Abstract
Intracranial meningiomas involving the major venous sinus (MVS) pose several complication risks upon performing radical resection. Some surgeons consider MVS invasion a contraindication for a complete resection of meningioma, and others suggest total resection followed by venous reconstruction. The aim of the study was to analyze our surgical results and discuss management strategy for intracranial meningiomas involving the MVS. Between 1993 and 2011, 107 patients with intracranial meningiomas involving MVS underwent surgery in our institution. Clinicoradiological features including pathological features and operative findings were retrospectively analyzed. Median follow-up duration was 60.2 months (range, 6.2–218.2 months). Distributions of tumor cases according to the involved sinus were as follows: 86% parasagittal, 10.3% tentorial, and 3.7% peritorcular. Simpson Grade I/II removal was achieved in 93 of 107 patients (87%). Partially or totally occluded MVS by their meningiomas (Sindou classification IV and V) was found in 39 patients (36%). Progression rate was 12% (13/107) and progression-free survival rates were 89%, 86%, and 80% at 5, 7, and 10 years, respectively. Sindou classification (IV/V) and Karnofsky performance status (KPS) score 6 month after the surgery (KPS < 90) were predictive factors for progression in our study (P = 0.044 and P = 0.001, respectively). The resection degree did not reach statistical significance (P = 0.484). Interestingly, there was no progression in patients that underwent radiation therapy or gamma knife radiosurgery for residual tumor. There were no perioperative deaths. Complication rate was 21% with brain swelling being the most common complication. There was no predictive factor for occurrence of postoperative complication in this study. In conclusion, complete tumor resection with sinus reconstruction did not significantly prevent tumor recurrence in intracranial meningioma involving MVS. Considering the complications from this procedure as it has possibly related with reduced postoperative KPS score, the tumor should be removed as much as possible while leaving remnant portion with significant invasion of sinus or drainage vein. Following radiation therapy or gamma knife radiosurgery for a remnant or recurred meningioma might then be justified.
Keywords: complication, intracranial meningioma, radical resection, recurrence, venous sinus
1. Introduction
Meningiomas are usually benign, slow-growing, and macroscopically well circumscribed tumors that constitute 14% to 19% of all primary intracranial tumors.[1,2] However, meningiomas often pose significant surgical obstacles based on their location.[3–5] When intracranial meningiomas invade the major venous sinus (MVS), they become challenging lesions to remove completely and without significant morbidity. Opening the sinus and radical resection of a tumor increases the risk of damage to the cerebral venous system causing significant neurological complications.[6,7] However, some previous reports showed that a subtotal resection is associated with a high rate of tumor recurrence.[8,9] As a result, it becomes important to achieve a balance between complete removal of the tumor and the resulting complication from this. Unfortunately, there is a lack of published large series regarding intracranial meningiomas invading the MVS and there are no definitive guidelines for the management of these complex cases.
The aim of the study was to retrospectively review the morbidity/mortality and long-term outcome and analyze the predictive factors for recurrence in our experience and finally discuss management strategy for intracranial meningiomas involving the MVS.
2. Methods
2.1. Patients and data
This study was conducted in compliance with the Declaration of Helsinki (sixth revision, 2008), and fulfilled all the requirements for patient anonymity. Our study was approved by the Institutional Review Board of the Chonnam National University Hwasun Hospital (CNUHH-2016–084). From a total of 912 patients with intracranial meningioma who were initially operated at our hospital between 1993 and 2011, 107 cases were confirmed as intracranial meningioma involving MVS, using brain magnetic resonance angiography (MRA) and/or conventional 4-vessel digital subtraction angiography (DSA) and reviewing operation notes. To define the clinical characteristics of these patients, we analyzed the medical record including admission notes, operative notes, notes from follow-up visit to the outpatient clinic, radiographic records, and pathological reports for the patient retrospectively.
