Supplemental Digital Content is available in the text
Keywords: gestational diabetes, metabolic syndrome, type 2 diabetes mellitus
Abstract
Metabolic syndrome (MetS) is an established predisposing condition for type 2 diabetes mellitus (T2DM). However, it is not thoroughly evaluated whether MetS increases the risk of T2DM in women with a previous history of gestational diabetes mellitus (GDM) who already at high risk of T2DM compared with the general population. We investigated the impact of MetS on the development of postpartum diabetes in women with a history of GDM.
This was a multicenter, prospective cohort study of women diagnosed with GDM. The follow-up evaluations, including the oral glucose tolerance test, were completed at 6 weeks postpartum and annually thereafter. MetS was diagnosed at the initial postpartum evaluation according to the revised criteria of the National Cholesterol Education Program-Adult Treatment Panel III. The risk of developing type 2 diabetes (T2DM) in the follow-up period was analyzed based on the presence of MetS, and the adjusted risk was calculated using a Cox proportional hazards model.
A total of 412 women without diabetes at the initial postpartum evaluation participated in the annual follow-up for median 3.8 years. MetS was prevalent in 66 (19.2%) women at the initial postpartum evaluation. The incidences of diabetes in women with and without MetS were 825 and 227 per 10,000 person-years, respectively (P < 0.001). The presence of MetS was an independent risk factor for T2DM, with a hazard ratio (HR) of 2.23 (95% confidence interval 1.04–5.08) in multivariate analysis after adjustment for clinical and metabolic parameters. When we considered MetS and impaired fasting glucose (IFG) separately, women with MetS, IFG, or both had an increased risk of T2DM, with HRs of 4.17, 4.36, and 6.98, respectively.
The presence of MetS during the early postpartum period is an independent risk factor for the development of T2DM in women with a previous history of GDM.
1. Introduction
Metabolic syndrome (MetS) is a well-established predisposing condition for type 2 diabetes (T2DM) in general population. It is a constellation of risk factors for atherosclerotic cardiovascular disease and insulin resistance is regarded as the most important underlying pathophysiology.[1] Numerous studies have demonstrated that people with MetS have increased risk of developing T2DM compared with the general population.[2–4] In the Framingham Offspring Study, MetS increased the risk of T2DM by nearly 7-fold in both men and women.[4] In addition, the risk of T2DM was increased by 5-fold even without impaired fasting glucose (IFG).[4] Gestational diabetes mellitus (GDM), which is defined as carbohydrate intolerance with onset or first recognition during pregnancy,[5] also increases the lifetime risk of type 2 diabetes mellitus (T2DM). Women with a history of GDM have more than a 7-fold increased risk of developing T2DM compared with those without a history of GDM.[6] In Korean women, the risk of developing T2DM is 3.5 times greater in women with a history of GDM than in the general population.[7,8] Because of this high incidence of T2DM, the identification and prediction of high-risk groups for T2DM are crucial for women with a history of GDM.
MetS and GDM share common clinical features. Women with a history of GDM tend to have higher prevalence of obesity, higher blood pressure, hyperglycemia, and dyslipidemia than women without a history of GDM.[9–11] The prevalence of MetS is increased by 3- to 4-fold in women with a history of GDM.[12] Reciprocally, MetS also increases the risk of GDM. It was reported that women with MetS in early gestational period have a 3.17-fold increased risk of developing GDM.[13] However, it is not thoroughly evaluated whether MetS increases the risk of T2DM in women with a previous history of GDM who are already at high risk of T2DM.
Considering this increased risk of T2DM in MetS and GDM and their interconnected nature, it would be of interest to investigate whether, and to what extent, MetS has an additional independent impact on the risk of T2DM in women with a history of GDM. We aimed to investigate the association of MetS with development of T2DM in Korean women with a previous history of GDM. In addition, we compared the effect of MetS and IFG on the development of T2DM in these women.
