Supplemental Digital Content is available in the text
Keywords: asthma, eczema, infection, meta-analysis
Abstract
Background:
The influence of maternal infection during pregnancy on allergic disorders in offspring is not well understood. We performed a systematic review and meta-analysis to evaluate current evidence on the association between maternal infection during pregnancy and asthma or eczema in offspring.
Methods:
We searched databases (PubMed, EMBASE, Medline, and Web of Science) for all relevant studies published before March 2016. Any cohort studies, case–control studies, or cross-sectional studies published in English and focused on the association between maternal infection during pregnancy and the risk of asthma or eczema in offspring were included. Random-effects models were used for combined analyses.
Results:
A total of 10 studies with 299,830 participants were included. Maternal infection was associated with an increased risk for asthma (odds ratio [OR]: 1.55; 95% confidence interval [CI]: 1.24–1.92; P < 0.01) and eczema (OR: 1.36; 95% CI: 1.13–1.64; P < 0.01). Further analyses showed associations between asthma and several specific maternal infections: fever episode (OR: 1.73; 95% CI: 1.35–2.23), chorioamnionitis (OR: 1.42; 95% CI: 0.96–2.11), respiratory infection (OR: 1.49; 95% CI: 0.94–2.36), and urogenital infection (OR: 1.39; 95% CI: 1.18–1.64).
Conclusion:
The results from this meta-analysis and systematic review provide evidence that maternal infection during pregnancy might be related to subsequent asthma and eczema in offspring. However, there was variation of included studies with regard to type of maternal infection, age of children, and methods of exposure ascertainment. Additional studies are needed to further confirm these associations.
1. Introduction
Asthma is characterized by an increased responsiveness of the airway and airway obstruction under various stimuli that is partially or completely reversible.[1] Eczema is characterized by epidermal dryness and itchiness that causes typical relapsing lesions and plaques.[2] Both are common chronic diseases that pose a global public health problem with significant impacts on direct, indirect, intangible, and opportunity costs. Worldwide prevalence rates of these disorders have remained high despite significant advances in disease prevention and therapy.[3,4]
The underlying mechanisms for asthma and eczema are unknown. Increasing evidence from animal and human studies suggest that fetal programming is a very critical stage for subsequent development of the immune and neuroendocrine systems; prenatal exposures to adverse events may induce epigenetic and immunologic modifications that may contribute to a higher risk for allergic disorders.[5,6] Maternal infections are one of the most common complications that occur during pregnancy. Maternal infection during pregnancy is associated with maternal morbidity, such as cesarean sections, and fetal and neonatal morbidity, such as premature rupture of membranes, low birth weight, bronchopulmonary dysplasia, and cerebral palsy.[7–9]
There is inconsistent evidence from studies that have investigated the association between maternal infection during pregnancy and asthma or eczema. The objective of this systematic review and meta-analysis was to determine if maternal infections during pregnancy are associated with an increased risk of asthma or eczema in offspring.
2. Methods
2.1. Search strategy and study selection
There is no registered protocol for this search. The PubMed, EMBASE, Medline, and Web of Science databases were searched for all relevant studies published before March 2016. Search terms included MeSH terms and free texts [e.g., (prenatal OR antenatal OR gestational OR maternal) AND (infection OR chorioamnionitis OR febrile) AND (asthma OR wheeze OR eczema)] (search strategy see in sTable 1 and sTable 2). The search was restricted to human studies published in the English language. We also identified additional relevant studies through manual searches of the reference lists of included studies. Titles and abstracts of the articles were initially screened; subsequently, 2 reviewers (ZDM and YQ) independently reviewed full-text articles to determine qualification based on selection criteria. Any differences in opinion were resolved through discussions with the study team.
Studies were included if they met the following criteria: they were on the topic of association between maternal infection during pregnancy and the risk of asthma or eczema in offspring; the study design was cohort, case–control, or cross-sectional; and they reported risk estimates (e.g., OR [ratio odd], RR [relative risk], HR [hazard ratios]) or original data from which risk estimates could be calculated. Studies that were case reports, case series, or letters were excluded. If the same research had duplicate reports, only the most recent one was included in the meta-analysis.
