Supplemental Digital Content is available in the text
Keywords: complementary medicine, manual medicine, nocebo, osteopathy, pain, ultrasound
Abstract
Background:
Placebo, defined as “false treatment,” is a common gold-standard method to assess the validity of a therapy both in pharmacological trials and manual medicine research where placebo is also referred to as “sham therapy.” In the medical literature, guidelines have been proposed on how to conduct robust placebo-controlled trials, but mainly in a drug-based scenario. In contrast, there are not precise guidelines on how to conduct a placebo-controlled in manual medicine trials (particularly osteopathy). The aim of the present systematic review was to report how and what type of sham methods, dosage, operator characteristics, and patient types were used in osteopathic clinical trials and, eventually, assess sham clinical effectiveness.
Methods:
A systematic Cochrane-based review was conducted by analyzing the osteopathic trials that used both manual and nonmanual placebo control. Searches were conducted on 8 databases from journal inception to December 2015 using a pragmatic literature search approach. Two independent reviewers conducted the study selection and data extraction for each study. The risk of bias was evaluated according to the Cochrane methods.
Results:
A total of 64 studies were eligible for analysis collecting a total of 5024 participants. More than half (43 studies) used a manual placebo; 9 studies used a nonmanual placebo; and 12 studies used both manual and nonmanual placebo. Data showed lack of reporting sham therapy information across studies. Risk of bias analysis demonstrated a high risk of bias for allocation, blinding of personnel and participants, selective, and other bias. To explore the clinical effects of sham therapies used, a quantitative analysis was planned. However, due to the high heterogeneity of sham approaches used no further analyses were performed.
Conclusion:
High heterogeneity regarding placebo used between studies, lack of reporting information on placebo methods and within-study variability between sham and real treatment procedures suggest prudence in reading and interpreting study findings in manual osteopathic randomized controlled trials (RCTs). Efforts must be made to promote guidelines to design the most reliable placebo for manual RCTs as a means of increasing the internal validity and improve external validity of findings.
1. Introduction
Placebo is defined as false treatment or false therapy and its effects are a well-recognized phenomenon in medicine.[1] Although an inert physically or pharmacologically therapy is administered,[2] placebos can produce improvements in patients’ symptomatology.[3] A robust body of literature clearly demonstrates the neurobiological correlates of placebo and placebo response in both healthy subjects and patients.[4–6] Placebo (and the related nocebo) effects seemed to be influenced by several psychosocial factors including patient expectation, patient–doctor relationship, and therapeutic rituals.[5,7] These factors seem to elicit neuropsychological mechanisms like conditioning,[1,8–10] expectation,[11–13] and reward,[8] which in turn drive modifications in the brain–body interactions[8] through endocrine,[2,10] immune,[8] and autonomic[2,8] systems.
Placebo has to be carefully considered in trial design[8,14] as it can mask the real effects of a given pharmacological or nonpharmacological treatment.[8] For this reason, placebos are considered fundamental control groups in randomized controlled trials (RCTs).[14,15] Critically, placebos have been tested and used in double blinded clinical trials investigating drug-based therapy effects. Using this study design, by definition, the therapist/drug administrator has to be blinded to the treatment. However, when applying this methodology to other health care areas, the scenario might be unsuitable. That is the case of manual complementary and alternative medicines, including osteopathy, where the use of placebos is inherently biased by the therapist, who actively administers the manual treatment.[16,17] In fact, these “drug-driven” scientific and methodological standards might have produced important consequences about the improper interpretation of studies in manual treatment with potential misinterpretation of its effects from a public health perspective.[18–20] Furthermore, the literature reports guidelines and recommendations to conduct nonpharmacological trials,[21] but there is still lack of a common and robust manual placebo paradigm.[22] Expected advantages from creating a paradigm will include an improvement of placebo awareness not only in the “core clinical practice curriculum of all health practitioners,”[1] but also in the field of research.
In the last decades, osteopathic clinical trials have been steadily increasing in number and type, addressing various diseases and patient populations with different methodologies. Considering comparison groups, osteopathic research often used the so-called “false treatment” as control arm.[23–27] This type of treatment can be referred to as sham therapy or placebo; however, its use and methodology are left to researchers’ discretion rather than to formal guidelines and/or recommendations. There is a need to clarify and codify the sham methods used in manual therapy research to assess and develop a robust paradigm that may become the standard placebo arm of a trial. Therefore, with the purpose to assess the use of sham therapy in manual medicine, primarily osteopathy, the aim of this systematic review was to describe the methods used for placebo/sham therapy defining similarities and diversities among osteopathic studies about different aspects: methodology, dosage, operator's characteristics, and type of patients. In addition, the clinical effectiveness of sham procedures was explored comparing control and intervention groups.
2. Methods
This systematic review included single- and multicenter RCT, quasi-RCT, controlled clinical trials, interrupted time series, and controlled before and after studies. Observational studies, cohort studies, cross-sectional studies, case-control, case-series, and case-report studies as well as abstract and animal studies were excluded. Study reports must have been written in English. Research including patients with any medical condition as well as healthy (asymptomatic) subjects of either sex and any age were considered eligible.
In addition, included studies had to have at least a control group in which a form of sham therapy was provided. Interventions and sham therapy could be applied alone or in addition to conventional treatments (i.e., pharmacological cointerventions, counseling, or advice prescription).
2.1. Data sources and searches
The identification of the studies was conducted by a comprehensive computerized search of MEDLINE (http://www.ncbi.nlm.nih.gov/pubmed), Scholar google (http://scholar.google.it), SCOPUS (http://www.elsevier.com/onlinetools/scopus), clinicaltrials.gov, chiloras/MANTIS, OSTMED.DR (http://ostmed-dr.com/), Osteopathic Research Web (http://www.osteopathic-research.com/), and the Cochrane Library (http://www.thecochranelibrary.com). Other sources included gray literature, national trials registers, web searching, and conference proceedings. Search terms are included in the Supplemental Content 1 (see Table, Supplemental Content, that reported the search strategy used). The search was conducted from journal inception to December 2015. Duplicate records were identified in EndNote and eliminated.
2.2. Study selection
Two reviewers (MV and LC) with expertise in the field of osteopathic medicine research independently conducted the study selection based on the explicit search strategy. Discrepancies were resolved by consensus with FC as an arbiter. There were discussions about 5 studies that were ultimately excluded. According to inclusion criteria, the reviewers independently screened titles and abstracts and full-text were retrieved and assessed.
2.3. Data extraction and quality assessment
Data extraction was performed independently by the 2 reviewers, in terms of population characteristics, type of interventions, type of sham, study results, and all the other descriptive characteristics of the included trials (i.e., nationality, year of publication). All disagreements were discussed and resolved by consensus. If data were not reported in the study, the author was contacted. The authors were emailed twice with the aim to obtain complete data. All analyzed data were stored in a dedicated hard disk, accessible only by the 2 reviewers.
