Supplemental Digital Content is available in the text
Keywords: body mass index, gastric high-grade dysplasia, serum total cholesterol
Abstract
Obesity is related to an increased risk of gastric cardia cancer. However, the influences of excess body weight and serum total cholesterol on the risk of gastric high-grade dysplasia have not been fully characterized.
A case–control study was conducted to explore the relationships between body mass index (BMI), serum total cholesterol level, and the risk of gastric high-grade dysplasia in Chinese adults. A total of 893 consecutive patients with gastric high-grade dysplasia (537 men and 356 women) and 902 controls (543 men and 359 women) were enrolled from January 2000 to October 2015. Odds ratios (ORs) with 95% confidence intervals (CIs) were estimated, and a multivariate analysis was conducted.
After adjusting for age, alcohol consumption, smoking status, family history of gastric cancer or esophageal cancer, and serum total cholesterol level, a BMI ranging from 27.5 to 29.9 was significantly related to an increased risk of gastric high-grade dysplasia in both men (adjusted OR = 1.87, 95% CI = 1.24–2.81) and women (adjusted OR = 2.72, 95% CI = 1.44–5.16). The 2 highest BMI categories (27.5–29.9 and ≥30.0) were identified as risk factors for gastric cardia high-grade dysplasia in both men (BMI = 27.5–29.9: adjusted OR = 1.78, 95% CI = 1.02–3.10; BMI ≥ 30.0: adjusted OR = 2.54, 95% CI = 1.27–5.08) and women (BMI = 27.5–29.9: adjusted OR = 2.88, 95% CI = 1.27–6.55; BMI ≥ 30.0: adjusted OR = 2.77, 95% CI = 1.36–5.64), whereas only a BMI ranging from 27.5 to 29.9 was a risk factor for gastric noncardia high-grade dysplasia in both men (adjusted OR = 1.98, 95% CI = 1.25–3.14) and women (adjusted OR = 2.88, 95% CI = 1.43–5.81). In addition, higher serum total cholesterol was associated with an increased risk of gastric noncardia high-grade dysplasia (adjusted OR = 1.83, 95% CI = 1.25–2.69) in women.
Increased BMI was associated with an increased risk of gastric high-grade dysplasia in both men and women, and higher serum total cholesterol increased the risk of gastric noncardia high-grade dysplasia in women.
1. Introduction
Gastric cancer (GC) was the fifth most common cancer and the third leading cause of cancer-related mortality worldwide in 2012; specifically, it was responsible for 723,000 deaths that year.[1,2] GC is a multifactorial disease, and both genetic and environmental factors are involved in its etiology. Risk factors for the noncardia and cardia regions of the stomach might differ. Common risk factors for both noncardia and cardia GC included the male sex,[3] older age,[4] radiation exposure,[5] cigarette smoking,[6] and a family history of GC.[7,8] Factors related to noncardia GC, but not cardia GC, included Helicobacter pylori infection,[9] low socioeconomic status,[10] and possible dietary factors such as the high intake of smoked and salty food and the low consumption of vegetables and fruits.[11–13] Risk factors that are exclusive to cardia GC include gastroesophageal reflux disease[14,15] and obesity.[16,17]
Atrophic gastritis, intestinal metaplasia, and gastric dysplasia are histologic premalignant lesions found to be multistep cascade precursors of gastric carcinogenesis.[18] Of these lesions, gastric dysplasia (especially gastric high-grade dysplasia) might be the last stage before GC development.[19] The Vienna classification of gastrointestinal epithelial neoplasia was the first source to clarify gastric high-grade dysplasia, and it has helpfully resolved many of the discrepancies between Western and Japanese pathologists regarding the diagnosis of gastrointestinal epithelial neoplastic lesions.[20] Approximately 85% of all patients with gastric dysplasia progress to GC annually[21,22]; however, the risk factors for gastric high-grade dysplasia are not well characterized.
