Abstract
Increasing evidence suggests the association between obesity and bone metabolism. However, whether excessive fat accumulation has a beneficial or adverse effect on bone health remains controversial. Chemerin is a novel adipocyte-derived hormone and a chemoattractant cytokine that regulates adipogenesis. This study was performed to investigate the associations of serum chemerin with bone mineral density (BMD) and serum pro-inflammatory cytokine levels in 543 Chinese obese postmenopausal women. BMD of the femoral neck and lumbar spine, lean mass, and fat mass were measured using dual energy X-ray absorptiometry. Anthropometric assessment and laboratory measurements were performed. The age, time after menopause, and fat mass were negatively correlated with femoral and lumbar BMD, whereas lean mass was positively correlated with aforementioned variables. Furthermore, BMD at the lumbar spine was inversely associated with serum chemerin and TNF-α levels (r = −0.155, P = 0.001; r = −0.147, P = 0.001). Multiple linear regression analyses showed that serum chemerin levels were negatively correlated with BMD at the lumbar site after controlling for the age, lean, and fat mass (β = −0.125, P = 0.001). Chronic low-grade inflammation state in obese population has an inverse effect on bone mass. Chemerin as an adipocytokine and chemoattractant negatively affects the bone mass of Chinese obese postmenopausal women. Further studies are needed to confirm the potential role of chemerin in the crosstalk between bone and fat accumulation in obese population.
Keywords: bone mineral density, chemerin, cytokines, obese, osteoporosis
1. Introduction
Obesity is positively associated with bone mass.[1] Many reports have supported the protective effect of obesity on bone mineral density (BMD) because mechanical loading conferred by body weight promotes bone formation.[2] However, adipose tissue, as the largest endocrine organ in the human body, functions differently in the bone.[3] Adipose tissue-associated hormone and adipose-modulated biochemical factors, such as estrogen[4] and adipokines, may influence the relationship between fat and bone.[5,6]
Obesity is strongly associated with chronic, low-grade, and systemic inflammation state. Inflammatory components have also been shown to significantly affect the bone. Increased circulating and tissue pro-inflammatory cytokines in obesity are involved in bone metabolism through multiple pathways.[1,7] Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) may promote osteoclast activity and bone resorption by modifying the receptor activator of NF-κB (RANK)/RANK ligand/osteoprotegerin pathway.[8,9]
Chemerin, an 18 kDa protein chemoattractant, is secreted by adipocytes mainly derived from fat[10] and other tissues.[11–13] This compound is known as a novel adipocytokine. Chemerin may be at the crossroads of inflammation and obesity because of its dual role in immune system and metabolism.[14] Serum chemerin concentrations are increased in overweight and obese subjects.[15–17] Moreover, chemerin levels are positively correlated with different obesity measures, such as BMI, and well-established markers of inflammation.[10,18,19] In vitro studies have shown that chemerin regulates adipocyte development and metabolic function.[20,21]
Recent studies, however, have shown that chemerin may have a role in regulating bone metabolism. Expression and secretion of chemerin increased dramatically with adipocyte differentiation of preosteoblast and primary bone marrow stromal cells (BMSCs). Chemerin/chemokine-like receptor 1 (CMKLR1) signaling pathway may play an important role in regulating adipogenesis and osteoblastogenesis of bone marrow-derived precursor cells.[22] Peroxisome proliferator-activated receptor gamma (PPARγ) significantly induced chemerin expression and secretion in BMSCs.[23] Chemerin and its receptor CMKLR1 are expressed in hematopoietic stem cell (HSC) and secreted into the extracellular media. In addition, chemerin promotes osteoclastogenesis.[24]
The relationship between chemerin and BMD has not been fully elucidated, although the potential effect of adipocytokine chemerin on bone metabolism has been suggested. We measured serum chemerin concentrations, BMD and pro-inflammatory cytokines in human obese population to investigate the possible role of chemerin in the relationship between obesity and bone metabolism. We recruited postmenopausal women to exclude possible confounding factors.
