Supplemental Digital Content is available in the text
Keywords: hepatocellular carcinoma, percutaneous ethanol injection therapy, prognosis, radiofrequency ablation
Abstract
Although percutaneous ethanol injection therapy (PEIT) is best indicated for patients with small hepatocellular carcinoma (HCC), the survival advantage of PEIT needs confirmation in real-world practice. This study was approved by the institutional review board, and the informed consent was waived. The study included 535 consecutive patients with newly diagnosed early stage (Barcelona Clinic Liver Cancer [BCLC] 0 or A) HCC who underwent initially radiofrequency ablation (RFA) (n = 288) or PEIT (n = 247) from January 2005 to December 2010. The primary outcome was overall survival (OS) and the secondary outcome was time to progression (TTP). The longest diameters of tumors of the groups differed significantly and larger for RFA group than PEIT group (P < 0.001; 1.94 ± 0.65 cm vs 1.60 ± 0.50 cm, respectively). The 5-year OS rates were 72.2% in the RFA group and 67.4% in the PEIT group (P = 0.608). Even after propensity score matching, OS rates between the 2 groups were similar (5-year OS: 72.8% with RFA [n = 175] and 68.0% with PEIT [n = 175]) (P = 0.709). Moreover, in patients with the longest diameter of tumors (≤1.5 cm), multivariate Cox regression analysis showed that the treatment modality was not a significant prognosticator for OS (hazard ratio [HR], 1.690; 95% confidence interval [CI], 0.828–3.449; P = 0.149) and time to progression (HR, 1.160; 95% CI, 0.773–1.740; P = 0.474). PEIT and RFA show equal effectiveness in treating HCCs <1.5 cm in terms of OS and time to progression.
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common etiologies of cancer-related morbidity and mortality.[1] Although resection offers a potential cure, most patients with HCC are not eligible for resection.[2] For some unresectable early stage HCCs, a variety of loco-regional therapies including microwave coagulation therapy,[3] percutaneous acetic acid injection,[4] cryoablation therapy,[5] laser interstitial thermal ablation therapy,[6] radiofrequency ablation (RFA), and percutaneous ethanol injection therapy (PEIT) have been widely applied.[7–9]
Among these therapies, PEIT had been widely applied as a standard treatment for small HCC (necrosis rate of 90%–100% of the HCC ≤2.0 cm and 70% in tumors >2.0 and ≤3.0 cm).[10,11] PEIT usually required multiple treatment session to achieve complete necrosis in tumors >3.0 cm due to the presence of intratumoral septa.[11] Although the rate of initial response is improved, development of viable intratumoral nests or distant recurrence after PEIT is nearly mandatory during follow-up. In this context, PEIT has been substituted by more effective thermal ablation techniques such as RFA in many centers.
RFA was first conducted for the treatment of HCC in 1999.[12] Its advantages over PEIT include ease of performance, effectiveness similar to that of surgical resection, high safety, and low invasiveness.[13] RFA has been reported to provide similar efficacy in tumors <2.0 cm and better local control rate compared with PEIT in tumors >2.0 cm.[11] However, a recent study showed that RFA had better survival benefits over PEIT regardless of tumors >2.0 cm or ≤2.0 cm.[14] Despite a systematic review of randomized trials comparing percutaneous ablation therapies demonstrated that RFA showed significantly enhanced 3-year survival rate over PEIT,[15–18] most trials performed in Europe have shown no significant differences between RFA and PEIT regarding overall survival (OS).[19–23] There are still controversies concerning the survival benefit of RFA over PEIT. Moreover, when compared with PEIT, RFA has significant possible shortcomings such as a lower application rate depending on the tumor location and a higher rate of major adverse events.[15]
This cohort study was aimed to compare the survival outcomes of RFA and PEIT in patients with early stage HCC.
2. Patients and methods
2.1. Patients
The study protocol got approval from the institutional review board of Seoul National University Hospital, and the informed consent was waived due to a retrospective design of this study. In this cohort study, the study population comprised of consecutive 535 patients who were diagnosed with early stage (stage 0 or A) HCC in accordance with the Barcelona Clinic Liver Cancer (BCLC) [11,24] underwent RFA or PEIT, as an initial treatment, between January 2005 and December 2010 at Seoul National University Hospital.