Obesity is defined as “abnormal or excessive fat accumulation to the extent that health is impaired,” and it is the fastest growing lethal disease in the Western and developing worlds.[23] The World Health Organization (WHO) estimated that more than 1.9 billion adults, 18 years old and older, were overweight worldwide in 2014. Of these individuals, over 600 million were obese. Obesity is associated with increased risks of various types of cancer including breast,[24] endometrium,[25] ovary,[26] colorectal,[27] esophageal,[28] liver,[29] gallbladder, pancreatic,[30] kidney,[31] and stomach cancer.[32] The relationship between obesity and GC was implicated through the positive association with cardia GC (but not noncardia GC) observed in several meta-analyses. Obesity was also related to an increased risk of early GC (both cardia and noncardia cancers) in a recent case–control study.[32] High serum cholesterol levels are a major risk factor for cardiovascular disease.[33] In addition, prospective and cross-sectional studies have suggested that an association exists between low serum cholesterol levels and subsequent cancer mortality.[34] Subsequently, low serum cholesterol levels were found to be an independent risk factor for GC, especially intestinal-type GC.[35] However, the effects of obesity and serum cholesterol levels on the incidence of gastric high-grade dysplasia have not been well elucidated.
We conducted a retrospective investigation among Chinese adults to determine the relations between body mass index (BMI), an acceptable proxy for thinness and fatness, and the risk of gastric high-grade dysplasia. The association of serum total cholesterol level and the risk of gastric high-grade dysplasia was also examined. To determine whether BMI or serum total cholesterol level was related to the risk of gastric high-grade dysplasia at different sites such as gastric cardia or noncardia high-grade dysplasia, subgroup analysis was performed.
2. Materials and methods
2.1. Study subjects
A retrospective, hospital-based, case–control study was conducted. Patients histologically diagnosed with gastric high-grade dysplasia were enrolled at Peking Union Medical College Hospital from January 2000 to October 2015. All patients with gastric high-grade dysplasia were histologically confirmed via endoscopic submucosal dissection or surgical specimen according to the Vienna classification of gastrointestinal epithelial neoplasia.[20] Patients were excluded as cases if they required prompt medical care, the site of the primary tumor was unknown, they had previously undergone gastric resection, they had a history of any type of wasting disease before the diagnosis of gastric high-grade dysplasia, or they had a history of cancer or concurrent cancer. The participants undergoing endoscopy who were free of cancer and any evidence of gastric dysplasia, atrophic gastritis, mucosa-associated lymphoid tissue lymphoma, or any type of wasting disease were recruited to the control groups. The controls were frequency matched by the sex and age (within 3 years) of the patients.
All of the participants provided written informed consent before enrolment and completed a questionnaire that included information on typical body weight and height over the last 3 years; a history of hypolipidemic therapy; demographic characteristics; H pylori infection status; personal and family histories of GC and other diseases; smoking and alcohol consumption habits; and environmental, occupational, and dietary exposures. Smoking status was classified into 2 categories: never smoked and smokers, the latter of which included former smokers and current smokers. Participants were also divided into 2 groups by drinking status: drinkers and never/rare drinkers. The Ethics Committee of Peking Union Medical College Hospital approved this study (S-k056).
2.2. Body mass index
BMI, a measure of adiposity, was calculated as weight in kilograms divided by height in meters squared (kg/m2). The heights and weights of patients were measured at hospital admission; these variables were measured for controls when they underwent endoscopy. The typical body weights and heights of all participants who lost weight over the last 3 years were collected before weight loss. BMIs were categorized as follows: 18.4 or lower, 18.5 to 22.9, 23.0 to 27.4, 27.5 to 29.9, and 30.0 or more.[36] These categories are more suitable for Asia–Pacific populations than those proposed by the WHO.[37] For our analyses and discussion, the range from 23.0 to 27.4 was referred to as “overweight”; that from 27.5 to 29.9 corresponded to “obese”; and values of 30.0 or more were referred to as “severely obese.” The BMI category ranging from 18.5 to 22.9 was considered as the reference group for all primary analyses.
2.3. Gastric high-grade dysplasia
A pathologist confirmed all of the cases diagnosed as gastric high-grade dysplasia based on the Vienna classification of gastrointestinal epithelial neoplasia. High-grade dysplasia denoted a group of lesions with features more severe than those considered as low-grade dysplasia but without evidential invasion[19] that included high-grade adenoma or dysplasia, noninvasive carcinoma (carcinoma in situ), and suspicion of invasive carcinoma. Neoplasia sites were identified via anatomic site, and gastric cardia high-grade dysplasia was defined as neoplasia located within 2 cm of the gastroesophageal junction. Neoplasia originating from the distal stomach was identified as gastric noncardia high-grade dysplasia.[32]
2.4. Serum total cholesterol determination
Fasting blood samples were taken from the patients at hospital admission and from controls at endoscopy. Serum total cholesterol was measured enzymatically using an analyser (AU5800, Beckman Coulter, Brea, CA). The typical serum total cholesterol of all participants who lost weight over the last 3 years or received hypolipidemic therapy were collected before weight loss or hypolipidemic therapy. The participants were divided into 2 groups based on serum total cholesterol level according to the largest Yueden index: ≤4.55 or >4.55 mmol/L.