Each study was independently evaluated. According to the Cochrane methods, the risk of bias was categorized in low, high, and unclear across the following domains: Sequence Generation; Allocation Concealment; Blinding to Personnel; Blinding to Outcome Analysis; and other bias.[18] Reviewers collected the quality assessment of reports of RCTs in terms of Jadad scores, considering the description and sequence of randomization, the double blind procedure, its appropriateness, and the description of withdrawals and dropouts (range 0–5).[28]
2.4. Data synthesis and analysis
Sham therapy was categorized into 3 groups: manual, nonmanual, and combined. The aim was to classify control groups based on the delivering of manual or non-manual manipulation. Therefore, the manual sham group was defined as studies delivering manual manipulations only; the nonmanual sham group was established as applying any type of control intervention without manual contact; and the combined sham group was represented by research using both manual and nonmanual procedures. For each study, the number of subjects receiving any form of sham therapy was computed. In addition, sham operators and type of sham were detailed and analyzed. The counts of the separate studies were summed to obtain the cumulative number of subjects/operators reporting each type of sham/therapy administered. The final counts were used to combine data from individual studies. Data were reported as mean, point estimate, percentage, and range. Dispersion was presented as standard deviation and 95% confidence interval (CI). For continuous data, mean differences with 95% CIs were used. For dichotomous outcomes, to compare the different classes of placebos for each given category, the observed total number of subjects were entered into 2 × 2 contingency table and results were presented as odds ratio (OR) with 95% CI. Nonoverlapping CIs of the rates of placebo groups indicate significant differences among the different categories. Moreover, to study the various type of sham therapies a conservative approach was chosen to analyze data. Therefore, χ2 test, Student t test, and ANOVA with Tukey post hoc analysis were used to compare groups. To control for multiple tests, a Bonferroni correction of α value was adopted. Furthermore, the Yates’ correction for continuity was used, required in one-degree-of-freedom situations.[29] Statistical significance was set at α < 0.05.
Other variables such as sample size, sex, age, weight, healthy subjects, withdrawals, and publication year were also considered in the analysis.
To explore the possibility to run a meta-analysis, studies were not pooled if there was significant heterogeneity. A stepwise heterogeneity assessment was performed. Clinical and methodological heterogeneity were assessed first considering the following levels: patients, intervention, outcome, control event rate/baseline risk, research setting, comparison conditions, early stopping rules, and population risk.[30] Statistical heterogeneity was assessed using the I2 statistic, only if clinical and methodological homogeneity were satisfied. The software used for statistical analyses was R v 3.2.0.
3. Results
3.1. Description of studies
Seventy full-text studies were assessed for eligibility (Fig. 1). Data and publications emanating from the same study were considered duplicates and therefore excluded. The final sample was 64 studies enrolling 5024 patients. Characteristics of the 3 placebo arms are shown in Table 1.
Figure 1.
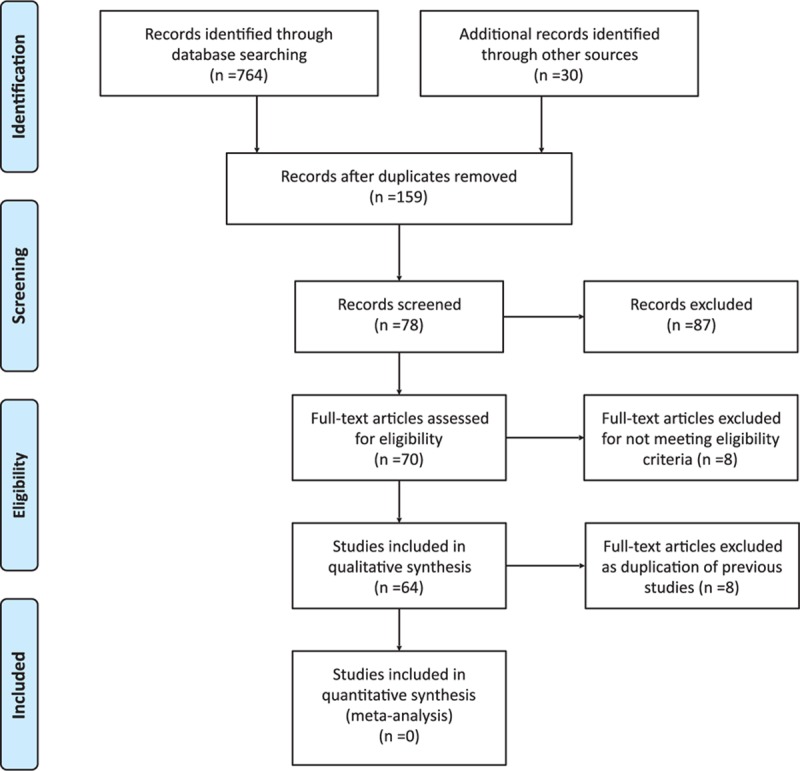
Flow diagram of the study selection.
Table 1.
Characteristics of the sham groups, divided into the manual, nonmanual, and combined (manual and nonmanual).
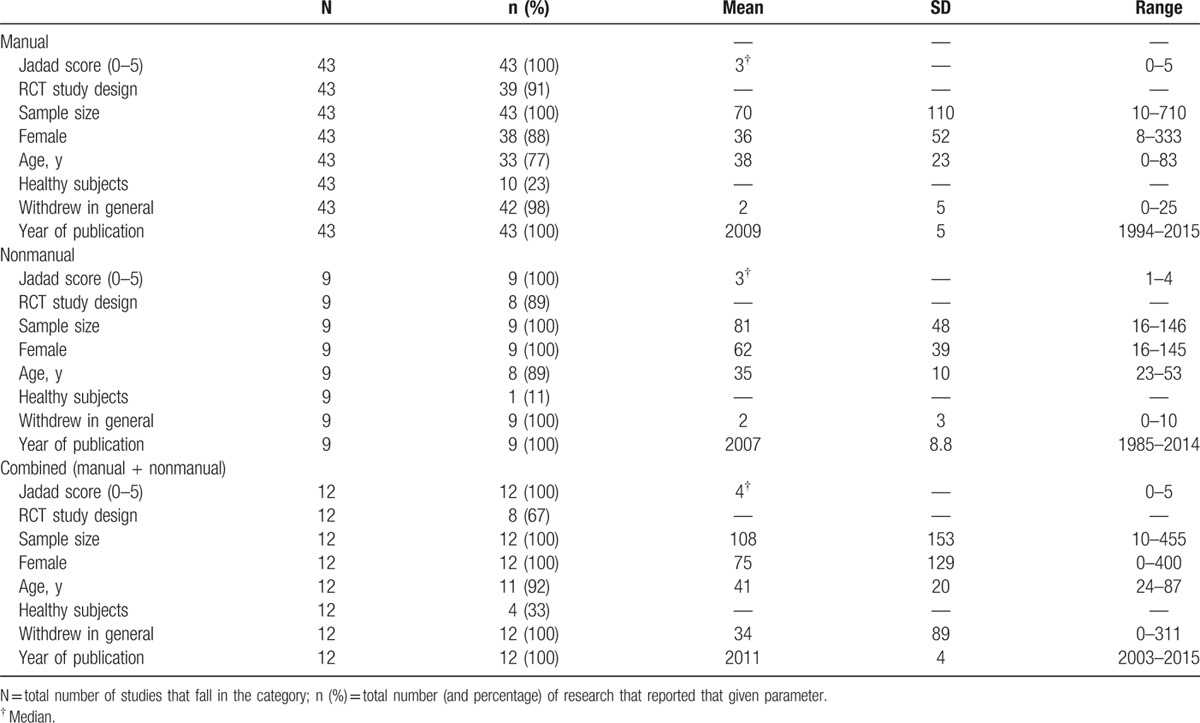
Descriptively, the majority of research was published between 2000 and 2015 (n = 61; 95%), mainly in the United States (n = 26; 41%) and Europe (n = 28; 44%), designed as RCT (n = 58; 91%) with 2 study arms (n = 40; 63%) and within the field of musculoskeletal care (n = 30; 47%).