The baseline information collected at the time of HCC diagnosis included demographic profiles, etiology of the chronic liver disease, laboratory findings, longest diameters of the tumors, severity and complications of liver cirrhosis, and treatment modality. All patients were given information about the details of RFA and PEIT. Initial treatment modality was selected according to the physician's advice and the patient's preference. Exclusion criteria of this study were as follows: the longest diameters of tumors >3.0 cm, the presence of extrahepatic metastasis or vascular invasion on imaging studies performed before the procedure, Child-Pugh class C liver cirrhosis, or history of other malignancies except HCC within 5 years.
HCC which was diagnosed by imaging modalities with/without alpha-fetoprotein (AFP) level results or by pathology based on the guidelines of the European Association for the Study of the Liver or the American Association for the Study of Liver Diseases (AASLD).[11,24] The degree of liver dysfunction was estimated based on the Child-Pugh classification. The presence of portal hypertension was assumed when the platelet count was <100,000/mm[3] and accompanying splenomegaly or esophageal/gastric varices were detected.[25] The longest diameters of tumors and tumor responses were measured according to dynamic computed tomography (CT) and/or magnetic resonance (MR) imaging by an experienced liver radiologist independently not knowing the survival information of the study patients.
2.2. RFA procedures
All RFA procedures were performed percutaneously under local anesthesia with moderate sedation (midazolam [Hana Pharm], fentanyl citrate [Hana Pharm, Seoul, South Korea], and ketamine [Huons; Hwaseong, Kyunggi, South Korea]) by 3 physicians (J.M.L., J.Y.L., and S.H.K., who had 11, 7, and 5 years of clinical experience in carrying out percutaneous ablation, respectively). Real-time US having a 3.5-MHz probe (IU22; Philips, Cleveland, OH) was selected as the first-line guidance modality in RFA group.[26] Right after the RFA procedures, contrast-enhanced, multiphase liver CT was checked in RFA group. These images were compared with those acquired before the RFA procedures to assess ablation success. Nodular areas of hypo-attenuation without contrast enhancement were considered to represent necrotic or treated tissue.[27] According to the immediate CT results, response to RFA was classified as complete or incomplete ablation.[26] In cases of incomplete ablation, another session of RFA was performed immediately after CT to accomplish complete ablation on that day. Primary technical success was defined as complete ablation of the target tumor, and secondary technical success was defined as achievement of complete ablation of the target tumor after repeat ablation.[27] Major and minor adverse events were assessed based on the Society of Interventional Radiology guidelines.[28]
2.3. PEIT procedures
PEIT was administered to each patient (1–3 sessions weekly) by 1–2 injections of 99.5% sterile ethanol (1.6–73.9 mL, mean 17.6 ± 16.7 mL) delivered to each lesion with a 21-gauge needle (Et-hanoject, TSK, Tokyo, Japan) having multiple-side-hole by 2 physicians (J.H.Y., and Y.J.K., who had 17 and 13 years of clinical experience in performing PEIT, respectively), depending on the size of the tumor and the distribution of the injected ethyl alcohol within the tumor.[29–31]
2.4. Assessment of treatment response
Follow-up examinations, including contrast-enhanced multiphase liver CT or MR imaging, measurement of serum AFP levels, and liver function tests, were performed in all patients 1 month after treatment. According to the 1-month follow-up CT or MR imaging results, the technical effectiveness of the RFA or PEIT procedures was assessed for each patient based on the standardized terminology of the Interventional Working Group on Image-Guided Tumor Ablation.[32] When persistent enhancing foci of tissue were observed at the site of the original lesion at 1-month follow-up, it was considered treatment failure.[32] If remnant or new HCCs were detected, a multidisciplinary approach, which included repeated loco-regional treatments, hepatic resection, liver transplantation, and TACE, was applied. Complete ablation observed on 1-month follow-up images was regarded as treatment success. In cases of treatment success, contrast-enhanced multiphase liver CT and/or MR imaging and the serum AFP level were followed up every 3 months.
OS was estimated from date of the 1st treatment to date of death or last contact. Time to progression (TTP) was measured from the date of treatment until the first documented tumor progression in imaging studies according to the modified Response Evaluation Criteria in Solid Tumors (modified Response Evaluation Criteria in Solid Tumors) [33] by independent radiologic assessment.