2.5. Information on covariates
Potential confounders included in data analyses were age (in single years), smoking status (never smoked and smokers, the latter of which included former smokers and current smokers), alcohol consumption (drinkers and never/rare drinkers), and family history of GC or esophageal cancer.
2.6. Statistical analyses
The associations between potential confounds and gastric high-grade dysplasia risk were assessed using chi-squared tests and Student t tests. The potential confounds included age, alcohol use, smoking status, and family history of GC or esophageal cancer (for gastric cardia high-grade dysplasia only). Univariate and multivariate logistic regression models were used to generate odds ratios (ORs) with corresponding 95% confidence intervals (CI) regarding BMI classifications and serum total cholesterol. The multivariate analyses were performed using multivariate logistic regression models to adjust for the following potential confounds with regard to all estimates of risk: age, alcohol consumption, smoking status, family history of GC or esophageal cancer, serum total cholesterol, and BMI. All statistical tests were 2-tailed, and the significance threshold was set at α = 0.05. All analyses were performed using SPSS 20.0[38] (IBM, Armonk, NY). With a sample of 893 patients and an expected OR of 1.7, we estimated that the trial would have 98% overall power to show a between-group difference in the primary outcome.
3. Results
3.1. Participant characteristics
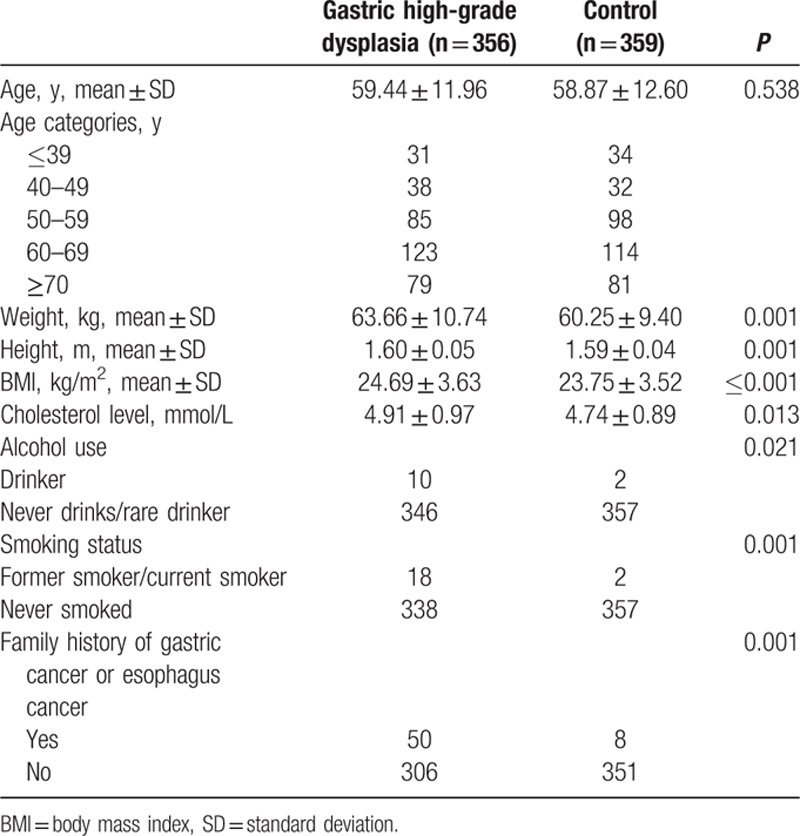
In total, 893 consecutive patients with gastric high-grade dysplasia (537 men and 356 women) and 902 controls (543 men and 359 women) were enrolled in the present study. The baseline characteristics of the patients with gastric high-grade dysplasia and the controls are depicted in Tables 1 and 2. The mean age of patients was 60.75 years for men and 59.44 years for women, whereas the controls had mean ages of 59.95 years for men and 58.87 years for women. In men, the serum total cholesterol levels were 4.58 ± 0.93 and 4.48 ± 0.96 mmol/L for patients and controls, respectively. The women with gastric high-grade dysplasia had a serum total cholesterol level of 4.91 ± 0.97 mmol/L, whereas the serum total cholesterol level of healthy women was 4.74 ± 0.89 mmol/L. Both male and female patients were more likely to have family histories of GC or esophageal cancer than their respective controls. However, women with gastric high-grade dysplasia were more likely to drink and smoke. Men with gastric high-grade dysplasia showed significantly higher BMIs (25.22 ± 3.36 kg/m2) than their respective controls (24.57 ± 3.04 kg/m2, P = 0.001). Women with gastric high-grade dysplasia significantly differed from controls with regard to BMI (24.69 ± 3.63 kg/m2 for patients vs 23.75 ± 3.52 kg/m2 for controls, P < 0.001).