3.2. Clinical comparison between the 3 placebo categories
Analyses revealed statistically significant differences between the 3 groups in terms of number of studies (χ2 = 40.55; P < 0.001). Marginal differences were shown regarding the type of study design used, with a larger number of RCTs in manual and nonmanual compared with combined sham group (χ2 = 4.56, P = .10). The number of withdrew subjects, that is the number of subjects who withdrew from the type of study that is reported (manual, nonmanual, combined), showed significant differences (F(2,54) = 3.331; P = .04). Post hoc comparison showed significantly more subjects dropped out in the combined placebo group than in either other group (−31.76; 95% CI −61.91, −1.61; P = 0.03).
No significant differences in sample size, age, Jadad score, number of healthy subjects and females enrolled, and years of publication were observed (Table 1).
Table 2 shows the number and percentage of sham characteristics across the different placebo groups. The rates of type of touch varied widely across trials and placebos. Light touch was the most used approach, particularly in the combined group. The latter showed a statistically significant percentage difference compared with the manual sham group (χ2 = 3.50, P = 0.06, Z = −2.26, P = 0.02). Nonmanual placebo used mainly ultrasound, whereas combined sham therapy preferred usual care or no intervention controls.
Table 2.
Different characteristics of sham scenario in manual, nonmanual, and combined groups.
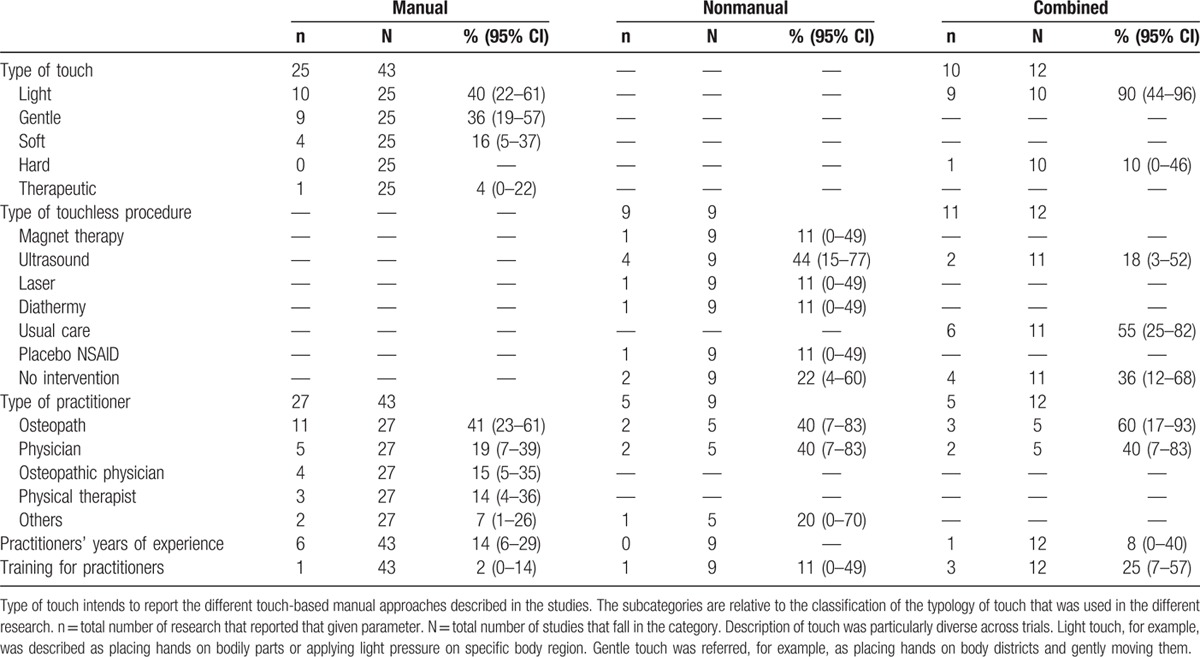
With regard to the type of practitioner who administered the sham therapy, osteopaths were the most prevalent group with higher percentage in the combined group. Years of experience were reported in few studies (7/64; 11%); however, considering the number of years of practitioners’ experience, data showed a significant difference between the 3 groups (F(2,50) = 6.75; P = 0.048). Tukey post hoc comparison demonstrated that manual sham group operators were older than those in the combined trials (9.67; 0.10, 19.23; P = 0.05). Only 5 studies (9%) described whether practitioners received consensus training before the commencement of the research, with 7 trials (8%) declaring the absence of preconsensus training. Moreover, in >50% of trials lack of comparability was showed between sham therapy and real intervention.
3.3. Differences in missing data reporting and sectorial publication
Data showed lack of reporting sham therapy information across studies. Dosage, number of session performed, period of sham treatment, type of procedures applied (protocol-based or personalized), and type of sham approach used were significantly underreported. Less than 30% of all included studies described at least 3 of the above-mentioned aspects.
On average, the manual sham group trials reported information on the 27.8% (range 2%–55%), nonmanual on 41.5% (0%–100%), and combined 83.3% (9%–100%) with a statistical significant difference between groups (χ2 = 7.38, P = 0.02). Specifically, ORs between placebo categories showed the followings: manual versus nonmanual (0.66; 0.32–1.40; P = 0.28), manual versus combined (0.43; 0.23–0.80; P < 0.01), and nonmanual versus combined (0.64; 0.27–1.50; P = 0.30). Thus, the probability of not reporting information is significantly higher in the manual trials than all other placebo groups.
