Abstract
This study aimed to investigate the potential misuse of novel oral anticoagulants (NOACs) and the physicians’ adherence to current European guideline recommendations in real-world using a large dataset from Real-life Multicenter Survey Evaluating Stroke Prevention Strategies in Turkey (RAMSES Study).
RAMSES study is a prospective, multicenter, nationwide registry (ClinicalTrials.gov identifier NCT02344901). In this subgroup analysis of RAMSES study, patients who were on NOACs were classified as appropriately treated (AT), undertreated (UT), and overtreated (OT) according to the European Society of Cardiology (ESC) guidelines. The independent predictors of UT and OT were determined by multivariate logistic regression.
Of the 2086 eligible patients, 1247 (59.8%) received adequate treatment. However, off-label use was detected in 839 (40.2%) patients; 634 (30.4%) patients received UT and 205 (9.8%) received OT. Independent predictors of UT included >65 years of age, creatinine clearance ≥50 mL/min, urban living, existing dabigatran treatment, and HAS-BLED score of <3, whereas that of OT were creatinine clearance <50 mL/min, ongoing rivaroxaban treatment, and HAS-BLED score of ≥3.
The suboptimal use of NOACs is common because of physicians’ poor compliance to the guideline recommendations in patients with nonvalvular atrial fibrillation (NVAF). Older patients who were on dabigatran treatment with good renal functions and low risk of bleeding were at risk of UT, whereas patients who were on rivaroxaban treatment with renal impairment and high risk of bleeding were at risk of OT. Therefore, a greater emphasis should be given to prescribe the recommended dose for the specified patients.
Keywords: anticoagulation, atrial fibrillation, off-label use, over-treatment, stroke, undertreatment
1. Introduction
Although vitamin K antagonist oral anticoagulants are currently recognized as the standard therapy for stroke prevention in atrial fibrillation (AF), their limitations prompted the development of non-vitamin K antagonist oral anticoagulants novel oral anticoagulants (NOACs). NOACs have been found to be safe and efficacious in large randomized controlled trials.[1–3] The efficacy and safety of dabigatran at a dose of 110 mg twice daily (bid) are similar to that of warfarin and they increase further at higher dose of 150 mg bid.[1] The stroke prevention activity and major bleeding rate of rivaroxaban are found to be comparable to that of warfarin. In addition, apixaban is found to be superior than warfarin in prevention of stroke with fewer incidences of bleeding compared with warfarin.[2,3]
Based on the results of phase III clinical studies, the ESC has updated the guideline for the management of AF and recommended NOACs for stroke prevention with a dose adjustment.[4] Dabigatran 110 mg BID is proposed to treat elderly (>80 years of age) patients with moderate chronic kidney disease (creatinine clearance 30–49 mL/min), risk of bleeding and concomitant use of interacting drugs like verapamil and dabigatran 150 mg bid is recommended for all other patients. For rivaroxaban, an adjusted once daily dose of 15 mg instead of 20 mg is recommended for the patients with creatinine clearance of 30 to 49 mL/min or risk of bleeding.[4] In case of apixaban, the recommended dose reduction criteria are same as in ARISTOTLE trial.[3,4] The overall trial results for the dose adjustment of rivaroxaban and apixaban according to the creatinine clearance are found to be consistent across subgroups and the RE-LY trial's post-hoc analysis using European label observed better net clinical benefit with dabigatran than warfarin.[5–7]
Use of NOACs in an inappropriate indication or at inappropriate doses is defined as off-label use or misuse. Although the dose adjustment is clearly recommended, NOACs may be used off-label. Earlier study evaluating prescription patterns of oral anticoagulants (OAC) found up to 47% of off-label use of NOACs.[8] Similarly, in a study conducted in Danish population, high prevalence of off-label prescription of dabigatran was noticed.[9] The misuse of NOACs indicated by the real-world data could be a possible reason for the adverse events.[10,11] There is a paucity of real-life data on the suboptimal use of OAC in patients with AF in Turkey; therefore, the present study was aimed to investigate the potential misuse of NOACs and effect of adherence to current recommendations in a real-world using large dataset from RAMSES Study in Turkey.
2. Methods
2.1. Study design
RAMSES study (ClinicalTrials.gov identifier NCT02344901) was a national, multicenter, cross-sectional registry of which design and methodology are detailed elsewhere.[12]
2.2. Setting and study population
The study was conducted in patients from 7 geographic regions of Turkey who have visited outpatient cardiology clinics at different hospitals including state, university, education and research, and private hospitals for the treatment within the different healthcare settings. The patients with age >18 years and electrocardiographically confirmed AF were enrolled into the study from February to May 2015. The patients with mechanical heart valve and mitral stenosis or with severe renal disease (creatinine clearance < 30 mL/min) or those who were not receiving NOAC therapy or in whom status of OAC therapy was unknown were excluded. In addition, the patients with a CHA2DS2VASc (congestive heart failure or left ventricular dysfunction, hypertension, age 65–75 years, diabetes mellitus, vascular disease, female sex [1 point for presence of each] thromboembolism or stroke history, age ≥75 years [2 points for presence of each]) score of 0 or 1 and females were excluded as OAC therapy is generally not recommended in these patients (Fig. 1). The HAS-BLED (hypertension, abnormal renal function, abnormal liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs predisposing to bleed, alcohol use [1 point for presence of each]) was calculated for each patient.