2. Materials and methods
2.1. Subjects
A total of 543 obese (BMI ≥30 kg/m2) postmenopausal women from health examination centers of the Affiliated Hospital of Jiangsu University and Nanjing First Hospital were recruited into the study from September 2011 to April 2015. The age was 62.88 ± 6.74 years (range, 50–77). All participants had to be in menopause for at least 12 months. Information on the medical history of the study population was obtained using a detailed questionnaire and physical examination. Exclusion criteria were as follows: secondary causes of osteoporosis (rheumatoid arthritis, thyroid and parathyroid disorders, Cushing disease, malabsorption, osteomalacia, steroid-induced osteoporosis, immobilization, malignancy, renal osteodystrophy, liver diseases, other metabolic diseases except obesity, addiction to smoking, or alcohol); and drug addiction or use of medication known to influence bone metabolism, adipocytokines, and pro-inflammatory cytokines levels (calcium, vitamin D, corticosteroids, calcitonin, bisphosphonates, anti-vitamin K agents, selective estrogen-modulating agents, diuretics, β-blockers, heparin, antiepileptic drugs, statins, thiarizonaolidinediones, and regular or frequent use of non-steroidal anti-inflammatory drugs). Written informed consents were obtained from all subjects prior to inclusion into the study. The study was conducted according to the principles outlined in the Declaration of Helsinki and was approved by the Medical Ethics Committee of Jiangsu University (Zhenjiang, China).
2.2. Anthropometric assessment
Body weight and height were recorded in light clothing and no shoes. Measurements were taken to the nearest 0.1 kg and 0.1 cm, respectively. BMI was calculated (body weight/height2). Waist circumference (WC) was measured midway between the costal margins and iliac crest. Hip circumference was defined at the level of the greater trochanters. Body composition (total body lean and fat masses), left hip, and lumbar spine (L1–L4) BMD (kg/m2) were measured by dual energy X-ray absorptiometry machine (Lumar Prodigy Advance, GE. Healthcare, Madison, WI) according to standard protocol. All subjects were tested by the same operator during the study to eliminate operator discrepancies. A total of 35 participants agreed to undergo three scans on the same day to calculate the precision. Coefficients of variation (precision) of the measurements were 0.9% for body composition, 0.8% for left hip BMD, and 1.0% for lumbar spine BMD. These anthropometric results were assessed in a fasting morning and measured in duplicate by the same operator.
2.3. Laboratory measurements
A fasting serum sample was taken on the same day of anthropometric assessment and stored at −80 °C prior to analysis. Analyses were performed at the Nuclear Medicine Research Center. Chemerin was measured using enzyme-linked immune sorbent assay (ELISA) (Uscn Life Science Inc., Wuhan, China) with a detection limit of 0.1 ng/mL. IL-6 was analyzed using an ELISA commercially available kit (R&D Systems, Inc., Minneapolis, MN) with a detection limit of 0.7 pg/mL. Serum TNF-α concentrations were measured using an ELISA commercially kit (R&D Systems, Inc., Minneapolis, MN). The detection limit of the assay was 0.191 pg/mL.
2.4. Statistical analysis
Descriptive data were presented as mean values with standard deviation (SD) or median with interquartile range. Normal distribution of data was evaluated by Kolmogorov–Smirnov test. Logarithmic transformations were performed for statistical analysis because the distribution of chemerin was skewed.
Pearson correlation analysis was used to evaluate the relationships between chemerin values and the anthropometric and pro-inflammatory cytokines. Partial correlation analysis was performed to assess the association with adjustment for age, lean mass, and fat mass. Standard multiple linear regression models were used to assess the association between circulation chemerin levels (independent variables) and BMDs at different sites (dependent variables). The models were adjusted for age, lean mass, and fat mass. P values <0.05 was considered statistically significant. Statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL).
Using the NCSS/PASS 2000 program, a sample size power calculation indicated that 543 participants were sufficient to perform the study with a power of 99% and an alpha error of 5%.