Taking into account the probability of publishing on sectorial (osteopathic) journals, χ2 test showed a marginal difference between the 3 categories (χ2 = 4.96, P = 0.08). In fact, manual placebo trials were more likely to be published in osteopathic journals compared with the other categories (manual: 20/43, 47%; nonmanual: 1/9, 11%; combined: 3/12, 25%).
3.4. Type of placebo used by age group
Considering the type of placebo according to age group, it was shown that there might be an age-related use (Fig. 2). All the 3 approaches were used in the adulthood (between 20 and 50 years old). Manual placebo was the only approach used in babies, whereas in the elderly only manual and combined were applied. A further analysis showed no statistically significant association between age group and type of placebo (χ2 = 5.02, P = 0.29).
Figure 2.
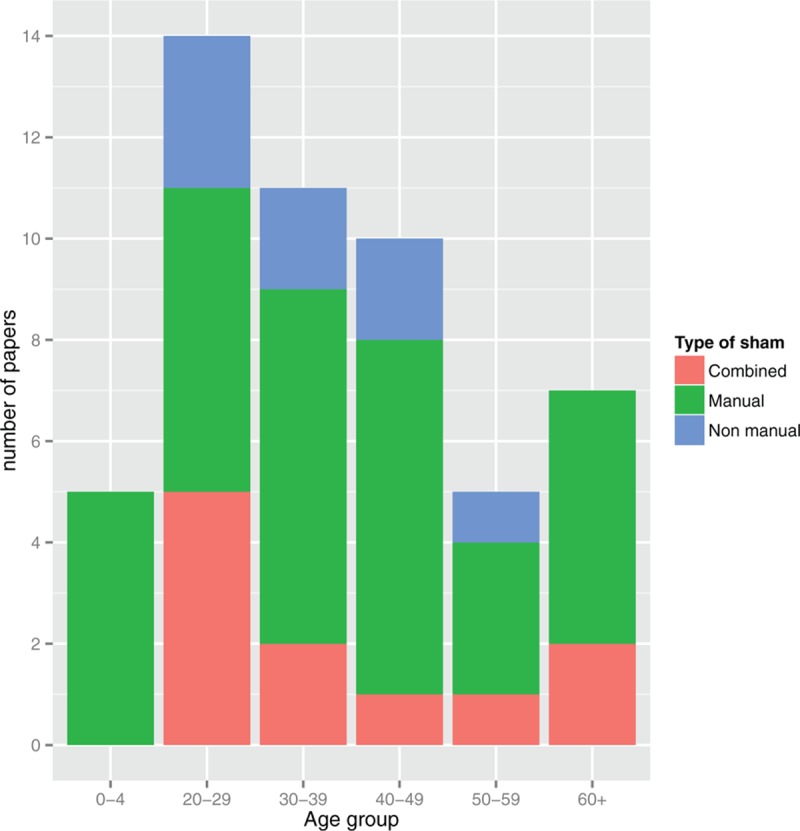
Number of papers included by type of sham and age group.
3.5. Adverse events across studies
Only 23% of research reported data on adverse events of which manual sham group showed the highest reporting percentage (12/43; 28%; range 16%–44%) compared with nonmanual (1/9; 6%; 0%–43%) and combined (2/12; 17%; 3%–49%), although not statistically significant (χ2 = 1.55, P = 0.46). As far as ORs are considered, data showed that the likelihood of reporting adverse events is larger in the manual sham group than both the combined group (1.67; 0.33–8.53; P = 0.54) and nonmanual sham trials (2.51; 0.29–21.84; P = 0.40). However, the combined category had higher probability than nonmanual sham group (1.50; 0.12–19.24; P = 0.76).
Among the 14 trials describing adverse events, 10 (71%) reported absence of symptoms whereas 5 showed mild side effects from the sham therapy applied. Furthermore, among a total of 720 reporting patients, 42 complained having adverse events (5.9%).
3.6. Sample size calculation
Only the 31% (20/64) of research preestablished a sample size considering statistical and clinical parameters. The vast majority of studies did not report any information or declare to not having calculated the sample size. Among the 3 placebo categories, the combined was the group with the highest percentage of trials with sample size calculation reporting (6/12; 50%). In manual and nonmanual sham groups, sample size calculation was reported in the 28% and 22%, respectively. No statistically significant difference was shown (χ2 = 2.53, P = 0.28).
3.7. Assessment of homogeneity across placebos groups
No research was considered acceptable for meta-analyses as did not satisfy inclusion criteria. Specifically, none of the studies used similar sham therapies, leading to high (clinical) heterogeneity of sham approach used, therefore preventing any further quantitative analysis. This limited, also, the possibility to compute statistical homogeneity through I2, and precluded additional pooling for exploring any clinical effectiveness of sham procedure used. Supplemental Content 2 (see Table, Supplemental Content, that reported information regarding eligible research) showed details regarding included studies eligible for meta-analysis. Supplemental Content 3 (see Table, Supplemental Content, that showed details on sham procedures) reported information on manual and nonmanual sham maneuvers used by trials.
3.8. Risk of bias evaluation
Reliability between reviewers was estimated. Kappa score was equal to 0.793 for quality assessment in terms of Jadad scores, whereas κ = 0.801 for risk of bias assessment. Therefore, reliability was considered high between reviewers. Out of 64 studies[31–92] included in the current systematic review, 9 were not RCTs.[31–39] The remaining 55 RCTs were evaluated in the different categories of bias.
The majority of the trials included presented a low risk of bias for sequence generation and allocation concealment. More than half of the studies claimed that the research personnel and/or patients involved in their respective trial were not aware of study design and outcomes, but did not report any information regarding blinding of outcome assessors. None of the RCT reported their protocols, thus the assessment for selection bias was not possible to perform. The quality of included studies was further assessed considering the followings: informed consent, conflict and declaration of interest, reporting funding source, ethical approval, confidentiality, access to data, trial registration, data collection, data management, and data monitor committee. A large number of the research did not describe sufficient information regarding the majority of the categories considered (see Table, Supplemental Content 4, that reported information regarding risk of bias).
4. Discussion
The results of the present review could be summarized as follows: high (clinical) heterogeneity regarding placebo/sham used between studies, lack of reporting information on placebo/sham methods, and within-study variability between sham and real treatment procedures.
First, interstudy clinical heterogeneity was significantly large; indeed, several types of sham therapies were reported. Some studies used a manual sham approach, whereas others used nonmanual contact or combined manual and nonmanual procedures. Interestingly, high variability within each category was revealed. In fact, several different types of touch were applied in manual sham studies. Consistently, many diverse instruments/physiotherapeutic tools were used in nonmanual sham trials. In addition, placebo pills and/or no intervention were used in other research. This scenario resulted in high clinical heterogeneity in sham manual procedures applied, which prevented a meta-analysis, although studies were homogeneous for other aspects (i.e., clinical field, intervention, participants). It can be argued that this great diversification in the control group could produce low internal and external validity of results. Therefore, lack of replicability could be a consequence of this methodological bias. These aspects might lead studies to decrease the likelihood of being compared and, thus, affecting the clinical validity.
