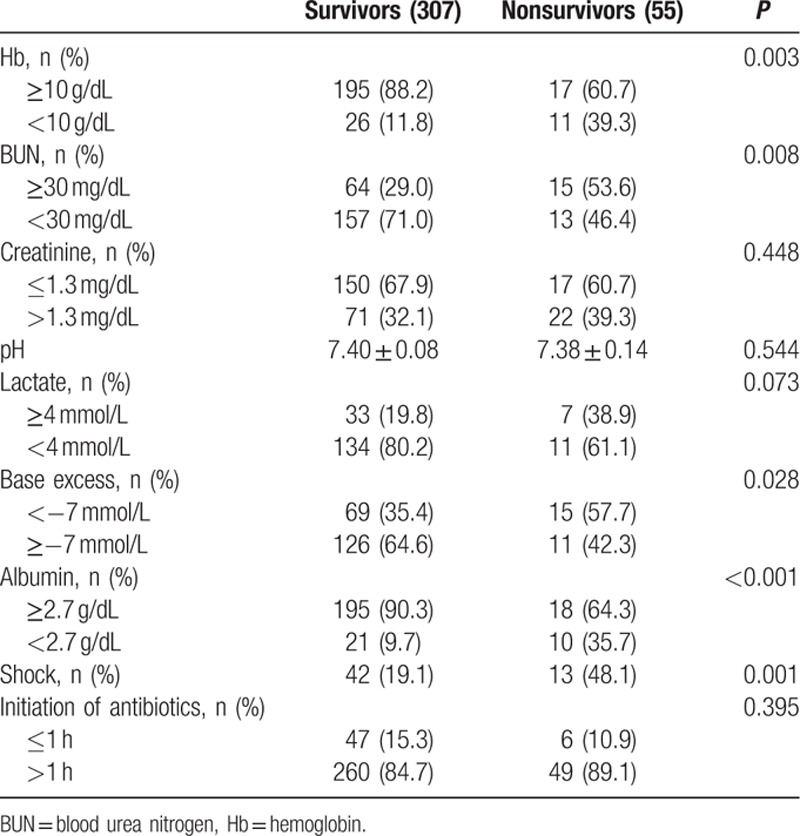
Table 4.
Intraoperative parameters by univariate analysis.
Univariate analysis of postoperative data identified anemia, elevated BUN, hyperlactatemia, base deficit, hypoalbuminemia, and shock as significant factors (Table 5).
Table 5.
Postoperative parameters by univariate analysis.
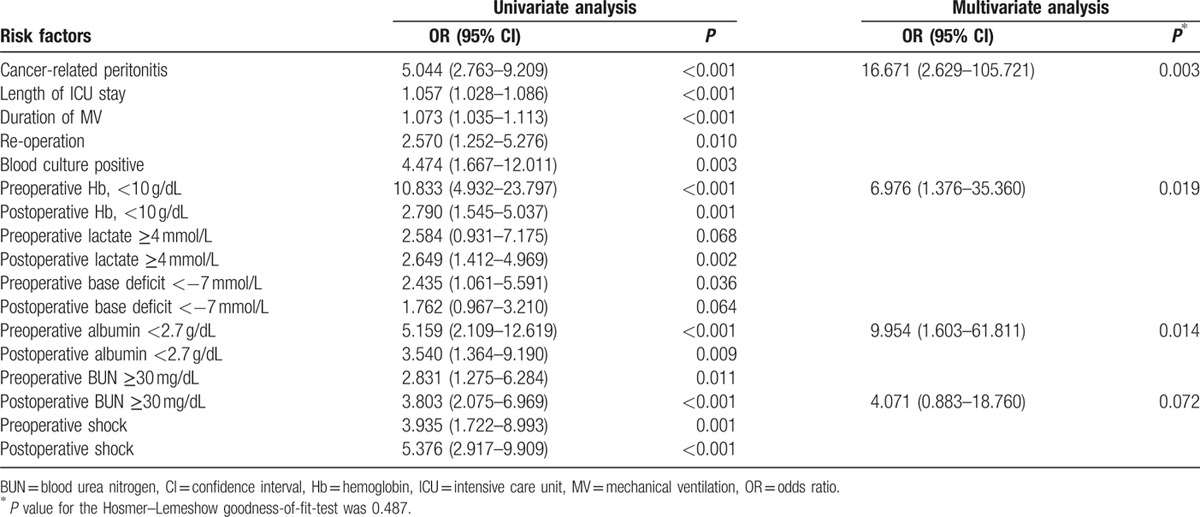
3.3. Independent risk factors by multivariate analysis
Multivariate analysis revealed preoperative anemia (odds ratio [OR] 5.109, 95% confidence interval [CI] 1.292–20.205, P = 0.020), preoperative hypoalbuminemia (OR 10.692, 95% CI 2.321–49.248, P = 0.002), cancer-related peritonitis (OR 9.664, 95% CI 2.304–40.533, P = 0.002), and postoperative hyperlactatemia (OR 5.337, 95% CI = 1.418–20.094, P = 0.013) independently predicted in-hospital mortality (Table 6).