2.2. Data extraction and study quality assessment
Two researchers (ZDM and YQ) independently extracted data from all included studies on first author's name, publication year, study location, study design, maternal infection, children's allergic disorder information, sample size, effect size and 95% confidence interval (CI), and adjusted or matched varieties. Disagreements about study information were resolved through discussing with a third author (TTZ).
All included studies were appraised independently by 2 authors using the Newcastle–Ottawa Scale (NOS).[10] This scale consists of 3 parts for cohort and case–control studies: selection (4 items), comparability (1 item), and outcome/exposure (3 items). The maximum score is 9 stars. The scores of 0 to 4, 5 to 7, and 8 to 9 stars correspond to studies that are considered as low, moderate, and high quality, respectively. Any disagreements between authors were resolved by discussion with a third author.
2.3. Data synthesis and statistical analysis
We conducted a meta-analysis to generate pooled ORs and 95% CI for the association between maternal infection during pregnancy and asthma or eczema in offspring using RevMan 5.3.5 and STATA Version 12.0.
Statistical heterogeneity across eligible studies was assessed by both the Cochran Q statistic test and the I2 heterogeneity test. The P-value for significance of the Cochran Q test was set to <0.10. Heterogeneity was divided into three levels according to the value of the I2 statistic: mild (I2 = 0–25%), moderate (I2 = 25–50%), and high (I2 = 50–100%). A random effects model was performed for all analyses in this study, as there was significant heterogeneity across studies in the pooled analysis and a small number of studies (<5 studies) in the subgroup meta-analyses for types of maternal infections.[11] In regards to study design, an outcome of asthma and adjustments for confounding factors were not consistent between studies; we conducted a sensitivity analysis to detect sources of heterogeneity and to examine the influence of various exclusion criteria on the overall risk estimate. We also conducted a sensitivity analysis by removing each study sequentially to examine the effect of elimination on the remaining pooled results. Publication bias was assessed by inspection of the funnel plots as well as Egger and Begg statistical test.
2.4. Ethical statement
As all analyses were based on previously published studies, ethical approval was not necessary.
3. Results
3.1. Literature search
A total of 426 potential, nonduplicate studies were identified from the online databases and screening reference lists of selected studies. After screening titles and abstracts of these articles, 373 records were excluded for being duplicate records, irrelevant topics, reviews, animal studies, in another language, or having no outcome data. Full texts of the remaining 32 studies were assessed for eligibility. Finally, we included 10 studies in this systematic review and meta-analysis. A flow diagram of study selection is presented in the supplemental data (PRISMA 2009 Flow Diagram).
3.2. Characteristics of included studies
Primary characteristics of included studies are summarized in Table 1. Seven cohort studies,[1,12–16,19] 2 case–control studies,[17,18] and 1 cross-sectional study[2] were included in the analysis. Publishing year ranged from 1999 to 2015. There was a pooled total of 299,830 participants from the 10 included studies. Half of the studies (n = 5) reported a positive association between maternal infection during pregnancy and asthma in offspring,[1,14,17–19] while the remaining 5 studies did not show a significant association. Five studies[2,15,17–19] were from Europe, 4[1,12,13,16] from America, and 1[14] from Australia. Nine studies[1,2,12–15,17–19] adjusted for or matched cases with potential confounding factors such as maternal age at delivery, maternal history of allergic disorders, maternal smoking during pregnancy, birth weight, gestational age, child's age, child's sex.
Table 1.
Characteristics of included studies.