Second, the analysis of missing data showed a consistent, structured, and significant lack of reporting information about sham procedure. In particular, trials systematically underreported details regarding the following aspects: sham dosage (i.e., duration, session, period); sham procedures (i.e., protocol-based or personalized, type of approach); and sham operator (type of practitioner, number of operators, operator's years of experience, training for practitioner, operator background, supervision/tutoring). This context might produce a reduction of robustness of placebo groups, increasing significantly the likelihood of having skewed results, high risk of bias (i.e., tools’ reliability and validity, performance bias), incomplete data reporting and, thus, reduced external validity of findings.
Third, sham treatment, by definition, should resemble the active treatment in any aspect except for technique. Using simplistically the placebo-drug paradigm, it can be argued that the real effect of “active ingredient” in osteopathic manipulative treatment (OMT) is equal to the effects of real OMT minus the effects of placebo/sham OMT. This equation might suggest that technique applied should be the only deterrent factor between real and placebo OMT. However, the results from the present review highlight that inconsistent procedures were applied between study and control groups. This might increase the probability of having unmatched groups and, therefore, change any estimation of OMT effect.
Furthermore, it can be claimed that, despite the effectiveness of a procedure, other “confounding” factors could be associated to results. Applying different touch strategies was argued to influence clinical outcomes. Noteworthy, light touch[93] is known to activate low-threshold mechanoreceptors through the so-called C-tactile fibers. This was demonstrated to modify the autonomic nervous system functions[94,95] (see McGlone 2014 for review).[96] Number of sessions, operators’ characteristics, and preconsensus training could all potentially interact with study outcomes. Therefore, several additional factors need to be considered to rate the quality of studies and estimate intervention effectiveness. From a clinical point of view, the assessment of these components is thus crucial to correctly evaluate the validity and reliability of research.
Interestingly, although the quality of studies was generally moderate, high risk of bias was showed in all items concerning the description of intervention, thus performance bias. This will possibly imply that patients did not receive a similar amount of attention, thus influencing the final outcome.
In general, the use of placebo is a key tool in any RCT.[14,15,97] It has been suggested that it is essential to shed light on the real effect of a given treatment taking into account several potential psychosocial confounders.[8] Methods of placebo were historically linked to pharmacological trials where the “sugar pill” was considered the placebo par excellence. In manual sham RCTs, it could, however, be very complex to isolate the “active ingredient” for several reasons.[16] In fact, as demonstrated by this review, the different “sham therapies” were significantly heterogeneous according to different aspects (population, operator, real treatment-like procedures, osteopathic techniques) and context (i.e., clinical). Moreover, after evaluating the quality and type of reporting information, osteopathic trialists lack to report key details on placebo and control group. For example, on the one hand Ruffini et al conducted a well-designed RCT including sufficient details of placebo procedures to affirm that OMT might have real parasympathetic and trophotropic effects.[26] This implies that findings could be considered valid and reliable at the light of sufficient methodological details and quality. On the other hand, Barnes et al carried out an RCT suggesting that OMT could be relevant in the improvement of cvical mobility.[41] Although the positive results described, the poor methodology as well as the insufficient information provided by the authors might question on the credibility and robustness of data.
Therefore, it can be clear that the above-mentioned research, although clinically positive, might produce completely different study assessments and effects of osteopathic intervention.
Moreover, published guidelines suggest ways to reduce risk of bias.[98,99] However, the concept of bias was itself built on pharmacological trials. It was difficult for nonpharmacological research to resist the compelling argument for the use of sham therapy demonstrated by pharmacological trials. As a consequence, this exposes the drug-free study designs to several methodological flaws, biases, for example, performance bias as not respecting the blinding of personnel,[21] and, thus, misinterpretation of quality and results. As a matter of fact, the present review highlighted this “paradox” of sham therapies calling for pragmatic methodological solutions. Practical recommendations for further studies included detailed description of sham procedures used, focusing on dosage, frequency, number of sessions, period of sham treatment, type of procedure, and approach applied. In case of manual sham treatment, description of type of manual approach used with details is required. Attention should be paid to the use of sham arms that mimic and are similar to the real intervention. Practitioners’ selection and consistency are another relevant aspects to be considered when planning an appropriate placebo-controlled RCT.
Collectively, results from the present systematic review suggest prudence in reading and interpreting study findings in manual osteopathic RCTs; the necessity to consider sham group as relevant part of osteopathic manual medicine trials; to accurate assess and plan sham procedures in RCTs; and the need to shift from a pharmacological research-based scenario to a multidisciplinary manual-based research guidance.
Several limitations could be pointed out in the present systematic review. There could be some potential sources of publication bias.[100] Although an attempt was made to identify unpublished research, which is more likely to have negative outcomes,[101,102] the search strategy may have left out relevant studies not currently indexed. Moreover, limiting the search to publications written in English could have skewed the general results.[103]
5. Conclusions
The aim of the present review was to explore the extent to which sham therapies were used in manual medicine, in particularly in osteopathic clinical trials. High heterogeneity regarding placebo used between studies, lack of reporting information on placebo methods and within-study variability between sham and real treatment procedures suggest prudence in reading and interpreting study findings in manual osteopathic RCTs. Efforts must be done to promote guidelines to design the most reliable “sugar pill” for manual RCT. Robust recommendations should be based on methodological aspects and on neurobiology of placebo. This will facilitate structured versus spontaneous sham therapy reporting. Therefore, effects are predicted on study validity and between-study homogeneity. Arguably, a valid, reliable, reasonable, and common placebo will increase the internal validity and improve external validity of findings.
Acknowledgments
The authors thank Dr Jorge Esteves for his help in critically reviewing the paper.
Supplementary Material
Footnotes
Abbreviations: OMT = osteopathic manipulative treatment, OR = odds ratio, RCT = randomized controlled trials.
