Abstract
Mammalian heat-shock-protein (HSP) 90α rapidly responses to environmental insults. We examined the hypothesis that not only serum HSP72 but also HSP90α is increased in the systemic inflammatory response syndrome (SIRS), severe-sepsis (SS), and/or sepsis (S) compared to healthy children (H); we assessed HSP90α relation to (a) multiple organ system failure (MOSF) and (b) inflammatory-metabolic response and severity of illness.
A total of 65 children with S, SS, or SIRS and 25 H were included. ELISA was used to evaluate extracellular HSP90α and HSP72, chemiluminescence interleukins (ILs), flow-cytometry neutrophil-CD64 (nCD64)-expression.
HSP90α, along with HSP72, were dramatically increased among MOSF patients. Patients in septic groups and SIRS had elevated HSP90α compared to H (P < 0.01). HSP90α was independently related to predicted death rate and severity of illness; positively to HSP72, nCD64, ILs, length of stay, days on ventilator, and fever; negatively to HDL and LDL (P < 0.05). The HSP72 was increased in SS/S and related negatively to HDL and LDL (P < 0.05).
Serum HSP90α is markedly elevated in children with severe sepsis and is associated with MOSF. Better than the HSP72, also increased in SS, SIRS, and MOSF, HSP90α is related to the inflammatory stress, fever, outcome endpoints, and predicted mortality and inversely related to the low-LDL/low-HDL stress metabolic pattern.
Keywords: children, fever, heat-shock-protein, metabolism, organ failure, sepsis, SIRS
1. Introduction
Severe infections kill >4.5 million children every year, representing a major public health problem. More patients with sepsis (S) (5.6%) and sepsis/septic shock (SS) (17.0%) die compared with mortality of all pediatric intensive care unit (PICU) (3.0%) admissions. The noninfectious systemic inflammatory response syndrome (SIRS) is also a major cause of disability or death. Although clinical adherence to the Pediatric Guidelines for septic shock has been shown to influence outcome, no targeted treatment to sepsis and/or SIRS underlying excessive immune response has been found so far.
An overwhelming immune response in infectious (S, SS) or noninfectious SIRS, as mediated by the uncontrolled release of proinflammatory mediators, can lead to shock, multiple organ system failure (MOSF), and even death. Elevated neutrophil CD64 (nCD64) expression was reported in adult patients and in children with sepsis.[1] The molecules, however, that promptly activate immune competent cells through the LPS-TLR4-CD14-dependent pathway, acting as “danger signals,” are extracellular heat shock proteins (HSPs). Extracellular HSPs released from stressed cells serve to signal an impending “danger signal” to neighboring and immune cells (danger hypothesis), presumably promoting immune responses and improving host defense.[2]
It has been previously shown that an intense stressor exposure may increase the 70-kDa family of HSP (inducible HSP72), which acts as a danger “alarm” to potentiate the nitric oxide (NO) stress-response and engender recovery from bacterial inflammation[3] or heat stress.[4] Contrasting to animal studies showing an HSP72 protective effect in experimental sepsis, human in vivo or in vitro studies are not conclusive, showing a possible relation to either protection or mortality and morbidity.[5] Although increased circulation of stress proteins signals danger to inflammatory cells and aid in immune surveillance, they have also been linked to a deleterious role in some diseases.[6] Thus, increased extracellular HSP72 levels were detected in the plasma of children admitted to the PICU with septic shock and correlated with worse outcome.[7] Extracellular HSP72 has previously been shown to be elevated not only in patients with septic shock, but also following cardio-pulmonary bypass or in children with acute lung injury.[8]
Another major molecular chaperone, 90-kDa family of HSP (inducible HSP90α), has been shown to interact with about 200 client proteins, mainly involved in transcription regulation and signaling-transduction pathways of glucocorticoid receptors, ribonucleoproteins, chromatin remodeling factors, transcription factors, and protein kinases.[9] The guarding role of this abundant “chaperone,” the intracellular HSP90α, is to launch a rapid response to various insults such as heat, hypoxia, reactive oxygen species, and injury-released growth factors.[10–11] Thus, a well-characterized function of secreted HSP90α is to boost cell motility, facilitating tissue healing.[12] Under hypoxia, HSP90α is secreted into extracellular space, binding and signaling through the low-density-lipoprotein (LDL)-receptor-related protein-1 to promote cell motility, finally leading to repair of damaged tissues.[13] In addition, the secretion of HSP90α, but not HSP90β, is increased in activated endothelial cells, promoting their angiogenic activities with the induction of matrix proteins.[14]
Following the contrasting results shown by HSP72 human studies,[5] it has been recently shown that the activation of the HSP90α/Akt pathway induces caspase-3 activation, provoking thereby apoptosis in septic mice.[15] In addition, inhibition of HSP90α prevented ischemia-reperfusion injury in the kidney donor and/or organ,[16] and suggested to open the route towards novel treatment modalities of severe sepsis associated acute lung injury.[11] Robust correlations, however, between extracellular HSP90α levels with clinically relevant outcome variables during the acute phase of SS or SIRS or with pro- or anti-inflammatory interleukins (IL), nCD64 or metabolic derangements are missing. Similarly, no studies in children have been conducted comparing HSP90α with HSP72, a HSP90α-machinery complement, in SS and/or SIRS, or relating their levels to organ failures, severity of illness and predicted or measured outcome endpoints.[17] We hypothesized that increased HSP90α levels could be detected in the serum of critically ill children, especially those with severe sepsis and/or septic shock and in SIRS compared to healthy controls (H). We also hypothesized that the abundant intracellular chaperone HSP90α—when released extracellularly as a chaperokine—might be better than the HSP72's correlated with MOSF, early inflammatory and metabolic acute stress, severity of illness, and outcome endpoints.
2. Materials and methods
2.1. Setting and research ethics
This prospective observational study was conducted in the PICU of the University Hospital, Heraklion, University of Crete, between January 2010 and September 2014. The study was approved by the Ethical Committee of the University Hospital, Heraklion, Crete, Greece. Written informed consent was obtained from patients’ guardians before inclusion in this study. All data were anonymous.