2. Materials and methods
2.1. Study subjects
This was a multicenter, prospective study of women with a previous history of GDM or gestational impaired glucose tolerance (GIGT). The subjects were recruited from August 1995 to May 1997 at 4 centers in Korea from among women who underwent screening for GDM. The diagnosis and follow-up protocol was described in previous reports.[14] Briefly, all pregnant women who were between 24 and 28 weeks of gestation were administered a 50 g oral glucose challenge test for the initial screening of GDM. Women with 1 h glucose levels ≥7.2 mmol/L were considered to be positive for the initial GDM screening and were administered a 3 h 100 g oral glucose tolerance test (OGTT) at 28 to 32 weeks of gestation. GDM and GIGT were diagnosed based on the criteria of the Third International Workshop-Conference on Gestational Diabetes Mellitus.[5] The criteria for glucose measurements were a fasting plasma glucose ≥5.8 mmol/L, 1 h glucose ≥10.6 mmol/L, 2 h glucose ≥9.2 mmol/L, and 3 h glucose ≥8.1 mmol/L. Two or more positive results were confirmatory for GDM and 1 positive result for GIGT. The first postpartum evaluations, including the 75 g OGTT, were performed at 6 weeks postpartum and follow-up evaluations were completed annually thereafter. The subjects were diagnosed with T2DM when the results of the75 g OGTT showed fasting plasma glucose levels ≥7.0 mmol/L or 2 h plasma glucose levels ≥11.1 mmol/L.[15]
A total of 1050 women with GDM or a single positive result in the 100 g OGTT and who completed the initial postpartum evaluation were eligible for this study. Women who were diagnosed with diabetes mellitus at the initial postpartum visit were excluded (n = 113). Among the remaining 937 subjects, 418 completed further follow-up evaluations more than once. Finally, 412 subjects were included for the analysis after the exclusion of 6 subjects with missing data for either blood pressure or plasma lipid measurements.
All participating women provided written informed consent. This study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB Number: B-1508/310-116) and conducted according to the Declaration of Helsinki.[16]
2.2. Postpartum evaluations
A face-to-face interview and physical examination by trained staff using a standardized protocol were conducted at each postpartum evaluation. Medical and reproductive history and lifestyle factors, including exercise and smoking, socioeconomic status, and educational level, were recorded. Height and weight were measured while the subjects were barefoot and wearing lightweight clothing. Waist circumference was measured at the level of the umbilicus. Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m). Blood pressure was measured in the left arm after 10 min of rest in the supine position with a mercury sphygmomanometer (Baumanometer, Baum, Copiague, NY) and averaged from 3 measurements. Family history of diabetes was considered positive if any first-degree relatives had diabetes. Physical activity was assessed at each postpartum evaluation and was classified as mild, moderate, or vigorous.[17] The subjects with moderate or vigorous physical activity were considered to be physically active.[18]
Laboratory assessment included the 75 g OGTT, serum insulin and C-peptide levels, and a fasting serum lipid profile (total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides). After 8 to 12 h of overnight fasting, all subjects underwent the 2 h 75 g OGTT. Fasting, 30 min, 1 h, 90 min, and 2 h blood samples were obtained.
Plasma glucose levels were measured using the glucose oxidase method (YSI 2300-STAT; YSI Life Sciences, Yellow Springs, OH). Plasma insulin was measured by radioimmunoassay (Linco Research Inc., St. Louis, MO). Total cholesterol and triglyceride levels were determined using an enzymatic assay (Beckman analyzer, Beckman Instruments, Brea, CA). HDL cholesterol levels were measured using the direct Sigma EZ-HDL assay (Sigma Diagnostics, St Louis, MO). LDL cholesterol levels were calculated using the Friedewald equation[19] in subjects with serum triglyceride levels ≤4.5 mmol/L.
To assess insulin sensitivity, the Matsuda index was calculated as 10,000/√(fasting glucose ∗ fasting insulin ∗ mean glucose ∗ mean insulin).[20] The insulinogenic index (IGI) ((insulin at 30 min − insulin at 0 min)/(glucose at 30 min − glucose at 0 min)) was calculated to estimate insulin secretion, and the disposition index (Matsuda index ∗ IGI) was calculated to assess insulin secretion considering the degree of insulin sensitivity.[21]
2.3. Definition of metabolic syndrome
The diagnosis of MetS was made at the first postpartum evaluation according to the revised criteria of the National Cholesterol Education Program (NCEP) definition[22] using the Asian criterion for waist circumference.[23] MetS was diagnosed when 3 or more of the following criteria were met: waist circumference ≥80 cm, systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, triglyceride level ≥1.7 mmol/L, HDL cholesterol level <1.29 mmol/L, and fasting plasma glucose ≥5.6 mmol/L.