Figure 1.
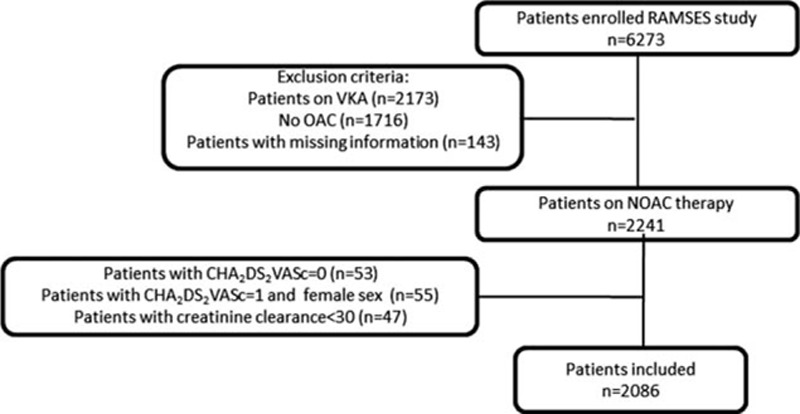
The flow of patients in RAMSES registry.
2.3. Data collection and outcomes
The survey was conducted to collect patient characteristics through well-designed questionnaire. Demographic data including age, sex, educational status, place of residence (rural or urban), and type of AF were noted. The information on patient's medical history, stroke, congestive heart failure and/or vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), or concurrent conditions with risk factors for stroke such as coronary artery disease, hypertension, and diabetes mellitus was collected. In addition, data on patients’ concurrent stroke prevention (antiplatelet and anticoagulant) and antiarrhythmic therapy and related hemorrhagic events and creatinine levels were recorded. The creatinine clearance was calculated using Cockroft-Gault formula and major bleeding was defined according to International Society of Thrombosis and Hemostasis criteria and incidences other than major bleeding were considered as non-major bleeding. CHA2DS2VASc and HAS-BLED scores were calculated based on history of hypertension, renal or liver failure, stroke, bleeding, labile international normalized ratio, age >65 years, concomitant drugs, or alcohol intake. The study was approved by ethics committee at Mugla Sitki Kocman University and written informed consent was obtained from all patients.
2.4. Definitions of under- and overtreatment
All of the 3 NOACs (dabigatran, rivaroxaban, and apixaban) in RAMSES study were used in 2 (low and high) doses. The lower dose of these drugs is recommended to treat patients with renal impairment and with high bleeding risk.[4] Dabigatran 110 mg bid and rivaroxaban 15 mg OD should be considered for patients with high risk of bleeding (HAS-BLED score ≥3) or moderate renal impairment (creatinine clearance 30–49 mL/min). The low dose of dabigatran should also be considered for elderly patients (age ≥80 years) and concomitant use of interacting drugs (e.g., verapamil). Apixaban 2.5 mg bid is recommended for patients complying with at least 2 of the following criteria: age ≥80 years, weight ≤60 kg, or serum creatinine ≥1.5 mg/dL. The patients were categorized into 3 groups according to these criteria:
AT: patients, those received recommended dose as per guideline
UT: patients, those were on the higher dose of NOAC, and
OT: patients, those were on the lower dose of NOAC.
AT: patients, those received recommended dose as per guideline,
UT: patients, those were on the lower dose of NOAC than the recommended dose, and
OT: patients, those were on the higher dose of NOAC than the recommended dose.
The parameters studied were compared among AT, UT, and OT groups.
2.5. Statistical analysis
The data collected were statistically analyzed using Statistical Package for Social Sciences software (SPSS 21, Chicago, IL). Continuous variables were summarized by median and interquartile range or mean ± standard deviation (SD). Categorical variables were expressed as frequencies and percentages. Student t test was applied to compare continuous variables and Fisher exact test or χ2 test was used to compare categorical variables. Multivariate logistic regression analysis was performed to detect independent predictors of UT and OT.
