Abstract
The aim of this study was to determine the features of triple-negative breast cancer (TNBC) using a large national database. TNBC is known to be an aggressive subtype, but national epidemiologic data are sparse. All patients with invasive breast cancer and known molecular subtype diagnosed in 2010 to 2011 were identified from the National Cancer Data Base (NCDB). Patients with and without TNBC were compared with respect to their sociodemographic and clinicopathologic features. TNBC was present in 38,628 of 295,801 (13%) female patients compared to 185 of 3136 (6%) male patients (P < 0.001). The incidence of TNBC varied by region from 10.8% in New England to 15.8% in the east south central US (P < 0.001), as well as by race with the highest rates in African-Americans (23.7%), and lowest in Filipino patients (8.9%). The incidence of TNBC also varied by histology, accounting for 76% of metaplastic cancers, but only 2% of infiltrating lobular carcinomas. TNBCs were significantly larger than non-TNBC (mean 2.8 cm vs 2.1 cm, P < 0.001), and more TNBC were poorly differentiated compared to other subtypes (79.7% vs 25.8%, P < 0.001). On univariate analysis, TNBC was no more likely than non-TNBC to have node-positive disease (32.0% vs 31.7%, respectively, P = 0.218) but in a multivariable analysis controlling for tumor size and grade, TNBC was associated with significantly less node-positivity (OR = 0.59; 95% confidence interval [CI]: 0.57–0.60). TNBC has distinct features regarding age, gender, geographic, and racial distribution. Compared to non-TNBC, TNBC is larger and higher grade, but less likely to have lymph node metastases.
Keywords: Breast cancer molecular type, national cancer database, triple-negative breast cancer
1. Introduction
Triple-negative breast cancer (TNBC) represents a subgroup of breast tumors defined by lack of expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (Her2). It tends to be biologically aggressive, and with a lack of commonly utilized targeting agents, it is often associated with a worse prognosis.[1] Despite unique identifying features, there is significant heterogeneity among TNBC patient populations and the results of small studies are sometimes contradictory.[2,3] Although many articles have been published describing its biology, behavior, and treatment, large scale epidemiologic studies based on national data are sparse.
The aim of this study was to determine the features of TNBC using a large national dataset. The National Cancer Database (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society and contains data on about 70% of the cancer cases in the United States. Her2 status has been collected since 2010, allowing determination of molecular type. In this study, 38,813 cases of TNBC diagnosed in 2010 and 2011 were compared to non-TNBC diagnosed in the same years with regard to sex, race/ethnicity, age, geographic distribution, histologic type, stage, pathologic characteristics, and treatment patterns.
2. Methods
The NCDB, established in 1989, is a nationwide, facility-based, comprehensive data set that captures about 70% of all newly diagnosed malignancies in the United States annually. After approval by the NCDB, patient deidentified data were downloaded from the website in January 2014 and analyzed in this study. Given the deidentified and retrospective nature of the data obtained from the NCDB, this study was deemed exempt by the Human Investigations Committee of Yale University.
2.1. Receptor data and study population
The instructions to the tumor registrars in the Collaborative Stage Coding Manual regarding estrogen and progesterone assays were to “record the pathologist's interpretation of the assay value.” However, it was noted that the College of American Pathologists had issued guidelines in late 2009 that if 1% or more of tumor cells stained positive, the ER/PR value was considered positive.[4]
With regard to Her2 assays, the NCDB contains data on immunohistochemistry, fluorescence in situ hybridization, and chromogenic in situ hybridization, but the field we used for this study was one where the individual local tumor registrars determined the best assay result for each individual patient. The registrars were instructed to use gene amplification assays first, and then use immunohistochemistry assay for cases where the amplification assay was borderline or not performed.
The population used for this study consisted of all patients with invasive breast cancer diagnosed in 2010 and 2011 whose ER, PR, and Her2 were known to be positive or negative. This represented 88.4% of all invasive breast cancers in the database for those years. Cases were categorized as triple negative (TNBC) if all 3 receptors were known to be negative, and non-TNBC if any one of the receptors was known to be positive.