Preoperatively, to define the extent and origin of the tumor and the degree of involvement of MVS, all patients received magnetic resonance imaging (MRI) and MRA, as described in previous our publication.[10] Fifty-eight patients also received conventional DSA to assess the patency of occlusion of the sinus, the extent of occlusion, and the number of collateral anastomoses close to the insertion zone of the meningioma. Tumor size from the largest diameter was assessed on the basis of preoperative images. Sinus involvement was graded according to the Sindou classification scheme,[4] as shown in Fig. 1: Type I, attachment to lateral wall of sinus; Type II, invasion of lateral recess; Type III, invasion of lateral wall; Type IV, invasion of lateral wall and roof; and Type V/VI, entire sinus occluded. The completeness of surgical removal was estimated according to the grading system proposed by Simpson.[11] Gross total resection (GTR) was defined as Simpson's Grades I/II resection without any visible tumor remnant on surgical finding and follow-up MRI. All the tumors were pathologically graded according to the World Health Organization (WHO) classification. The postoperative outcome was analyzed using the Karnofsky Performance Status (KPS) score to measure the degree of disability.[12] KPS score was evaluated in all patients before surgery, at discharge, and 6 months after surgery. Surgery-related complication was defined as a newly developed neurological deficit or an aggravated preexisting deficit. To evaluate tumor recurrence, postoperative follow-up MRI was obtained from all patients 6 months after surgery and then every year for the rest of the patient's life. On follow-up MRI, a newly enhanced mass in a completely resected case or a regrowing symptomatic mass in an incompletely resected case was regarded as the recurrence.
Figure 1.
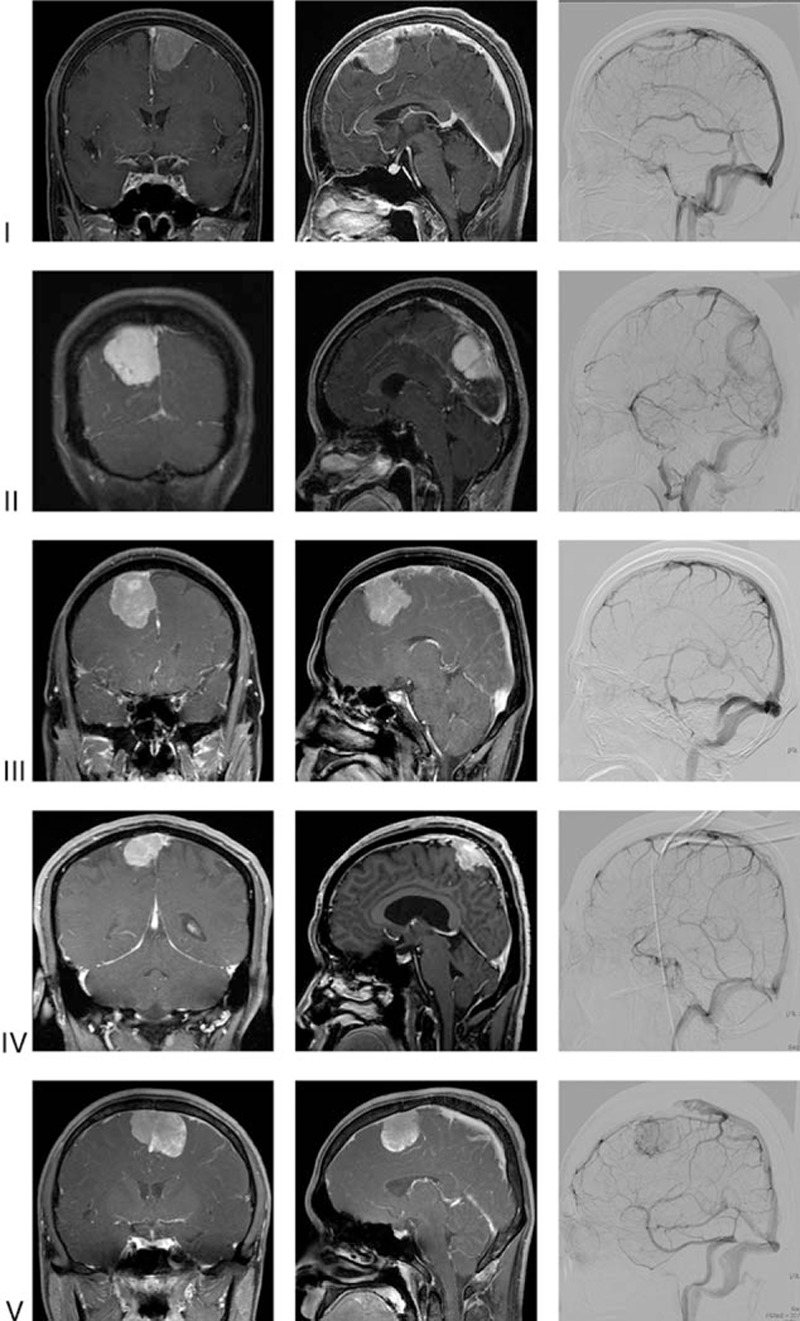
Representative radiographic images according to Sindou classification. Coronal (left) and sagittal (middle) T1-enhanced MR with gadolinium and sagittal images in venous phase (right) of conventional angiography for intracerebral artery were used for classification with relevant operation record.