3. Results
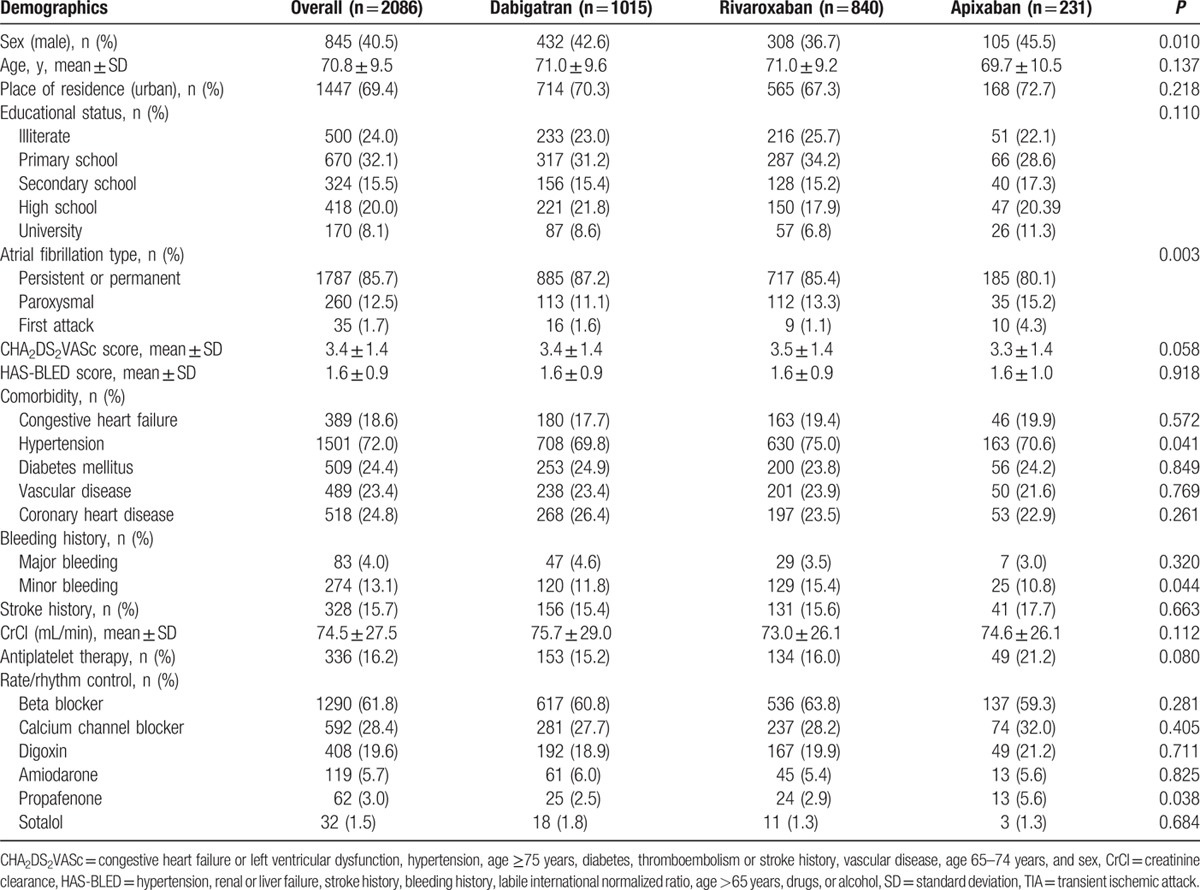
RAMSES study enrolled a total of 6273 patients across 57 sites from 29 provinces of Turkey. The data used in this subgroup analysis were from 2086 patients: dabigatran (dose 110 mg [n = 626] and dose 150 mg [n = 389]), rivaroxaban (dose 15 mg [287] and 20 mg [n = 553]), and apixaban (dose 2.5 mg [n = 45] and 5 mg [n = 186] (Fig. 2). The baseline demographics according to NOAC use are presented in Table 1.
Figure 2.
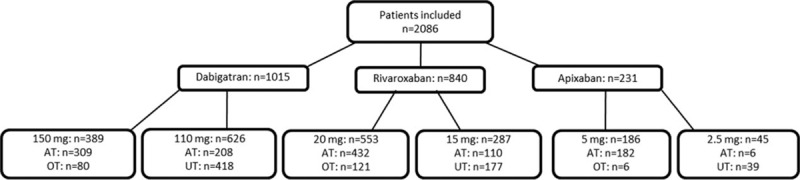
The novel oral anticoagulant (NOAC) dose for patients per undertreated (UT), appropriately treated (AT), and overtreated (OT) groups.
Table 1.
Baseline characteristics of the patients.
3.1. Guideline based use of NOACs
Of the 2086 patients studied, 1247 (59.8%) were treated with recommended dose (AT group) and remaining 839 (40.2%) were treated with off-label doses that included 634 (30.4%) patients in UT and 205 (9.8%) patients in OT groups. The comparison of characteristics of patients from AT, UT, and OT groups is shown in Table 2. The mean age of patients from AT, UT, and OT groups were 70.0 ± 9.9, 71.4 ± 8.9, and 73.9 ± 8.3 years, respectively (P < 0.001). There was a parabolic relationship between age and UT. However, OT was gradually increased with increase in patient's age (Fig. 3A). The mean creatinine clearance recorded for AT, UT, and OT groups was 76.0 ± 28.0, 75.4 ± 23.1, 62.6 ± 34.1 mL/min (P < 0.001), respectively. Figure 3B illustrates creatinine clearance according to off-label use of NOACs. Treatment adequacy, UT and OT rates according to preferred NOAC are shown in Figure 3C. UT was most commonly observed with dabigatran, whereas OT was most common with rivaroxaban. The mean CHA2DS2VASc score of OT group was significantly higher than AT and UT groups (4.2 ± 2.5 vs. 3.4 ± 1.4, 3.4 ± 1.3, respectively; P < 0.001). There were significant differences in HAS-BLED scores between the AT, UT, and OT groups (1.6 ± 1.0, 1.3 ± 0.6, 2.5 ± 1.1, respectively; P < 0.001). For AT, UT, and OT groups, the respective mean CHA2DS2VASc scores were significantly different 3.4 ± 1.4, 3.4 ± 1.3, and 4.2 ± 2.5 (P < 0.001) and the respective mean HAS-BLED scores were also significantly different 1.6 ± 1.0, 1.3 ± 0.6, and 2.5 ± 1.1 (P < 0.001), respectively. The proportions of UT and OT per CHA2DS2VASc and HAS-BLED scores are illustrated in Figure 4. The use of antiplatelet therapy was most prevalent in the OT group (26%) followed by AT (16.8%) and UT (11.6%) and it was significantly different (P < 0.001) from each other.