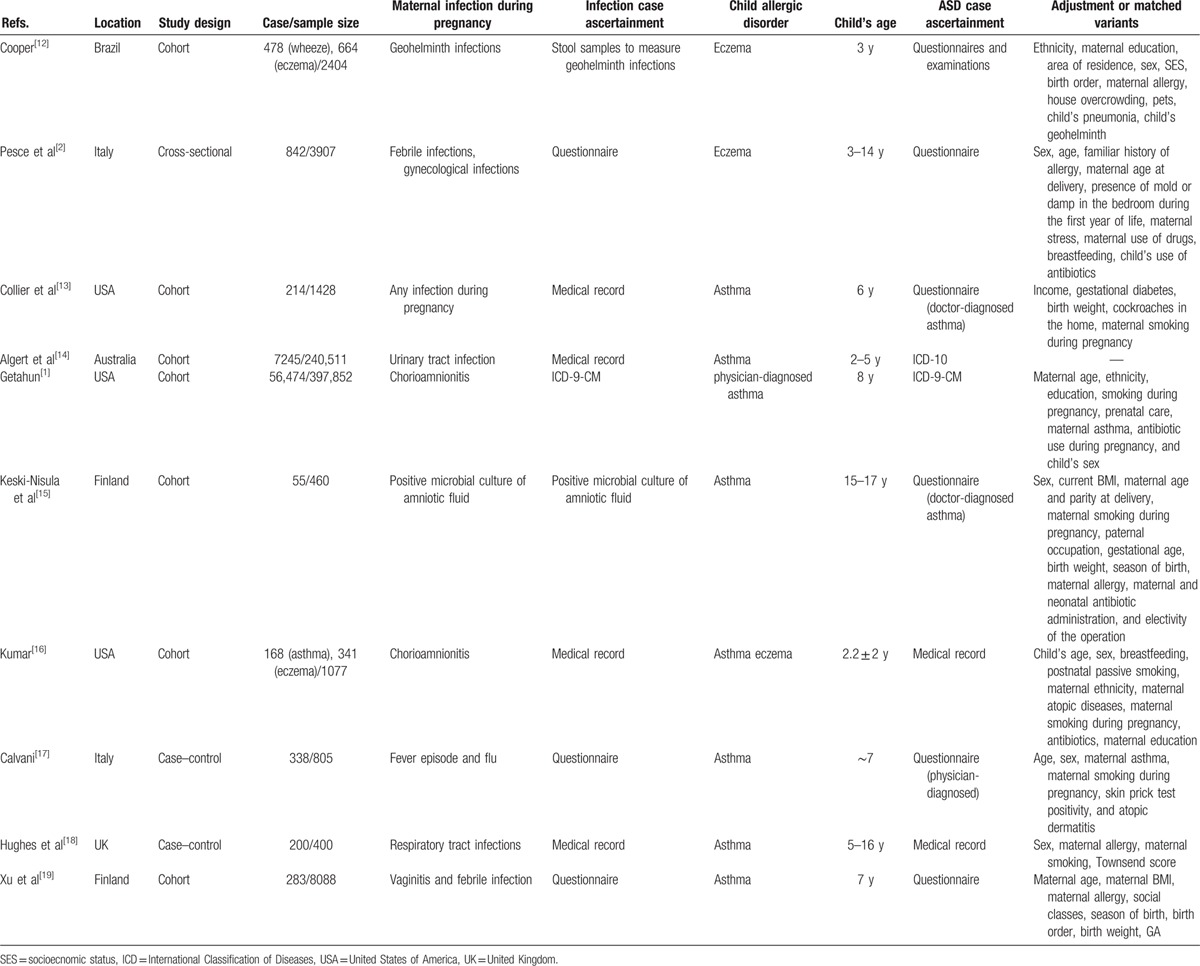
Included studies diagnosed maternal infection in a variety of ways: 7 studies were based on medical record or questionnaires,[2,12,14,16–19] 1 diagnosed cases according to the International Classification of Diseases (ICD-9),[1] 1 according to stool samples to measure geohelminth infection,[11] and 1 cultured microbes from amniotic fluid samples.[15] In all studies, maternal infection occurred before delivery.
Outcome assessment varied between studies. Five studies[2,12,13,15,17] used questionnaires (of which 3 studies[13,15,17] specified “report of physician-diagnosed asthma or eczema”), and 5[1,14,16,18,19] studies diagnosed by physician or medical record review. Participant age range varied between studies, including 2 to 5 years,[14] 15 to 17 years,[15] 3 to 14 years,[2] and 5 to 16 years.[18] Others were at a single time ranging from 1 to 8 years.
3.3. Quality assessment
The NOS scores for included studies ranged from 6 to 9 (median NOS score = 7), suggesting overall methodological quality of eligible studies was moderate to high (sTable 3). The cross-sectional[2] and case–control studies[17,18] were considered high quality, while the cohort studies[1,12–16,19] were considered moderate or high quality.