2.5. Statistical analysis
Conventional clinical characteristics at the time of enrollment were analyzed to identify predictors that influenced survival as determined by Kaplan–Meier method and compared using the log-rank test. Stepwise, multivariate analysis was performed with the Cox proportional hazards model to identify independent risk factors that influenced survival. Factors found to be significantly related with clinical outcome by univariate analysis were included in the multivariate analysis. Propensity scores were computed for each patient by using age, sex, and clinical measurements as covariates for sample matching to balance between RFA and PEIT treatment. All statistical analyses were performed by R language ver. 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria) and PASW statistical software version 18.0 (IBM, Chicago, IL). P values of <0.05 were considered significant.
3. Results
A total of 535 patients newly diagnosed with HCC were included in this study; patient characteristics are summarized in Table S1. Two hundred eighty-eight (288/535, 53.8%) patients underwent RFA and 247/535 (46.2%) patients underwent PEIT as the initial treatment. RFA group had significantly larger longest tumor size and had lower platelet counts (P < 0.001 and P = 0.015, respectively) than PEIT group. However, PEIT group had significantly poorer hepatic function (higher model for end-stage liver disease score) (P = 0.013) than RFA group. These 2 groups similar in age, sex, etiologies of chronic liver disease, serum transaminase levels, prothrombin time, serum total bilirubin level, serum albumin level, Child-Pugh scores, AFP levels, prothrombin induced by vitamin K absence-II level, tumor number, and portal hypertension.
3.1. Survival analyses of all study patients
During a median follow-up period of 56.0 months (range, 0.5–93.0 months), 77 (26.7%) patients in the RFA group and 75 (30.4%) patients in the PEIT group died. The 1-, 3-, and 5-year cumulative probabilities of OS rates of the RFA group and the PEIT group were 94.0%, 81.0%, 60.0% and 95.0%, 79.0%, 61.0%, respectively. The survival rates between the RFA and the PEIT groups were similar (P = 0.650). In multivariate Cox regression analysis, the treatment modality was not a significant predictor for long-term survival. Age (hazard ratio [HR], 1.053; 95% confidence interval [CI], 1.035–1.071; P < 0.001) and the longest diameters of tumors (HR, 1.516; 95% CI: 1.092–2.105; P = 0.013) were independent predictors associated with poor survival (Table S2). The 1-, 3-, and 5-year cumulative probabilities of OS rates of small tumor group (the longest diameters of tumors <1.5 cm) and large tumor group (the longest diameter of tumors >1.5 and ≤3.0 cm) were 94.0%, 84.0%, 69.0% and 95.0%, 76.0%, 54.0%, respectively. The survival rates between the small tumor group (the longest diameter of tumors <1.5 cm) and the large tumor group (the longest diameter of tumors >1.5 and ≤3.0 cm) were significantly different (P = 0.014) (Fig. 1A).
Figure 1.

Kaplan–Meier analyses of overall survival and time to progression in all patients. Patients with the longest diameter of tumors ≤1.5 cm group showed (A) a higher overall survival rate (P = 0.014) and (B) longer time to progression (P = 0.024) than patients with the longest diameter of tumors >1.5 and ≤3.0 cm group.
Recurrence was diagnosed in 173 of 288 (53.8%) patients in the RFA group and 184 of 247 (46.2%) in the PEIT group. The 1-, 3-, and 5-year cumulative probabilities of recurrence rates of the RFA group and the PEIT group were 48.0%, 78.0%, 96.0% and 50.0%, 84.0%, 94.0%, respectively. The recurrence rates between the RFA and the PEIT groups were similar (P = 0.283). In multivariate Cox regression analysis, the treatment modality was not a significant risk factor for rapid time to progression. Child-Pugh score (HR, 1.149; 95% CI, 1.032–1.279; P = 0.011) and the longest diameter of tumors (HR, 1.302; 95% CI: 1.054–1.608; P = 0.014) were independent predictors for rapid progression (Table S3). The 1-, 3-, and 5-year cumulative probabilities of recurrence rates of small tumor group (the longest diameter of tumors <1.5 cm) and large tumor group (the longest diameter of tumors >1.5 and ≤3.0 cm) were 43.0%, 78.0%, 94.0% and 54.0%, 85.0%, 95.0%, respectively. The survival rates between the small tumor group (the longest diameter of tumors <1.5 cm) and the large tumor group (the longest diameter of tumors >1.5 and ≤3.0 cm) were significantly different (P = 0.024) (Fig. 1B).