3. Results
3.1. Descriptive statistics
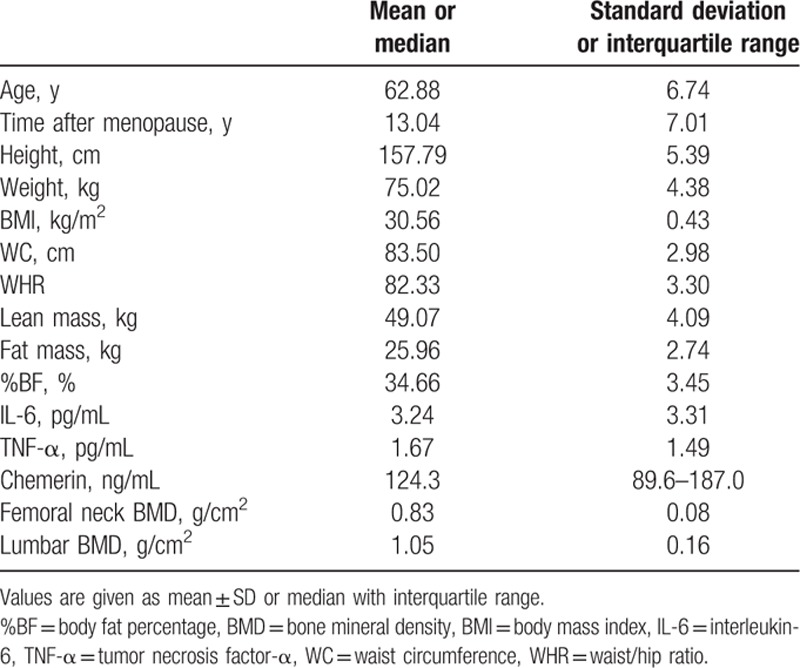
The general characteristics, anthropometric assessment, chemerin, and pro-inflammatory cytokine results are shown in Table 1. Information from 543 Chinese obese postmenopausal women, with ages ranging from 50 to 77 years, is provided in Table 1. The time after menopause (mean ± SD) of the women was 13.04 ± 7.01 years. According to the World Health Organization criteria, 97 women (17.9%) were considered osteoporotic.
Table 1.
General characteristics of obese Chinese postmenopausal population (543 subjects).
3.2. Association of chemerin with age and obese anthropometric parameter
Table 2 shows unadjusted and adjusted correlations between chemerin and obese anthropometric parameters. Serum chemerin levels showed positive correlations with age, fat mass, %BF, and WC. Correlations between serum chemerin levels and fat mass (r = 0.107, P = 0.012) and %BF (r = 0.130, P = 0.002) persisted after adjustment for the age. Correlation between serum chemerin levels and WC (r = 0.130, P = 0.002) was even after adjustment for age and fat mass.
Table 2.
The correlation between chemerin and bone-related parameters in obese Chinese postmenopausal women.

3.3. Association of chemerin and pro-inflammatory cytokines
Chemerin showed a positive correlation with TNF-α after adjustment for age and fat mass (r = 0.099, P = 0.022, Table 2). However, no significant correlation was found between chemerin and IL-6 in postmenopausal women.
3.4. Association of chemerin and BMD
Bivariate correlation analysis showed negative correlations between chemerin and BMD at the lumbar spine (L1–L4) (r = −0.155, P = 0.001, Table 2). The correlation remained significant (r = −0.131, P = 0.002 and r = −0.117, P = 0.006) even after adjustment for age and fat mass. No significant correlation was found between chemerin and BMD at the femur neck in postmenopausal women.
Table 3 shows the results of multiple linear regression analyses for the correlation between circulating chemerin levels and BMD at both sites after controlling for age, lean mass, and fat mass.
Table 3.
Multiple linear regression analysis for the association between chemerin (independent variables) and bone mineral density (dependent variables) in obese postmenopausal women.

In multiple regression analyses, serum chemerin levels showed significant negative correlation with BMD at the lumbar site after controlling for the age, lean mass, and fat mass (β = −0.125, P = 0.001).
3.5. Association of body composition, pro-inflammatory cytokines, and BMD
BMD measurements of the femur neck and lumbar spine were inversely correlated with age, time after menopause, and fat mass but positively correlated with lean mass (Table 3). The relationship of TNF-α to BMD was inverse in the lumbar spine (r = −0.147, P = 0.001), whereas no significant correlation was found in the femur neck. However, no significant correlations were found between IL-6 and BMD at both sites in postmenopausal women (Table 4).
Table 4.