FC, MV, LC, and GDA equally contributed to the paper.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Colloca L, Jonas WB, Killen J, et al. Reevaluating the placebo effect in medical practice. Z Psychol 2014; 222:124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 2015; 16:403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaptchuk TJ, Miller FG. Placebo effects in medicine. N Engl J Med 2015; 373:8–9. [DOI] [PubMed] [Google Scholar]
- 4.Finniss DG, Kaptchuk TJ, Miller F, et al. Biological, clinical, and ethical advances of placebo effects. Lancet 2010; 375:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedetti F, Carlino E, Pollo A. How placebos change the patient's brain. Neuropsychopharmacology 2011; 36:339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedetti F. Placebo Effects: Understanding the Mechanisms in Health and Disease. New York: Oxford University Press; 2008. [Google Scholar]
- 7.Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev 2013; 93:1207–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollo A, Carlino E, Benedetti F. Placebo mechanisms across different conditions: from the clinical setting to physical performance. Philos Trans R Soc Lond B Biol Sci 2011; 366:1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen K, Kirsch I, Odmalm S, et al. Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proc Natl Acad Sci U S A 2015; 112:7863–7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol 2008; 48:33–60. [DOI] [PubMed] [Google Scholar]
- 11.Shahar E, Shahar DJ. Causal diagrams, the placebo effect, and the expectation effect. Int J Gen Med 2013; 6:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linde K, Fassler M, Meissner K. Placebo interventions, placebo effects and clinical practice. Philos Trans R Soc Lond B Biol Sci 2011; 366:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedetti F. Placebo-induced improvements: how therapeutic rituals affect the patient's brain. J Acupunct Meridian Stud 2012; 5:97–103. [DOI] [PubMed] [Google Scholar]
- 14.Hall KT, Loscalzo J, Kaptchuk TJ. Genetics and the placebo effect: the placebome. Trends Mol Med 2015; 21:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espay AJ, Norris MM, Eliassen JC, et al. Placebo effect of medication cost in Parkinson disease: a randomized double-blind study. Neurology 2015; 84:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaibi A, Saltyte Benth J, Bjorn Russell M. Validation of placebo in a manual therapy randomized controlled trial. Sci Rep 2015; 5:11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koes BW. How to evaluate manual therapy: value and pitfalls of randomized clinical trials. Man Ther 2004; 9:183–184. [DOI] [PubMed] [Google Scholar]
- 18.Kaptchuk TJ. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann Intern Med 2002; 136:817–825. [DOI] [PubMed] [Google Scholar]
- 19.Kaptchuk TJ, Stason WB, Davis RB, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ 2006; 332:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teixeira MZ, Guedes CH, Barreto PV, et al. The placebo effect and homeopathy. Homeopathy 2010; 99:119–129. [DOI] [PubMed] [Google Scholar]
- 21.Kelley JM, Kraft-Todd G, Schapira L, et al. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2014; 9:e94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholten-Peeters GG, Thoomes E, Konings S, et al. Is manipulative therapy more effective than sham manipulation in adults: a systematic review and meta-analysis. Chiropr Man Therap 2013; 21:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson T, Grahame R, Harkness J, et al. Controlled comparison of short-wave diathermy treatment with osteopathic treatment in non-specific low back pain. Lancet 1985; 1:1258–1261. [DOI] [PubMed] [Google Scholar]
- 24.Martelli M, Cardinali L, Barlafante G, et al. Do placebo effects associated with sham osteopathic procedure occur in newborns? Results of a randomized controlled trial. Complement Ther Med 2014; 22:197–202. [DOI] [PubMed] [Google Scholar]
- 25.Patterson MM. Research in OMT: what is the question and do we understand it? J Am Osteopath Assoc 2007; 107:8–11. [Google Scholar]
- 26.Ruffini N, D’Alessandro G, Mariani N, et al. Variations of high frequency parameter of heart rate variability following osteopathic manipulative treatment in healthy subjects compared to control group and sham therapy: randomized controlled trial. Front Neurosci 2015; 9:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sergueef N, Nelson KE, Glonek T. The effect of cranial manipulation on the Traube-Hering-Mayer oscillation as measured by laser-Doppler flowmetry. Altern Ther Health Med 2002; 8:74–76. [PubMed] [Google Scholar]
- 28.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 29.Runyon RP, Haber A. Fundamentals of behavioral statistics. Minnesota: Addison-Wesley; 1971. [Google Scholar]
- 30.Gagnier JJ, Moher D, Boon H, et al. Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Med Res Methodol 2012; 12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bockenhauer SE, Julliard KN, Lo KS, et al. Quantifiable effects of osteopathic manipulative techniques on patients with chronic asthma. J Am Osteopath Assoc 2002; 102:371–375. [PubMed] [Google Scholar]
- 32.Degenhardt BF, Johnson JC, Fossum C, et al. Changes in cytokines, sensory tests, and self-reported pain levels after manual treatment of low back pain. Clin Spine Surg 2016. 28. [DOI] [PubMed] [Google Scholar]
- 33.Giles PD, Hensel KL, Pacchia CF, et al. Suboccipital decompression enhances heart rate variability indices of cardiac control in healthy subjects. J Altern Complement Med 2013; 19:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henley CE, Ivins D, Mills M, et al. Osteopathic manipulative treatment and its relationship to autonomic nervous system activity as demonstrated by heart rate variability: a repeated measures study. Osteopath Med Prim Care 2008; 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karason AB, Drysdale IP. Somatovisceral response following osteopathic HVLAT: a pilot study on the effect of unilateral lumbosacral high-velocity low-amplitude thrust technique on the cutaneous blood flow in the lower limb. J Manipulative Physiol Ther 2003; 26:220–225. [DOI] [PubMed] [Google Scholar]
- 36.Lougee H, Johnston RG, Thomson OP. The suitability of sham treatments for use as placebo controls in trials of spinal manipulative therapy: a pilot study. J Bodyw Mov Ther 2013; 17:59–68. [DOI] [PubMed] [Google Scholar]
- 37.Miana L, Bastos VH, Machado S, et al. Changes in alpha band activity associated with application of the compression of fourth ventricular (CV-4) osteopathic procedure: a qEEG pilot study. J Bodyw Mov Ther 2013; 17:291–296. [DOI] [PubMed] [Google Scholar]
- 38.Shi X, Rehrer S, Prajapati P, et al. Effect of cranial osteopathic manipulative medicine on cerebral tissue oxygenation. J Am Osteopath Assoc 2011; 111:660–666. [PubMed] [Google Scholar]