Table 6.
Univariate and multivariate logistic regression model for in-hospital mortality.
4. Discussion
In our cohort of critically ill surgical patients of mean age of 60 years who had undergone emergency GI surgery, overall in-hospital mortality was 15.2% which concurs with previous studies.[1,3,15,16] Although previous studies have found age and sex are risk factors of mortality among peritonitis patients,[1,17,18] we observed no significant effect.
Elevated blood lactate levels have been used to define the prognostic value of occult hypoperfusion and tissue hypoxemia in critically ill patients. A lactate level of ≥4 mmol/L has been reported to be highly specific (89%–99%) for predicting acute-phase mortality and in-hospital mortality.[5] In recent years, several reports have been issued on the use of lactate level as a prognostic factor for technical surgery, particularly cardiovascular surgery or for patients with sepsis due to colorectal perforation.[19] In the present study, postoperative hyperlactatemia (≥4 mmol/L) was observed in the nonsurvivor group (37.7%) and had a specificity of 81.4%. However, multivariate analysis did not show it as a significant independent risk factor.
Acidosis, base deficit, and bicarbonate levels have been considered important outcome markers in conventionally resuscitated patients,[7] and acidosis at admission has been reported to be associated with higher mortality in the ICU.[7,20] Serum lactate levels are closely related to metabolic acidosis in septic patients, and lactic acidosis also has been found to predict mortality in patients with severe sepsis and septic shock.[21] However, in present study, multivariate analysis did not identify base deficit as an independent risk factor.
Cancer-related peritonitis was developed in 21% of 362 study subjects, and univariate analysis showed it to be related to mortality (P < 0.001). Malignancy is included in MPI as a risk factor,[12] and the presence of malignant disease is known to be associated with mortality in peritonitis.[3] We could not get the parameter to calculate the MPI due to the missing data. In the present study, GI cancer was one of them main causes of bowel perforation, and its mortality rate was higher than other causes of peritonitis.
Hypoalbuminemia is commonly developed in acute disease with several mechanisms.[22] Because the half-life of albumin is about 20 days, it is not a good parameter for identifying or quantifying malnutrition. However, several studies have reported preoperative hypoalbuminemia is a risk factor of postoperative complications or death.[22–24] On the contrary, unlike preoperative albumin, albumin at 24 hours postoperatively seems to inadequately predict mortality.[25] Our findings regarding the relation between hypoalbuminemia and in-hospital mortality concur with previous studies.[22–25]
Preoperative anemia is known to be independently associated with an increased risk of mortality in patients undergoing cardiac and noncardiac surgery,[26] and intraoperative hemorrhage and transfusions are associated with poor prognosis.[27,28] In the present study, preoperative anemia was also found to be an independent risk factor of mortality. It might be associated with the poor patient condition, such as malnutrition, underlying malignancies, or sepsis.
The present study has a number of limitations, which are listed as follows:
It was conducted at a single tertiary university hospital, so many of the study subjects had an underlying malignancy, and their mean age was relatively high at 60 years. And thus, as mentioned above, age was not found to be a risk factor of mortality.
Because of the limited patient numbers and groups, risk factors showed wide confidence indices and our results cannot represent all patients who undergo emergency surgery for secondary peritonitis.
The study was conducted using a retrospective design, and thus, some data were missing, and the timing sampling was not well-controlled. Furthermore, preoperative resuscitation was not performed under the protocol, and emergency department management processes were not adequately recorded and monitored.
Time from arrival to surgery was not controlled, and the study subjects included emergency room admissions and in-patients, which prevented analysis of the relation between time from arrival and surgery in many cases.
The MPIs were not calculated, and duration of the symptoms could not be included in the analysis due to missing records.
5. Conclusions
In critically ill surgical patients who underwent emergency GI surgery, cancer-related peritonitis, preoperative anemia (Hb <10 g/dL), and preoperative hypoalbuminemia (<2.7 mg/dL) had been found by multivariate analysis to be independent risk factors of in-hospital mortality. The recognition of risk factors at an early stage could aid risk stratification and the provision of optimal perioperative care.
Footnotes
Abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II, ASA = American Society of Anesthesiologists, BUN = blood urea nitrogen, GI = gastrointestinal, Hb = hemoglobin, ICU = intensive care unit, MPI = Mannheim Peritonitis Index, SAPS II = Simplified Acute Physiology Score II, SOFA = Sequential Organ Failure Assessment.