2.4. Statistical analysis
Data are expressed as means ± standard deviations for continuous variables or as percentages for categorical variables. The subjects were classified based on the diagnosis of MetS at the initial postpartum evaluation. The differences between the subjects with MetS and those without MetS were tested using Student t test for continuous variables and chi-squared test for categorical variables. The Masuda index, IGI, and disposition index showed skewed distribution, and the statistical tests were performed using log transformed values. A Cox proportional hazards model was used to determine the independent risk factors for the development of T2DM. Subjects were censored at the onset of diabetes or at the last postpartum evaluation. In the first multivariate Cox model, the established risk factors for T2DM (age at delivery, postpartum BMI, family history of diabetes, and physical activity) were included as covariates. In the second Cox model, the covariates of the first model and pregnancy associated parameters (breast feeding, multiparity, and fasting plasma glucose during pregnancy) were included. Lastly, the covariates of the first and second models and metabolic parameters (the Matsuda index and disposition index) were included in the third multivariate Cox model. Hazard ratios (HRs) are presented with their corresponding 95% confidence intervals (CIs) and P values. Results with P values lower than 0.05 were considered significant. The statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM, Armonk, NY).
3. Results
3.1. Baseline characteristics of subjects based on the presence of metabolic syndrome
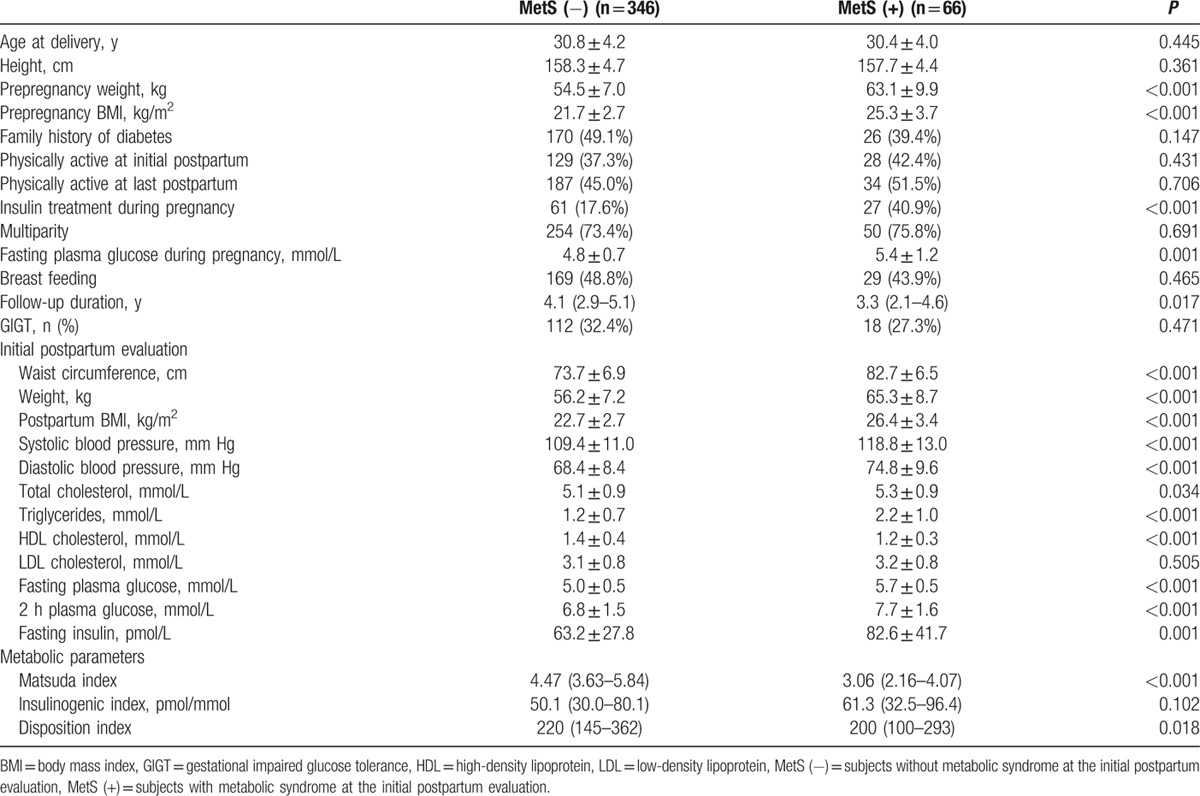
Table 1 summarizes the baseline characteristics of the women who participated in this study. Among the 412 women included in the analysis, 66 (19.1%) women had MetS at the initial postpartum evaluation. The frequency of each component in the diagnostic criteria of MetS was ranged from 8% (blood pressure criteria) to 46% (HDL cholesterol criteria) (Supplemental Table 1). The parameters included in the diagnostic criteria of MetS—waist circumference, blood pressure, triglyceride, HDL cholesterol, and fasting plasma glucose—were significantly higher in women with MetS, as expected. Other potential risk factors for diabetes, including age, family history of diabetes, physical activity, multiparity, and breast feeding, were not significantly different between women with and without MetS. Fasting plasma glucose levels during pregnancy and the prevalence of insulin treatment during pregnancy were significantly higher in women with MetS. Metabolic parameters, the Matsuda index, and the disposition index were significantly lower in women with MetS.
