Abstract
We performed a study to investigate whether contamination of hemostasis samples with a glucose-containing solution might generate spurious results in rotational thromboelastometry (ROTEM) tests.
Venous blood was taken from 12 healthy volunteers and divided into 4 specimen bottles, which were contaminated with different concentrations (0%, 5%, 10%, and 20%) of glucose solution.
Significant lengthening of INTEMCT was observed in the 10% and 20% groups compared with baseline values (7.7% and 9%, P = 0.041 and P = 0.037, respectively). INTEMCFT increased by 20.1% in the 20% group (P = 0.005). INTEMα-angle and INTEMMCF decreased by 3.9% and 2.7%, respectively, in the 20% group (P = 0.010 and P = 0.049, respectively). EXTEMCFT was prolonged significantly, by 10.2%, 15.5%, and 25.6%, in the 5%, 10%, and 20% groups, respectively (P = 0.004, P < 0.001, and P < 0.001, respectively). EXTEMα-angle decreased significantly by 1.9%, 3.2%, and 4.0% in the 5%, 10%, and 20% groups, respectively (P = 0.014, P = 0.001, and P = 0.005, respectively). EXTEMMCF decreased by 3.4% in the 20% group (P = 0.023). FIBTEMMCF decreased by 9.2% and 17.5% in the 10% and 20% groups, respectively (P = 0.019 and P = 0.021, respectively). A significant correlation was observed between standard glucose solution contamination in the specimens and percentage variation of EXTEMCFT, EXTEMMCF, and FIBTEMMCF.
To obtain accurate data from the ROTEM test regarding the hemostatic status of patients, specimens with suspected or known contamination should not be analyzed.
Keywords: glucose, hemostasis, rotational thromboelastometry
1. Introduction
Blood coagulation analysis is very important for resuscitation with fluid and blood components in critical situations, especially in patients injured by multiple trauma or operated on with massive bleeding, because accurate information about the hemostatic status of patients can contribute to guiding appropriate management and improving the patients’ outcome.[1,2] Ideally, real-time monitoring of hemostasis is helpful for treating coagulopathy; however, conventional hemostatic test results are available about 60 minutes after blood sampling.[3]
Rotational thromboelastometry (ROTEM; Tem International GmbH, Munich, Germany), which is known as a point-of-care (POC) tool, can detect coagulation cascade abnormalities very rapidly and provides comprehensive assessment of the patient's hemostatic status.[4–6] ROTEM test results have also been used to guide coagulation therapy.[7–9] In recent years, a number of articles have reported the usefulness of ROTEM as a POC device in numerous clinical situations such as emergency care, cardiovascular surgery, liver transplantation, trauma surgery, and septic disseminated intravascular coagulation.[7,10–13]
In critical care, patient management is performed aggressively, and numerous fluids and monitoring devices are established, such as arterial line placement. Therefore, the risks of blood sampling errors (e.g., collection of blood from the same limb where intravenous fluid is being administered or the risk of blood sample contamination from wrong arterial line infusions such as glucose-containing fluid) still exist.[14] Recently, Lippi et al[15] reported that contamination of blood samples with glucose could cause spurious results in conventional coagulation tests including prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen concentration.
For this reason, we performed a study to investigate whether contamination of blood samples with a standard glucose solution might generate spurious results in the ROTEM tests: INTEM, EXTEM, and FIBTEM.
2. Methods
This study was approved by the Institutional Review Board at Seoul National University Bundang Hospital (Seongnam-si, South Korea, approval on December 15, 2015, B-1512/326-301), this observational study was registered in the Clinical Research Information Service (CRiS, http://cris.nih.go.kr, KCT0001749). Written informed consent was obtained from 12 healthy volunteers (5 males and 7 females) who had not been taking medications known to interfere with hemostasis such as anticoagulants, antiplatelets, or nonsteroidal antiinflammatory drugs. All volunteers fasted for 6 hours before blood sampling.