3.4. Maternal infection and the risk of asthma and eczema in offspring
Pooled results identified a positive association between maternal infection and the risk of asthma in offspring (pooled OR: 1.55; 95% CI: 1.24–1.92) (Fig. 1). However, significantly high heterogeneity across studies was noted in the overall analyses (I2 = 88%; P < 0.01). A funnel plot (Fig. 2) was visually asymmetrical, but the Egger test and Begg test (all P > 0.05) confirmed that there was no publication bias in this meta-analysis.
Figure 1.
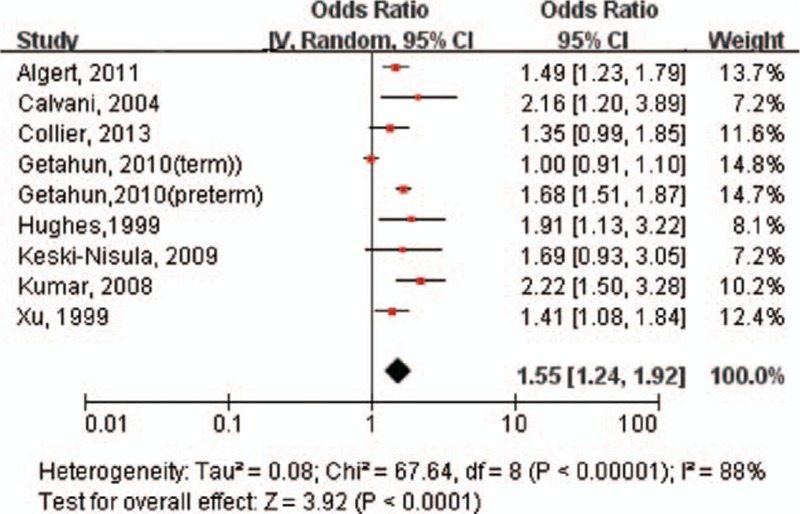
Forest plot shows association between maternal infection during pregnancy and risk of asthma. CI = confidence interval, OR = odds ratio.
Figure 2.
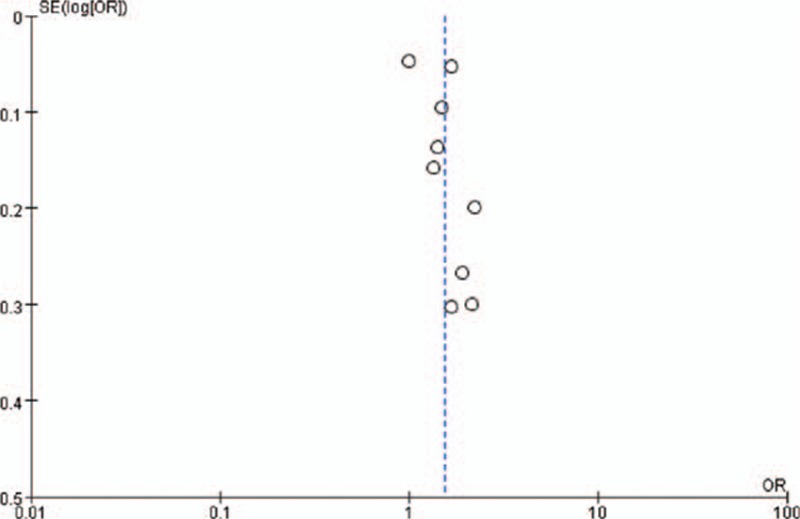
Funnel plot of overall asthma risk in offspring with maternal infection during pregnancy exposure. SE = standard error.
In the sensitivity analyses, exclusion of 2 case–control studies[17,18] yielded decreased results (OR: 1.47; 95% CI: 1.16–1.87; P < 0.01), with evidence of significant heterogeneity (I2 = 90%; P < 0.01). Exclusion of 2 correlated estimates from Getahun et al[1] showed similar results (OR: 1.57; 95% CI: 1.38–1.79; P < 0.01), but heterogeneity decreased to a nonsignificant level (I2 = 9%; P = 0.36). Additional exclusion of any single study did not significantly alter the overall pooled OR.