2.2. Operative technique (sinus management)
Tumors were resected completely (Simpson's grade I/II) if possible. Management of the sinus depended on the extent of sinus occlusion (Sindou type) and the tumor location and collateral anastomoses. For type II tumors, the sinus was not opened and the remaining residual tumor was observed for regrowth. For type III to IV tumors with demonstrated sinus flow, the sinus was opened with the goal of complete tumor resection and then the opened sinus wall was reconstructed. If we predicted that sinus opening and total tumor removal would lead to a serious complication, we would decide to resect the tumor subtotally to obtain a Simpson grade III/IV. For type V/VI tumors with no demonstrated patency, the sinus was ligated and resected along with the tumors. However, for type V tumors that demonstrated patency of sinus or considered as not fully developed of collateral anastomoses, the tumor within the sinus was left and observed.
Small remnant was left in 14 patients, who received radiation therapy (RT) or gamma knife radiosurgery (GKRS), or were followed up. For the patients with WHO grade II or III meningioma, radiation therapies were recommended. Except the patient with poor compliance or tiny remnant, not detected on MRI, radiosurgery was used for remnant mass.
2.3. Statistical analysis
Progression-free survival (PFS) rates calculated from date of surgical operation to date of first recurrence in complete resection case or regrowth in remnant case, or last follow-up in patients with no recurrence or regrowth were estimated with the Kaplan–Meier method and compared with the log-rank test. For the multivariate analysis, independent prognostic factors for recurrence were determined using the Cox proportional hazards model. The comparison of a permanent complication occurrence and a categorical variable was done by a chi-square test or Fisher-exact probability test. Furthermore, binary logistic regression test was applied for multivariate analysis. All statistical analyses were performed using SPSS version 20.0 software program for Windows (SPSS, Chicago, IL, USA). P value of less than 0.05 was considered significant.
3. Results
3.1. Clinical presentation
Clinical characteristics of the enrolled 107 patients with intracranial meningiomas invading MVS are summarized in Table 1. Median follow up period was 60.2 months (range, 6.2–218.2 mo). The first symptoms were usually limb paresis, seizures and visual disturbance. Anterior and middle 1/3 parasagittal location was the most common (84.8%). Tumor size ranged from 1.3 to 7 cm (median, 4 cm). Intratumoral calcification was documented in 27 patients (25.2%). On the basis of preoperative imaging (MRA and/or DSA) and intraoperative confirmation, the sinus was completely obstructed (Type V) in 15 patients (14%) and only partially invaded (Type IV) in 24 patients (22.4%). The sinus was not invaded intraluminally or only the sinus wall was invaded (Types I, II, and III) in 68 patients (63.6%). In WHO classification, majority of the patients (90.7%) were diagnosed as a benign, mostly fibroblastic type (WHO grade I).
Table 1.
Clinical characteristics of 107 patients having intracranial meningioma involving major venous sinus.
3.2. Treatment
The extent of tumor removal according to the sinus invasion are summarized in Table 2. A diagram presents schematic constitution of the patients and surgical outcome according to the state of sinus invasion and degree of tumor resection (Fig. 2). Of 24 patients with tumors that only partially invaded the sinus (Sindou type IV), Simpson grade I/II resection (complete resection with sinus reconstruction) was performed in 17 patients (70.8%). In 7 patients (29.2%) whom total tumor removal would have required sacrificing the sinus or the cortical bridging veins, we decided to resect the tumor subtotally to obtain a Simpson grade III/IV removal. Of these 7 patients, 3 patients underwent GKRS and 4 patients were only observed by follow-up brain MRI. After surgery, 2 patients (11.7%) experienced recurrence in Simpson grade I/II group and 2 patients (28.5%) experienced regrowth in observation group of Simpson grade III/IV group.
Table 2.
Extent of tumor removal according to sinus invasion.
Figure 2.