In total, 12 mL of fresh venous blood was obtained from an antecubital vein and placed in citrate-containing polypropylene tubes (Vacutainer, Becton Dickinson, Plymouth, UK). A 2-syringe sampling technique was used to avoid tissue thromboplastin contamination of blood specimens. The 1st blood sample was discarded, and then the study blood sample was taken immediately. Based on the study protocol of Lippi et al,[15] the collected specimens were pooled and then divided into 4 bottles of 2.0 mL each. To create final contamination levels of 0%, 5%, 10%, and 20%, 100, 200, and 400 μL of a standard 5% glucose solution (50 g glucose monohydrate in 1000 mL water, 278 mOsm/L; JW Pharmaceutical, Seoul, South Korea) was added to 3 bottles, respectively, and 1 sample was used as a control (baseline). The individual mixing process was performed about 5 minutes before the ROTEM analysis.
ROTEM analyses were conducted according to the manufacturer's recommendations. We obtained the following 4 ROTEM parameters: clotting time (CT), clot formation time (CFT), α-angle, and maximum clot firmness (MCF). INTEM and EXTEM values, which offer information on the intrinsic and extrinsic pathway, respectively, were assessed using the recommended reagents (in-TEM: 0.2 M CaCl2 20 μL and thromboplastin-phospholipid 20 μL; ex-TEM: 0.2 M CaCl2 20 μL and tissue factor 20 μL). The change of fibrin polymerization was examined using a FIBTEM test with the fib-TEM reagent (0.2 M CaCl2 20 μL with cytochalasin D and tissue factor 20 μL).
Data were analyzed using SPSS for Windows software (ver. 22; IBM Corp., Armonk, NY). All continuous data were assessed for normality using the Shapiro–Wilk test. Repeated measures analysis of variance test was used to compare the baseline values with values after glucose contamination. Pearson correlation coefficient was used to determine the correlation between the degree of glucose contamination and the percentage changes. Data are expressed as means (SD). A P-value less than 0.05 was considered to indicate statistical significance.
3. Results
Measured plasma glucose concentrations increased with increasing glucose contamination (Table 1). With increasing glucose contamination, ROTEM parameters showed a hypocoagulable pattern as follows (Table 1): prolonged CT and CFT values, and decreased α-angle and MCF values. Significant lengthening of INTEMCT was observed in the 10% and 20% contamination groups compared with baseline values (7.7% and 9%, P = 0.041 and P = 0.037, respectively) (Fig. 1A). INTEMCFT was significantly increased, by 20.1%, in the 20% contamination group (P = 0.005) (Fig. 1A). INTEMα-angle and INTEMMCF were significantly decreased, by 3.9% and 2.7%, respectively, in the 20% contamination group (P = 0.010 and P = 0.049, respectively) (Fig. 1A). EXTEMCFT was prolonged significantly, by 10.2%, 15.5%, and 25.6%, in the 5%, 10%, and 20% contamination groups, respectively (P = 0.004, P < 0.001, and P < 0.001, respectively) (Fig. 1B). EXTEMα-angle decreased significantly, by 1.9%, 3.2%, and 4.0%, in the 5%, 10%, and 20% contamination groups, respectively (P = 0.014, P = 0.001, and P = 0.005, respectively) (Fig. 1B). EXTEMMCF was significantly decreased, by 3.4%, in the 20% contamination group (P = 0.023) (Fig. 1B). FIBTEMMCF was significantly decreased, by 9.2% and 17.5%, in the 10% and 20% contamination groups, respectively (P = 0.019 and P = 0.021, respectively) (Fig. 1C).
Table 1.
ROTEM parameters and glucose levels (n = 12).