Table 1.
Baseline characteristics of the subjects based on the presence of metabolic syndrome.
3.2. Metabolic syndrome and development of T2DM
Over 1640 person-years of follow-up, 51 women developed T2DM. The overall incidence was 311 per 10,000 person-years. Among 346 women without MetS at the initial postpartum evaluation, 32 (9.2%) women developed T2DM, whereas 19 of 66 (28.8%) women with MetS developed T2DM. The estimated incidence was 227 per 10,000 person-years in women without MetS and 825 per 10,000 person-years in women with MetS (Fig. 1).
Figure 1.
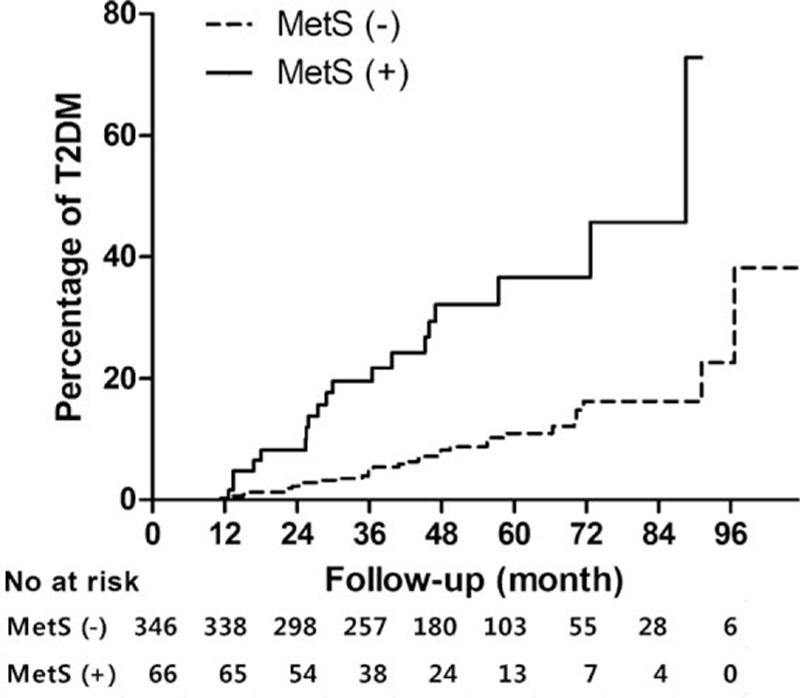
Cumulative incidence of T2DM according to the presence of MetS. The Kaplan–Meier curves show the incidence of T2DM in subjects with or without MetS at the initial postpartum evaluation. The P value of the log-rank test was <0.001. MetS = metabolic syndrome, T2DM = type 2 diabetes mellitus.
In the univariate analysis using the Cox proportional hazards model, the presence of MetS, postpartum BMI, physical activity at the last postpartum evaluation, fasting plasma glucose during pregnancy, and insulin treatment during pregnancy were significantly associated with development of T2DM (see Supplemental Table 2, which shows the results of univariate analysis of risk factors). Among metabolic parameters, the IGI and disposition index were significantly associated with T2DM. The Matsuda index had an HR of 0.56, but did not reach statistical significance level (P = 0.068).