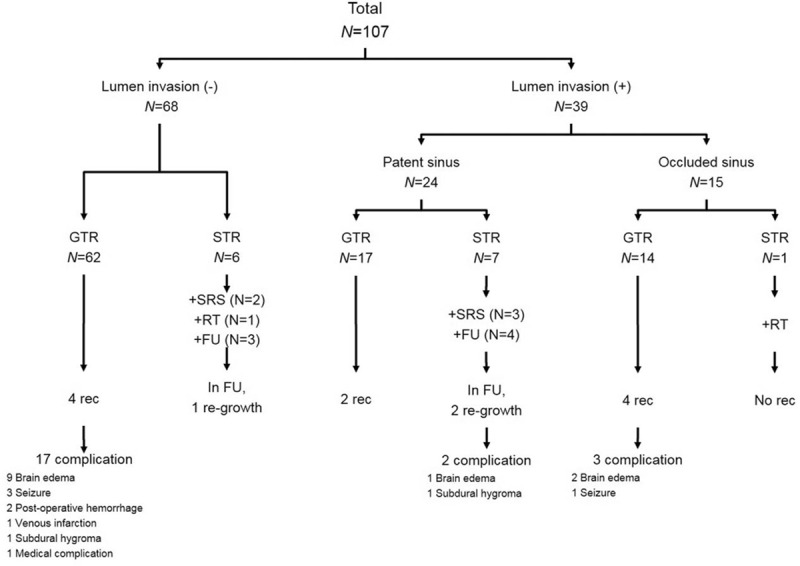
A tree diagram of the frequency of sinus invasion and sinus occlusion in our patients, and the methods by which their tumor was managed. Rates of recurrence and complication for each group are noted. FU = follow-up, GTR = gross total resection, rec = recurrence, RT = radiotherapy, SRS = stereotactic radiosurgery, STR = subtotal resection.
In 15 patients with complete occlusion of the sinus (Sindou type V), tumors were resected together with the portion of sinus involved to achieve Simpson grade I/II removal in 14 patients (93.4%) and Simpson grade IV removal in 1 patient (6.6%). The patient with residual tumor was diagnosed with malignant meningioma and received postoperative RT. As a result, 4 patients (28.5%) experienced recurrence in Simpson grade I/II group.
In the remaining 68 patients with tumors that had not invaded intralumen of sinus (Sindou I, II, and III), tumors were resected to achieve Simpson grade I/II removal in 62 patients (91.2%), Simpson grade III/IV removal in 6 patients (8.8%). Of these 6 patients, 2 patients underwent GKRS, 1 patient underwent RT, and 3 patients were just observed by follow-up MRI. As a result, 4 patients (6.4%) experienced recurrence in Simpson grade I/II group and 1 patient (16.6%) experienced regrowth in observation group of Simpson grade III/IV group. For 2 patients with malignant meningioma, they received immediate postoperative RT, regardless of invasion and resection degree (Sindou type I and Simpson grade I, Sindou type V and Simpson grade III). These patients were stable after 5 and 10 years of follow-up.
3.3. Recurrence
PFS rates for MVS involving meningiomas were 89%, 86%, and 80% at 5, 7, and 10 years, respectively (Fig. 3A). The results of analyses of the factors that correlate with recurrence are shown in Table 3 and Fig. 3B and C. PFS was 95%, 92%, and 86% at 5, 7, and 10 years for Sindou type I-III tumors and 77% and 70% at 5 and 7 years for Sindou type IV/V tumors. This difference was statistically significant (P = 0.023). At 6 months after the operation, high KPS (KPS≥90) was related with longer PFS than low KPS (P < 0.001). Multivariate analysis also confirmed that Sindou classification of extent of sinus involvement (P = 0.044, hazard ratio [HR] 0.308, 95% confidential index [CI] 0.098–0.970) and KPS 6 months after the operation (P = 0.001, HR: 0.142, 95% CI: 0.045–0.443) were significant as independent prognostic factor for recurrence.
Figure 3.
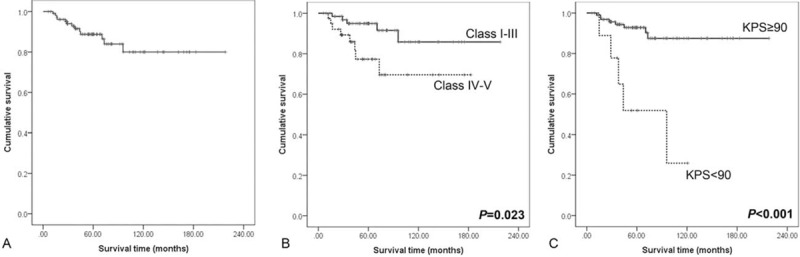
Kaplan–Meier curves showing PFS of 107 study patients according to different predictors (overall comparison was estimated using a log-rank test). (A) Overall PFS curve, (B) PFS curve for Sindou classification, and (C) PFS curve for KPS score 6 months after the operation. KPS = Karnofsky performance status, PFS = progression-free survival.
Table 3.
Predictive factors of 13 patients having recurrence on univariate and multivariate analysis.