2.2. Tumor size
The NCDB variable, “tumor size,” is a combination of clinical and pathological size. If surgery was performed before any other treatments, the pathological size was used, but if neoadjuvant therapy was employed, the clinical tumor size before treatment was used. The size is coded in 1 mm increments, but for this study the size was converted to 1 cm increments, that is, from 1 to 10 mm, 11 to 20 mm, 21 to 30 mm, etc, so that positive lymph node rates could be calculated for each size interval.
2.3. Race, histology, and treatment variables
The NCDB classified race and histology into many categories, with relatively few cases in each group. To allow a valid analysis, only racial categories with greater than 400 cases and histology categories with greater than 200 cases were included; categories with less than 400 and 200 cases, respectively, were recoded as “other.” For determination of surgical treatment, only cases that had definitive surgery, either mastectomy or lumpectomy, were included. For patients who had neoadjuvant chemotherapy, pathologic complete response was defined as no remaining invasive tumor in either the breast or the axillary lymph nodes.
2.4. Statistical analysis
Statistical analysis was performed with IBM SPSS version 22, Chicago, Ill. USA. Bivariate comparisons were performed with chi-square tests, and multivariable analysis was performed with binary logistic regression. Statistical tests were 2-sided, and P values <0.05 were considered significant.
3. Results
3.1. Completeness of data
The Her2 variable was complete for 91% of the 2010 to 2011 cases, and was positive in 14.2%, negative in 83.1%, and borderline in 2.7%. The cases of borderline Her2 expression were excluded, resulting in 88% of all invasive cancers being positive or negative for Her2. Essentially all of the cases with complete data on Her2 also had complete data for ER and PR. It appears that the 2009 guidelines changing the definition of hormone receptor positivity from 10% to 1% were adopted fairly quickly. Out of all invasive cancers diagnosed in 2010 and 2011, 79.1% were positive for ER, 18.0% were negative, and 2.9% were unknown/not done; whereas for 2004 to 2009, only 73.6% were positive, 20.1% negative, and 6.3% unknown/not done.
3.2. Incidence of triple-negative tumors by sex, race/ethnicity, age, and geographic region
Table 1 shows incidence data for TNBC based on sex, race/ethnicity, age, and geographic region. TNBC was present in 38,628 of 295,801 (13%) female patients compared to 185 out of 3136 (6%) male patients (P < 0.001). Non-Hispanic black and Hispanic patients had higher incidence of TNBC, and TNBC was more common amongst younger patients (P < 0.001 for both). Across all age groups, African-American women were more likely to have TNBC than their white counterparts; however, at the youngest ages (< 30), the 2 racial groups had roughly equivalent proportions of TNBC (Fig. 1A). Based on 9 geographic regions of the United States, TNBC was highest in the east south central (15.8%), the west south central (14.5%), and the south Atlantic regions (14.0%) and lowest in New England (10.8%, P < 0.001).
Table 1.
Incidence of triple-negative tumors by sex, race/ethnicity, age, and geographic region.