2.2. Study Patients
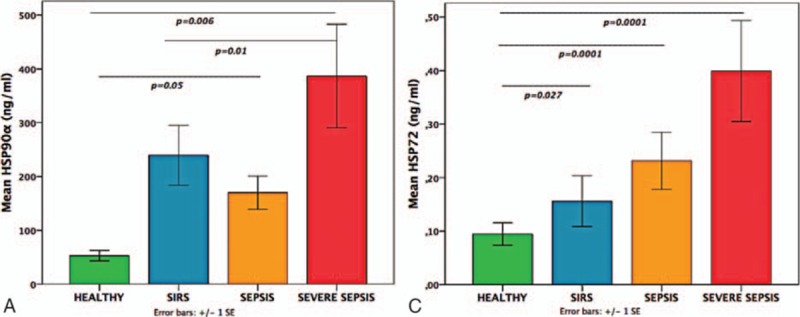
Sixty-five critically ill children, consecutively admitted to the PICU, with noninfectious (SIRS) (n=23) or infectious SIRS [S (n = 20), and SS (n = 22)] were enrolled in the study. Twenty-five healthy children undergoing screening tests for minor elective surgery were consisted in the control group. Allocation into groups was carried out within 6 hours of admission by the attending physician, who was unaware of the results of laboratory assays, until at least 20 patients were included in each group. Fifteen of the severe sepsis group fulfilled the criteria of septic shock; all septic shock and severe sepsis patients had infection, SIRS, and at least 2 organ failures, thereby representing a homogenous group (SS). Patients with immunodeficiency, autoimmunity, malignancies, and receiving steroids or with metabolic or chronic diseases were excluded (criteria predefined). Enrolled SIRS patients had trauma without infection (fractures, liver, spleen, or lung contusions). None of the patients received parenteral nutrition. After initial blood studies, patients were put on enteral nutrition according to our PICU protocol. The Pediatric Logistic Organ Dysfunction Score (PELOD),[18] the simplified Therapeutic Intervention Scoring System (TISS),[19] and the Pediatric Risk of Mortality Score (PRISM)[20] were the recorded markers of severity of disease on the day of blood sampling. The predicted death rate was calculated after PRISM. Length of stay (LOS), days on mechanical ventilation (DOMV), and PICU-mortality were the outcome endpoints recorded. Sepsis, severe sepsis, septic shock, and SIRS were defined according to the International Pediatric Sepsis Consensus Conference definitions. The MOSF was defined using the criteria by Wilkinson (presence of at least 2 organ system failures).[21] According to the exclusion criteria, patients with cancer, immune-deficiencies (immunosuppressive agents but not stress-dose hydrocortisone), and metabolic diseases or endocrinopathies (diabetes, hypercholesterolemia) were not included.
2.3. Laboratory evaluations
Admission blood and urine cultures, pharyngeal and deep tissue swabs, and bronchoalveolar lavage (BAL) were routinely taken in all patients to the PICU, despite the fact that most of the critically ill patients had already received antibiotics prior to their admission to the intensive care unit.
Blood was drawn for routine exams at the time of admission. Venous blood was processed to measure white blood count (WBC), procalcitonin (PCT), and C-reactive protein (CRP) levels. Samples for enzyme-linked immunosorbent assay (ELISA) or chemiluminescence were collected in tubes and were centrifuged immediately at 4000 rpm 10 minutes to separate serum from the cellular components. Samples were stored at −80°C until later analysis. Investigators were blinded to the patients’ group assignment and available clinical data until after the analysis.
2.4. Elisa immunoassay
Extracellular protein levels of HSP90α and HSP72 were determined by ELISA commercial kits obtained from Enzo Life Sciences (Lausanne, Switzerland) according to manufacturer's instructions. Samples underwent a 1:25 dilution for HSP90α and 1:1 for HSP72 and then were pipetted into the wells precoated with specific mouse-monoclonal antibody. Following incubation and washing, a horseradish peroxidase antibody specific for HSP90α was added to the wells, whereas this phase was performed in 2 steps for the HSP72. Following washing, the substrate solution was added and color developed. The development of color was stopped and the optical density of each well was measured in an automated microplate reader set at 450 nm. All measurements were performed in duplicate. The sensitivities of the assays were 50 pg/mL for HSP90α and 90 pg/mL for Hsp72.
2.5. Chemiluminescence
Tumor necrosis factor alpha (TNF-α), IL-6, IL-10, and IL-8 were measured using a commercially available chemiluminescence kit (IMMULITE 100 Siemens Medical Solutions Diagnostics Limited, Surrey, UK). The sensitivities of the assays were <2 pg/mL for IL-6, <1 pg/mL for IL-10, 2 pg/mL for IL-8, and 4 pg/mL for TNF-α. All samples were processed in duplicate with mean value used if the coefficient of variance was <20%.
2.6. Flow cytometry
Whole blood, EDTA-anticoagulated samples were immediately transported to the laboratory for flow cytometric CD64 analysis. In brief, 100 μl of the whole blood sample were incubated with phycoerythrin (PE)-conjugated CD64 (Clone 22) for 20 minutes at room temperature, in the dark. PE-mouse IgG was used as the isotype control (antibodies from IMMUNOTECH-BECKMAN COULTER, Marseille, France). Labeled cells were thoroughly washed with PBS+2 % FCS+ sodium azide. Following lysis in immunoprep reagent system (BECKMAN COULTER, Mervue, Galway, Ireland), the cells were analyzed on an Epics-Coulter cytometer. They were initially gated according to their morphology (forward- and side-scatter characteristics) so that >30,000 cells were acquired in each sample. Results are presented as the percentage (%) of positive staining cells. Analyses were performed in monocyte, lymphocyte, and neutrophil populations.