We further analyzed the association between several specific kinds of maternal infection and asthma. Strong associations of asthma were found with fever episode (OR: 1.73; 95% CI: 1.35–2.23), chorioamnionitis (OR: 1.42; 95% CI: 0.96–2.11), respiratory infection (OR: 1.49; 95% CI: 0.94–2.36), and urogenital infection (OR: 1.39; 95% CI: 1.18–1.64) (Fig. 3). There were a small number of included studies provided the data of eczema, so that we could not analyze the specific kinds of maternal infection and eczema.
Figure 3.
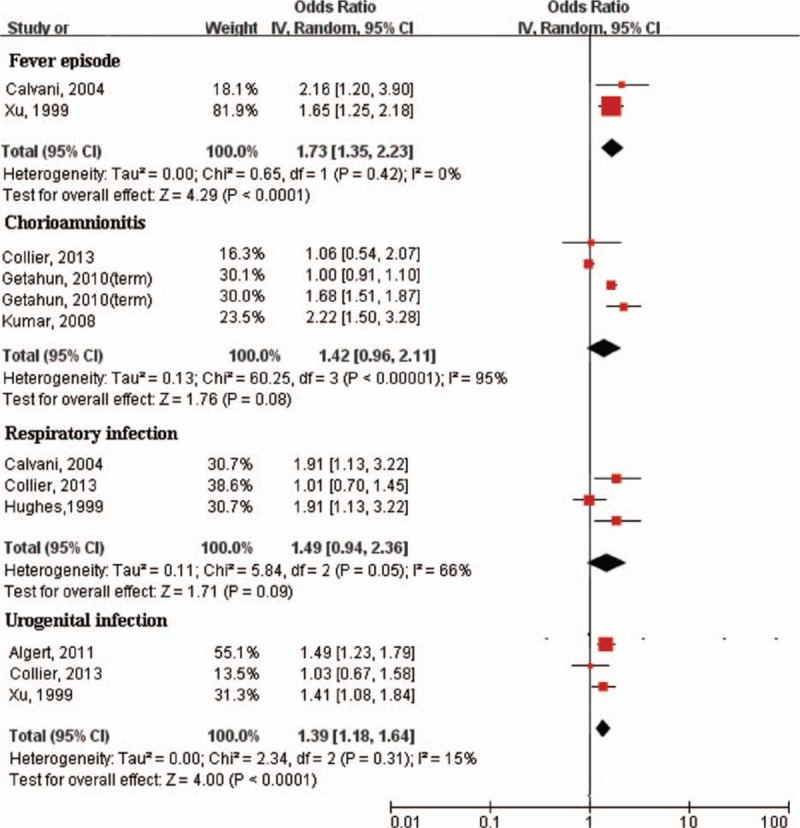
Forest plot of different kinds of maternal infection during pregnancy and risk of asthma. Fever episode and urogenital infection during pregnancy was significantly associated with asthma. CI = confidence interval, OR = odds ratio.
There was a statistically significant association of maternal infection with eczema (OR: 1.36; 95% CI: 1.13–1.64; P < 0.01) (Fig. 4). No significant heterogeneity was detected in the combined analysis (I2 = 0%; P = 0.87). The funnel plot indicated no publication bias and the Egger test was not significant (P = 0.482). In sensitivity analyses, exclusion of any single study did not significantly alter the overall combined OR.
Figure 4.
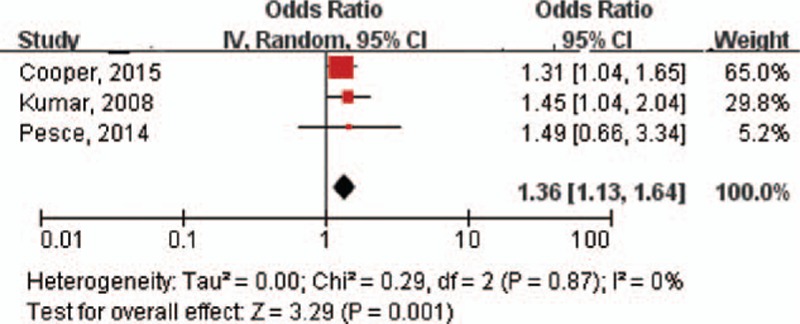
Forest plot shows association between maternal infection during pregnancy and risk of eczema. CI = confidence interval, OR = odds ratio.