3.2. Survival analyses after propensity score matching
One-to-one propensity score matching was performed to minimize confounding factors in survival analyses. A total of 175 patients from each group were matched, and the demographic and baseline clinical characteristics were well balanced between the 2 groups (Table 1).
Table 1.
Patient characteristics after propensity score matching.
The 1-, 3-, and 5-year cumulative probabilities of OS rates of the RFA group and the PEIT group were 93.0%, 82.0%, 70.0% and 92.0%, 76.0%, 67.0%, respectively. OS distributions between the 2 groups did not differ significantly (P = 0.709) (Fig. 2A). In multivariate Cox regression analysis, the treatment modality was not a significant risk factor for survival. Age (HR, 1.056; 95% CI, 1.028–1.084; P < 0.001) and baseline serum AFP level (HR, 1.516; 95% CI: 1.171–1.963; P = 0.002) were independent predictors associated with poor survival (Table 2).
Figure 2.
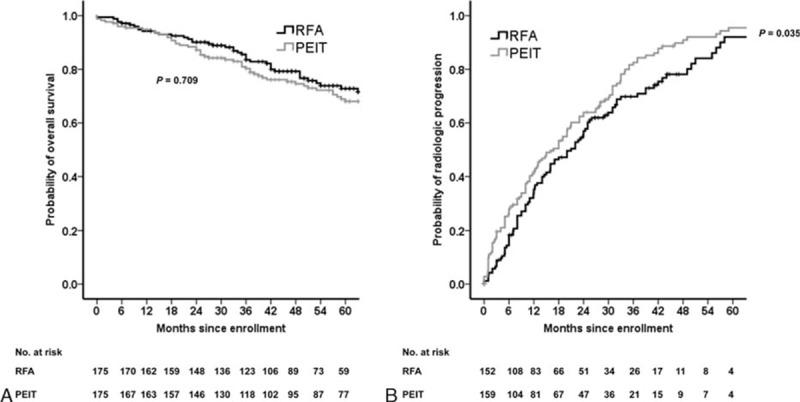
Kaplan–Meier analyses of overall survival and time to progression after propensity score matching. (A) PEIT was not inferior to RFA in overall survival (P = 0.709). (B) RFA had significantly longer time to progression than PEIT (P = 0.035).
Table 2.
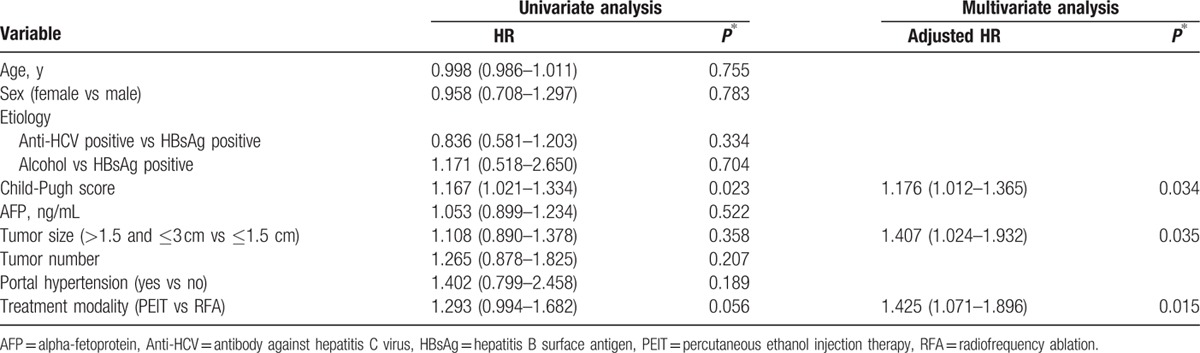
Factors identified on univariate and multivariate analyses that affect overall survival in HCC patients undergoing RFA or PEIT after matching by propensity score.

The 1-, 3-, and 5-year cumulative probabilities of recurrence rates of the RFA group and the PEIT group were 48.0%, 78.0%, 96.0% and 50.0%, 84.0%, 94.0%, respectively. The recurrence rates between the RFA and the PEIT groups were significantly different (P = 0.035) (Fig. 2B). In multivariate Cox regression analysis, the treatment modality was significant predictor for time to progression (HR, 1.425; 95% CI: 1.071–1.896; P = 0.015). Child-Pugh score (HR, 1.176; 95% CI, 1.012–1.365; P = 0.034) and the longest diameter of tumors (HR, 1.407; 95% CI: 1.024–1.932; P = 0.035) were also independent predictive factors associated with rapid progression (Table 3).