2.7. Routine laboratory measurements
CRP assay was performed on the immunonephelometer Dade Behring BN II (Dade Behring Diagnostics Inc., Somerville, NJ). Abnormal were considered CRP concentrations >0.8 mg/dL. Procalcitonin (PCT) was measured by an immunoluminometric assay (Lumitest-PCT, Brahms-Diagnostica, Berlin, Germany) with a detection threshold at 0.1 ng/mL. According to the laboratory cut-off values, PCT levels >0.5 ng/mL were considered abnormal. Plasma concentrations of glucose, triglycerides (TG), total cholesterol (TC), high-density lipoproteins (HDL) or LDL, and hematological markers were measured by routine laboratory methods.
2.8. Statistical analysis
The 1-sample Kolmogorov–Smirnoff test was used in order to determine the data distribution from measured variables. Data are expressed as median values with range for continuous parameters and as frequencies for categorical variables. The Kruskal–Wallis and the Mann–Whitney tests were used to perform comparisons among groups, as appropriate. We ran multiple comparison analyses using post hoc Dunn's pairwise tests with Bonferroni corrections for the Kruskal–Wallis H test; the x2 test statistic was adopted to calculate the effect size (H2 = x2/(n – 1)) in order to avoid the inflation of overall type I error. Respectively, the Mann–Whitney U-values with z-scores were used to calculate the effect size (r = z/√n) in order to measure the size of differences. Between-group comparisons were conducted using the χ2 test for categorical parameters and Spearman rank correlation coefficient for correlation between 2 continuous variables. Although the correlation coefficients usually serve as the effect size index, to further estimate the effect size, we converted the correlations to Fisher's z scales to calculate the sizes of associations. Finally, a multivariate linear regression analysis (stepwise method) was adopted to examine whether HSP90α was an independent predictor of outcome endpoints. A 2-sided significance level of 0.05 was used for statistical inference. All statistical analyses were performed using the IBM SPSS Statistics (version 22.0; Chicago, IL).
3. Results
There were no statistical differences regarding gender in the studied groups. Septic, but not severely septic, patients were younger compared to healthy and SIRS groups. Two patients in the SIRS group—both having suffered multiple injuries from motor vehicle accidents (the patients with the highest PRISM and TISS)—and 2 patients with septic shock (multiple organ systems failure) died. Patients with severe sepsis had higher first-day temperature, severity scores, organ failures, and longer LOS, and DOMV compared to the sepsis or SIRS groups (Table 1). The calculated effect size was larger for MOSF and fever, whereas it was better for predicted than actual mortality. Would we had applied the recently updated SEPSIS-3 definition[22] for adults in our pediatric population, we would have found the same statistical differences among groups. The only difference noted by running such a pilot study has been that SEPSIS-3 septic shock should have replaced SS group, and SEPSIS-3 sepsis would correspond to our S group (except 1 patient moving from the SS to S group).
Table 1.
Baseline characteristics on study enrollment.
In the 2 septic groups, pathogens isolated in the PICU were as follows: blood: Streptococcus pneumonia, Acinetobacter baumannii, Enterococcus faecalis), Escherichia coli, (n = 1 each), Neisseria meningitidis and Candida species (n = 2 each) and Staphylococcus haemolyticus (n = 4); BAL: Pseudomonas aeruginosa (n = 5), Serratia marcescens, Stenotrophomonas maltophilia, Neisseria, Mycoplasma pneumonia (n = 1 each); urine: Pseudomonas aeruginosa (n = 2), Escherichia coli (n = 1); Central nervous system: N meningitidis (n = 1). Patients without isolates had received antibiotics before PICU admission in another hospital (cultures not sent or results unavailable). No pathogens were isolated from the blood and samples from other sites in SIRS patients.
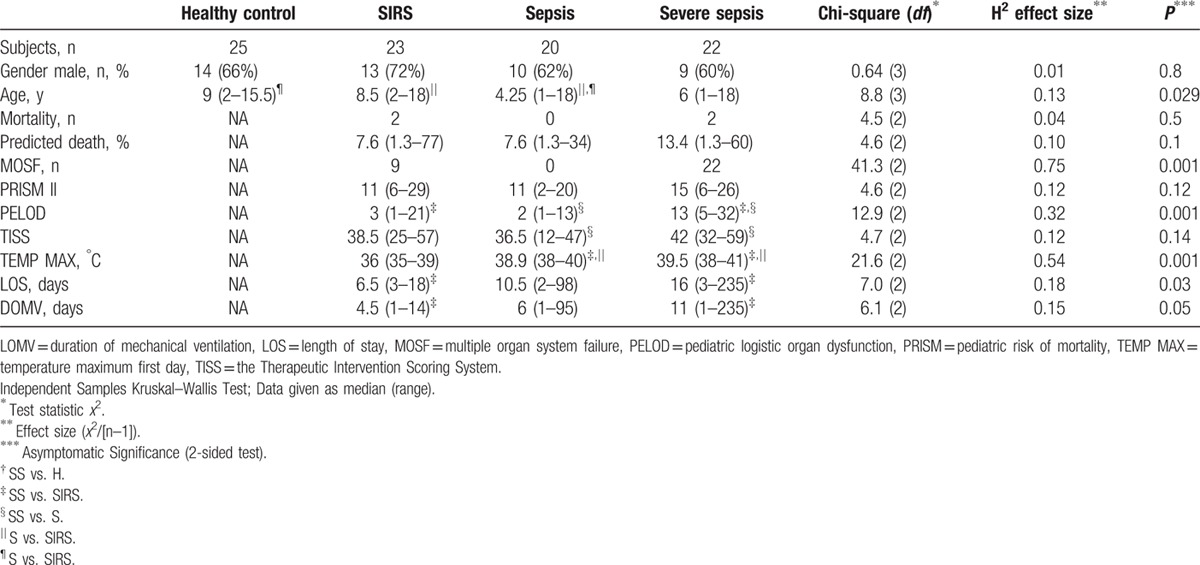
Patients in S, SS, and SIRS groups had elevated HSP90α compared to H (Fig. 1A). Septic groups, especially SS, had also increased HSP72 compared to H and/or SIRS (Fig. 1B). The HSP90α elevation paralleled: an increase of HSP72, IL-6, IL-8, and IL-10 levels in septic groups compared to H; elevated HSP72, IL6, and TNF-α in SS compared to SIRS; elevated IL-6 and IL-8 in SIRS compared to H (Table 2). The calculated effect size was better for HSP90α and IL-6.