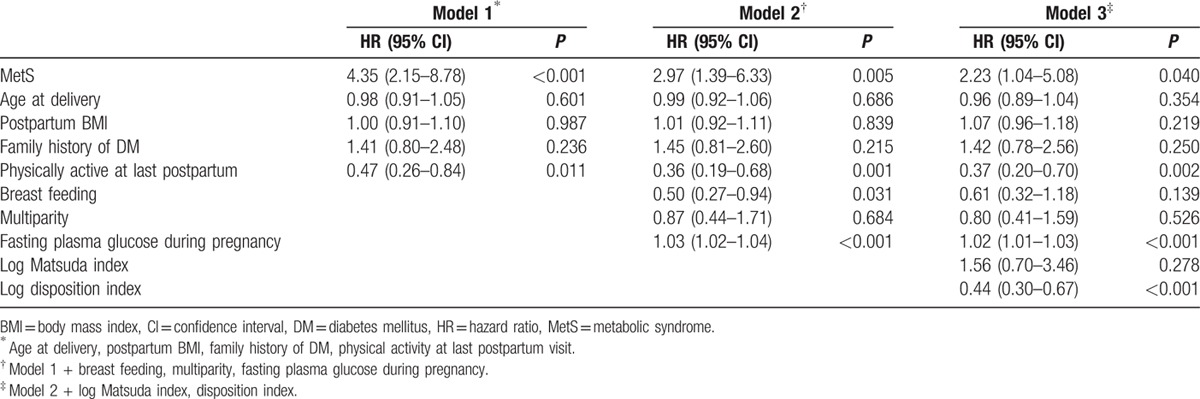
Table 2 summarizes the results of the multivariate analysis to determine the independent risk factors for T2DM. In the first multivariate model, which was adjusted for established risk factors for T2DM, including age, postpartum BMI, family history of diabetes, and physical activity at the last postpartum evaluation, the presence of MetS had an HR of 4.35 (95% CI 2.15–8.78, P < 0.001). Even after adjustment for pregnancy associated factors (multiparity, breast feeding, fasting plasma glucose during pregnancy) (model 2), the presence of MetS had a significant impact on the development of T2DM (HR 2.97, 95% CI 1.39–6.33, P = 0.005). After further adjustment for metabolic parameters, including the Matsuda index and disposition index (model 3), the HR of MetS for the development of T2DM was 2.23 (95% CI 1.04–5.08, P = 0.040).
Table 2.
Multivariate analysis of risk factors for the development of type 2 diabetes mellitus.
To assess whether the number of positive MetS criteria in each subject increases the risk of T2DM, in addition to the presence of MetS, we calculated the HR of the number of positive MetS criteria as a continuous variable. The risk of T2DM was significantly increased by the number of positive MetS criteria (HR 1.40, 95% CI 1.06–1.85, P = 0.018) after adjustment for age, postpartum BMI, family history of diabetes, physical activity at the last postpartum evaluation, multiparity, breastfeeding, and fasting plasma glucose during pregnancy. However, the statistical significance disappeared after adjustment for metabolic parameters (see Supplemental Table 3, which shows the result of multivariate analysis with MetS as a continuous variable).
We further investigated the association between each component of MetS and risk of T2DM. In univariate analysis, blood pressure ≥130/80 mm Hg, triglyceride ≥1.7 mmol/L, and fasting plasma glucose ≥5.6 mmol/L were associated with T2DM. In multivariate analysis, triglyceride and fasting plasma glucose criteria were still significantly associated with T2DM (Supplemental Table 4).
3.3. Independent and additive impact of IFG and MetS
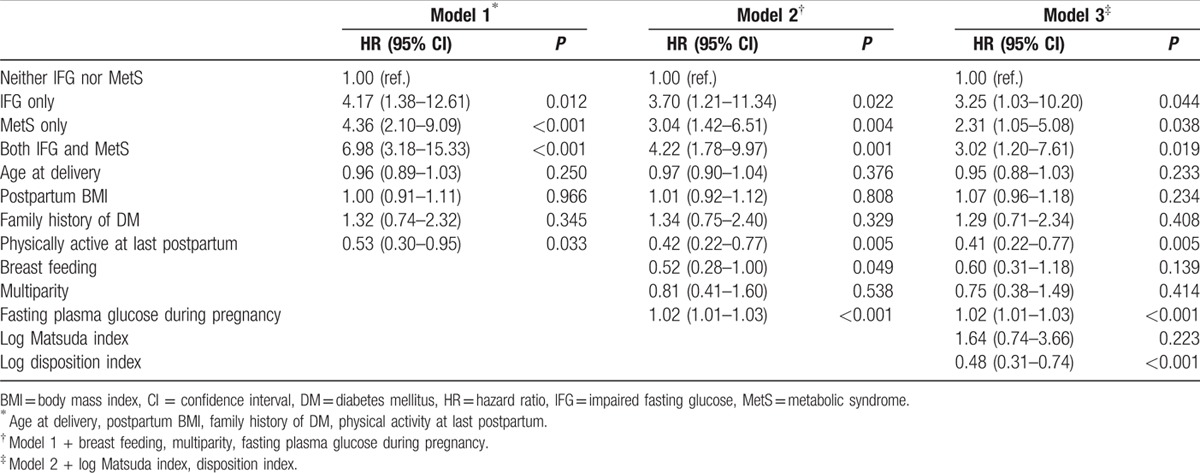