Table 3.
Factors identified on univariate and multivariate analyses that affect time to progression in HCC patients undergoing RFA or PEIT after matching by propensity score.
3.3. Survival analyses according to the longest diameter of tumors in propensity-matched cohort
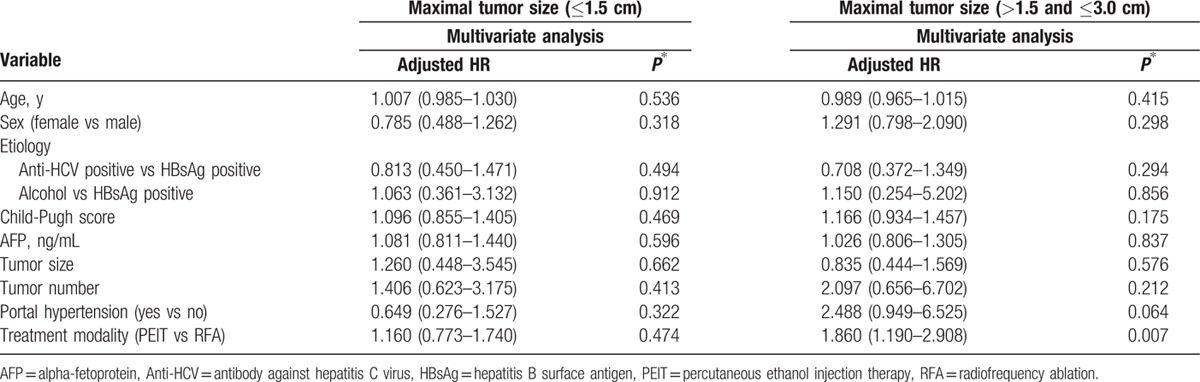
Since the longest diameter of tumors was an independent prognosticator both in all study patients and in propensity-matched cohort, we next performed survival analyses according to the longest diameter of tumors in our propensity-matched cohort. In patients with the longest diameter of tumors (≤1.5 cm), multivariate Cox regression analysis demonstrated that age was an independent predictive factors associated with poor survival (HR, 1.051; 95% CI: 1.010–1.093; P = 0.015) but the treatment modality was not significantly associated with survival (P = 0.149) (Fig. 3A). The treatment modality was not significantly associated with survival in patients with the longest diameter of tumors (>1.5 and ≤3.0 cm) (P = 0.850) (Fig. 3B) and multivariate Cox regression analysis demonstrated that age (HR, 1.054; 95% CI, 1.011–1.099; P = 0.013), sex (HR, 2.255; 95% CI, 1.136–4.478; P = 0.020), and baseline serum AFP level (HR, 1.795; 95% CI: 1.263–2.550; P = 0.001) were independent predictors associated with poor survival (Table 4).
Figure 3.
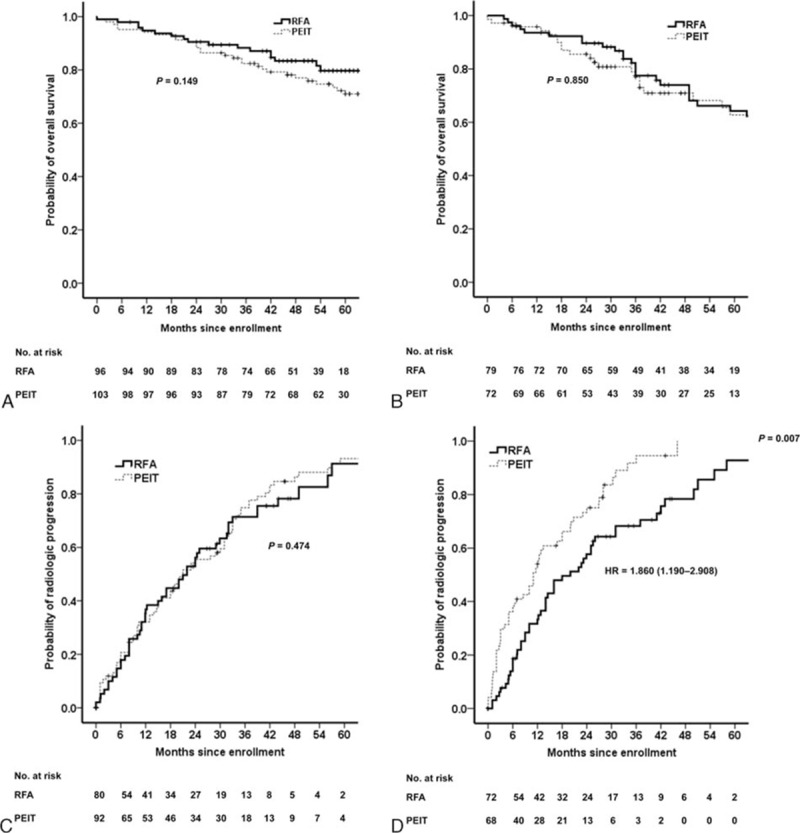
Kaplan–Meier analyses of overall survival and time to progression according to the longest diameter of tumors after propensity score matching. PEIT was similar to RFA in overall survival both in (A) patients with the longest diameter of tumors ≤1.5 cm (P = 0.149) and in (B) patients with the longest diameter of tumors >1.5 and ≤3.0 cm group (P = 0.850). In terms of time to progression, RFA had not significantly longer time to progression than PEIT in (C) patients with the longest diameter of tumors ≤1.5 cm (P = 0.474) but had significantly longer time to progression in (D) patients with the longest diameter of tumors >1.5 and ≤3.0 cm group (P = 0.007).