Figure 1.
(A) Patients with severe sepsis, sepsis, or SIRS had increased extracellular HSP90α in comparison to healthy controls; (B) patients with severe sepsis or sepsis had increased extracellular HSP72 in comparison to healthy controls and/or SIRS (Mann–Whitney tests). HSP = heat shock protein, SIRS = systemic inflammatory response syndrome.
Table 2.
First-day serum concentrations and group differences.
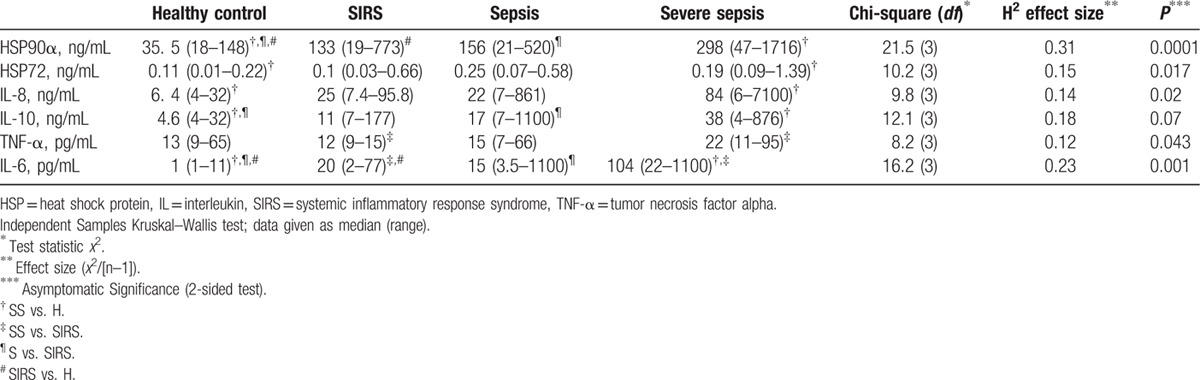
HSP90α, along with HSP72, were dramatically increased among MOSF patients (Fig. 2A and B). The HSP90α and HSP72 increases in the MOSF group paralleled: (a) significant increases of the inflammatory biomarkers nCD64, IL-6, CRP, and PCT; (b) significant decreases of the nutritional indices cholesterol, HDL, and LDL, but not glucose (Table 3). The effect sizes (r) were larger for HSP90α, CRP, PCT, IL-6, LDL, HSP72, and nCD64.
Figure 2.
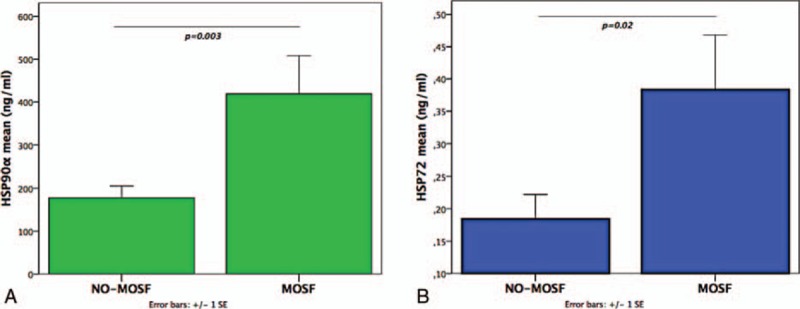
Patients with MOSF had increased. (A) Extracellular HSP90α and (B) extracellular HSP72 compared to non-MOSF patients (Mann–Whitney test). HSP = heat shock protein, MOSF = multiple organ system failure.
Table 3.
Acute stress (first 24 h) levels of HSPs, inflammatory biomarkers, and nutritional indices in mechanically ventilated children with sepsis or SIRS classified according to their MOSF status.
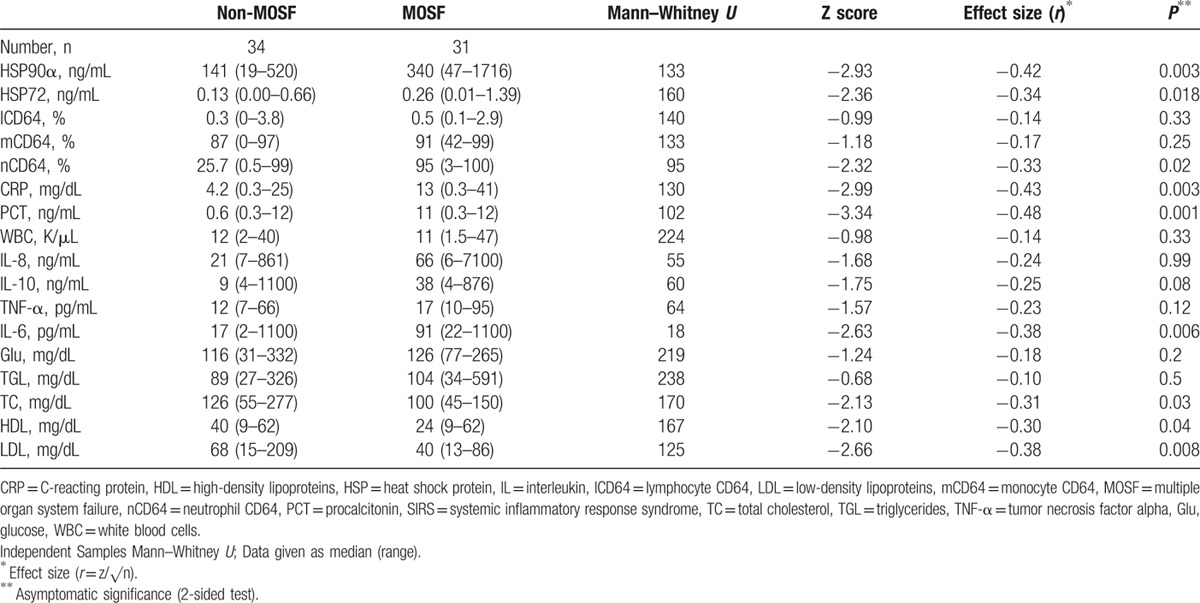
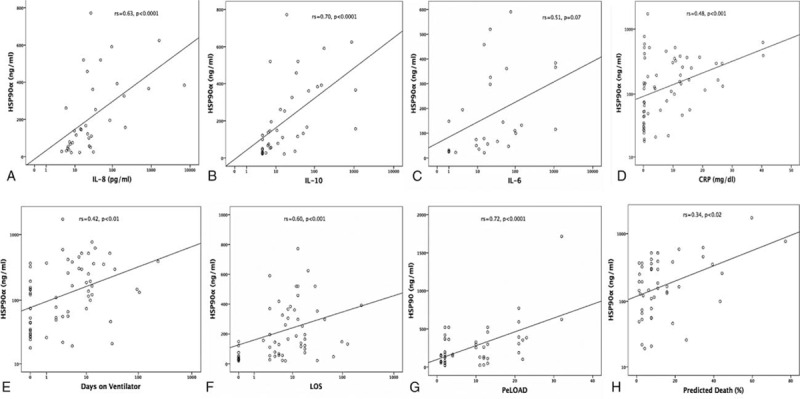
HSP90α related positively to IL-8, IL-10, IL-6, and CRP (Fig. 3A–D). HSP90α was also related to ventilator days, LOS, organ dysfunction (PELOD), PRISM-predicted death rate (Fig. 3E–H), PCT (rs = 0.51, P < 0.001), TISS (rs = 0.44, P < 0.02), and first-day maximum temperature (rs = 0.38, P < 0.045). TNF (rs = 0.47, P < 0.03), nCD64 (rs = 0.64, P < 0.001), CRP (rs = 0.67, P < 0.001), and PCT (rs = 0.72, P < 0.03), but not HSP72, IL-8, or IL-10, were also related to the first-day maximum temperature. In all cases, renal clearance was adjusted for in the associations between HSP and illness severity /mortality. The calculated mostly large effect size Fisher's Zr scales were for Fig. 3A = 0.74, b = 0.87, c = 0.56, d = 0.52, e = 0.45, f = 0.69, g = 0.91, h = 0.35. Despite a wide scattering, HSP90α exhibited a positive relation to HSP72, nCD64 and stress-related metabolic molecules glucose and triglycerides (Fig. 4A–D). Even more importantly, HSP90α and HSP72 related negatively to both lipoproteins (Fig. 4E–H). The calculated effect size by Fisher's Zr scales was rather medium in this case: Fig. 4A = 0.38, b = 0.51, c = 0.55, d = 0.43, e = 0.52, f = 0.39, g = 0.42, h = 0.30.
Figure 3.
Positive correlations of HSP90α serum concentrations with serum levels of: (A–D) Interleukins and CRP; (E–H) days on ventilator, length of stay (LOS), organ dysfunction (PELOD), and PRISM-predicted death rate as indicated. Spearman rank correlation test, correlation coefficient r, and P values are given. CRP = C-reacting protein, HSP = heat shock protein, IL = interleukin, LOS = length of stay, PELOD = Pediatric Logistic Organ Dysfunction Score, PRISM = Pediatric Risk of Mortality Score.