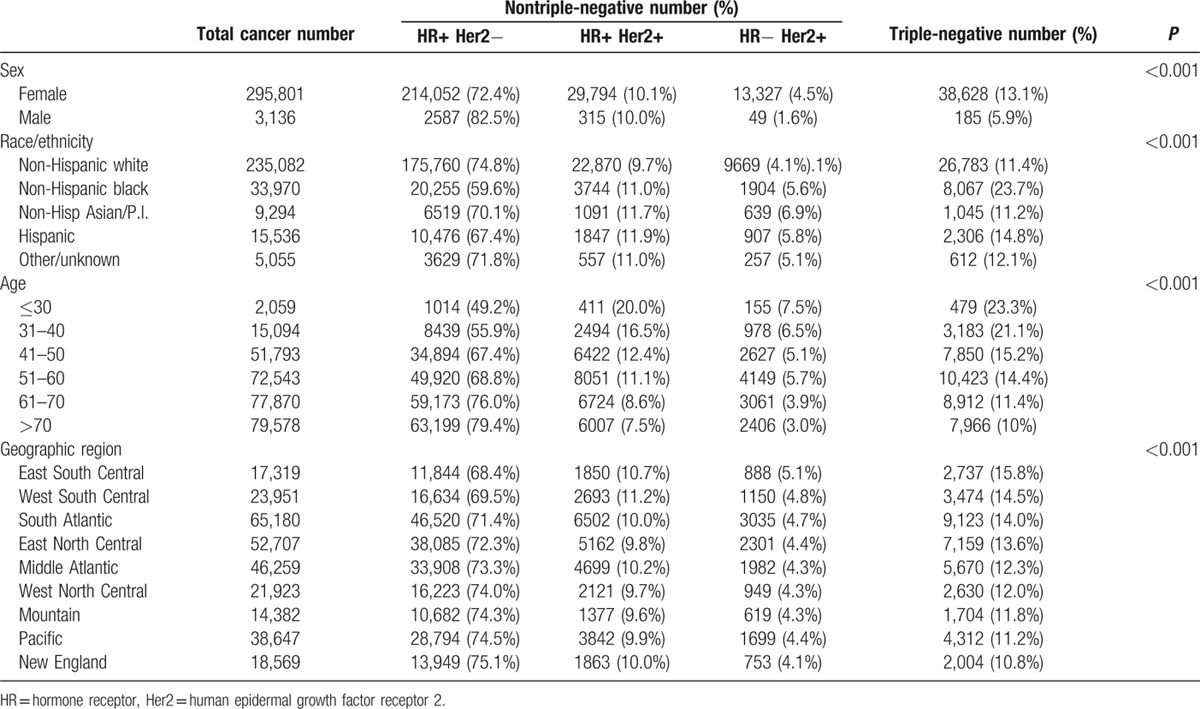
Figure 1.
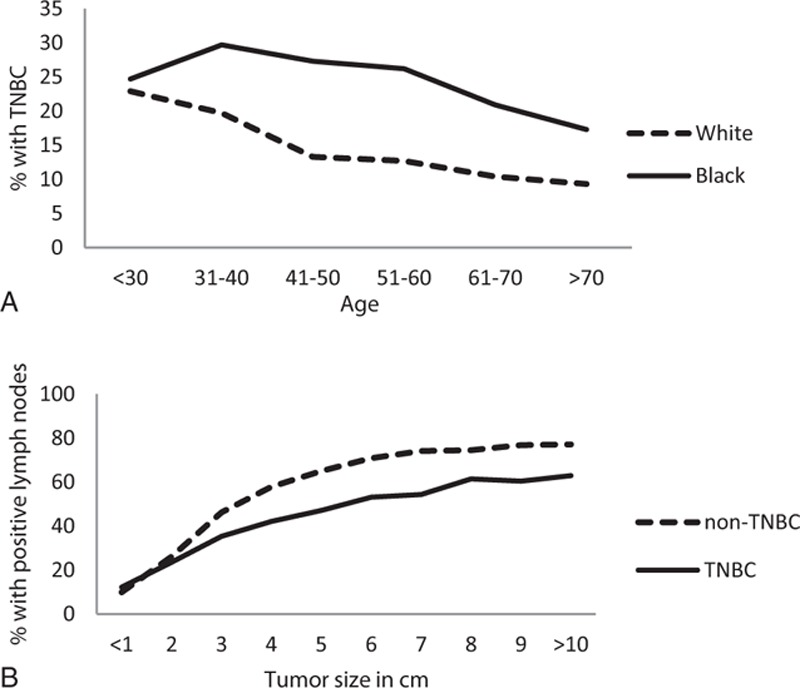
(A) Percentage of cancers that are triple negative by patient age and race. (B) Percentage of cancers that are lymph node positive by tumor size and molecular type.