- 39.Wells MR, Giantinoto S, D’Agate D, et al. Standard osteopathic manipulative treatment acutely improves gait performance in patients with Parkinson's disease. J Am Osteopath Assoc 1999; 99:92–98. [DOI] [PubMed] [Google Scholar]
- 40.Ajimsha MS, Daniel B, Chithra S. Effectiveness of myofascial release in the management of chronic low back pain in nursing professionals. J Bodyw Mov Ther 2014; 18:273–281. [DOI] [PubMed] [Google Scholar]
- 41.Barnes PL, Laboy F, 3rd, Noto-Bell L, et al. A comparative study of cervical hysteresis characteristics after various osteopathic manipulative treatment (OMT) modalities. J Bodyw Mov Ther 2013; 17:89–94. [DOI] [PubMed] [Google Scholar]
- 42.Burns DK, Wells MR. Gross range of motion in the cervical spine: the effects of osteopathic muscle energy technique in asymptomatic subjects. J Am Osteopath Assoc Mar 2006; 106:137–142. [PubMed] [Google Scholar]
- 43.Cardoso-de-Mello EM-RAP, Rodriguez-Blanco C, Riquelme-Agullo I, et al. Effects of the fourth ventricle compression in the regulation of the autonomic nervous system: a randomized control trial. Evid Based Complement Alternat Med 2015; 2015:148285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cleary C, Fox JP. Menopausal symptoms: an osteopathic investigation. Complement Ther Med 1994; 2:181–186. [Google Scholar]
- 45.Curtis P, Gaylord SA, Park J, et al. Credibility of low-strength static magnet therapy as an attention control intervention for a randomized controlled study of CranioSacral therapy for migraine headaches. J Altern Complement Med 2011; 17:711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.da Silva RC, de Sa CC, Pascual-Vaca AO, et al. Increase of lower esophageal sphincter pressure after osteopathic intervention on the diaphragm in patients with gastroesophageal reflux. Dis Esophagus 2013; 26:451–456. [DOI] [PubMed] [Google Scholar]
- 47.Florance BM, Frin G, Dainese R, et al. Osteopathy improves the severity of irritable bowel syndrome: a pilot randomized sham-controlled study. Eur J Gastroenterol Hepatol 2012; 24:944–949. [DOI] [PubMed] [Google Scholar]
- 48.Fryer G, Carub J, McIver S. The effect of manipulation and mobilisation on pressure pain thresholds in the thoracic spine. J Osteopath Med 2004; 7:8–14. [Google Scholar]
- 49.Fryer G, Ruszkowski W. The influence of contraction duration in muscle energy technique applied to the atlanto-axial joint. J Osteopath Med 2004; 7:79–84. [Google Scholar]
- 50.Goldstein FJ, Jeck S, Nicholas AS, et al. Preoperative intravenous morphine sulfate with postoperative osteopathic manipulative treatment reduces patient analgesic use after total abdominal hysterectomy. J Am Osteopath Assoc 2005; 105:273–279. [PubMed] [Google Scholar]
- 51.Guiney PA, Chou R, Vianna A, et al. Effects of osteopathic manipulative treatment on pediatric patients with asthma: a randomized controlled trial. J Am Osteopath Assoc 2005; 105:7–12. [PubMed] [Google Scholar]
- 52.Haller H, Ostermann T, Lauche R, et al. Credibility of a comparative sham control intervention for Craniosacral Therapy in patients with chronic neck pain. Complement Ther Med 2014; 22:1053–1059. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton L, Boswell C, Fryer G. The effects of high-velocity, low-amplitude manipulation and muscle energy technique on suboccipital tenderness. Int J Osteopath Med 2007; 10:42–49. [Google Scholar]
- 54.Hensel KL, Pacchia CF, Smith ML. Acute improvement in hemodynamic control after osteopathic manipulative treatment in the third trimester of pregnancy. Complement Ther Med 2013; 21:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howell JN, Cabell KS, Chila AG, et al. Stretch reflex and Hoffmann reflex responses to osteopathic manipulative treatment in subjects with Achilles tendinitis. J Am Osteopath Assoc 2006; 106:537–545. [PubMed] [Google Scholar]
- 56.Knebl JA, Shores JH, Gamber RG, et al. Improving functional ability in the elderly via the Spencer technique, an osteopathic manipulative treatment: a randomized, controlled trial. J Am Osteopath Assoc 2002; 102:387–396. [PubMed] [Google Scholar]
- 57.Licciardone JC, Stoll ST, Cardarelli KM, et al. A randomized controlled trial of osteopathic manipulative treatment following knee or hip arthroplasty. J Am Osteopath Assoc 2004; 104:193–202. [PubMed] [Google Scholar]
- 58.Licciardone JC, Stoll ST, Fulda KG, et al. Osteopathic manipulative treatment for chronic low back pain: a randomized controlled trial. Spine (Phila Pa 1976) 2003; 28:1355–1362. [DOI] [PubMed] [Google Scholar]
- 59.McPartland JM, Giuffrida A, King J, et al. Cannabimimetic effects of osteopathic manipulative treatment. J Am Osteopath Assoc 2005; 105:283–291. [PubMed] [Google Scholar]
- 60.Noll DR, Shores JH, Gamber RG, et al. Benefits of osteopathic manipulative treatment for hospitalized elderly patients with pneumonia. J Am Osteopath Assoc 2000; 100:776–782. [PubMed] [Google Scholar]
- 61.Nourbakhsh MR, Fearon FJ. The effect of oscillating-energy manual therapy on lateral epicondylitis: a randomized, placebo-control, double-blinded study. J Hand Ther 2008; 21:4–13. [DOI] [PubMed] [Google Scholar]
- 62.Philippi H, Faldum A, Schleupen A, et al. Infantile postural asymmetry and osteopathic treatment: a randomized therapeutic trial. Dev Med Child Neurol 2006; 48:5–9. [DOI] [PubMed] [Google Scholar]
- 63.Snider KT, Snider EJ, Johnson JC, et al. Preventative osteopathic manipulative treatment and the elderly nursing home resident: a pilot study. J Am Osteopath Assoc 2012; 112:489–501. [PubMed] [Google Scholar]
- 64.Tozzi P, Bongiorno D, Vitturini C. Fascial release effects on patients with non-specific cervical or lumbar pain. J Bodyw Mov Ther 2011; 15:405–416. [DOI] [PubMed] [Google Scholar]
- 65.Tozzi P, Bongiorno D, Vitturini C. Low back pain and kidney mobility: local osteopathic fascial manipulation decreases pain perception and improves renal mobility. J Bodyw Mov Ther 2012; 16:381–391. [DOI] [PubMed] [Google Scholar]
- 66.Wynne MM, Burns JM, Eland DC, et al. Effect of counterstrain on stretch reflexes, hoffmann reflexes, and clinical outcomes in subjects with plantar fasciitis. J Am Osteopath Assoc 2006; 106:547–556. [PubMed] [Google Scholar]
- 67.Attali TV, Bouchoucha M, Benamouzig R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: short-term and long-term results of a randomized trial. J Dig Dis 2013; 14:654–661. [DOI] [PubMed] [Google Scholar]
- 68.Boët C, Fugier S, Marsault J, et al. High-velocity low-amplitude thrust manipulation of the lumbar spine immediately modifies soleus T reflex in asymptomatic adults. Int J Osteopath Med 2013; 16:131–142. [Google Scholar]
- 69.Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-Armed randomized controlled trial. Complement Ther Med 2015; 23:149–156. [DOI] [PubMed] [Google Scholar]