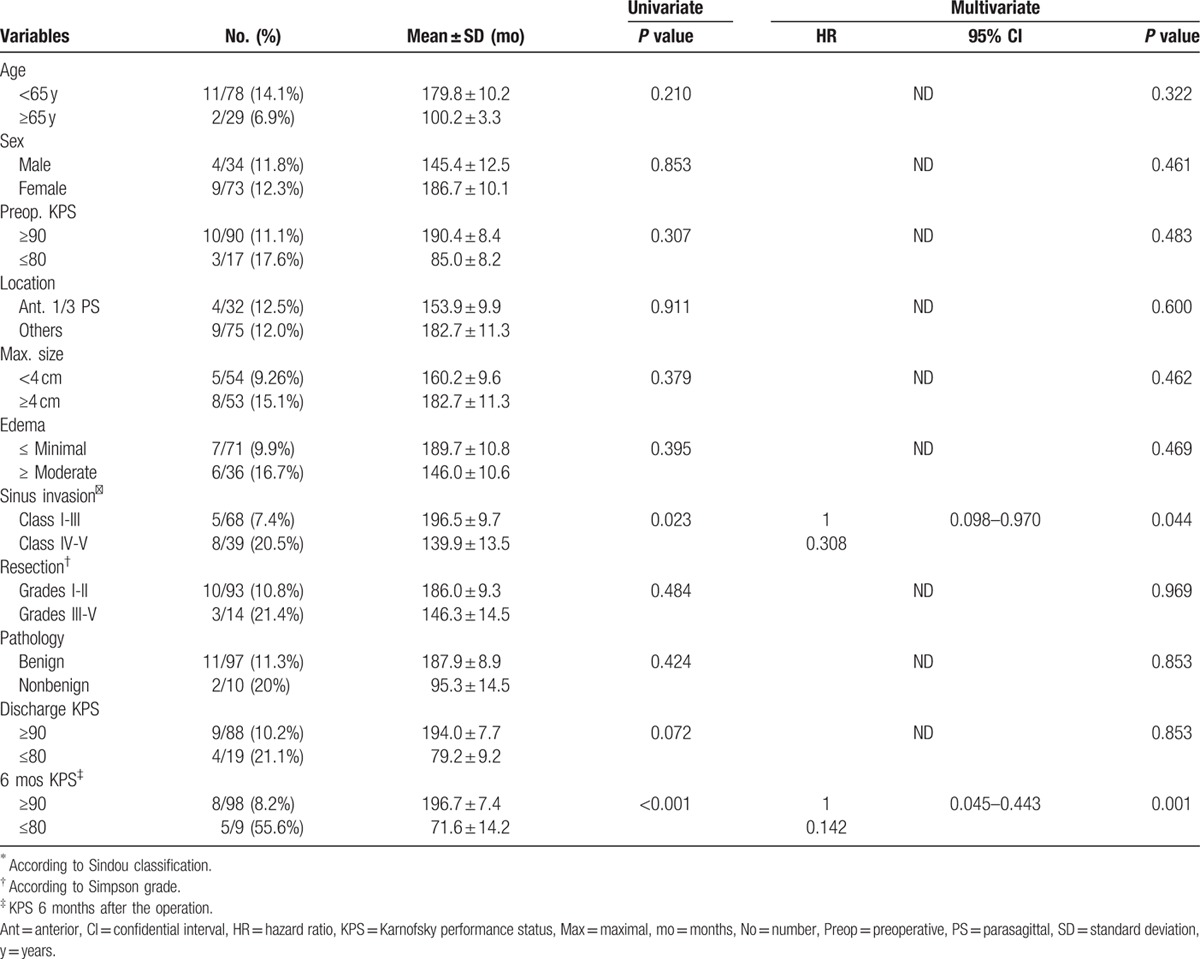
However, factors such as resection degree, histological type, tumor size, and peritumoral edema did not show statistical significance in the statistical analyses. Three patients (12.5%) with Simpson grade I removal recurred. Seven patients (10.1%) with Simpson grade II removal and 1 patient (12.5%) with Simpson grade III and 2 patients (33.3%) with Simpson grade IV also recurred. Mean time for recurrence was 2.9 years (3.5 years after grade I/II removal, 1 year after grade III/IV removal); however, the resection degree did not reach statistical significance (P = 0.484). Two atypical (25.3%) and 11 benign (11.3%) meningiomas recurred. Of these 11 patients with benign meningiomas, 9 patients underwent Simpson grade I/II removal, 2 patients underwent Simpson grade III/IV removal. WHO grade also did not reach to statistical significance (P = 0.424). There was no recurrence in patients that underwent RT or GKRS for residual tumor. The overall repeat recurrence (“re-recurrence”) rate was 4%. After a recurrence, addition of RT or GKRS with surgery prevented a “re-recurrence” of the tumor; for patients that receiving only surgery for their recurrence, a certain percentage developed a “re-recurrence” of their tumor.