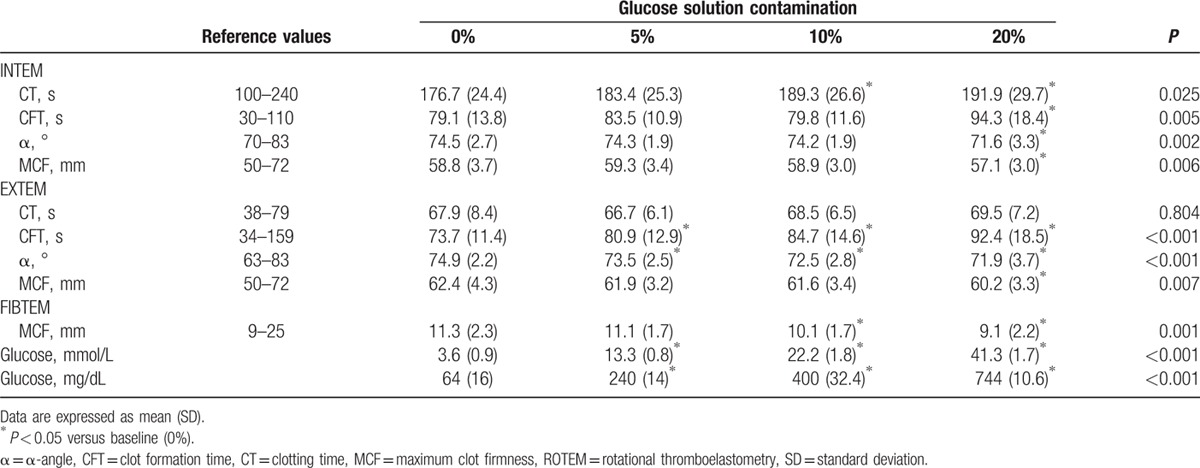
Figure 1.
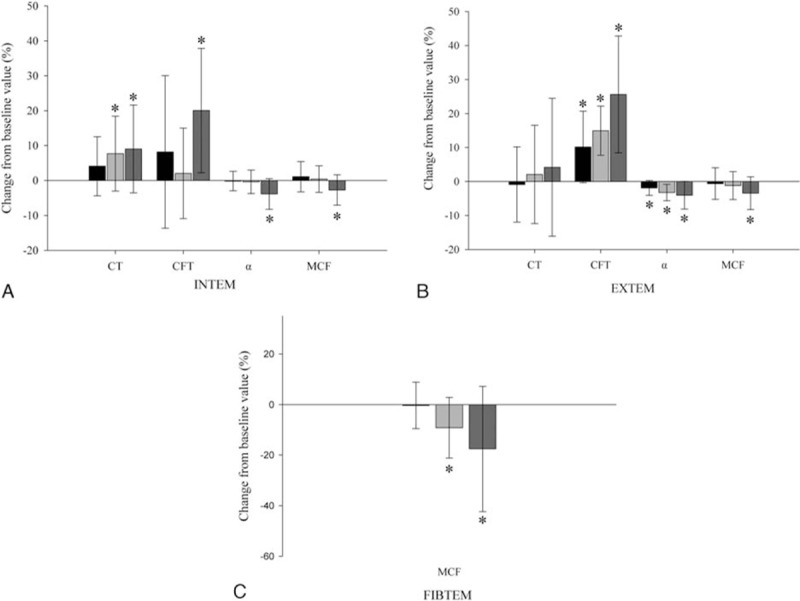
Percentage change from preoperative values for ROTEM parameters: (A) INTEM, (B) EXTEM, and (C) FIBTEM; 5% (black), 10% (pale grey), and 20% (dark gray) diluted groups. Values are means (SD). ∗Significant differences from 5% diluted group (P < 0.05). α = α-angle (°), CFT = clot formation time (second), CT = clotting time (second), MCF = maximum clot firmness (mm), ROTEM = rotational thromboelastometry.
A significant correlation was observed between standard glucose solution contamination in the specimens and percentage variation of EXTEMCFT, EXTEMMCF, and FIBTEMMCF (Fig. 2A–C, respectively).
Figure 2.