3.5. Timing of antenatal maternal infection and risk of asthma and eczema
Most studies lacked data on the “sensitive period,” that is, the time period during pregnancy where maternal infection may be most harmful to the fetus. However, Hughes et al[18] found a stronger association of childhood asthma with respiratory infection during the first trimester compared with the second and third trimesters. A recent study conducted by Algert et al[14] observed that gestational age at time of first antenatal urinary tract infection had no effect on the risk of childhood asthma. Pesce et al[2] reported that the risk of eczema was significantly higher in children born to mothers who reported febrile infections during the first trimester or gynecological infections during the third trimester of pregnancy.
4. Discussion
In the present study, we performed a meta-analysis and systematic review of publications that evaluated the strength of association between maternal infection and asthma or eczema in their offspring. We showed that maternal infection was associated with an increased risk of asthma and eczema in offspring compared with control groups. The magnitude of pooled risk remained almost unchanged in the sensitive analysis. We observed that the substantial heterogeneity changed to be a rather lower level after excluding the study with 2 effect estimates, indicating that the overall heterogeneity could likely be explained by the nonindependent estimates in study. Heterogeneity was also reduced in the further separate analysis according to type of maternal infection. However, the conclusion of the adverse effect of maternal infection on asthma and eczema should be treated with caution for that maternal chorioamnionitis and respiratory infection during pregnancy showed a trend toward increased ORs but did not reach significant difference statistically.
Maternal infections during pregnancy not only have an adverse impact on the mothers, but also are likely to be an independent risk factor for allergic disorders in their children. Fetal programming is a biological mechanism that may explain why maternal infection during pregnancy is associated with children's allergic disorders, and prenatal or postnatal factors may have dramatic consequences in the early programming of the developing immune system.[20] Maternal infection is associated with a strong proinflammatory response that increases inflammatory cytokines. Studies based on animal models and human samples clearly showed that chorioamnionitis increased the expression of interleukin (IL)-6, tumor necrosis factor (TNF)-α, interferon (IFN)-β, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1β, and IL-8 in uterus.[21–23] Tracheal aspirate samples collected from ventilated preterm infants showed higher concentrations of IL-1α, IL-1β, and IL-8 in a group of infants with chorioamnionitis.[24]
Among these inflammatory cytokines, serum TNF-α has been found to have a significant negative correlation with forced expiratory volume in 1 second (FEV1) in asthmatic children.[25] IL-8 levels were increased in children who developed wheezing illnesses in response to rhinovirus[26]; in another cohort, individuals who wheezed with rhinovirus were at additional risk of persistent wheezing at follow up at age 3 years.[27] Fetal exposure to inflammatory cytokines, including IL-6, IL-8, and TNF-α, is increased with chorioamnionitis and has been linked to chronic lung disease.[28] Since fetal lung and skin are in constant contact with amniotic fluid, exogenous toxins or mediators of inflammation released through or from the placenta may lead to fetal exposure. It is also possible that early neonatal infection caused by bacterial colonization during delivery could be more common in children who have a genetic predisposition to allergy and asthma through a defective innate immune response in the early phase of life.
Although atopy plays an important role in the pathogenesis of asthma, fewer than half of childhood asthma cases are attributable to atopy.[29] Other potential risk factors associated with childhood asthma include preterm birth, low birth weight, and maternal antibiotic use during pregnancy.[30–32] Previous studies found an increased risk of preterm birth and low birth weight due to maternal infection during pregnancy.[7–9] A comprehensive meta-analysis by Zhao et al[32] showed that antenatal antibiotics were significantly associated with asthma or wheezing in childhood. In the present study, only 3 included studies (1 for eczema and 2 for asthma) reported that maternal infection was significantly associated with asthma and eczema after adjustment for maternal antibiotic usage,[1,2,15] suggesting that maternal infection might be an independent risk factor for asthma in children. However, we cannot completely rule out some residual confounding, as data were not available on specific types of antibiotics used or frequency of administration.