Because IFG itself increases the risk of T2DM,[24] we attempted to compare the effect of IFG and MetS on risk of T2DM. We categorized the subjects into 4 groups: neither IFG nor MetS, MetS only, IFG only, and both IFG and MetS. When we compared the Matsuda index and disposition index in these subgroups, MetS only group showed decreased Matsuda index and preserved disposition index (see Supplemental Fig. 1, which shows the Matsuda index and disposition index in subgroups). In contrast, IFG only group had a value of preserved Matsuda index and decreased disposition index (see Supplemental Fig. 1, which shows the Matsuda index and disposition index in subgroups). The incidence of T2DM was 154 per 10,000 person-years in the neither IFG nor MetS group, 565 per 10,000 person-years in the MetS only group, 725 per 10,000 person-years in the IFG only group, and 987 per 10,000 person-years in the both IFG and MetS groups (Fig. 2). Compared with the neither IFG nor MetS group, the HRs of T2DM adjusted for clinical diabetes risk factors (age, postpartum BMI, family history of diabetes, physical activity) were 4.17 (95% CI 1.38–12.6, P = 0.012) for the MetS only group, 4.36 (2.10–9.09, P < 0.001) for the IFG only group, and 6.98 (95% CI 3.18–15.3, P < 0.001) for the both IFG and MetS groups (Table 3).
Figure 2.
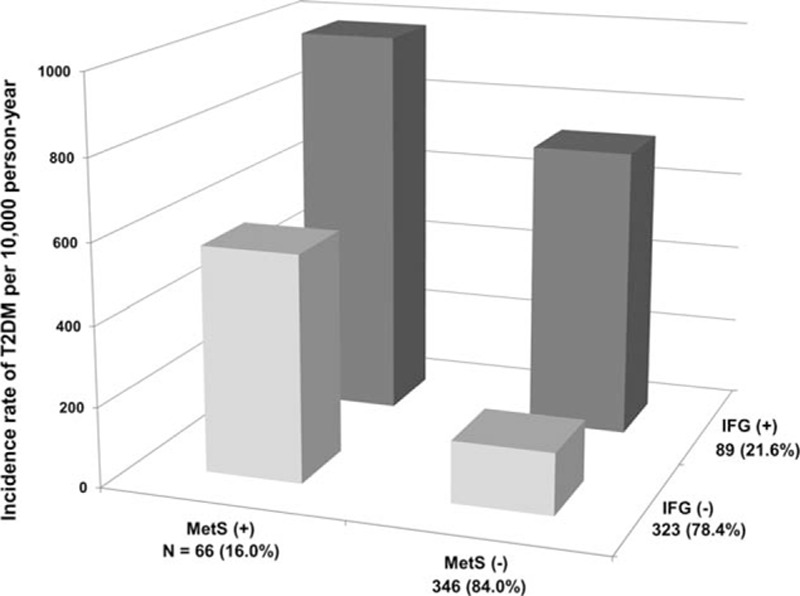
Incidence of T2DM according to the presence of MetS and IFG. The incidence of T2DM in the 4 groups: no IFG and MetS, MetS only, IFG only, and both IFG and MetS. The MetS only, IFG only, and both IFG and MetS groups had significantly higher incidences of T2DM than the no IFG and MetS group (P value of the log-rank test <0.05). IFG = impaired fasting glucose, MetS = metabolic syndrome, T2DM = type 2 diabetes mellitus.
Table 3.
Multivariate analysis of risk factors for the development of type 2 diabetes mellitus according to the presence of impaired fasting glucose and metabolic syndrome.
4. Discussion
In this prospective study, MetS was an independent risk factor for the development of T2DM in Korean women with a previous history of GDM. MetS increased the risk of T2DM by more than 4-fold after adjustment for clinical diabetes risk factors and more than 2-fold even after further adjustment for pregnancy associated factors and metabolic parameters. The number of positive MetS criteria in each subject was also significantly associated with the development of T2DM. Each positive component of MetS resulted in an incremental increase in the risk of T2DM by 40%.