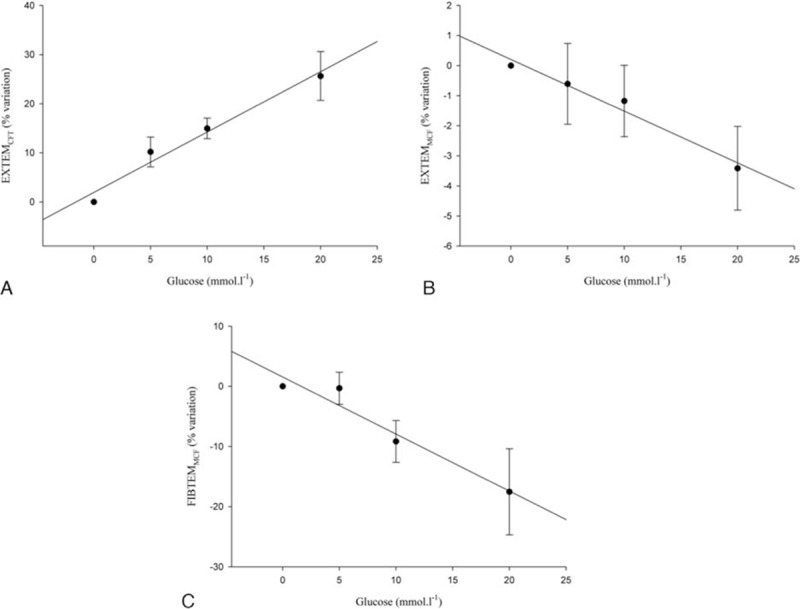
Correlations between the standard glucose solution contamination in the specimens and percentage variation of ROTEM parameters: (A) EXTEMCFT (R = 0.9860, P = 0.0140), (B) EXTEMMCF (R = 0.9855, P = 0.0145), and (C) FIBTEMMCF (R = 0.9705, P = 0.0295). CFT = clot formation time, MCF = maximum clot firmness, ROTEM = rotational thromboelastometry.
4. Discussion
Interesting findings were observed in the present study regarding the effect of acute hyperglycemic contamination on the blood coagulation profile analyzed with ROTEM. All coagulation pathways, including the intrinsic, extrinsic, and fibrinolytic systems, seemed to be impaired with increasing glucose contamination.
Volume replacement therapy is one of the most common clinical practices, and various fluids (e.g., crystalloid and colloids) and blood components (e.g., red blood cells, fresh frozen plasma) are generally used. Laboratory findings play an important role in establishing the plan of resuscitation, transfusion, or fluid therapy. In particular, monitoring the change of hemostatic profiles is essential to guide appropriate management of massive bleeding, disseminated intravascular coagulation, or coagulopathy of unknown origin.
There are several reports related to the risk of errors during blood collection[14,16] and the effect of blood sample contamination with glucose-containing solutions on the interpretation of chemistry parameters.[17] Conventional coagulation tests could also show spurious results because of iatrogenic acute hyperglycemia caused by hemodilution with a glucose-containing solution.[15] Lippi et al[15] performed an in vitro study of the relation between glucose contamination and results of conventional coagulation testing. They reported that the value of PT was prolonged (hypocoagulable pattern), and aPTT (hypercoagulable pattern) and fibrinogen concentration were decreased with increasing glucose contamination. PT, aPTT, and fibrinogen concentration represent the extrinsic, intrinsic, and fibrinolytic pathways in the coagulation cascade, respectively. On the other hand, in the present study, INTEM, EXTEM, and FIBTEM parameters showed a hypocoagulable pattern. These conflicting results in the extrinsic coagulation pathway (PT vs EXTEM) might be due to the different techniques used to measure the blood coagulation state. Hemostasis is modulated by various factors such as platelet function, tissue factor, and procoagulant factors.[18] Because the conventional coagulation test is performed using the patient's plasma, it cannot reflect the complexity of the hemostatic process. In contrast, ROTEM analyses the viscoelasticity of whole blood and interprets the interaction of various factors participating in hemostasis. Another possible causal factor that may explain the difference in changes between PT and EXTEM is the inconsistent turnaround time (time delay from blood sampling to laboratory analysis). In the present study, we performed the ROTEM test within 10 minutes after blood sampling. Nevertheless, the important point of our report, and of that by Lippi el al, is that glucose contamination of blood samples could cause interpretation errors in both the conventional test and ROTEM. Therefore, blood samples suspected of contamination should not be analyzed.