Figure 4.
Positive correlations of HSP90α serum concentrations with: (A–D) Serum levels of HSP72, nCD64 expression, glucose and triglycerides; (E–H) negative correlations of HSP90α and HSP72 serum concentrations with LDL and HDL as indicated. Spearman rank correlation test, correlation coefficient r, and P values are given. HDL = high-density lipoproteins, HSP = heat shock protein, LDL = low-density lipoproteins, nCD64 = neutrophil CD64.
Despite the small mortality rate, not only HSP90α (682 [520–1716] vs. 121 [18–625] ng/mL, P < 0.0001) but also HSP72 (0.41 [0.24–1.4] vs. 0.13 [0–1] ng/mL, P < 0.015) were increased among nonsurvivors. The small mortality rate in this study could not allow performing a binary logistic analysis. Instead, as predicted mortality was related to mortality (r = 0.53, P < 0.01) and the 2 variables did not differ (P < 0.06), multiple linear regression analysis was performed (stepwise method) to examine if any of the HSPs, ILs, inflammatory biomarkers (CD64, CRP, PCT), and nutritional indices could independently predict outcome (predicted mortality). Only HSP90α was an independent predictor of predicted mortality (Beta 0.55, 95% CI 0.02–0.04, P < 0.001). HSP90α was also independently associated with MOSF (PELOD) (Beta 0.51, 95% CI 0.01–0.02, P < 0.001) as were TNF (Beta 0.45, 95% CI 0.9–0.3, P < 0.002) and IL-10 (Beta –0.31, 95% CI –0.02– (–0.002), P < 0.02). Finally, HSP90α was independently associated with the therapeutic intervention score (TISS) (Beta 0.38, 95% CI 0.005–0.02, P < 0.002) as was IL-10 (Beta –0.37, 95% CI –0.02– (–0.005), P < 0.002).
4. Discussion
We showed that serum concentrations of HSP90α are markedly elevated in children with severe sepsis and to a lesser degree in critically ill children with sepsis or SIRS. We also showed that HSP90α is dramatically increased among patients with organ failures, an increase better associated with early inflammatory-metabolic stress than HSP72. However, a parallel increase of extracellular HSP72 in patients with sepsis or MOSF was also shown. Finally, we showed that both HSPs are inversely related to the low-LDL/low-HDL stress metabolic pattern, revealing a possible role for the heat shock response in the acute metabolic derangement in sepsis and SIRS. Not irrelevant to early HSP90α-response to stress severity and MOSF-association, we showed that extracellular HSP90α was independently related to PRISM-predicted death rate and the severity of organ dysfunction. In addition, HSP90α was related to fever, nCD64, IL-6, IL-8, IL-10, acute phase proteins, and length of stay or mechanical ventilation significantly more than extracellular HSP72.