3.4. Complications and outcomes
Postoperative complications for all studied patients are shown in Table 4. Overall, complications occurred in 22 patients (21%). The most common complications was brain swelling (11 patients; 10.3%) followed by seizure (4 patients; 3.7%). Only one of these 11 patients with brain swelling underwent surgical management. In 20 of the 22 patients (91%) with complications, the tumor had been removed with Simpson's grade I/II, as shown in Fig. 2. However, factors such as age, sex, preoperative KPS, tumor location, resection degree of tumor, tumor size, perilesional edema, extent of sinus invasion and histological type did not show statistical significance in the statistical analyses (data not shown).
Table 4.
Incidence of operative complication.

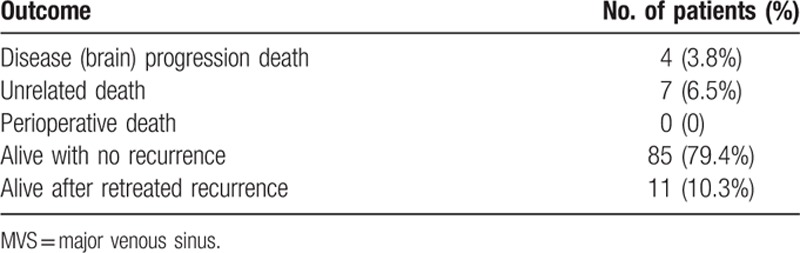
Immediate after the operation, 15 patients (14%) had worse KPS (3 patients had their KPS decrease from 100 to 80, 9 patients went from 90 to 80, and 3 patients went from 80 to 70). Most other patients (86%), however, exhibited improvement in their conditions, and an overall outcome assessment of 107 patients demonstrated 9 patient having a KPS score of 80, 15 patients a score of 90, 83 patients a score of 100 (Table 5). A review of outcomes for 107 patients with intracranial meningiomas invading MVS is in Table 6.
Table 5.
KPS of 107 patients before and after operation.
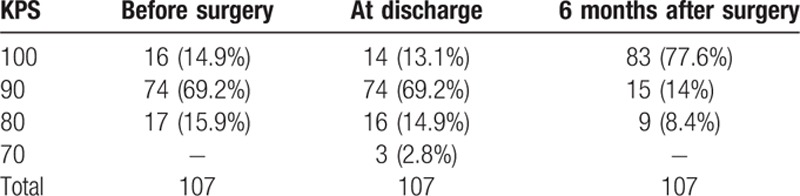
Table 6.
Outcomes of 107 patients after surgical resection for intracranial meningiomas invading MVS.
4. Discussion
Despite the availability of better preoperative brain imaging and intraoperative technical advances, significant challenges remain in the management of intracranial meningiomas involving MVS. The invasion of the meningioma with the cerebral venous circulation system makes it difficult to safely and completely remove the tumors. Attempts, to open the sinus and resect a tumor radically, carry the risk of damage to the cerebral venous system and subsequent significant neurological complications such as brain edema and cerebral venous infarction.[6,7] Some studies suggest that intracranial meningioma invading MVS contraindicate a complete removal of tumor, that a subtotal removal is warranted.[3,15] However, some previous reports showed that a subtotal resection is associated with a high rate of tumor recurrence.[8,9] For these reasons, intracranial meningioma involving MVS have been a challenge in management decision making and there is need to develop better management guidelines. To achieve this, it becomes necessary to better understand the risk factors for operative complications and the predictive factors influencing recurrence.