Two possible factors contributing to the results of the present study can be suggested. The 1st is the effect of hemodilution on blood coagulation. In general, crystalloids such as saline and lactated Ringer solution could induce a hypercoagulable state in the patient.[19–21] On the other hand, hemodilution with hydroxyethyl starches (HESs) could cause hypocoagulability.[22,23] Starch is a carbohydrate consisting of long chains of glucose units.[24] Although HES and 5% glucose solution differ in composition, they might exert similar hemodilution effects on blood coagulation as fluid solutions. The 2nd consideration is the glucose effect per se on blood coagulation. There are various results regarding the effect of acute hyperglycemia on the coagulation cascade. In an animal study, acute moderate hyperglycemia (10.0–13.3 mmol/L) maintained for 6 hours did not show a detectable influence on blood coagulation as measured by ROTEM.[25] In an in vivo study, platelets and endothelial cells were not activated during 6 hours of hyperglycemia with concentrations of 11.1 and 16.7 mmol/L in healthy volunteers.[26] In contrast, hyperglycemia produced increases in tissue factor procoagulant and platelet activity in healthy volunteers.[27] Our ROTEM results indicated a hypocoagulable state. These conflicting findings might be explained as follows. First, our study was conducted in the hyperacute hyperglycemic state (i.e., within 10 minutes). In contrast, other studies were performed about 6 or 24 hours after the hyperglycemic condition.[25–27] Therefore, there is a possibility that our study may not reflect the glucose effect on the coagulation cascade during 6 or 24 hours. Second, we evaluated only the hyperglycemic effect on blood coagulation. However, not only hyperglycemia but also hyperinsulinemia are the hallmarks of type 2 diabetes mellitus. Vaidyula et al[27] reported that exposure to combined hyperglycemia/hyperinsulinemia caused an approximate 9-fold rise in circulating tissue factor procoagulant activity. Similarly, Meigs et al[28] demonstrated that an elevated level of fasting insulin is related to impaired fibrinolysis and hypercoagulability.
Our study has a few limitations. First, the number of sample was small (12 volunteers). Although, there were significant findings in relation between glucose contamination and the ROTEM values in the present study, further study would be needed to show more potent findings. Second, the study population consisted mostly of young adults (mean age: 32 years). To extent our findings to the general population, further studies will be required aimed at clarifying the role of age on the effect of glucose contamination in blood coagulation analysis.
In conclusion, this study showed that hemostatic analysis could be influenced by contamination with a glucose-containing solution that resulted in hemodilution and a hyperglycemic state of a blood sample, as judged by ROTEM. To obtain accurate data from the ROTEM test regarding the hemostatic status of patients, blood samples suspected of contamination should not be analyzed.
Acknowledgments
The authors thank the grant (No. 02-2014-057) from the Research Fund of Seoul National University Bundang Hospital, South Korea for the support.
Footnotes
Abbreviations: CFT = clot formation time, CT = clotting time, MCF = maximum clot firmness, ROTEM = rotational thromboelastometry.
Funding/support: This study was supported by a grant (No. 02-2014-057) from the Research Fund of Seoul National University Bundang Hospital, South Korea.
The authors have no conflicts of interest to disclose.
References
- 1.Abdelfattah K, Cripps MW. Thromboelastography and rotational thromboelastometry use in trauma. Int J Surg 2015; pii: S1743-9191 (15)01223-6. [DOI] [PubMed] [Google Scholar]
- 2.Naik BI, Pajewski TN, Bogdonoff DI, et al. Rotational thromboelastometry-guided blood product management in major spine surgery. J Neurosurg Spine 2015; 23:239–249. [DOI] [PubMed] [Google Scholar]
- 3.Toulon P, Ozier Y, Ankri A, et al. Point-of-care versus central laboratory coagulation testing during haemorrhagic surgery. A multicenter study. Thromb Haemost 2009; 101:394–401. [PubMed] [Google Scholar]
- 4.Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010; 251:604–614. [DOI] [PubMed] [Google Scholar]