Correlation between bone-related parameters and BMD in obese Chinese postmenopausal women.

4. Discussion
Chemerin, which is released by adipose tissue, is strongly correlated with obesity.[10] The relationship between serum concentrations of chemerin and BMD has not been extensively investigated despite the close association of obesity and BMD.[1] In the current study, we found that serum chemerin level was positively associated with TNF-α and negatively associated with BMD of the lumbar spine. Moreover, chemerin served as an independent negative predictor of BMD. These findings support the hypothesis that chemerin may play a significant role in regulating bone mass in obesity.
Chemerin has shown a strong association with BMD. Several characteristics of chemerin provide plausible pathways for modulation of bone metabolism. Chemerin is widely expressed by various cell types such asadipocytes, osteoblasts, HSC, mesenchymal stem cells (MSCs), and macrophages.[21–24] The multipotent role of MSCs has been proposed as a key mediator in the pathogenesis of osteoporosis, and the reduction in bone mass is partly believed to result from common precursor cells that become fat instead of bone.[25] Chemerin is a negative regulator during bone formation.[3] Chemerin stimulates adipocyte differentiation of BMSCs by activation of CMKLR1. Knockdown of chemerin gene increased osteoblast marker gene expression and mineralization after osteoblastogenic stimulation.[22] In vivo, rosiglitazone elevated chemerin mRNA levels in adipose tissue and bone marrow along with an increase in circulating chemerin levels in mice.[23] Chemerin regulated osteoclast differentiation of HSCs by modulating intracellular calcium and NFAT2 expression/activation.[24] These results confirm that chemerin may negatively affect bone metabolism by inhibiting bone formation in the bone marrow.
Some studies have shown that PPARγ agonist rosiglitazone increased marrow fat, deteriorated trabecular architecture, and diminished bone density.[26,27] PPARγ induced chemerin expression and partially rescued the loss of adipogenesis caused by knockdown of chemerin.[22] Overexpression of PPARγ in obese peripheral blood mononuclear cells (PBMCs) of an obese individual may have a critical role in the relationship between obesity and bone loss.[28] Thus, PPARγ agonist-induced bone mass loss maybe partially and indirectly caused by increased chemerin level which would stimulate more fat than osteoblasts in bone marrow and decrease bone formation.
Chemerin, which is newly identified as an adipokine in human adipose tissue, modulates adipogenesis and adipocyte metabolism by its paracrine or endocrine functions.[20,21] Chemerin and chemerinR mRNA expression were dramatically upregulated during the differentiation from human preadipocytes into adipocytes.[20] Chemerin levels were positively correlated with measures of obesity and metabolic syndrome.[10,17,29] Chemerin also served as a significant predictor of metabolically healthy obesity.[30] Obesity is associated with low-grade inflammation.[31] Chemerin, as a chemo-attractant protein, also has a complex role in inflammation.[32] Increased levels of chemerin in obesity were also related to inflammation.[19] We showed that chemerin was significantly positively correlated with TNF-α in obese population, which is consistent with the result from a previous study.[33] PBMC is proposed as the representative of the inflammatory status in obesity, and PBMC gene expression levels of chemerin are strongly upregulated in obesity.[34] These phenomena indicated that chemerin may contribute to the low-grade chronic inflammation that characterizes obesity. Chemerin activated the intracellular signaling cascades MAPKs and Akt, followed by an enhanced secretion of pro-inflammatory cytokines IL-6 and TNF-α, incultured human articular chondrocytes.[35] TNF-α treatment of 3T3-L1 adipocytes increases bioactive chemerin levels and may enhance prochemerin synthesis and secretion from adipocytes.[36] Anti-TNF therapy reduces serum levels of chemerin in rheumatoid arthritis, which is associated with the decrease in serum levels of IL-6 and macrophage migration inhibitory factor.[37] Dual role of chemerin in metabolism and inflammation may provide a link between chronic obesity and inflammation.[14]
Chronic low-grade systemic inflammatory state has been associated with osseous metabolism.[38] Chronic inflammation and increased pro-inflammatory cytokines TNF-α and IL-6 induce bone resorption and bone loss in a number of diseases, such as periodontitis,[39] rheumatoid arthritis,[40] and inflammatory bowel disease.[41] These pro-inflammatory cytokines in obesity stimulates osteoclastactivity and bone resorption through RANKL/RANK/OPG pathways.[7–9] In this study, serum levels of chemerin, as well as TNF-α, were negatively associated with BMD measurements of the lumbar spine in obese population, which is in line with the study by He et al,[42] who also found negative effect of chemerin on BMD in osteoporotic patients. Moreover, our results showed that chemerin levels were independently associated with BMD at the lumbar spine site. Thus, results show a relationship of obesity–chemerin–pro-inflammatory cytokines–osteoporosis, that is, fat-derived chemerin and pro-inflammatory cytokines crosstalked with each other. This process exerts a complex effect on bone loss in obese postmenopausal women.