3.3. Incidence of triple-negative tumors by detailed racial groups
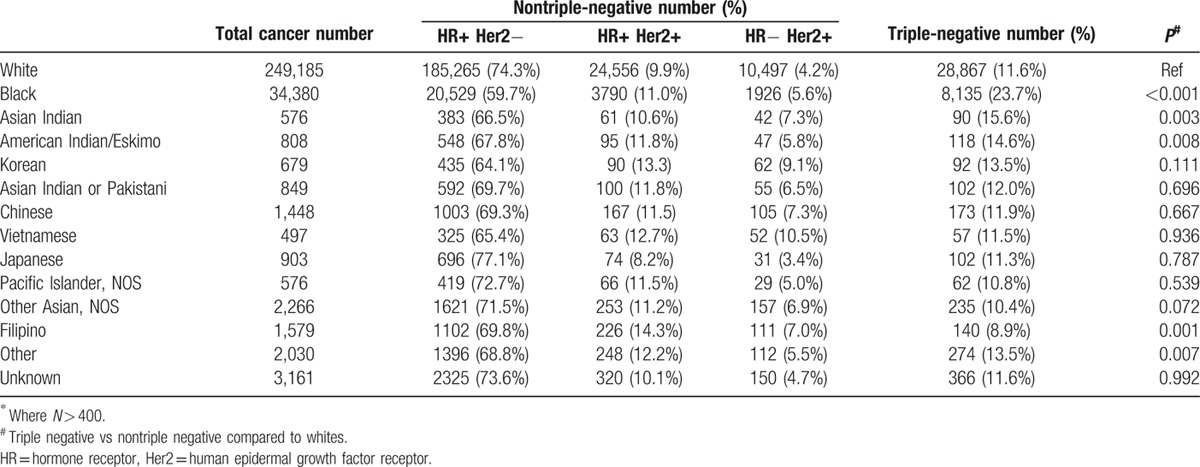
The NCDB included detailed information on racial group distribution. Table 2 shows the incidence of triple-negative tumors for all racial groups that contained more than 400 individuals. Compared to the white population, black, American Indian/Eskimo, and Asian Indian patients had a higher incidence of TNBC, and Filipino patients had a lower incidence of TNBCs.
Table 2.
Incidence of triple-negative tumors by detailed racial groups∗.
3.4. Incidence of triple-negative tumors by detailed histologic type
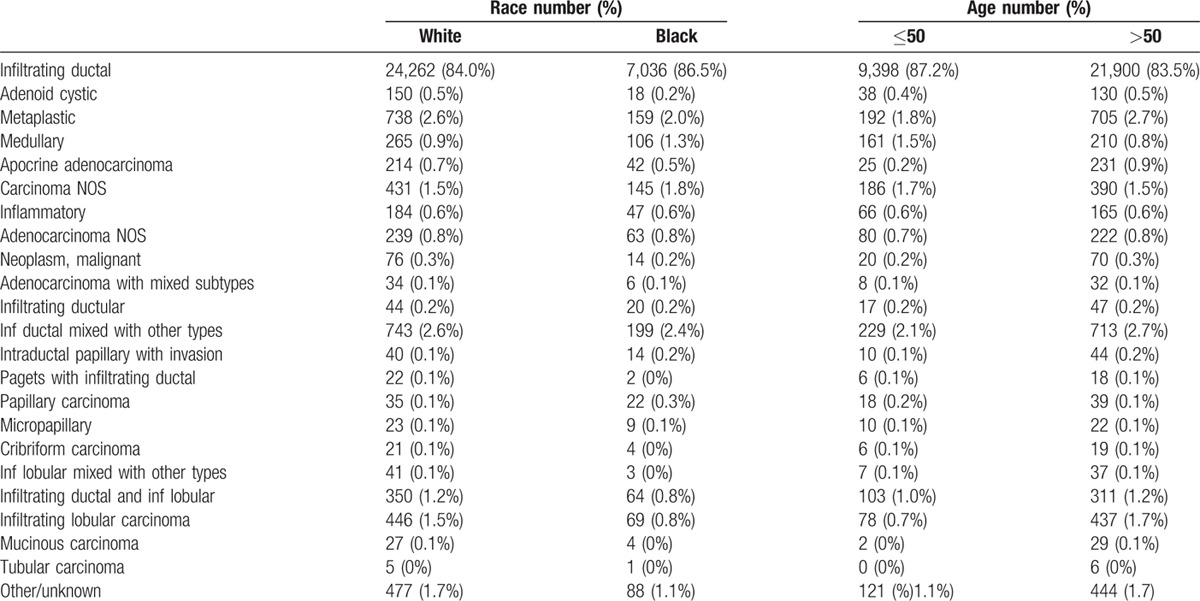
Table 3 shows the incidence of TNBC among all histologic types with more than 200 cases. Infiltrating ductal carcinoma was the most common histologic type and contained 14.6% TNBC. However, there was an extremely wide range from adenoid cystic, which was 78% TNBC to tubular that was 0.3%. Histologic types with a higher percent TNBC compared to infiltrating ductal were adenoid cystic, metaplastic, medullary, apocrine