Table 4.
Factors identified on multivariate analyses according to maximal tumor size that affect overall survival in HCC patients undergoing RFA or PEIT after matching by propensity score.

The treatment modality (PEIT vs RFA) was not a significant predictor for rapid progression in patients with the longest diameter of tumors (≤1.5 cm) (P = 0.474) (Fig. 3C). However, in patients with the longest diameter of tumors (>1.5 and ≤3.0 cm), the treatment modality (PEIT vs RFA) was an independent predictor for rapid progression (HR, 1.860; 95% CI: 1.190–2.908; P = 0.007) (Fig. 3D; Table 5).
Table 5.
Factors identified on multivariate analyses according to maximal tumor size that affect time to progression in HCC patients undergoing RFA or PEIT after matching by propensity score.
3.4. Major complications
There was no procedure-related death in both groups. Major complications were observed in 5 RFA group patients (2.9%) (bile duct injuries [n = 1], colon perforation [n = 1], and stomach wall burn [n = 3]). One RFA group patient experienced cholangitis associated with a treatment-related bile duct injury. The patient fully recovered after a course of broad-spectrum antibiotics. One patient had colon injury adjacent to the ablation zone, as well as abscess formation, both of which were treated with colon resection. After surgery, the patient recovered completely. Three patient experienced stomach wall burn after RFA treatment and fully recovered with conservative management. Major complications were frequently occurred in RFA group than in PEIT group (2.9% vs 0.0%; P = 0.029).
4. Discussion
In this study of patients with early stage HCC (BCLC 0 & A), PEIT showed similar survival benefit compared to RFA both in all study patients and in propensity score matched cohort. Especially, in patients with the longest diameter of tumors (≤1.5 cm), clinical outcomes including OS and time to progression were not significantly different between the PEIT group and the RFA group. Although, RFA was superior to PEIT with respect to time to progression in patients with the longest diameter of tumors (>1.5 and ≤3.0 cm), OS was not significantly different between 2 groups. To our best knowledge, this is first study demonstrating that both PEIT and RFA provide excellent and comparable survival in patients with the longest diameter of tumors <1.5 cm.