- 5.Johansson PI, Stensballe J. Effect of haemostatic control resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang 2009; 96:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll RC, Craft RM, Langdon RJ, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res 2009; 154:34–39. [DOI] [PubMed] [Google Scholar]
- 7.Schochl H, Nienaber U, Hofer G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care 2010; 14:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schochl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care 2011; 15:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahe-Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg 2009; 138:694–702. [DOI] [PubMed] [Google Scholar]
- 10.Momeni M, Carlier C, Baele P, et al. Fibrinogen concentration significantly decreases after on-pump versus off-pump coronary artery bypass surgery: a systematic point-of-care ROTEM analysis. J Cardiothorac Vasc Anesth 2013; 27:5–11. [DOI] [PubMed] [Google Scholar]
- 11.Alamo JM, Leon A, Mellado P, et al. Is “intra-operating room” thromboelastometry useful in liver transplantation? A case-control study in 303 patients. Transplant Proc 2013; 45:3637–3639. [DOI] [PubMed] [Google Scholar]
- 12.Koami H, Sakamoto Y, Ohta M, et al. Can rotational thromboelastometry predict septic disseminated intravascular coagulation? Blood Coagul Fibrinolysis 2015; 26:778–783. [DOI] [PubMed] [Google Scholar]
- 13.Schochl H, Cotton B, Inaba K, et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care 2011; 15:R265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta KJ, Cook TM. Accidental hypoglycaemia caused by an arterial flush drug error: a case report and contributory causes analysis. Anaesthesia 2013; 68:1179–1187. [DOI] [PubMed] [Google Scholar]
- 15.Lippi G, Buonocore R, Musa R, et al. The effect of hyperglycaemia on haemostasis testing – a volunteer study. Anaesthesia 2015; 70:549–554. [DOI] [PubMed] [Google Scholar]
- 16.Thirugnanam M, French J. Accidental hypoglycaemia caused by an arterial flush drug error. Anaesthesia 2014; 69:524–525. [DOI] [PubMed] [Google Scholar]
- 17.Lippi G, Avanzini R, Sandei F, et al. Blood sample contamination by glucose-containing solutions: effects and identification. Br J Biomed Sci 2013; 70:180–183. [PubMed] [Google Scholar]
- 18.Favaloro EJ, Funk DM, Lippi G. Pre-analytical variables in coagulation testing associated with diagnostic errors in hemostasis. Lab Med 2012; 43:1–10. [Google Scholar]
- 19.Roche AM, James MF, Grocott MP, et al. Coagulation effects of in vitro serial haemodilution with a balanced electrolyte hetastarch solution compared with a saline-based hetastarch solution and lactated Ringer's solution. Anaesthesia 2002; 57:950–955. [DOI] [PubMed] [Google Scholar]
- 20.Roche AM, James MF, Bennett-Guerrero E, et al. A head-to-head comparison of the in vitro coagulation effects of saline-based and balanced electrolyte crystalloid and colloid intravenous fluids. Anesth Analg 2006; 102:1274–1279. [DOI] [PubMed] [Google Scholar]
- 21.Ruttmann TG, James MF, Viljoen JF. Haemodilution induces a hypercoagulable state. Br J Anaesth 1996; 76:412–414. [DOI] [PubMed] [Google Scholar]
- 22.Ekseth K, Abildgaard L, Vegfors M, et al. The in vitro effects of crystalloids and colloids on coagulation. Anaesthesia 2002; 57:1102–1108. [DOI] [PubMed] [Google Scholar]
- 23.Egli GA, Zollinger A, Seifert B, et al. Effect of progressive haemodilution with hydroxyethyl starch, gelatin and albumin on blood coagulation. Br J Anaesth 1997; 78:684–689. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Zhang X, Tian Y. Ionic liquids as novel solvents for biosynthesis of octenyl succinic anhydride-modified waxy maize starch. Int J Biol Macromol 2016; 86:119–125. [DOI] [PubMed] [Google Scholar]
- 25.McGovern KF, Lascola KM, Smith SA, et al. Assessment of acute moderate hyperglycemia on traditional and thromboelastometry coagulation parameters in healthy adult horses. J Vet Emerg Crit Care (San Antonio) 2012; 22:550–557. [DOI] [PubMed] [Google Scholar]
- 26.Kotzailias N, Graninger M, Knechtelsdorfer M, et al. Acute effects of hyperglycaemia on plasma concentration of soluble P-selectin and von Willebrand factor in healthy volunteers – a prospective randomised double blind controlled study. Thromb Res 2009; 123:452–459. [DOI] [PubMed] [Google Scholar]
- 27.Vaidyula VR, Rao AK, Mozzoli M, et al. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes 2006; 55:202–208. [PubMed] [Google Scholar]
- 28.Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA 2000; 283:221–228. [DOI] [PubMed] [Google Scholar]