adenocarcinoma, and inflammatory carcinoma. Carcinoma not otherwise specified (NOS) and adenocarcinoma NOS were also more likely to be TNBC, probably because these categories are used for very undifferentiated cancers. Histologic types with a lower percent of TNBC than infiltrating ductal included tubular carcinoma, mucinous carcinoma, infiltrating lobular, infiltrating ductal and infiltrating lobular, infiltrating lobular mixed with other types, cribriform carcinoma, micropapillary and papillary carcinoma, Paget's disease with infiltrating ductal, intraductal papillary with invasion, and infiltrating ductal mixed with other types. To see whether differences in histological type may account for the racial and age differences observed, Table 4 shows histology by race and age for triple-negative cancers. The racial and age differences were mainly due to infiltrating ductal cancers and not due to the very small contribution of other histologic types.
Table 3.
Incidence of tripl-negative tumors by histologic type∗.
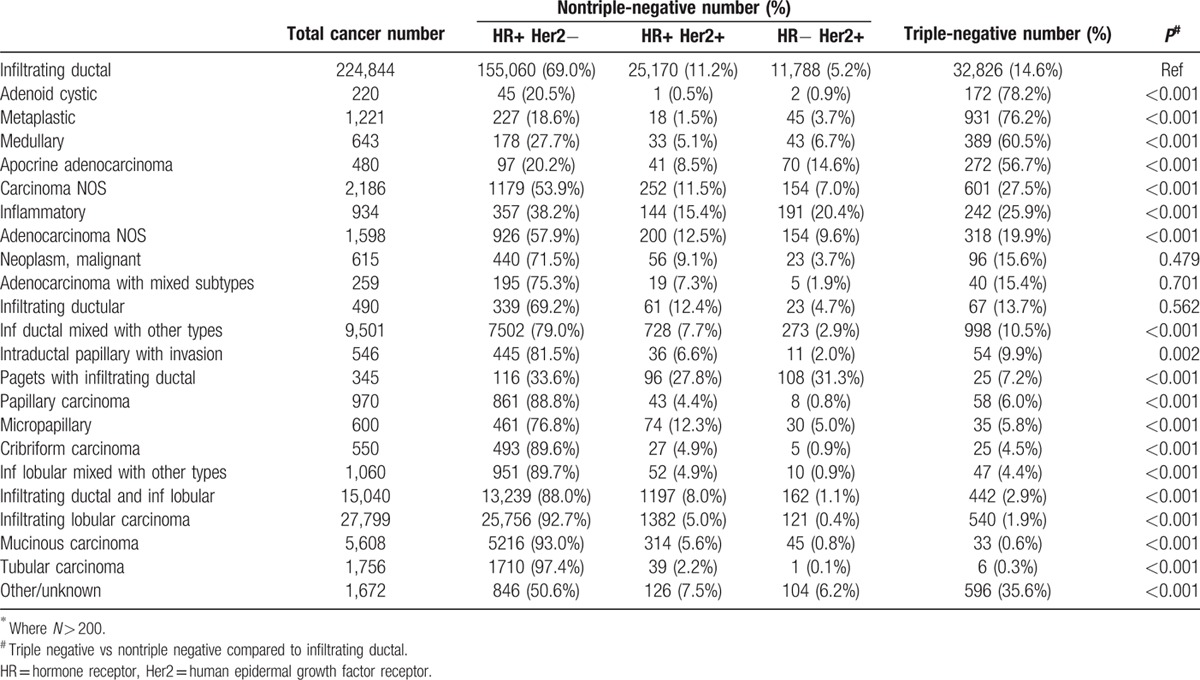
Table 4.