There were several strengths in this meta-analysis including the use of sensitivity analyses to further assess significant results and a large sample size. However, some potential limitations should be discussed. First, we only included studies published in English. A systemic bias might exist in this study due to the exclusion of languages other than English. Second, the results are lack of power, since the relatively small number of studies in the combined analysis. Third, eligible studies contained a variety of types of maternal infection during pregnancy, and analysis stratified by types of maternal infection showed insignificant results for chorioamnionitis and respiratory infection. Finally, although major confounding factors were adjusted in the included studies, we were unable to remove potential confounding factors, such as antibiotic exposure, birth weight, and breastfeeding.
On the basis of our findings, several questions arise. First, is there a strong link between maternal infection and allergic disorders in their offspring? Future studies need to consider many issues, including adequate control for confounding factors, use of a validated outcome measurement, and assessment of different types of allergic disorders. Second, is the association of maternal infection with asthma and eczema influenced by different pathogens or in different organ systems? Collier et al demonstrated that urinary tract infections are associated with a 60% increase in the odds of childhood asthma. Other types of antepartum infection, including gynecologic infections and respiratory infections, were not associated with an increased risk of childhood asthma.[12] There is no clear explanation for how prenatal urinary tract infections are more likely to lead to persistent asthma in children over other infections. Third, whether or not there is a “sensitive period” for vulnerability is an issue that must be resolved to understand the underlying biological mechanisms of allergic disorders in children and to consider novel therapeutic interventions; this was challenging to interpret due to significant variation among studies in design, exposure, and outcome measurements.
In conclusion, this meta-analysis and systematic review provides evidence that maternal infection during pregnancy may increase the risk of asthma and eczema in offspring. Given the limitations existing in current article, more studies are needed to further this confirmed this association and explore the underlying questions.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, NOS = Newcastle–Ottawa Scale, OR = odds ratio.
Funding: This work was supported by the National Science Foundation of China (no. 81330016 and 31171020 to DM; no. 81172174 and 81270724 to YQ), the Major State Basic Research Development Program (2013CB967404), the Grants from Ministry of Education of China (IRT 0935, 313037, 20110181130002), the Grant from State Commission of Science Technology of China (2012BAI04B04), the Grants from Science and Technology Bureau of Sichuan province (2014SZ0149), and the Grant of clinical discipline program (neonatology) from the Ministry of Health of China (1311200003303).
Author contributions: TTZ and LZ contributed equally to this work. TTZ and LZ conceived and designed the study. ZDM and YQ conducted literature searches and data collection. TTZ and LZ performed the statistical analysis and wrote manuscript. ZDM revised the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Getahun D, Strickland D, Zeiger RS, et al. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med 2010; 164:187–192. [DOI] [PubMed] [Google Scholar]
- 2.Pesce G, Marcon A, Marchetti P, et al. Febrile and gynecological infections during pregnancy are associated with a greater risk of childhood eczema. Pediatr Allergy Immunol 2014; 25:159–165. [DOI] [PubMed] [Google Scholar]
- 3.de Korte-de Boer D, Mommers M, Gielkens-Sijstermans CM, et al. Stabilizing prevalence trends of eczema, asthma and rhinoconjunctivitis in Dutch schoolchildren (2001–2010). Allergy 2015; 70:1669–1673. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Zhou Y, Li S, et al. Prevalence and risk factors of childhood allergic diseases in eight metropolitan cities in China: a multicenter study. BMC Public Health 2011; 11:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harpsoe MC, Basit S, Bager P, et al. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol 2013; 131:1033–1040. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig IR, Sly PD, Schmidt LA, et al. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol 2014; 134:160–169. [DOI] [PubMed] [Google Scholar]
- 7.Gagliardi L, Rusconi F, Bellù R, et al. Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics 2014; 134:e154–e161. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson L, Haglund B, Odlind V, et al. Prenatal inflammatory risk factors for development of bronchopulmonary dysplasia. Pediatr Pulmonol 2014; 49:665–672. [DOI] [PubMed] [Google Scholar]