Table 2.
Comparison of patient characteristics for recommended, undertreatment and overtreatment groups.
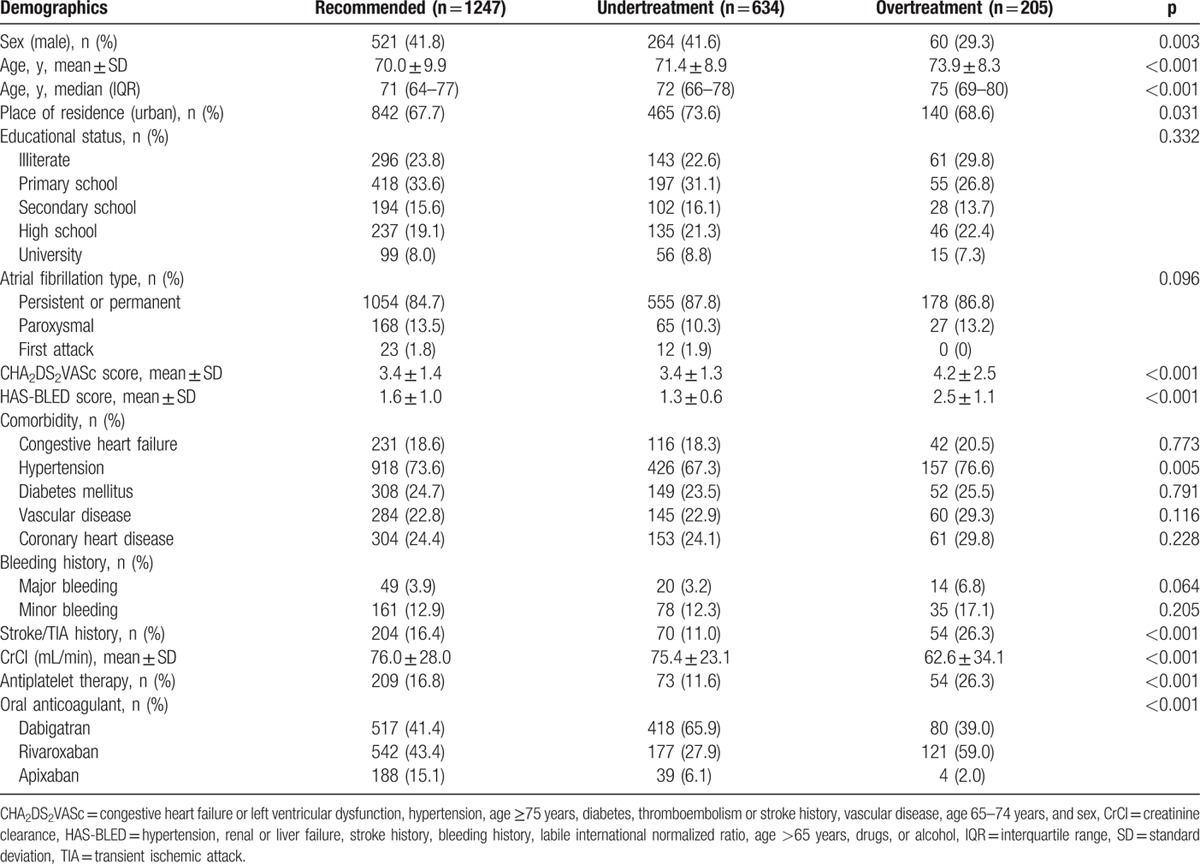
Figure 3.
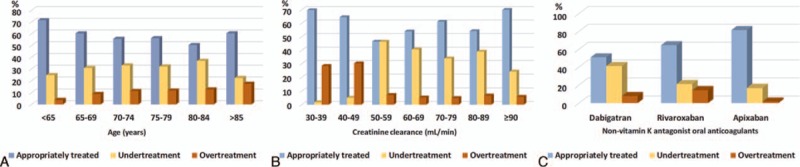
Rate of age categories (A), creatinine clearance (CrCl) categories (B), and novel oral anticoagulant (NOAC) therapies (C) in relation to undertreated (UT), appropriately treated (AT), and overtreated (OT) groups.