The “danger hypothesis” suggests the release of stress proteins from severely stressed or damaged cells which serve as a danger signal to neighboring cells.[23] HSP90α membrane translocation and cell secretion is through exosome protein secretory pathway[24] or in a specific and inhibitable manner through the endo-lysosomal pathway from viable cells.[25] Then, immediately after complex DNA damage the threonine 7 residue of HSP90α is phosphorylated[26] and accumulated at sites of DNA double-strand breaks forming repair foci.[24] An important finding of this study is a trend for an escalating response to stress (assuming DNA damage) of not only HSP90α, but also HSP72 in the SIRS and in the 2 septic groups compared to healthy children. In accordance to our findings of increased HSP72 serum levels associated with predicted death rate in children with SS and SIRS, increased extracellular HSP72 levels with septic shock have been previously correlated with mortality in adults[27] and in children.[7] Verifying its alarming role, circulating HSP72 has also been associated with organ dysfunction or complications in coronary artery disease,[28] liver disease, vaso-occlusive sickle cell disease-crisis, and in pre-eclampsia.[29] Extending these publications, we have now shown that higher extracellular HSP72 levels correlate with MOSF in critically ill children with SIRS and/or sepsis.
There are limited data describing extracellular HSP90α levels in adults, children, or neonates with severe sepsis. We have recently shown that extracellular HSP90α levels are increased in adult SS and SIRS and that they are related to HSP72, interleukins, and cortisol.[30] We are now extending this observation by revealing increased HSP90α in critically ill children, especially those with severe sepsis. Furthermore, we found a wide HSP90α correlation to the nCD64, IL-6, IL-8, IL-10, severity of illness, LOS, days on ventilator and predicted death rate. These findings might simply indicate a proportionally greater level of physiologic stress associated with SS compared to SIRS. Thus, extracellular HSP90α, acting as a “chaperokine,” could have potentiated an already active systemic inflammatory response in SS. Our finding that HSP90α is inversely related to an early septic metabolic pattern indicates that HSP90α may induce a strong acute phase nCD64—interleukin inflammatory response resulting in an unbalanced metabolic status. We have previously shown that an early-onset sepsis-related stress-metabolic pattern is characterized by an acute increased [nCD64, glucose, triglyceride]—decreased [total-cholesterol, HDL, LDL] biomarker pattern, contrasting the moderate combination of noninfectious SIRS in which glucose increase is combined with a nonsignificant cholesterol-lipoprotein decrease.[31] This is the first time that higher extracellular HSP90α levels are shown to be independently associated with mortality (predicted), MOSF, severity of illness scores, and therapeutic interventions (TISS). Plasma HSP90α has recently been reported to be a potential predictor for poor outcome in septic adults.[32] Also, in a murine model of sepsis, HSP90α inhibitors improved pulmonary and circulatory function and attenuated systemic and pulmonary inflammation.[11]
Our data, revealing an alarming increase of HSP90α among MOSF patients, assume that HSP90α may have a central clinical role to play in septic and/or SIRS patients. It has been previously proposed that MOSF in sepsis may be due to an early hyper-inflammatory “cytokine storm” response or to an early activation of innate immunity and suppression of adaptive immunity.[33] Although most patients restore innate and adaptive immunity and survive the infectious or noninfectious stress, some patients enter a state of either profound immunosuppression or persistent activation of the innate immunity, with resultant secondary infections or intractable inflammation and multiple organ failure, respectively.[33] In our study, serum HPS90α differences suggest a double pathogenic mechanism not only in severe sepsis but also in noninfectious SIRS, roughly discriminating between healthy and critically ill, and between MOSF and non-MOSF patients. It has been suggested that dendritic and immune cell receptors capture HSP released from HSP-containing exosomes and release chemokines and interleukins.[34] This may explain the strong HSP90α–IL relation shown in this study. The host innate immune response occurs through an NF-êB-dependent pro-inflammatory gene expression via TLR4 and TLR2, similar to an LPS-mediated signal transduction.[35] Similarly, the inducible isoform HSP90α mediates LPS-signaling as a part of the LPS receptor cluster, involving reactive oxygen species, cell apoptosis,[36] and the regulation of IL-1 gene expression.[37]
In our study, extracellular HSP90α was significantly correlated with IL-8, previously shown to be associated with LPS-induced caspase-3 activation and apoptosis.[38] Furthermore, intracellular HSP72 and HSP90α exhaustion, which occurs as sepsis progresses,[39] results in a progressive decrease of HLA-DR on CD14+ monocytes in severe sepsis.[39] Importantly, intracellular HSP90α expression was recently shown to improve cellular resistance to heat-shock and apoptosis, a positive effect attenuated by HSP90α pre-stress inhibition.[40] Similarly, HSP72 mRNA and intracellular HSP72 proteins were higher in sepsis, further induced by heat-shock and related to serum ILs.[41] In our study, HSP90α, TNF, IL-6, and nCD64 were also correlated with fever. Hypoxia-inducible factor-1 alpha and HSP90α can orchestrate temperature-dependent gene transcription and involve in thermal adaptation.[42]
Several limitations of our experimental approach should be mentioned. Given the number of strict exclusion criteria applied to assure homogeneity of our subcohorts and the rarity of nonimmune-compromised patients with severe sepsis, our sample size is relatively small, although it is larger compared to the sample size of similar studies.[27,43] Accordingly, it is not possible to establish the function of HSP90α in circulation during disease in such a translational study. Subanalyses of interacting effects of specific therapeutic interventions on extracellular HSP90α concentrations were not conducted due to the size of patients in our cohorts. However, by using adrenaline/noradrenaline as first choice inotropes/vasoacting agents, we did not have patients receiving dobutamine, recently shown to induce DNA-binding of HSF-1 and mRNA-expression of HSP90α.[44] During the study period, none of our patients was receiving thiopental that specifically and differentially induces a heat shock response, mediating thereby cytoprotection via the expression of HSP72 in human T-lymphocytes.[45] Another limitation of the study is that we did not determine extracellular HSP concentrations beyond the first day of hospitalization in PICU. The possible fluctuations of extracellular and intracellular HSP90α and HSP72 concentrations over time are currently under examination by our group.