It is usually accepted that postoperative recurrence is related to the completeness of the original surgical removal.[16–18] Using aggressive radical tumor resection with reconstruction of the sinus and its flows, Sindou[19] reported the recurrence rate of only 4%. Dimeco et al[14] reported that if sinus was predicted to have patency, marginal resection of the tumor was performed until the tumor was completely freed from its attachment, followed by reconstruction of the affected sinus. In addition, if sinus demonstrated complete occlusion, the involved sinus was ligated and then cut out. Consequently, they reported recurrence rate of 13.9% and Simpson grade became a significant independent predictive factor for recurrence (recurrence rate for grade I of 13.5%, grade II 24%, and grade IV 49% at 10-year follow-up). These studies, however, reported relatively high postoperative mortality and morbidity rates (Sindou[19] with 3% mortality and 8% morbidity, Dimeco et al[14] with 1.85% mortality and 28.7% morbidity). Since there are many cortical bridging veins near the sinus, these venous structures can be closely associated with a tumor mass, or even enveloped within the tumor. Sacrifice of a cortical bridging vein can cause venous engorgement and a decrease in vascular flow that encourages venous stasis with a resultant thrombosis.[8,20]
Another analysis advocated not only aiming for a Simpson grade I/II resection but also preserving the venous structure.[21] In this study, Simpson grade and sinus management were not found to be predictive for recurrence and they reported 11% recurrence rate and a relatively low postoperative mortality (0.9%) and morbidity (3.6%) rate. Similar result was derived from other studies about sphenoid wing meningioma invading cavernous sinus.[22] We demonstrated that Simpson grade I/II resection and removal of invaded sinus wall did not prevent recurrence with any statistically significance like recent reports (Table 3).[21,22] Our results also demonstrate that the degree of sinus involvement (Sindou types IV and V) significantly influences the recurrence or regrowth (P = 0.0026). This may be due to the invasion of tumor cells in the meninges outside the limits of a macroscopic attachment.[23]
Previous studies,[13,14,21,24] except that of Sindou,[19] indicated that recurrence or regrowth could be delayed or even avoided by stereotactic radiosurgery (SRS) for residual tumor. Considering the tumor recurrence rate for 3 categories of patients in our study [GTR (10/93, 11%), STR+SRS/RT (0/7, 0%), STR (43%)], GTR patients can be observed and STR patients should receive SRS/RT. Our treatment strategy, similar to Raza et al[21] has been to achieve Simpson grade I/II resection but with the goal of preservation of cerebral venous structures and if necessary, postoperative RT or GKRS to be performed for control of residual tumor. SRS, including GKRS should probably be used as adjuvant surgical procedure.[25,26]
In our study, a 12.1% progression and 21% morbidity rate were represented. There was no operative mortality and no recurrence after undergoing RT or GKRS for residual tumor. In 20 of the 22 patients (91%) with complications, the tumor had been removed with Simpson's grade I/II. Unlike previous studies, factors such as age, sex, preoperative KPS, tumor location, resection degree of tumor, tumor size, perilesional edema, extent of sinus invasion, and histological type did not show statistical significance in the statistical analyses.[6,7] It is notable that 91% of the patients with complications had their tumor removed at a Simpson grade I/II. Our results should be interpreted with caution because this was a retrospective study including a small number of patients. And limited follow-up period is an unavoidable difficulty with clinical series of benign tumors. Further studies need to be performed prospectively with large numbers of subjects for establishing treatment strategy of meningiomas invading MVS.
In conclusion, based on the results of other studies and our results, a complete tumor resection and reconstruction of the sinus does not significantly influence tumor recurrence and at the same time it is possibly related with increases rates of mortality and morbidity in the management of intracranial meningioma invading MVS. We suggest that the safest strategy is the resection as much of the tumor as possible while leaving remnant portion invading sinus or adjacent drainage vein, treated by RT or GKRS.
Footnotes
Abbreviations: GKRS = gamma knife radiosurgery, GTR = gross total resection, KPS = Karnofsky performance status, MRI = magnetic resonance image, MVS = major venous sinus, RT = radiation treatment, SRS = stereotactic radiosurgery, STR = subtotal resection.
M-SH and Y-JK contributed equally to this work.
This study was supported by a grant (HCRI15014–21) of Chonnam National University Hospital Biomedical Research Institute.
MSH, YKK, JIY, and KSM analyzed the data and drafted manuscript. KHL and SHL revised manuscript critically for important intellectually content. KHL, JIY, and KSM performed the statistical analysis. WDK, TYJ, and WYJ helped acquisition and interpretation of data. WDK, TYJ, and IYK participated in reviewing literatures and helped in conception and design of the study. KSM and SJ conceived the study, participated in the design of it and coordination. All authors read and approved the final manuscript.
The authors have no conflicts of interest to declare.