Histology of triple-negative cancers by race and age.
3.5. Tumor characteristics of triple-negative and nontriple-negative cancers
Tumor characteristics for TNBC and non-TNBCs are shown in Table 5. TNBC and Her2 positive patients had larger tumors than HR+ Her2− patients and were more likely to be high grade, have lymphovascular invasion, and to present with clinically metastatic disease.
Table 5.
Tumor characteristics of triple-negative and nontriple-negative cancers.
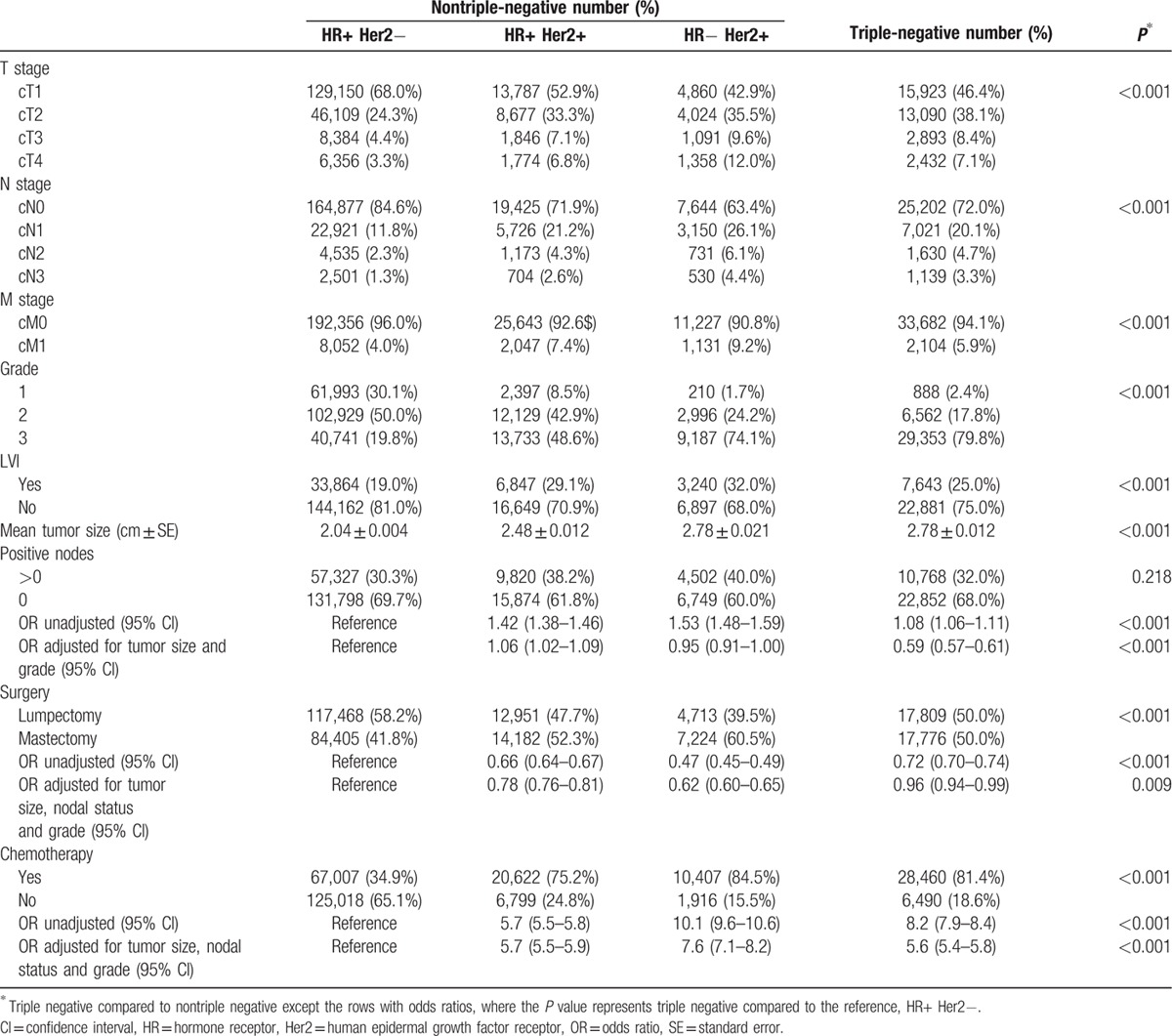
In unadjusted analysis TNBC were more likely than HR+ Her2– patients but less likely than Her2+ patients to have positive nodes. However, when stratified by tumor size, as seen in Fig. 1B, patients with TNBC were significantly less likely to have positive nodes than their non-TNBC counterparts (P < 0.001). Adjusting for tumor size and grade in a multivariable logistic regression model, TNBCs had a much lower rate of lymph node positivity than any other molecular type (OR = 0.59; 95% CI: 0.57–0.61).