Novel immune-metabolic and neurophysiological mechanisms underlie altered cellular bioenergetics and organ failure.[46] In particular, the secretion and extracellular action of HSP90α has been proposed to be a critical step downstream of the hypoxia-inducible factor-1 alpha pathway, leading to enhanced cell migration and wound healing.[47] Our study findings, showing an early increase of extracellular HSP90α in acute severe stress and its relation not only to the protective nCD64, IL-10 but also to the inflammatory IL-6, IL-8, PCT, CRP, and PELOD, and to the suppressed LDL and HDL, might imply HSP90α as a contributing factor not only in the remodeling of damaged organ tissues but also in provoking apoptosis in severe sepsis.[15] This is in accordance to experimental studies showing that HSP90 inhibitors improve survival and ameliorate inflammation, shock, and organ dysfunction.[48] It has been already reported that targeting HSP90α provides protection against intestinal inflammation and leakage and has been proposed to be a useful strategy to ameliorate intestinal failure in polymicrobial sepsis.[49] Risk models for all-ages’ critical illness, based on multimarker genomics, lipidomics and metabolomics,[50] could possibly include HSP72 and HSP90α, thus making HSP90α a promising tool in the future improvement of the therapeutic approach of severe sepsis and MOSF.
5. Conclusions
In the present study, we report for the first time that the extracellular HSP90α is significantly elevated in the critically ill with SIRS, especially in pediatric patients with severe sepsis. More importantly, we showed the extracellular “chaperokine” HSP90α is associated with MOSF, inflammatory stress, severity of illness, organ dysfunction, length of stay, days on ventilator, and predicted mortality. The HSP90α and HSP72 inverse relations to the low-LDL/low-HDL early septic metabolic derangement might well imply a coordinator role of their increased extracellular expressions at the onset of the host response in severe sepsis. Future studies are required to further advance our understanding of mechanisms that regulate the immune responses during severe sepsis and SIRS-related MOSF that may lead to the identification of new diagnostic, prognostic, or treatment targets.
Footnotes
Abbreviations: HDL = high-density-lipoprotein, HSP = heat shock protein, ILs = interleukins, LDL = low-density lipoprotein, LOMV = length of mechanical ventilation, LOS = length of stay, MOSF = multiple organ system failure, PELOD = pediatric logistic organ dysfunction, PRISM = pediatric risk of mortality, SIRS = the systemic inflammatory response syndrome, TISS = the Therapeutic Intervention Scoring System, TLR4 = toll-like-receptor-4.
Authorship: DMF and HD have generated, collected, and assembled the data; GB and DMF concept and designed the study; MV and MK substantially contributed to assembly and analyze samples; MV, HD, and SI have substantially contributed to the interpretation of data and revision and editing of the draft; DMF and GB organized patient recruitment, analyzed and interpreted the data and drafted the manuscript; all authors edited and approved the final version of the manuscript. All authors read and approved the manuscript.
Funding: This research has been co-financed by the European Union (European Social Fund (ESF)) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)-Research Funding Program: THALES
The authors have no conflicts of interest to disclose.
References
- 1.Groselj-Grenc M, Ihan A, Derganc M. Neutrophil and monocyte CD64 and CD163 expression in critically ill neonates and children with sepsis: comparison of fluorescence intensities and calculated indexes. Mediators Inflamm 2008; 2008:202646.doi:10.1155/2008/202646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 2006; 79:425–434.doi:10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- 3.Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones 2003; 8:272–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma HS, Muresanu D, Sharma A, et al. Cerebrolysin treatment attenuates heat shock protein overexpression in the brain following heat stress: an experimental study using immunohistochemistry at light and electron microscopy in the rat. Ann NY Acad Sci 2010; 1199:138–148.doi:10.1111/j.1749-6632.2009.05330.x. [DOI] [PubMed] [Google Scholar]
- 5.Briassoulis G, Briassouli E, Fitrolaki D-M, et al. Heat shock protein 72 expressing stress in sepsis: unbridgeable gap between animal and human studies—a hypothetical “comparative” study. BioMed Res Int 2014; 2014:101023.doi:10.1155/2014/101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderwood SK, Mambula SS, Gray PJ, et al. Extracellular heat shock proteins in cell signaling. FEBS Lett 2007; 581:3689–3694.doi:10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler DS, Fisher LE, Catravas JD, et al. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med 2005; 6:308–311. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler DS, Wong HR. Heat shock response and acute lung injury. Free Radic Biol Med 2007; 42:1–14.doi:10.1016/j.freeradbiomed.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA 2000; 97:10832–10837.doi:10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol 2001; 188:281–290.doi:10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, et al. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med 2007; 176:667–675.doi:10.1164/rccm.200702-291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Sahu D, Tsen F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim Biophys Acta 2012; 1823:730–741.doi:10.1016/j.bbamcr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaprakash P, Dong H, Zou M, et al. HSP90α and HSP90β co-operate a stress-response mechanism to cope with hypoxia and nutrient paucity during wound healing. J Cell Sci 2015; 128:1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song X, Luo Y. The regulatory mechanism of Hsp90alpha secretion from endothelial cells and its role in angiogenesis during wound healing. Biochem Biophys Res Commun 2010; 398:111–117.doi:10.1016/j.bbrc.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Luo R, Jiang R, et al. The role of the Hsp90/Akt pathway in myocardial calpain-induced caspase-3 activation and apoptosis during sepsis. BMC Cardiovasc Disord 2013; 13:8.doi:10.1186/1471-2261-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill S, Ross JA, Wigmore SJ, et al. The role of heat shock protein 90 in modulating ischemia-reperfusion injury in the kidney. Expert Opin Investig Drugs 2012; 21:1535–1548.doi:10.1517/13543784.2012.713939. [DOI] [PubMed] [Google Scholar]