Authors’ contributions: JGL and SHL designed the study; JYL and SHL performed data collection; JGL and MJJ contributed to the data interpretation; and JYL and SHL wrote the manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Singh R, Kumar N, Bhattacharya A, et al. Preoperative predictors of mortality in adult patients with perforation peritonitis. Indian J Crit Care Med 2011; 15:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider CP, Seyboth C, Vilsmaier M, et al. Prognostic factors in critically ill patients suffering from secondary peritonitis: a retrospective, observational, survival time analysis. World J Surg 2009; 33:34–43. [DOI] [PubMed] [Google Scholar]
- 3.Wacha H, Hau T, Dittmer R, et al. Risk factors associated with intra-abdominal infections: a prospective multicenter study. Peritonitis Study Group. Langenbecks Arch Surg 1999; 384:24–32. [DOI] [PubMed] [Google Scholar]
- 4.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109. [DOI] [PubMed] [Google Scholar]
- 5.Okorie ON, Dellinger P. Lactate: biomarker and potential therapeutic target. Crit Care Clin 2011; 27:299–326. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004; 32:1637–1642. [DOI] [PubMed] [Google Scholar]
- 7.Smith I, Kumar P, Molloy S, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med 2001; 27:74–83. [DOI] [PubMed] [Google Scholar]
- 8.Pupelis G, Drozdova N, Mukans M, et al. Serum procalcitonin is a sensitive marker for septic shock and mortality in secondary peritonitis. Anaesthesiol Intensive Ther 2014; 46:262–273. [DOI] [PubMed] [Google Scholar]
- 9.Ho KM, Dobb GJ, Knuiman M, et al. A comparison of admission and worst 24-hour Acute Physiology and Chronic Health Evaluation II scores in predicting hospital mortality: a retrospective cohort study. Crit Care 2006; 10:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270:2957–2963. [DOI] [PubMed] [Google Scholar]
- 11.Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: a systematic review. Crit Care 2008; 12:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder MM, Wacha H, Feldmann U, et al. The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis. Chirurg 1987; 58:84–92. [PubMed] [Google Scholar]
- 13.Gunning K, Rowan K. ABC of intensive care: outcome data and scoring systems. BMJ 1999; 319:241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes A, Moreno RP, Metnitz B, et al. Epidemiology and outcome following post-surgical admission to critical care. Intensive Care Med 2011; 37:1466–1472. [DOI] [PubMed] [Google Scholar]
- 16.Azoulay E, Adrie C, De Lassence A, et al. Determinants of postintensive care unit mortality: a prospective multicenter study. Crit Care Med 2003; 31:428–432. [DOI] [PubMed] [Google Scholar]
- 17.Moller MH, Shah K, Bendix J, et al. Risk factors in patients surgically treated for peptic ulcer perforation. Scand J Gastroenterol 2009; 44:145–152.[2 p following 152]. [DOI] [PubMed] [Google Scholar]
- 18.Notash AY, Salimi J, Rahimian H, et al. Evaluation of Mannheim peritonitis index and multiple organ failure score in patients with peritonitis. Indian J Gastroenterol 2005; 24:197–200. [PubMed] [Google Scholar]
- 19.Shimazaki J, Motohashi G, Nishida K, et al. Postoperative arterial blood lactate level as a mortality marker in patients with colorectal perforation. Int J Colorectal Dis 2014; 29:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasubramanyan N, Havens PL, Hoffman GM. Unmeasured anions identified by the Fencl-Stewart method predict mortality better than base excess, anion gap, and lactate in patients in the pediatric intensive care unit. Crit Care Med 1999; 27:1577–1581. [DOI] [PubMed] [Google Scholar]
- 21.Lee SW, Hong YS, Park DW, et al. Lactic acidosis not hyperlactatemia as a predictor of in hospital mortality in septic emergency patients. Emerg Med J 2008; 25:659–665. [DOI] [PubMed] [Google Scholar]
- 22.Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr 2001; 20:265–269. [DOI] [PubMed] [Google Scholar]
- 23.Yap FH, Joynt GM, Buckley TA, et al. Association of serum albumin concentration and mortality risk in critically ill patients. Anaesth Intensive Care 2002; 30:202–207. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg 1999; 134:36–42. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Jang JY, Lee JG. Clinical significance of postoperative prealbumin and albumin levels in critically ill patients who underwent emergency surgery for acute peritonitis. Korean J Crit Care Med 2013; 28:247–254. [Google Scholar]
- 26.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 2011; 378:1396–1407. [DOI] [PubMed] [Google Scholar]
- 27.Bernard AC, Davenport DL, Chang PK, et al. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg 2009; 208:931–937.[937 e931–932; discussion 938–939]. [DOI] [PubMed] [Google Scholar]
- 28.Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg 2010; 252:11–17. [DOI] [PubMed] [Google Scholar]