Figure 4.
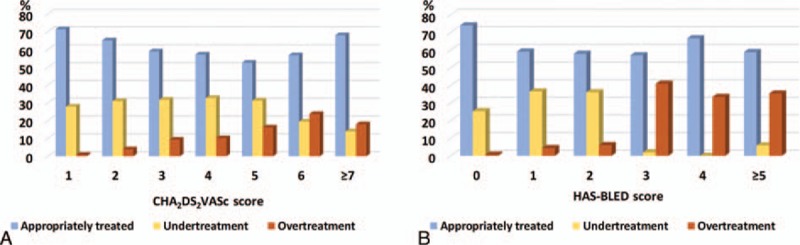
Distribution of CHA2DS2VASc and HAS-BLED scores based on undertreated, appropriately treated, and overtreated groups.
3.2. Risk factors for UT and OT
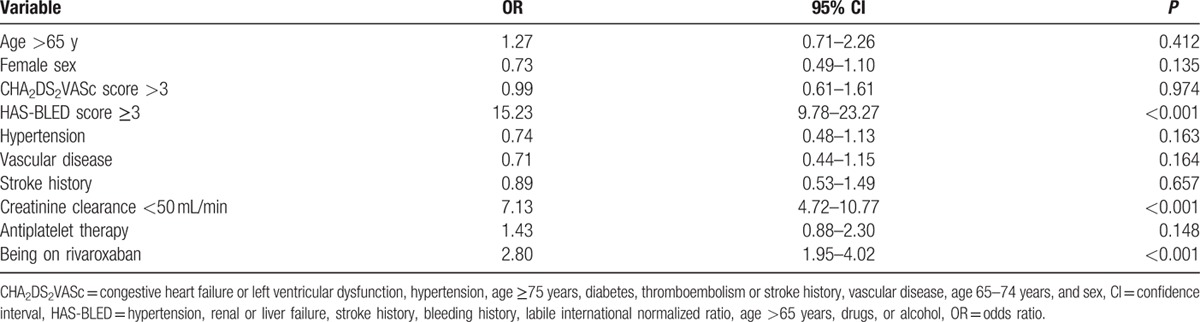
The variables with significant difference in univariate analysis were included in multivariate logistic regression analysis. The independent predictors of UT were >65 years of age, creatinine clearance ≥50 mL/min, HAS-BLED score <3, living in urban area, and treatment with dabigatran and that of OT were creatinine clearance <50 mL/min, treatment with rivaroxaban treatment, and HAS-BLED score ≥3 (Tables 3 and 4).
Table 3.
Predictors associated with undertreatment; results of the logistic regression analysis.
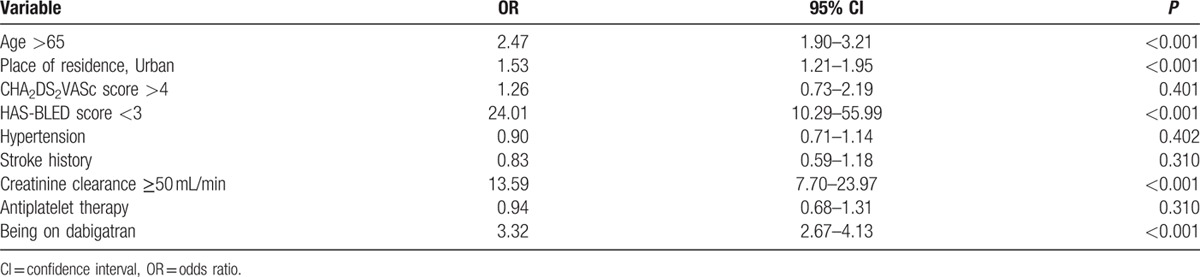
Table 4.
Predictors associated with overtreatment, results from the logistic regression analysis.
4. Discussion
In this subgroup analysis of RAMSES study, we evaluated off-label use of NOACs among NVAF patients with high risk of stroke. The ESC guideline-recommended doses were chosen for 59.8% of the patients, whereas 40.2% were in off-label group (30.4% UT and 9.8% OT). Older patients from urban areas with good renal functions and low HAS-BLED scores and receiving dabigatran treatment were at increased risk of having UT. However, patients with moderate renal impairment and high HAS-BLED score who were on rivaroxaban treatment were at increased risk of having OT.