Tumor size is an important factor in the selection of local therapy. AASLD guidelines state that RFA is more effective than PEIT for HCC >2.0 cm, but the efficacy of PEIT and RFA may be equal in treating tumors ≤2.0 cm [11]. For patients with tumors >2.0 cm, previous studies have consistently showed that the survival benefit of RFA surpasses that of PEIT.[16–18,34] However, for patients with tumors >2.0 cm, there are still controversies about the survival advantage of RFA.[14,35] In this context, the therapeutic efficacy of PEIT is mainly dependent on tumor size.[36] Strictly speaking, tumor biology is significantly different between tumors 1.5 and 1.6–2.0 cm in diameter.[30,37] Pathologic findings identified in 106 resected HCCs <2.0 cm in diameter have demonstrated local intrahepatic metastases (located ≤1.0 cm from the initial tumor), and microscopic portal vein invasion among the most frequently occurring tumors (the so-called distinctly nodular types).[30] The frequency of portal vein invasion has been reported as significantly higher in HCC 1.6–2.0 cm in diameter (40%) than in HCC 1.1–1.5 cm in diameter (25%, P < 0.01).[30,36] Therefore, we performed survival analysis comparing the efficacy of PEIT and RFA using reference tumor size 1.5 cm in this study. There have been 2 studies considering tumor size of 1.5 cm prognostic factor.[30,38] In one small single arm study (n = 31), the authors argued that PEIT was best indicated for patients with HCC <1.5 cm but they did not analyze the survival benefit of PEIT with RFA.[30] Another small study (n = 23) demonstrated the difference in the incidence of local recurrence of HCC <1.5 cm in patients treated with RFA or PEIT.[38] In that study, the authors argued that PEIT was more effective than RFA in terms of the period between treatment and recurrence but they did not perform survival analysis.[38] Therefore, our study had several superiorities over previous studies, including the large number of patients with HCC (535 patients) and the survival analyses including OS and time to progression both in all study patients and in propensity score matched cohort.
Indeed, the effectiveness of PEIT-induced tumor ablation is less predictable than RFA-induced ablation, especially in large HCCs, and this is due to the inhomogeneous diffusion of ethanol within the nodule because of the presence of fibrous septa and the better effectiveness of thermal ablation in the treatment of extracapsular invasion or satellitosis.[35] However, in our propensity score matched cohort, recurrence after PEIT did not negatively affect survival in patients with the longest diameter of tumors (>1.5 and ≤3.0 cm). This finding may be related to the timely and effective treatment of the locally recurrent tumor. In addition, it should not be overlooked that most patients (91.2%, 488/535) had portal hypertension and that progression of HCC was not the only cause of death in the whole study patients, whereas, death was caused by hepatic failure without HCC progression or by extrahepatic comorbidities.[39] Therefore, PEIT can be regarded as an effective alternative treatment option to RFA in patients with HCCs >1.5 cm in case of severely impaired clotting parameters or with HCC nodule located superficially close to the abdomen wall or in a site that would be dangerous for thermal ablation, such as near the gallbladder, major bile ducts or bowel loops, or decreasing the effectiveness of RFA-induced thermal ablation, such as close to large intrahepatic vessels.
This study has some limitations. Firstly, this is a single-center-based retrospective study. Second, the prolonged enrollment period of the study could be additional sources of bias leading to time-related variability in pretreatment staging and posttreatment effectiveness assessment. Lastly, a rigorous cost-effectiveness analysis concerning the best therapeutic approach for this subgroup of patients was not performed in this study.
In conclusion, PEIT and RFA are equally effective for treating HCCs <1.5 cm in terms of OS and time to progression but cumulative HCC recurrence is significantly higher in patients with the longest diameter of tumors (>1.5 and ≤3.0 cm) who undergo PEIT. Therefore, RFA should be considered the standard treatment, whereas PEIT should be reserved as effective alternative option to patients with the longest diameter of tumors <1.5 cm.
Supplementary Material
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Diseases, AFP = alpha-fetoprotein, BCLC = Barcelona Clinic Liver Cancer, CI = confidence interval, CT = computed tomography, HCC = hepatocellular carcinoma, HR = hazard ratio, MR = magnetic resonance, OS = overall survival, PEIT = percutaneous ethanol injection therapy, RFA = radiofrequency ablation, TTP = time to progression.