MetS is an established risk factor for T2DM in the general population.[4,25] In the Framingham Offspring Study of 1163 men and 1386 women, MetS, according to the revised NCEP criteria, increased the risk of T2DM by 6.92- and 6.90-fold in men and women, respectively, during 8 years of follow-up.[4] In another prospective cohort study, the HR of MetS for the development of T2DM was 3.30 after multivariate adjustment.[3] In our study, the risk of T2DM was increased by 2.97-fold after adjustment for clinical- and pregnancy-related parameters and was increased by 2.23-fold after additional adjustment for metabolic indices in women who had MetS at the initial postpartum evaluation. The effect size of MetS on T2DM in our study was somewhat small than in the general population. This may reflect that women with a history of GDM already have a high risk of T2DM. However, even in this high-risk population, MetS still had an additional impact on the development of T2DM.
Women with a history of GDM have decreased pancreatic beta cell function.[26] In our previous study, women who developed T2DM in the postpartum period had significantly lower beta cell function than women who retained normal glucose tolerance, and women who developed T2DM in the early postpartum period had even lower beta cell function than those who developed T2DM in the late postpartum period.[27] On the other hand, insulin resistance is considered the hallmark of MetS.[1] The indices of insulin sensitivity and beta cell function, the Matsuda index, and the disposition index, were significantly lower in women with MetS than women without MetS in our study. MetS, along with a history of GDM, may accelerate the development of T2DM by combining insulin resistance and beta cell dysfunction.
The inclusion of IFG, which is a well-known predisposing condition of T2DM, in the definition of MetS might be a confounding factor, especially when diabetes is the endpoint of the analysis. To discern the impact of IFG and MetS, we categorized subjects into 4 groups based on the presence of IFG and MetS. Interestingly, the IFG only group had decreased pancreatic beta cell function and preserved insulin sensitivity, while the MetS only group had decreased insulin sensitivity and preserved pancreatic beta cell function. In spite of different characteristics of these 2 groups, they had similarly increased risk of T2DM after adjustment for clinical diabetes risk factors. The group with both IFG and MetS had decreased insulin sensitivity and pancreatic beta cell function, and exhibited the highest risk of T2DM. This implies the independent and additive effects of MetS and IFG on the development T2DM. Insulin resistance and pancreatic beta cell dysfunction might underlie their differential impacts on the development of T2DM in women with a previous history of GDM.
Both pharmacologic and nonpharmacologic interventions have been shown to prevent or delay the development of T2DM.[28] Lifestyle interventions aimed at reducing weight, decreasing fat intake, and increasing physical activity reduced T2DM incidence by 58%.[29] These lifestyle interventions were shown to reduce insulin resistance and were also recommended for patients with MetS.[22] For women in the postpartum period, breast feeding is another nonpharmacologic intervention for reducing the risk of T2DM.[30] Each additional year of lactation reduced the maternal risk of T2DM by 15%.[31] In our study, maintaining moderate to vigorous physical activity was associated with a significantly lower risk of T2DM, even after adjustment for various diabetes risk factors. Breast feeding was also associated with a lower risk of T2DM, but this association lost statistical significance after adjustment for metabolic indices. The usual age when women get pregnant is lower than the age at which screening for diabetes is recommended by the American Diabetes Association or the Korean Diabetes Association.[32,33] Thus, the postpartum evaluation is an opportunity for earlier detection and risk management for high-risk women. In our study, women who had MetS and a history of GDM exhibited an exceptionally high risk of T2DM. The aforementioned interventions to prevent the development of T2DM should be vigorously applied to these high-risk women.
There are several limitations to our study. First, more than 50% of subjects who underwent the initial postpartum evaluation did not have further follow-up evaluations. However, clinical and laboratory parameters, including the prevalence of MetS, age at delivery, and postpartum BMI, were similar between the groups with or without long-term follow-up (see Supplemental Table 5, which shows similar characteristics between the 2 groups). The subjects who had long-term follow-up generally represented the eligible subjects. Second, the subjects may not have fully recovered from pregnancy state at the initial postpartum evaluation, and this might affect the metabolic parameters of the subjects. The weight at the initial postpartum visit was higher than the prepregnancy weight by 1.6 kg (95% CI 1.3–1.9). The diagnosis of MetS may be different in the prepregnancy period. Our results should be confined to the diagnosis of MetS in the early postpartum period. In addition, we were not able to include normal control group with normoglycemic pregnancy in our analysis. We have previously investigated the risk of T2DM in women with a history of GDM compared with the general population.[8] In present study, we mainly focused on women with a previous history of GDM who are at high risk of future T2DM, and investigated whether MetS additionally increases the risk of T2DM.