Table 1.
Characteristics of male patients and controls.
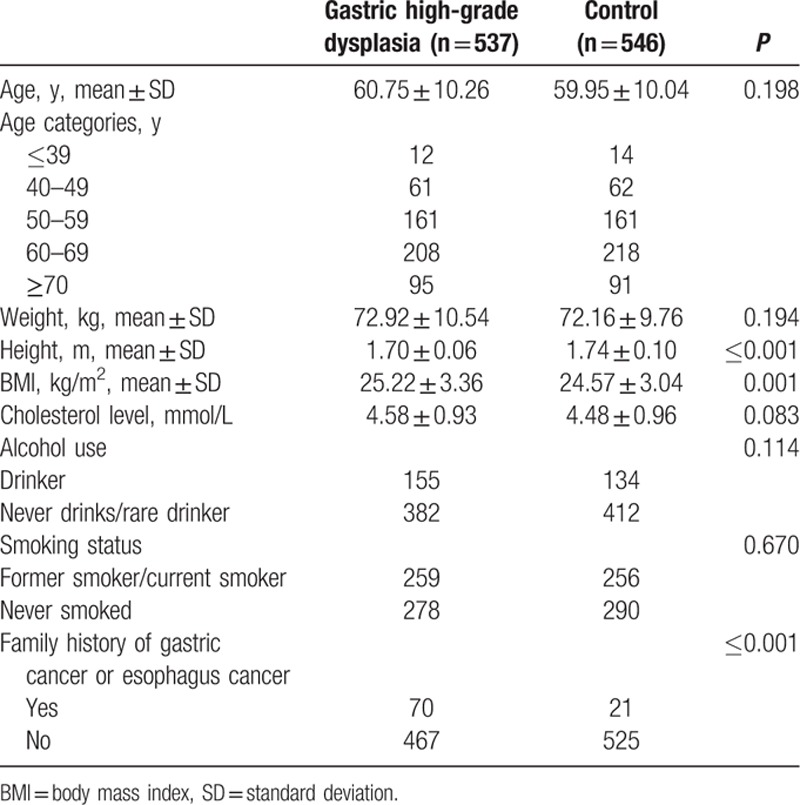
Table 2.
Characteristics of female patients and controls.
3.2. The relationship between BMI and the risk of gastric high-grade dysplasia
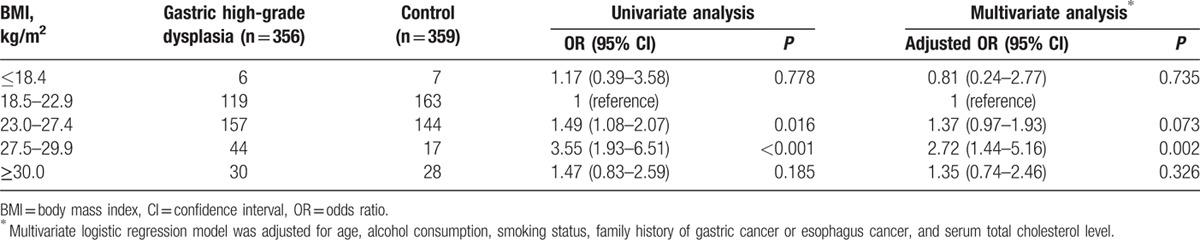
The ORs (adjusted for age, alcohol consumption, smoking status, family history of GC or esophageal cancer, and serum total cholesterol) for gastric high-grade dysplasia based on the BMI categories for men and women are presented in Tables 3 and 4. After adjusting for age, alcohol consumption, smoking status, family history of GC or esophageal cancer, and serum total cholesterol, a BMI ranging from 27.5 to 29.9 was a risk factor for gastric high-grade dysplasia in both men and women. The adjusted ORs were 1.87 (95% CI = 1.24–2.81) for men and 2.72 (95% CI = 1.44–5.16) for women (BMI = 27.5–29.9 vs 18.5–22.9). However, no significant associations were observed between a BMI ranging from 23.0 to 27.4 and the risk of gastric high-grade dysplasia or between the highest BMI category (30.0 or more) and the risk of gastric high-grade dysplasia in either men or women (adjusted for age, alcohol consumption, smoking status, family history of GC or esophageal cancer, and serum total cholesterol).