3.6. Treatment of triple-negative and nontriple-negative cancers
Table 5 also shows differences in treatment. On univariate analysis, TNBC and Her2 positive patients received less breast-conserving surgery compared with HR+ Her2− patients. However, after adjusting for tumor size, nodal status, and grade, Her2+ patients continued to have significantly less breast conservation whereas the difference largely disappeared for TNBC patients. TNBC and Her2+ patients were also much more likely to receive chemotherapy; and among patients who received chemotherapy, they were more likely to receive neoadjuvant chemotherapy (28% vs 22%, P < 0.001) and to have a pathologic complete response (40% vs 28%, P < 0.001).
4. Discussion
To our knowledge, this is the largest study regarding the epidemiology, tumor characteristics, and treatment patterns of TNBCs in the United States. We found that the incidence of TNBC was 13% among females and 6% among males. The incidence of TNBC was highest among non-Hispanic black and Hispanic patients (23.7% and 14.8%). These data are in agreement with previously published smaller studies.[1,5,6,7] In addition, however, we found an increased incidence of TNBC in American Indian/Eskimo (14.6%) and Asian Indian patients (15.6%). Interestingly, the incidence of TNBC was lowest in Filipino patients (8.9%). It has been previously reported that Filipino woman have an increased risk of Her2 positive breast cancers, regardless of ER and PR status.[8] In addition, we found that the highest incidence of TNBC is in the southern regions of the United States, which might reflect the racial/ethnic distribution of the population.
In all racial/ethnic groups, TNBC was associated with increased incidence among young patients. However, as seen in Fig. 1A, the shape of the curves differs by race. In white patients, TNBC was highest under age 40, whereas in black patients, it did not really drop off until after age 60.
The data illustrate the broad histological heterogeneity of TNBCs. There were 7 different histologic subtypes where TNBC was more prevalent and 12 types where it was less prevalent than infiltrating ductal carcinoma. This is consistent with smaller studies that showed TNBC was associated with some special histologic subtypes such as adenoid cystic,[9,10] medullary or metaplastic subtypes,[11,12] and not with other subtypes such as tubular or lobular carcinoma.[13]
This study clarifies the relationship between TNBC and lymph node metastases. Although the overall percentage of positive nodes for TNBC and non-TNBC is equivalent, TNBC tends to be larger and of higher grade; hence, it would be predicted to have a higher incidence of lymph node metastases. When adjusted for these factors, however, the incidence of positive nodes with TNBC is considerably less than non-TNBC. This is in agreement with other recent studies.[14–16] However the finding in this study of increased lymphovascular invasion in TNBCs is contrary to previously published reports.[15,16]
We found that TNBC and Her2+ patients were treated more frequently with mastectomy than were HR+ Her2− patients. However, upon adjusting for larger tumor size, nodal status, and grade, TNBCs had about the same breast conservation rate as HR+ Her2− patients. While TNBC patients have a higher risk for both local and distant recurrence, histologic or molecular subtype is not an indication for mastectomy and standard criteria should be applied to select the surgical approach in TNBC.[1,2,7,17]
The majority (80%) of TNBC patients received systemic chemotherapy and the odds of receiving chemotherapy were much greater for TNBC than for non-TNBC even when adjusted for stage and grade. Furthermore, TNBC was more likely to be treated with neoadjuvant chemotherapy, and more likely to have a pathologic complete response. This is what we would expect based on the biology and clinicopathologic features of TNBC.[18,19] More detailed analyses of neoadjuvant chemotherapy use in the NCDB have recently been published.[20,21]
In summary, TNBC has distinct features regarding age, gender, geographic, and racial/ethnic distribution. Compared to non-TNBC, TNBC is larger and higher grade, but less likely to have lymph node metastases. When the stage is taken into account, the surgical treatment is similar to that of non-TNBC, but treatment with chemotherapy is much more common.