In conclusion, our prospective study of women with a previous history of GDM showed that the presence of MetS in the early postpartum period had a significant impact on the development of T2DM. After adjustment for age, BMI, family history of diabetes, physical activity, and pregnancy-related and metabolic parameters, MetS was independently associated with T2DM. Given the additional risk due to MetS, women who have a history of GDM and are diagnosed with MetS in the early postpartum period should be counseled about the benefit of intervention to prevent or delay the development of T2DM.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, GDM = gestational diabetes mellitus, HR = hazard ratio, IFG = impaired fasting glucose, MetS = metabolic syndrome, OGTT = oral glucose tolerance test, T2DM = type 2 diabetes mellitus.
NHC and CHA have equally contributed to this work.
This work was supported by the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare (Grant No. A111362) and the Seoul National University Bundang Hospital Research Fund (02-2015-043).
HCJ and NHC designed the study and recruited the study participants. NHC and CHA analyzed the data and drafted the manuscript. JHM, SHK, SHC, SL, KSP, BEM, and HCJ contributed to the discussion and reviewed and edited the manuscript. All authors approved the final version of the manuscript. HCJ is the guarantor of this work, had full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 2.Laaksonen DE, Lakka HM, Niskanen LK, et al. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol 2002; 156:1070–1077. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo C, Okoloise M, Williams K, et al. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care 2003; 26:3153–3159. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005; 112:3066–3072. [DOI] [PubMed] [Google Scholar]
- 5.Metzger BE. Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes 1991; 40 suppl 2:197–201. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009; 373:1773–1779. [DOI] [PubMed] [Google Scholar]
- 7.Jang HC. Gestational diabetes in Korea: incidence and risk factors of diabetes in women with previous gestational diabetes. Diabetes Metab J 2011; 35:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Jang HC, Park HK, et al. Prevalence of type 2 diabetes among women with a previous history of gestational diabetes mellitus. Diabetes Res Clin Pract 2008; 81:124–129. [DOI] [PubMed] [Google Scholar]
- 9.Meyers-Seifer CH, Vohr BR. Lipid levels in former gestational diabetic mothers. Diabetes Care 1996; 19:1351–1356. [DOI] [PubMed] [Google Scholar]
- 10.Pallardo F, Herranz L, Garcia-Ingelmo T, et al. Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care 1999; 22:1053–1058. [DOI] [PubMed] [Google Scholar]
- 11.Tobias DK, Hu FB, Forman JP, et al. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care 2011; 34:1582–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab 2005; 90:4004–4010. [DOI] [PubMed] [Google Scholar]
- 13.Chatzi L, Plana E, Pappas A, et al. The metabolic syndrome in early pregnancy and risk of gestational diabetes mellitus. Diabetes Metab 2009; 35:490–494. [DOI] [PubMed] [Google Scholar]
- 14.Cho NH, Jang HC, Park HK, et al. Waist circumference is the key risk factor for diabetes in Korean women with history of gestational diabetes. Diabetes Res Clin Pract 2006; 71:177–183. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33 suppl 1:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 17.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 1985; 121:91–106. [DOI] [PubMed] [Google Scholar]
- 18.Jeon CY, Lokken RP, Hu FB, et al. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007; 30:744–752. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22:1462–1470. [DOI] [PubMed] [Google Scholar]
- 21.Retnakaran R, Qi Y, Goran MI, et al. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med 2009; 26:1198–1203. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet 2005; 366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 24.Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glucose to type 2 diabetes. Diabetes Care 2007; 30:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aschner P. Metabolic syndrome as a risk factor for diabetes. Expert Rev Cardiovasc Ther 2010; 8:407–412. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab 2001; 86:989–993. [DOI] [PubMed] [Google Scholar]
- 27.Kwak SH, Choi SH, Jung HS, et al. Clinical and genetic risk factors for type 2 diabetes at early or late post partum after gestational diabetes mellitus. J Clin Endocrinol Metab 2013; 98:E744–E752. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344:1343–1350. [DOI] [PubMed] [Google Scholar]
- 30.Jager S, Jacobs S, Kroger J, et al. Breast-feeding and maternal risk of type 2 diabetes: a prospective study and meta-analysis. Diabetologia 2014; 57:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA 2005; 294:2601–2610. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2010; 33 suppl 1:S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko SH, Kim SR, Kim DJ, et al. Clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J 2011; 35:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