- 70.Hubert D, Soubeiran L, Gourmelon F, et al. Impact of osteopathic treatment on pain in adult patients with cystic fibrosis—a pilot randomized controlled study. PLoS One 2014; 9:e102465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noll DR. The short-term effect of a lymphatic pump protocol on blood cell counts in nursing home residents with limited mobility: a pilot study. J Am Osteopath Assoc 2013; 113:520–528. [DOI] [PubMed] [Google Scholar]
- 72.Papa L, Mandara A, Bottali M, et al. A randomized control trial on the effectiveness of osteopathic manipulative treatment in reducing pain and improving the quality of life in elderly patients affected by osteoporosis. Clin Cases Miner Bone Metab 2012; 9:179–183. [PMC free article] [PubMed] [Google Scholar]
- 73.Sandhouse ME, Shechtman D, Sorkin R, et al. Effect of osteopathy in the cranial field on visual function—a pilot study. J Am Osteopath Assoc 2010; 110:239–243. [PubMed] [Google Scholar]
- 74.Wieting JM, Beal C, Roth GL, et al. The effect of osteopathic manipulative treatment on postoperative medical and functional recovery of coronary artery bypass graft patients. J Am Osteopath Assoc 2013; 113:384–393. [PubMed] [Google Scholar]
- 75.Castro-Sanchez AM, Mataran-Penarrocha GA, Sanchez-Labraca N, et al. A randomized controlled trial investigating the effects of craniosacral therapy on pain and heart rate variability in fibromyalgia patients. Clin Rehabil 2011; 25:25–35. [DOI] [PubMed] [Google Scholar]
- 76.Haller H, Lauche R, Cramer H, et al. Craniosacral therapy for the treatment of chronic neck pain: a randomized sham-controlled trial. Clin J Pain 2015; 32:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hensel KL, Buchanan S, Brown SK, et al. Pregnancy research on osteopathic manipulation optimizing treatment effects: the PROMOTE study. Am J Obstet Gynecol 2015; 212:108.e1–108.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Licciardone JC, Kearns CM, Hodge LM, et al. Osteopathic manual treatment in patients with diabetes mellitus and comorbid chronic low back pain: subgroup results from the OSTEOPATHIC Trial. J Am Osteopath Assoc 2013; 113:468–478. [PubMed] [Google Scholar]
- 79.Schwerla F, Bischoff A, Nurnberger A, et al. Osteopathic treatment of patients with chronic non-specific neck pain: a randomised controlled trial of efficacy. Forsch Komplementmed 2008; 15:138–145. [DOI] [PubMed] [Google Scholar]
- 80.Cerritelli F, Pizzolorusso G, Ciardelli F, et al. Effect of osteopathic manipulative treatment on length of stay in a population of preterm infants: a randomized controlled trial. BMC Pediatr 2013; 13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cerritelli F, Pizzolorusso G, Renzetti C, et al. A multicenter, randomized, controlled trial of osteopathic manipulative treatment on preterms. PLoS One 2015; 10:e0127370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pizzolorusso G, Cerritelli F, Accorsi A, et al. The effect of optimally timed osteopathic manipulative treatment on length of hospital stay in moderate and late preterm infants: results from a RCT. Evid Based Complement Alternat Med 2014; 2014:243539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Antolinos-Campillo PJ, Oliva-Pascual-Vaca A, Rodriguez-Blanco C, et al. Short-term changes in median nerve neural tension after a suboccipital muscle inhibition technique in subjects with cervical whiplash: a randomised controlled trial. Physiotherapy 2014; 100:249–255. [DOI] [PubMed] [Google Scholar]
- 84.Klein R, Bareis A, Schneider A, et al. Strain-counterstrain to treat restrictions of the mobility of the cervical spine in patients with neck pain: a sham-controlled randomized trial. Complement Ther Med 2013; 21:1–7. [DOI] [PubMed] [Google Scholar]
- 85.Swender DA, Thompson G, Schneider K, et al. Osteopathic manipulative treatment for inpatients with pulmonary exacerbations of cystic fibrosis: effects on spirometry findings and patient assessments of breathing, anxiety, and pain. J Am Osteopath Assoc 2014; 114:450–458. [DOI] [PubMed] [Google Scholar]
- 86.Mansilla-Ferragut P, Fernandez-de-Las Penas C, Alburquerque-Sendin F, et al. Immediate effects of atlanto-occipital joint manipulation on active mouth opening and pressure pain sensitivity in women with mechanical neck pain. J Manipulative Physiol Ther 2009; 32:101–106. [DOI] [PubMed] [Google Scholar]
- 87.Wahl RA, Aldous MB, Worden KA, et al. Echinacea purpurea and osteopathic manipulative treatment in children with recurrent otitis media: a randomized controlled trial. BMC Complement Altern Med 2008; 8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc 2014; 114:678–685. [DOI] [PubMed] [Google Scholar]
- 89.Brose SW, Jennings DC, Kwok J, et al. Sham manual medicine protocol for cervical strain-counterstrain research. PM R 2013; 5:400–407. [DOI] [PubMed] [Google Scholar]
- 90.Licciardone JC, Buchanan S, Hensel KL, et al. Osteopathic manipulative treatment of back pain and related symptoms during pregnancy: a randomized controlled trial. Am J Obstet Gynecol 2010; 202:43.e1–43.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noll DR, Degenhardt BF, Johnson JC, et al. Immediate effects of osteopathic manipulative treatment in elderly patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc 2008; 108:251–259. [PubMed] [Google Scholar]
- 92.Licciardone JC, Minotti DE, Gatchel RJ, et al. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Ann Fam Med 2013; 11:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perini I, Olausson H, Morrison I. Seeking pleasant touch: neural correlates of behavioral preferences for skin stroking. Front Behav Neurosci 2015; 9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Craig AD. How Do You Feel? An Interoceptive Moment With Your Neurobiological Self. Princeton, NJ: Princeton University Press; 2014. [Google Scholar]
- 95.Janig W. The Integrative Action of the Autonomic Nervous System, Neurobiology of Homeostasis. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 96.McGlone F, Wessberg J, Olausson H. Discriminative and affective touch: sensing and feeling. Neuron 2014; 82:737–755. [DOI] [PubMed] [Google Scholar]
- 97.Brim RL, Miller FG. The potential benefit of the placebo effect in sham-controlled trials: implications for risk-benefit assessments and informed consent. J Med Ethics 2013; 39:703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013; 346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 1990; 263:1385–1389. [PubMed] [Google Scholar]
- 101.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull 1979; 86:638. [Google Scholar]
- 102.Cook DJ, Guyatt GH, Ryan G, et al. Should unpublished data be included in meta-analyses?: current convictions and controversies. JAMA 1993; 269:2749–2753. [PubMed] [Google Scholar]
- 103.Moher D, Fortin P, Jadad AR, et al. Completeness of reporting of trials published in languages other than English: implications for conduct and reporting of systematic reviews. Lancet 1996; 347:363–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