We found a negative association between fat mass and BMD at the lumbar spine in obese postmenopausal women. Positive association between body weight and BMD was previously demonstrated in many populations.[43] However, the specific role of lean and fat masses, which constitute the body weight, in BMD remains controversial.[44,45] Some studies showed that fat mass maybe a negative independent predictor of BMD,[46] and lean mass has a strong positive influence on BMD regardless of age or sex.[47] Meta-analysis revealed that exercise exerted a significant positive effect on BMD with increased lean body mass in postmenopausal women[48] and in men.[49] At the same time, exercise induced fat mass loss with reduced serum chemerin and improved systemic inflammation in obese men.[50] These studies may indicate that excessive body fat accumulation accompanied by elevated chemerin and related chronic inflammation state may in turn had a negative effect on BMD. Large-scale studies, both in vivo and in vitro, are warranted to evaluate the relation among chemerin, obesity, and osteoporosis.
Some limitations of our study should be noted. First, the cross-sectional nature of this study limited the interpretation of our results, and no inferences of causality could be formulated. The hypothesis we made to attempt to explain the relationship between chemerin and BMD needs further and more detailed investigations. Second, the study included only Chinese obese postmenopausal women. Thus, the results may not be generalized to other populations, such as women in other ethnic groups and ages or men and adolescents. Third, we were unable to directly measure sex hormone, namely, estrogens and androstendione, which changes with age and duration of postmenopausal and affects fat redistribution and bone mass. Fourth, bone density scan (DXA) measurements are influenced by body position, body size, and bone size.[51,52] Bias from the difference of areal BMD (by DXA) from the real BMD will affect the relationship between chemerin and bone mass. Fifth, given that chemerin is involved in bone, fat, and inflammation, we did not measure other adipocytokines, inflammatory markers besides TNF-α and IL-6, and bone metabolic markers. Lastly, the data of the lifestyle of participants, such as exercise, which may affect both circulation chemerin levels and BMD were not evaluated.
The relationship between obesity and BMD in obese population is clinically important because excessive body fat might induce local and systemic low-grade inflammation, causing a risk factor for bone loss. Any therapeutic interventions for obesity that modify body fat metabolism may affect BMD. We speculated that chemerin, known as an adipokine and chemoattractant, might provide a link among fat, inflammation, and bone. Few studies have attempted to investigate the effects of fat and chemerin on BMD in obese population. Therefore, in this study we investigated the relationship between chemerin, BMD, and pro-inflammatory cytokines in obese population, aiming to determine whether adipokine chemerin and systemic low-grade inflammation are associated with BMD in obese postmenopausal women.
In conclusion, this is the first report that identified unique associations between BMD, fat mass, inflammation, and chemerin. Chemerin is a novel adipocyte-derived hormone previously implicated in obesity. This compound is also a chemokine and plays a role in systemic immune diseases. Our findings show that serum chemerin levels were positively associated with pro-inflammatory cytokines and negatively associated with BMD of the lumbar spine in Chinese obese postmenopausal women. Chemerin apparently plays a negative role in the relationship between fat mass and BMD. Further prospective investigations in different population groups are necessary to elucidate these associations. Chemerin is a promising biomarker and target for the prevention and treatment of osteoporosis.
Footnotes
Abbreviations: %BF = body fat percentage, BMD = bone mineral density, BMI = body mass index, IL-6 = interleukin-6, PPARγ = peroxisome proliferator-activated receptor γ, TNF-α = tumor necrosis factor-α, WC = waist circumference, WHR = waist/hip ratio.