Footnotes
Abbreviations: ER = estrogen receptor, Her2 = human epidermal growth factor receptor 2, NCDB = National Cancer Database, PR = progesterone receptor, TNBC = triple-negative breast cancer.
Presented at the Society of Surgical Oncology, March 26 to 29, 2015, Houston, TX.
The authors have no conflicts of interest to declare.
References
- 1.Newman LA, Reis-Filho JS, Morrow M, et al. The 2014 society of surgical oncology susan g. Komen for the cure symposium: triple-negative breast cancer. Ann Surg Oncol 2015; 22:874–882. [DOI] [PubMed] [Google Scholar]
- 2.Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat 2011; 127:713–720. [DOI] [PubMed] [Google Scholar]
- 3.Ueno NT, Zhang D. Targeting EGFR in triple negative breast cancer. J Cancer 2011; 2:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy MJ, McGowan PM, Crown J. Targeted therapy for triple-negative breast cancer: where are we? Int J Cancer 2012; 131:2471–2477. [DOI] [PubMed] [Google Scholar]
- 6.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012; 118:5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal J, Ginsburg O, Rochon PA, et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015; 313:165–173. [DOI] [PubMed] [Google Scholar]
- 8.Gangi A, Chung A, Mirocha J, et al. Breast-conserving therapy for triple-negative breast cancer. JAMA Surg 2014; 149:252–258. [DOI] [PubMed] [Google Scholar]
- 9.Wetterskog D, Lopez-Garcia MA, Lambros MB, et al. Adenoid cystic carcinomas constitute a genomically distinct subgroup of triple-negative and basal-like breast cancers. J Pathol 2012; 226:84–96. [DOI] [PubMed] [Google Scholar]
- 10.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 2012; 23 suppl 6:vi7–vi12. [DOI] [PubMed] [Google Scholar]
- 11.Amirikia KC, Mills P, Bush J, et al. Higher population-based incidence rates of triple-negative breast cancer among young African-American women: implications for breast cancer screening recommendations. Cancer 2011; 117:2747–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parise C, Caggiano V. Disparities in the risk of the ER/PR/HER2 breast cancer subtypes among Asian Americans in California. Cancer Epidemiol 2014; 38:556–562. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat 2014; 144:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangi A, Mirocha J, Leong T, et al. Triple-negative breast cancer is not associated with increased likelihood of nodal metastases. Ann Surg Oncol 2014; 21:4098–4103. [DOI] [PubMed] [Google Scholar]
- 15.Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol 2009; 16:2705–2710. [DOI] [PubMed] [Google Scholar]
- 16.Ugras S, Stempel M, Patil S, et al. Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann Surg Oncol 2014; 21:3780–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol 2011; 29:2852–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384:164–172. [DOI] [PubMed] [Google Scholar]
- 19.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363:1938–1948. [DOI] [PubMed] [Google Scholar]
- 20.Killelea BK, Yang VQ, Wang SY, et al. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol 2015; 33:4267–4276. [DOI] [PubMed] [Google Scholar]
- 21.Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 2015; 121:2544–2552. [DOI] [PubMed] [Google Scholar]