This study was funded by the Industrial Strategic Technology Development Program (#10045352) (MSIP & MKE, Korea) and the Liver Research Foundation of Korea. The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Iavarone M, Colombo M. HBV-related HCC, clinical issues and therapy. Dig Liver Dis 2011; 43 suppl 1:S32–S39. [DOI] [PubMed] [Google Scholar]
- 2.Lai EC, Fan ST, Lo CM, et al. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg 1995; 221:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337. [DOI] [PubMed] [Google Scholar]
- 4.Ohnishi K, Yoshioka H, Ito S, et al. Prospective randomized controlled trial comparing percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology 1998; 27:67–72. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Akpinar E, Johann M, et al. Place of cryosurgery in the treatment of malignant liver tumors. Ann Surg 1997; 225:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izzo F. Other thermal ablation techniques: microwave and interstitial laser ablation of liver tumors. Ann Surg Oncol 2003; 10:491–497. [DOI] [PubMed] [Google Scholar]
- 7.Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am 1995; 1:73–81. [PubMed] [Google Scholar]
- 8.Livraghi T. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. Hepatogastroenterology 2001; 48:20–24. [PubMed] [Google Scholar]
- 9.Poon RT, Fan ST, Tsang FH, et al. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg 2002; 235:466–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology 1995; 197:101–108. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology 2010; 78 suppl 1:113–124. [DOI] [PubMed] [Google Scholar]
- 13.Yang B, Zan RY, Wang SY, et al. Radiofrequency ablation versus percutaneous ethanol injection for hepatocellular carcinoma: a meta-analysis of randomized controlled trials. World J Surg Oncol 2015; 13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin ZZ, Shau WY, Hsu C, et al. Radiofrequency ablation is superior to ethanol injection in early-stage hepatocellular carcinoma irrespective of tumor size. PLoS One 2013; 8:e80276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009; 49:453–459. [DOI] [PubMed] [Google Scholar]
- 16.Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology 2004; 127:1714–1723. [DOI] [PubMed] [Google Scholar]
- 17.Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005; 54:1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005; 129:122–130. [DOI] [PubMed] [Google Scholar]
- 19.Giorgio A. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis: a simple, effective and cheap procedure for percutaneous ablation. J Gastrointestin Liver Dis 2010; 19:461–462. [PubMed] [Google Scholar]
- 20.Signoriello S, Annunziata A, Lama N, et al. Survival after locoregional treatments for hepatocellular carcinoma: a cohort study in real-world patients. Sci World J 2012; 2012:564706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol 2008; 43:727–735. [DOI] [PubMed] [Google Scholar]
- 22.Giorgio A, Di Sarno A, De Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res 2011; 31:2291–2295. [PubMed] [Google Scholar]
- 23.Forner A, Bruix J. Ablation for hepatocellular carcinoma: is there need to have a winning technique? J Hepatol 2010; 52:310–312. [DOI] [PubMed] [Google Scholar]
- 24.European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908–943. [DOI] [PubMed] [Google Scholar]
- 25.Lee YB, Lee DH, Cho Y, et al. Comparison of transarterial chemoembolization and hepatic resection for large solitary hepatocellular carcinoma: a propensity score analysis. J Vasc Interv Radiol 2015; 26:651–659. [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014; 270:900–909. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009; 20:S377–390. [DOI] [PubMed] [Google Scholar]
- 28.Gervais DA, Goldberg SN, Brown DB, et al. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 2009; 20:S342–347. [DOI] [PubMed] [Google Scholar]
- 29.Bartolozzi C, Lencioni R. Ethanol injection for the treatment of hepatic tumours. Eur Radiol 1996; 6:682–696. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi M, Kim SR, Imoto S, et al. Long-term outcome of percutaneous ethanol injection therapy for minimum-sized hepatocellular carcinoma. World J Gastroenterol 2008; 14:1997–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang HW, Kim YJ, Kim KM, et al. [Efficacy of percutaneous ethanol injection therapy in Korean with hepatocellular carcinoma]. Korean J Gastroenterol 2003; 42:502–509. [PubMed] [Google Scholar]
- 32.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 2005; 235:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205–216. [DOI] [PubMed] [Google Scholar]
- 34.Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010; 52:380–388. [DOI] [PubMed] [Google Scholar]
- 35.Pompili M, De Matthaeis N, Saviano A, et al. Single hepatocellular carcinoma smaller than 2 cm: are ethanol injection and radiofrequency ablation equally effective? Anticancer Res 2015; 35:325–332. [PubMed] [Google Scholar]
- 36.Vilana R, Bruix J, Bru C, et al. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology 1992; 16:353–357. [DOI] [PubMed] [Google Scholar]
- 37.Kim SR, Imoto S, Nakajima T, et al. Well-differentiated hepatocellular carcinoma smaller than 15 mm in diameter totally eradicated with percutaneous ethanol injection instead of radiofrequency ablation. Hepatol Int 2009; 3:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakui N, Iida K, Ikehara T, et al. Recurrence incidence of small HCC in cirrhosis patients by ablation versus injection. Hepatogastroenterology 2010; 57:195–201. [PubMed] [Google Scholar]
- 39.Yu SJ, Lee JH, Jang ES, et al. Hepatocellular carcinoma: high hepatitis B viral load and mortality in patients treated with transarterial chemoembolization. Radiology 2013; 267:638–647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