Older age is usually associated with several comorbidities, decreased creatinine clearance, increased risk of bleeding, and AF-related ischemic stroke. The risk–benefit ratio should be carefully evaluated before prescribing OACs in elderly patients. This study showed that age >65 was an independent predictor of UT and these elderly patients received low dose of NOAC. The suboptimal use of OACs in elderly patients can be attributed to high risk of bleeding and physicians should be aware of a potential risk for underdosing in the elderly.[13,14] Although the age >65 years was not identified as an independent predictor of OT, the risk of OT increased with age possibly because of increased serum concentrations of NOACs.[15,16] Hence, a dose reduction is recommended for patients with age >80 years.[4]. The patients older than 75 years and receiving dabigatran are at higher risk of bleeding compared with warfarin as observed in post hoc analysis of RE-LY trial.[17] Although OAC therapy offers a net clinical benefit in older patients, specific patient characteristics such as renal function and risk of bleeding should be considered before prescribing a NOAC.[18]
Since a decade warfarin is the only available drug for prevention of stroke in patients with AF with a complicated dosing regimen. A strict monitoring is necessary to maintain therapeutic range of warfarin to prevent undesired outcomes. The invention of NOACs has changed strategies of stroke prevention; however, the pharmacokinetic properties of these drugs have restricted their use in patients with renal failure. Warfarin has been associated with a favorable outcome in patients with chronic kidney disease.[19] The anticoagulant effect and safety of recommended dose of NOACs is found to be comparable with warfarin in patients with moderate renal insufficiency.[20] In this study, overdosing was commonly observed in patients with moderate renal impairment that may lead to potential hemorrhagic complications. As per the ESC guidelines, the renal functions in patients with a creatinine clearance >80 mL/min should be assessed annually and 2 to 3 times per year for patients with moderate renal impairment.[4] During every patient visit, the clinicians should re-evaluate the dose of the NOAC based on the renal clearance. A creatinine clearance ≥50 mL/min was observed as an independent predictor of UT; and therefore, physicians should be aware of underdosing especially in patients with mild renal impairment.
The CHA2DS2VASc score is used to estimate the risk of stroke in patients with AF.[21] The incidences of stroke increase with increase in CHA2DS2VASc scores; however, the recommended dose of NOAC is not associated with this risk category and only OAC without antiplatelet therapy is recommended for patients at risk of stroke. Similarly, in this study, the prevalence of OT was increased with the increase of CHA2DS2VASc score and antiplatelet agents were more often used in patients from OT group than UT and AT groups. Therefore, use of intensive antithrombotic therapy in patients at higher risk of stroke is not recommended. A HAS-BLED score of ≥3 was also found as an independent predictor of OT while HAS-BLED score of <3 was an independent predictor of UT. There was a positive correlation between CHA2DS2VASc and HAS-BLED scores. The dose of NOAC should be decided based on patients’ thrombotic risk and bleeding complications.
Of the 3 NOACs evaluated in this study, only dabigatran has previously been evaluated in a randomized, prospective trial with a large population for both doses (dabigatran 110 mg: 6015 patients, dabigatran 150 mg: 6076 patients, against warfarin: 6022 patients).[1] The results of this study might have led the physicians to have a cautious approach, which could be a possible contributory factor for the higher prevalence of UT with dabigatran. The use of low-dose dabigatran in patients at risk of hemorrhage is reasonable. However, a post-hoc analysis of RE-LY trial showed better outcomes, when dabigatran was used in accord with EU label.[7] The high risk of bleeding is not a criterion for the dose adjustment of factor Xa inhibitors, and hence clinicians might prefer to use a higher dose of NOACs in these patients. The efficacy and safety data available for low doses of factor Xa inhibitors are from their phase III trials. In ROCKET-AF trial, the low-dose rivaroxaban is used in a relatively small number of patients with moderate renal impairment (rivaroxaban 15 mg: 1474 patients, rivaroxaban 20 mg: 5657 patients, and warfarin: 7133 patients) and even smaller number of patients were treated with low dose of apixaban (apixaban 2.5 mg: 831 patients, apixaban 5 mg: 8289 patients, warfarin: 9081 patients) in ARISTOTLE trial.[2,3] A typical NVAF patient is 70- to73-year old with a creatinine clearance of 67 to 69 mL/min.[1–3] Thus, the higher doses of NOACs can be suitable for the most of NVAF patients. However, physicians should keep in mind that OT may be a problem especially in the frail elderly patients who are prone to the potential harmful outcomes of overdosing.[22,23]
It is of utmost important to prescribe the appropriate dose to the patients to prevent from undesired outcomes. Physicians should be cognizant of the latest treatment recommendations and medication algorithms promulgated by professional organizations.
5. Limitations
This study had following limitations:
Owing to its cross-sectional design, the safety and efficacy outcomes with the different doses of NOACs could not be assessed. This can be assessed through a large prospective trial.
There was a relatively small number of patients on apixaban treatment, as apixaban has only recently been approved for prevention of stroke in NVAF.
6. Conclusion
This subgroup analysis of RAMSES study showed that 40.2% of NVAF patients had off-label dose of NOACs. Independent predictors of UT included age >65 years, creatinine clearance ≥50 mL/min, urban living, existing dabigatran treatment, and HAS-BLED score of <2, whereas that of OT were creatinine clearance <50 mL/min, ongoing rivaroxaban treatment, and HAS-BLED score of ≥3. Suboptimal management or lack of adherence to dose regimen recommended in guidelines may lead to ineffective or potentially harmful outcomes with OAC. Therefore, a greater emphasis should be given to prescribe the recommended dose for the specified patients.