- 17.Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol 2001; 188:281–290.doi:10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 18.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet Lond Engl 2003; 362:192–197.doi:10.1016/S0140-6736 (03)13908-6. [DOI] [PubMed] [Google Scholar]
- 19.Miranda DR, de Rijk A, Schaufeli W. Simplified therapeutic intervention scoring system: the TISS-28 items—results from a multicenter study. Crit Care Med 1996; 24:64–73. [DOI] [PubMed] [Google Scholar]
- 20.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996; 24:743–752. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson JD, Pollack MM, Glass NL, et al. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr 1987; 111:324–328. [DOI] [PubMed] [Google Scholar]
- 22.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:762–774.doi:10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang A, Benke D, Eitner F, et al. Heat shock protein 60 is released in immune-mediated glomerulonephritis and aggravates disease: in vivo evidence for an immunologic danger signal. J Am Soc Nephrol 2005; 16:383–391.doi:10.1681/ASN.2004040276. [DOI] [PubMed] [Google Scholar]
- 24.Cheng C-F, Fan J, Fedesco M, et al. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol Cell Biol 2008; 28:3344–3358.doi:10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol Baltim Md 19502006; 177:7849–7857. [DOI] [PubMed] [Google Scholar]
- 26.Quanz M, Herbette A, Sayarath M, et al. Heat shock protein 90α (HSP90α) is phosphorylated in response to DNA damage and accumulates in repair foci. J Biol Chem 2012; 287:8803–8815.doi:10.1074/jbc.M111.320887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelain DP, de Bittencourt Pasquali MA, M Comim C, et al. Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock 2011; 35:466–470.doi:10.1097/SHK.0b013e31820fe704. [DOI] [PubMed] [Google Scholar]
- 28.Dybdahl B, Slørdahl SA, Waage A, et al. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart Br Card Soc 2005; 91:299–304.doi:10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukushima A, Kawahara H, Isurugi C, et al. Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia. J Obstet Gynaecol Res 2005; 31:72–77.doi:10.1111/j.1447-0756.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 30.Vardas K, Apostolou K, Briassouli E, et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. BioMed Res Int 2014; 2014:803561.doi:10.1155/2014/803561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitrolaki D-M, Dimitriou H, Kalmanti M, et al. CD64-Neutrophil expression and stress metabolic patterns in early sepsis and severe traumatic brain injury in children. BMC Pediatr 2013; 13:31.doi:10.1186/1471-2431-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R1, Wan XJ1, Zhang X1, et al. Plasma HSPA12B is a potential predictor for poor outcome in severe sepsis. PLoS One 2014; 9:e101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13:862–874.doi:10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 2000; 6:435–442.doi:10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 35.Anderson KM, Srivastava PK. Heat, heat shock, heat shock proteins and death: a central link in innate and adaptive immune responses. Immunol Lett 2000; 74:35–39. [DOI] [PubMed] [Google Scholar]
- 36.Kim SO, Ono K, Han J. Apoptosis by pan-caspase inhibitors in lipopolysaccharide-activated macrophages. Am J Physiol Lung Cell Mol Physiol 2001; 281:L1095–L1105. [DOI] [PubMed] [Google Scholar]
- 37.Hsu H-Y, Wen M-H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem 2002; 277:22131–22139.doi:10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 38.Schneider EM, Lorenz I, Ma X, et al. G-CSF modulates LPS-induced apoptosis and IL-8 in human microvascular endothelial cells: involvement of calcium signaling. Ann N Y Acad Sci 2003; 1010:78–85. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos P, Pistiki A, Christodoulopoulou T, et al. Heat shock proteins 70/90 and associations with immunosuppression along with sepsis: preliminary data. Crit Care 2015; 19 suppl 1:42.doi:10.1186/cc14122.25879803 [Google Scholar]
- 40.Zhang X-H, Wu H, Tang S, et al. Apoptosis in response to heat stress is positively associated with Hsp90 expression in chicken myocardial cells in vitro. J Vet Sci 2016; June 13. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briassouli E, Tzanoudaki M, Goukos D, et al. Glutamine may repress the weak LPS and enhance the strong heat shock induction of monocyte and lymphocyte HSP72 proteins but may not modulate the HSP72 mRNA in patients with sepsis or trauma. BioMed Res Int 2015; 2015:806042.doi:10.1155/2015/806042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leach MD, Farrer RA, Tan K, et al. Hsf1 and Hsp90 orchestrate temperature-dependent global transcriptional remodelling and chromatin architecture in Candida albicans. Nat Commun 2016; 7:11704.doi:10.1038/ncomms11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fehrenbach E, Niess AM, Veith R, et al. Changes of HSP72-expression in leukocytes are associated with adaptation to exercise under conditions of high environmental temperature. J Leukoc Biol 2001; 69:747–754. [PubMed] [Google Scholar]
- 44.Roesslein M, Froehlich C, Jans F, et al. Dobutamine mediates cytoprotection by induction of heat shock protein 70 in vitro. Life Sci 2014; 98:88–95.doi:10.1016/j.lfs.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Roesslein M, Schibilsky D, Muller L, et al. Thiopental protects human T lymphocytes from apoptosis in vitro via the expression of heat shock protein 70. J Pharmacol Exp Ther 2008; 325:217–225.doi:10.1124/jpet.107.133108. [DOI] [PubMed] [Google Scholar]
- 46.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 2014; 40:463–475.doi:10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Li Y, Guan S, et al. Extracellular heat shock protein-90 alpha: linking hypoxia to skin cell motility and wound healing. EMBO J 2007; 26:1221–1233.doi:10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y-L, Shen H-H, Cheng P-Y, et al. 17-DMAG, an HSP90 inhibitor, ameliorates multiple organ dysfunction syndrome via induction of HSP70 in endotoxemic rats. PloS One 2016; 11:e0155583.doi:10.1371/journal.pone.0155583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Huang Z-J, Rahman M, et al. Radicicol, an Hsp90 inhibitor, inhibits intestinal inflammation and leakage in abdominal sepsis. J Surg Res 2013; 182:312–318.doi:10.1016/j.jss.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 50.Mickiewicz B, Vogel HJ, Wong HR, et al. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am J Respir Crit Care Med 2013; 187:967–976.doi:10.1164/rccm.201209-1726OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