Liang Shi and Chaoming Mao contributed equally to the article.
The present study was supported by grants from the National Natural Science Foundation of China (No. NSFC81370889), Jiangsu Provincial Key Research and Development Program (No. BE2016721), and Zhenjiang Health and Science Programs (No. SH2013075). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have declared that no competing interests exist.
The authors report no conflicts of interest.
References
- 1.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res 2011; 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crepaldi G, Romanato G, Tonin P, et al. Osteoporosis and body composition. J Endocrinol Invest 2007; 30 suppl 6:42–47. [PubMed] [Google Scholar]
- 3.Liu Y, Song CY, Wu SS, et al. Novel adipokines and bone metabolism. Int J Endocrinol 2013; 2013:895045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 2006; 116:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenchik L, Register TC, Hsu FC, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 2003; 33:646–651. [DOI] [PubMed] [Google Scholar]
- 6.Barbour KE, Zmuda JM, Boudreau R, et al. The effects of adiponectin and leptin on changes in bone mineral density. Osteoporos Int 2012; 23:1699–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mundy GR. Osteoporosis and inflammation. Nutr Rev 2007; 65 (Pt 2):S147–S151. [DOI] [PubMed] [Google Scholar]
- 8.Pfeilschifter J, Koditz R, Pfohl M, et al. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 2002; 23:90–119. [DOI] [PubMed] [Google Scholar]
- 9.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001; 142:5050–5055. [DOI] [PubMed] [Google Scholar]
- 10.Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007; 148:4687–4694. [DOI] [PubMed] [Google Scholar]
- 11.Nagpal S, Patel S, Jacobe H, et al. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J Invest Dermatol 1997; 109:91–95. [DOI] [PubMed] [Google Scholar]
- 12.Wittamer V, Franssen JD, Vulcano M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 2003; 198:977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol 2005; 174:244–251. [DOI] [PubMed] [Google Scholar]
- 14.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab 2010; 21:660–667. [DOI] [PubMed] [Google Scholar]
- 15.Weigert J, Neumeier M, Wanninger J, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf) 2010; 72:342–348. [DOI] [PubMed] [Google Scholar]
- 16.El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med 2011; 28:1194–1200. [DOI] [PubMed] [Google Scholar]
- 17.Neuparth MJ, Proenca JB, Santos-Silva A, et al. Adipokines, oxidized low-density lipoprotein, and C-reactive protein levels in lean, overweight, and obese portuguese patients with type 2 diabetes. ISRN Obes 2013; 2013:142097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozaoglu K, Segal D, Shields KA, et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab 2009; 94:3085–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: tumor necrosis factor-alpha stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg Obes Relat Dis 2013; 9:306–314. [DOI] [PubMed] [Google Scholar]
- 20.Roh SG, Song SH, Choi KC, et al. Chemerin—a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun 2007; 362:1013–1018. [DOI] [PubMed] [Google Scholar]
- 21.Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 2007; 282:28175–28188. [DOI] [PubMed] [Google Scholar]
- 22.Muruganandan S, Roman AA, Sinal CJ. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J Bone Miner Res 2010; 25:222–234. [DOI] [PubMed] [Google Scholar]
- 23.Muruganandan S, Parlee SD, Rourke JL, et al. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J Biol Chem 2011; 286:23982–23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muruganandan S, Dranse HJ, Rourke JL, et al. Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis. Stem Cells 2013; 31:2172–2182. [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014; 156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazeli PK, Horowitz MC, MacDougald OA, et al. Marrow fat and bone—new perspectives. J Clin Endocrinol Metab 2013; 98:935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rzonca SO, Suva LJ, Gaddy D, et al. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 2004; 145:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirzaei K, Hossein-Nezhad A, Eshaghi SM, et al. The relationship between obesity and bone mineral density: evidence for a role of peroxisome proliferator-activated receptor gamma. Minerva Endocrinol 2015; 40:177–185. [PubMed] [Google Scholar]