Acknowledgments
The authors would like to thank Ekrem Bilal Karaayvaz, MD, Bağcılar Education and Research Hospital, Department of Cardiology, Mevlut Koc, MD, Assoc. Prof, Adana Numune Education and Research Hospital, Department of Cardiology, Durmus Yıldıray Şahin, MD, Assoc. Prof, Adana Numune Education and Research Hospital, Department of Cardiology, Tolga Çimen, MD, Dışkapı Yıldırım Beyazıt Education and Research Hospital, Department of Cardiology, Tolga Sinan Güvenç, MD, Siyami Ersek Heart Education and Research Hospital, Department of Cardiology, Nihat Pekel, MD, Assist Prof, İzmir Medikal Park Hospital, Department of Cardiology, Kerem Temel, MD, Acıbadem Eskişehir Hospital, Department of Cardiology, and Vehip Keskin, MD, Muğla Private Cardiology Clinic, for their contribution to the study.
Footnotes
Abbreviations: AF = atrial fibrillation, ARISTOTLE = apixaban versus warfarin in patients with atrial fibrillation, AT = appropriately treated, CHA2DS2VASc = congestive heart failure or left ventricular dysfunction, hypertension, age 65–75 years, diabetes, vascular disease, female sex, thromboembolism or stroke history, age ≥75 years, ESC = European Society of Cardiology, HAS-BLED = hypertension, abnormal renal function, abnormal liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs predisposing to bleed, alcohol use, NOAC = novel oral anticoagulant, NVAF = nonvalvular atrial fibrillation, OAC = oral anticoagulants, OT = overtreated, RAMSES = Real-life Multicenter Survey Evaluating Stroke Prevention Strategies in Turkey, RE-LY = Dabigatran versus warfarin in patients with atrial fibrillation, ROCKET-AF = rivaroxaban versus warfarin in nonvalvular atrial fibrillation, SD = standard deviation, UT = undertreated.
Collaborators: Fatma Özpamuk Karadeniz, MD1, Ahmet İlker Tekkesin, MD2, Yasin Çakıllı, MD3, Ceyhan Türkkan, MD2, Mehmet Hamidi, MD4, Vahit Demir, MD5, Mustafa Ozan Gürsoy, MD6, Müjgan Tek Öztürk, MD7, Gökhan Aksan, MD8, Sabri Seyis, MD9, Mehmet Ballı, MD10, Mehmet Hayri Alıcı, MD11, Serdar Bozyel, MD12, Ibrahim Altun, MD, Assistant Prof13, Feyza Çalık, MD14, Oğuz Karaca, MD, Assist. Prof15, Füsun Helvacı, MD8, Kadriye Akay, MD16, Yiğit Çanga, MD17, Savaş Çelebi, MD18, Emine Altuntas, MD19, Mehmet Aytürk, MD7, Hacı Murat Güneş, MD, Assistant Prof15, Tahir Bezgin, MD20, Aytekin Aksakal, MD21, Beytullah Çakal, MD, Assistant Prof2, Ayşe Çolak, MD22, Özgür Kaplan, MD23, Adem Tatlısu, MD24, Gökhan Gözübüyük, MD23, Selami Demirelli, MD25, Adnan Kaya, MD26, İbrahim Rencüzoğulları, MD, Assist Prof27, Zübeyde Bayram, MD17, Zeki Şimşek, MD17, Murat Civan, MD28, Ulaankhu Batgharel, MD 29, Ali Ekber Ata, MD 30, Gökhan Göl, MD 31, Gurbet Özge Mert, MD 13, Kadir Ugur Mert, MD 13, Aleks Değirmencioğlu, MD 32, Özkan Candan, MD 33, Özlem Özcan Çelebi, MD 34, Cem Doğan, MD 23, Fethi Yavuz, MD 35, Şeref Ulucan, MD, Assistant Prof 36, Arif Arısoy, MD, Assist Prof 37, Bingül Dilekçi Şahin, MD 25, Emrah Ermiş, MD 25, Serkan Gökaslan, MD 38, İdris Pektaş, MD 39, Aslı Tanındı, MD, Assist Prof 40, Kamuran Tekin, MD 41, Kadriye Memic Sancar, MD 13, Edip Güvenç Çekiç, MD, Assist Prof 42, Nesrin Filiz Başaran, MD, Assist Prof 42
1 Şanlıurfa Balıklıgöl State Hospital, Department of Cardiology, 2 Siyami Ersek Heart Education and Research Hospital, Department of Cardiology, 3 Tuzla State Hospital, Department of Cardiology, 4 Bandırma State Hospital, Department of Cardiology, 5 Yozgat State Hospital, Department of Cardiology, 6 Gaziemir State Hospital, Department of Cardiology, 7 Ankara Keçiören Education and Research Hospital, Department of Cardiology, 8 Şişli Hamidiye Etfal Education and Research Hospital, Department of Cardiology, 9 Mersin Private Dogus Hospital, Department of Cardiology, 10 Mersin Toros State Hospital, Department of Cardiology, 11 Gaziantep 25 Aralık State Hospital, Department of Cardiology, 12 Kocaeli Derince Education and Research Hospital, Department of Cardiology, 13 Mugla Sitki Kocman University, Faculty of Medicine, Department of Cardiology, 14 Mersin State Hospital, Department of Cardiology, 15 İstanbul Medipol Üniversity Faculty of Medicine, Department of Cardiology, 16 Kocaeli State Hospital, Department of Cardiology, 17 Kartal Yavuz Selim State Hospital, Department of Cardiology, 18 TOBB ETÜ Hospital, Department of Cardiology, 19 Bingöl State Hospital, Department of Cardiology, 20 Gebze Fatih State Hospital, Department of