- 9.Miller JE, Pedersen LH, Streja E, et al. Maternal infections during pregnancy and cerebral palsy: a population-based cohort study. Paediatr Perinat Epidemiol 2013; 27:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G, Shea B, O’Connell J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2011) Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed July 4, 2014. [Google Scholar]
- 11.IntHout J, Ioannidis JP, Borm GF, et al. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014; 14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper PJ, Chico ME, Amorim LD, et al. Effects of maternal geohelminth infections on allergy in early childhood. J Allergy Clin Immunol 2016; 137:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collier CH, Risnes K, Norwitz ER, et al. Maternal infection in pregnancy and risk of asthma in offspring. Matern Child Health J 2013; 17:1940–1950. [DOI] [PubMed] [Google Scholar]
- 14.Algert CS, Bowen JR, Lain SL, et al. Pregnancy exposures and risk of childhood asthma admission in a population birth cohort. Pediatr Allergy Immunol 2011; 22:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keski-Nisula L, Katila ML, Remes S, et al. Intrauterine bacterial growth at birth and risk of asthma and allergic sensitization among offspring at the age of 15 to 17 years. J Allergy Clin Immunol 2009; 123:1305–1311. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Yu Y, Story RE, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol 2008; 121:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvani M, Alessandri C, Sopo SM, et al. Infectious and uterus related complications during pregnancy and development of atopic and nonatopic asthma in children. Allergy 2004; 59:99–106. [DOI] [PubMed] [Google Scholar]
- 18.Hughes CH, Jones RC, Wright DE, et al. A retrospective study of the relationship between childhood asthma and respiratory infection during gestation. Clin Exp Allergy 1999; 29:1378–1381. [DOI] [PubMed] [Google Scholar]
- 19.Xu B, Pekkanen J, Järvelin MR, et al. Maternal infections in pregnancy and the development of asthma among offspring. Int J Epidemiol 1999; 28:723–727. [DOI] [PubMed] [Google Scholar]
- 20.Jones CA, Kilburn SA, Warner JA, et al. Intrauterine environment and fetal allergic sensitization. Clin Exp Allergy 1998; 28:655–659. [DOI] [PubMed] [Google Scholar]
- 21.Uchide N, Ohyama K, Bessho T, et al. Possible roles of proinflammatory and chemoattractive cytokines produced by human fetal membrane cells in the pathology of adverse pregnancy outcomes associated with influenza virus infection. Mediators Inflamm 2012; 2012:270670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taki A, Abe M, Komaki M, et al. Expression of angiogenesis-related factors and inflammatory cytokines in placenta and umbilical vessels in pregnancies with preeclampsia and chorioamnionitis/funisitis. Congenit Anom (Kyoto) 2012; 52:97–103. [DOI] [PubMed] [Google Scholar]
- 23.Toti P, Arcuri F, Tang Z, et al. Focal increases of fetal macrophages in placentas from pregnancies with histological chorioamnionitis: potential role of fibroblast monocyte chemotactic protein-1. Am J Reprod Immunol 2011; 65:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aghai ZH, Camacho J, Saslow JG, et al. Impact of histological chorioamnionitis on tracheal aspirate cytokines in premature infants. Am J Perinatol 2012; 29:567–572. [DOI] [PubMed] [Google Scholar]
- 25.Koksal BT, Ozbek OY, Bayraktar N, et al. Evaluation of angiopoietin 1 and 2, vascular endothelial growth factor, and tumor necrosis factor alpha levels in asthmatic children. Allergy Asthma Proc 2014; 35:482–488. [DOI] [PubMed] [Google Scholar]
- 26.Gern JE, Martin MS, Anklam KA, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol 2002; 13:386–396. [DOI] [PubMed] [Google Scholar]
- 27.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005; 116:571–577. [DOI] [PubMed] [Google Scholar]
- 28.Prendergast M, May C, Broughton S, et al. Chorioamnionitis, lung function and bronchopulmonary dysplasia in prematurely born infants. Arch Dis Child Fetal Neonatal Ed 2011; 96:F270–F274. [DOI] [PubMed] [Google Scholar]
- 29.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax 1999; 54:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol 2014; 133:1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.den Dekker HT, Sonnenschein-van der Voort AM, de Jongste JC, et al. Early growth characteristics and the risk of reduced lung function and asthma: a meta-analysis of 25,000 children. J Allergy Clin Immunol 2016; 137:1026–1035. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D, Su H, Cheng J, et al. Prenatal antibiotic use and risk of childhood wheeze/asthma: a meta-analysis. Pediatr Allergy Immunol 2015; 26:756–764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