- 29.Flehmig G, Scholz M, Kloting N, et al. Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PLoS One 2014; 9:e99785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010; 299:E506–E515. [DOI] [PubMed] [Google Scholar]
- 31.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 2003; 112:1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fatima SS, Rehman R, Baig M, et al. New roles of the multidimensional adipokine: chemerin. Peptides 2014; 62:15–20. [DOI] [PubMed] [Google Scholar]
- 33.Lehrke M, Becker A, Greif M, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol 2009; 161:339–344. [DOI] [PubMed] [Google Scholar]
- 34.Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Peripheral mononuclear blood cells contribute to the obesity-associated inflammatory state independently of glycemic status: involvement of the novel proinflammatory adipokines chemerin, chitinase-3-like protein 1, lipocalin-2 and osteopontin. Genes Nutr 2015; 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg V, Sveinbjornsson B, Bendiksen S, et al. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin (21–157). Arthritis Res Ther 2010; 12:R228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parlee SD, Ernst MC, Muruganandan S, et al. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-{alpha}. Endocrinology 2010; 151:2590–2602. [DOI] [PubMed] [Google Scholar]
- 37.Herenius MM, Oliveira AS, Wijbrandts CA, et al. Anti-TNF therapy reduces serum levels of chemerin in rheumatoid arthritis: a new mechanism by which anti-TNF might reduce inflammation. PLoS One 2013; 8:e57802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietschmann P, Mechtcheriakova D, Meshcheryakova A, et al. Immunology of osteoporosis: a mini-review. Gerontology 2016; 62:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res 2003; 82:82–90. [DOI] [PubMed] [Google Scholar]
- 40.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone 2002; 30:340–346. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein CN, Leslie WD, Taback SP. Bone density in a population-based cohort of premenopausal adult women with early onset inflammatory bowel disease. Am J Gastroenterol 2003; 98:1094–1100. [DOI] [PubMed] [Google Scholar]
- 42.He J, Li JC, Xie H, et al. Serum chemerin levels in relation to osteoporosis and bone mineral density: a case-control study. Dis Markers 2015; 2015:786708.Epub 2015 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoffl I, Kemmler W, Kladny B, et al. In healthy elderly postmenopausal women variations in BMD and BMC at various skeletal sites are associated with differences in weight and lean body mass rather than by variations in habitual physical activity, strength or VO2max. J Musculoskelet Neuronal Interact 2008; 8:363–374. [PubMed] [Google Scholar]
- 44.Sheng Z, Xu K, Ou Y, et al. Relationship of body composition with prevalence of osteoporosis in central south Chinese postmenopausal women. Clin Endocrinol (Oxf) 2011; 74:319–324. [DOI] [PubMed] [Google Scholar]
- 45.Chang KH, Tseng SH, Lin YC, et al. The relationship between body composition and femoral neck osteoporosis or osteopenia in adults with previous poliomyelitis. Disabil Health J 2015; 8:284–289. [DOI] [PubMed] [Google Scholar]
- 46.Rhie YJ, Lee KH, Chung SC, et al. Effects of body composition, leptin, and adiponectin on bone mineral density in prepubertal girls. J Korean Med Sci 2010; 25:1187–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang MC, Bachrach LK, Van Loan M, et al. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone 2005; 37:474–481. [DOI] [PubMed] [Google Scholar]
- 48.Kelley GA, Kelley KS, Kohrt WM. Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 2012; 13:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in men: a meta-analysis of randomized controlled trials. Bone 2013; 53:103–111. [DOI] [PubMed] [Google Scholar]
- 50.Khoo J, Dhamodaran S, Chen DD, et al. Exercise-induced weight loss is more effective than dieting for improving adipokine profile, insulin resistance and inflammation in obese men. Int J Sport Nutr Exerc Metab 2015; 25:566–575. [DOI] [PubMed] [Google Scholar]
- 51.Patel R, Blake GM, Rymer J, et al. Long-term precision of DXA scanning assessed over seven years in forty postmenopausal women. Osteoporos Int 2000; 11:68–75. [DOI] [PubMed] [Google Scholar]
- 52.Havill LM, Mahaney MC, L Binkley T, Specker BL. Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res 2007; 22:737–746. [DOI] [PubMed] [Google Scholar]