Cardiology, 21 Samsun Education and Research Hospital, Department of Cardiology, 22 Mut State Hospital, Department of Cardiology, 23 Malatya State Hospital, Department of Cardiology, 24 Sivas Numune State Hospital, Department of Cardiology, 25 Erzurum Bölge Education and Research Hospital, Department of Cardiology, 26 Suruç State Hospital, Department of Cardiology, 27 Kafkas University Faculty of Medicine, Department of Cardiology, 28 İstanbul Private Liv Hospital, Department of Cardiology, 29 Acıbadem Private Hospital, Department of Cardiology, 30 Samsun Medikal Park Hospital, Department of Cardiology, 31 Süreyyapaşa Education and Research Hospital, Department of Cardiology, 32 İstanbul Acıbadem University Faculty of Medicine, Department of Cardiology, 33 Uşak State Hospital, Department of Cardiology, 34 Private Medicana International Ankara Hospital, Department of Cardiology, 35 Dr. Ersin Arslan State Hospital, Department of Cardiology, 36 Mevlana University Faculty of Medicine, Department of Cardiology, 37 Gaziosmanpaşa University Faculty of Medicine, Department of Cardiology, 38 İstanbul Haydarpaşa Numune Education and Research Hospital, Department of Cardiology, 39 Mersin University Faculty of Medicine, Department of Cardiology, 40 Ufuk University Faculty of Medicine, Department of Cardiology, 41 Batman Bölge State Hospital, Department of Cardiology, 42 Muğla Sıtkı Koçman University Faculty of Medicine, Department of Pharmacology.
The authors report no conflicts of interest.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 5.Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014; 35:1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011; 32:2387–2394. [DOI] [PubMed] [Google Scholar]
- 7.Lip GYH, Clemens A, Noack H, et al. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost 2014; 111:933–942. [DOI] [PubMed] [Google Scholar]
- 8.Basaran O, Filiz Basaran N, Cekic EG, et al. PRescriptiOn PattERns of Oral Anticoagulants in Nonvalvular Atrial Fibrillation (PROPER study). Clin Appl Thromb Hemost 2015; pii:1076029615614395. [DOI] [PubMed] [Google Scholar]
- 9.Sørensen R, Gislason G, Torp-Pedersen C, et al. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open 2013; 3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camm AJ, Amarenco P, Haas S, et al. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J 2016; 37:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyer-Westendorf J, Förster K, Pannach S, et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood 2014; 124:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rationale, design and methodology of the RAMSES Study: ReAl-life Multicenter Survey Evaluating Stroke Prevention Strategies. Turk Kardiyol Dern Ars 2016; 44:215–220. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med 1999; 131:927–934. [DOI] [PubMed] [Google Scholar]
- 14.Fumagalli S, Said SAM, Laroche C, et al. Age-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe. JACC Clin Electrophysiol 2015; 1:326–334. [DOI] [PubMed] [Google Scholar]
- 15.Girgis IG, Patel MR, Peters GR, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non-valvular atrial fibrillation: results from ROCKET AF. J Clin Pharmacol 2014; 54:917–927. [DOI] [PubMed] [Google Scholar]
- 16.Stangier J, Stähle H, Rathgen K, et al. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008; 47:47–59. [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011; 123:2363–2372. [DOI] [PubMed] [Google Scholar]
- 18.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009; 119:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sardar P, Chatterjee S, Herzog E, et al. Novel oral anticoagulants in patients with renal insufficiency: a meta-analysis of randomized trials. Can J Cardiol 2014; 30:888–897. [DOI] [PubMed] [Google Scholar]
- 21.Odum LE, Cochran KA, Aistrope DS, et al. The CHADS-versus the new CHA2DS2-VASc scoring systems for guiding antithrombotic treatment of patients with atrial fibrillation: review of the literature and recommendations for use. Pharmacotherapy 2012; 32:285–296. [DOI] [PubMed] [Google Scholar]
- 22.Escobar C, Barrios V, Fellows SE, et al. Dabigatran and bleeding risk: the importance of a correct prescription. J Emerg Med 2014; 46:831–832. [DOI] [PubMed] [Google Scholar]
- 23.Harper P, Young LE. Merriman, bleeding risk with dabigatran in the frail elderly. N Engl J Med 2012; 366:864–866. [DOI] [PubMed] [Google Scholar]