References
- 1.Quest DO. Meningiomas: an update. Neurosurgery 1978; 3:219–225. [DOI] [PubMed] [Google Scholar]
- 2.Wara WM, Sheline GE, Newman H, et al. Radiation therapy of meningiomas. Am J Roentgenol Radium Ther Nucl Med 1975; 123:453–458. [DOI] [PubMed] [Google Scholar]
- 3.Sindou M. Meningiomas invading the sagittal or transverse sinuses, resection with venous reconstruction. J Clin Neurosci 2001; 8 suppl 1:8–11. [DOI] [PubMed] [Google Scholar]
- 4.Sindou M, Auque J, Jouanneau E. Neurosurgery and the intracranial venous system. Acta Neurochir Suppl 2005; 94:167–175. [DOI] [PubMed] [Google Scholar]
- 5.Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet 2004; 363:1535–1543. [DOI] [PubMed] [Google Scholar]
- 6.Schmid-Elsaesser R, Steiger HJ, Yousry T, et al. Radical resection of meningiomas and arteriovenous fistulas involving critical dural sinus segments: experience with intraoperative sinus pressure monitoring and elective sinus reconstruction in 10 patients. Neurosurgery 1997; 41:1005–1016.discussion 1016-1008. [DOI] [PubMed] [Google Scholar]
- 7.Giombini S, Solero CL, Lasio G, et al. Immediate and late outcome of operations for Parasagittal and falx meningiomas. Report of 342 cases. Surg Neurol 1984; 21:427–435. [DOI] [PubMed] [Google Scholar]
- 8.Murata J, Sawamura Y, Saito H, et al. Resection of a recurrent parasagittal meningioma with cortical vein anastomosis: technical note. Surg Neurol 1997; 48:592–595.discussion 595-597. [DOI] [PubMed] [Google Scholar]
- 9.Marks SM, Whitwell HL, Lye RH. Recurrence of meningiomas after operation. Surg Neurol 1986; 25:436–440. [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Jung S, Moon KS, et al. Preoperative evaluation of venous systems with 3-dimensional contrast-enhanced magnetic resonance venography in brain tumors: comparison with time-of-flight magnetic resonance venography and digital subtraction angiography. Surg Neurol 2005; 64:128–133.discussion 133-124. [DOI] [PubMed] [Google Scholar]
- 11.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957; 20:22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karnofsky DA. The bases for cancer chemotherapy. Stanford Med Bull 1948; 6:257–269. [PubMed] [Google Scholar]
- 13.Sughrue ME, Rutkowski MJ, Shangari G, et al. Results with judicious modern neurosurgical management of parasagittal and falcine meningiomas. Clinical article. J Neurosurg 2011; 114:731–737. [DOI] [PubMed] [Google Scholar]
- 14.DiMeco F, Li KW, Casali C, et al. Meningiomas invading the superior sagittal sinus: surgical experience in 108 cases. Neurosurgery 2004; 55:1263–1272.discussion 1272-1264. [DOI] [PubMed] [Google Scholar]
- 15.Bonnal J, Brotchi J. Surgery of the superior sagittal sinus in parasagittal meningiomas. J Neurosurg 1978; 48:935–945. [DOI] [PubMed] [Google Scholar]
- 16.Black PM. Meningiomas. Neurosurgery 1993; 32:643–657. [DOI] [PubMed] [Google Scholar]
- 17.Crompton MR, Gautier-Smith PC. The prediction of recurrence in meningiomas. J Neurol Neurosurg Psychiatry 1970; 33:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirimanoff RO, Dosoretz DE, Linggood RM, et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 1985; 62:18–24. [DOI] [PubMed] [Google Scholar]
- 19.Sindou MP, Alvernia JE. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg 2006; 105:514–525. [DOI] [PubMed] [Google Scholar]
- 20.Fries G, Wallenfang T, Hennen J, et al. Occlusion of the pig superior sagittal sinus, bridging and cortical veins: multistep evolution of sinus-vein thrombosis. J Neurosurg 1992; 77:127–133. [DOI] [PubMed] [Google Scholar]
- 21.Raza SM, Gallia GL, Brem H, et al. Perioperative and long-term outcomes from the management of parasagittal meningiomas invading the superior sagittal sinus. Neurosurgery 2010; 67:885–893.discussion 893. [DOI] [PubMed] [Google Scholar]
- 22.Ivan ME, Cheng JS, Kaur G, et al. Association of morbidity with extent of resection and cavernous sinus invasion in sphenoid wing meningiomas, Journal of neurological surgery. J Neurol Surg B Skull Base 2012; 73:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borovich B, Doron Y. Recurrence of intracranial meningiomas: the role played by regional multicentricity. J Neurosurg 1986; 64:58–63. [DOI] [PubMed] [Google Scholar]
- 24.Nowak A, Dziedzic T, Czernicki T, et al. Surgical treatment of parasagittal and falcine meningiomas invading the superior sagittal sinus. Neurol Neurochir Pol 2014; 48:174–180. [DOI] [PubMed] [Google Scholar]
- 25.Kondziolka D, Flickinger JC, Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery 1998; 43:405–413.discussion 413-404. [DOI] [PubMed] [Google Scholar]
- 26.Mathiesen T, Pettersson-Segerlind J, Kihlstrom L, et al. Meningiomas engaging major venous sinuses. World Neurosurgery 2014; 81:116–124. [DOI] [PubMed] [Google Scholar]