Table 3.
Relationship between BMI and the risk of gastric high-grade dysplasia in men.
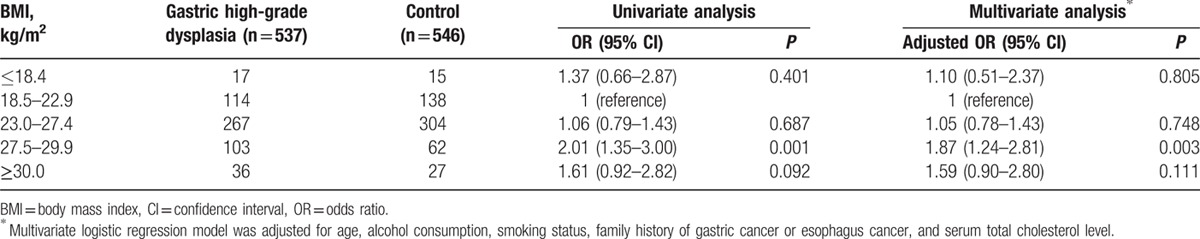
Table 4.
Relationship between BMI and the risk of gastric high-grade dysplasia in women.
3.3. The relationship between BMI and the risk of gastric high-grade dysplasia stratified by the location of the tumor
As shown in Tables 5 and 6, we also examined the relationship between excess BMI and gastric high-grade dysplasia in different anatomic sites in men and women. In men, the adjusted ORs of the BMIs ranging from 27.5 to 29.9 and those above 30.0 regarding gastric cardia high-grade dysplasia compared with the normal weight group (BMI = 18.5–22.9) were 1.78 (95% CI = 1.02–3.10) and 2.54 (95% CI = 1.27–5.08). Similarly, the adjusted OR for BMIs ranging from 27.5 to 29.9 for gastric noncardia high-grade dysplasia was 1.98 (95% CI = 1.25–3.14) in men. In contrast, no associations were observed between a BMI ranging from 23.0 to 27.4 and the risks for gastric noncardia high-grade dysplasia or gastric cardia high-grade dysplasia in men. The highest BMI category (30.0 or more) was also not related to the risk of gastric noncardia high-grade dysplasia in men.
Table 5.
Relationship between BMI and the risk of gastric high-grade dysplasia stratified by the location of the tumor in men.
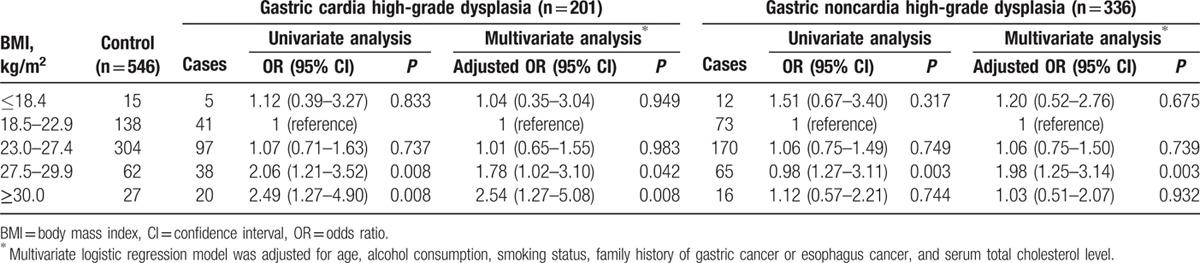
Table 6.
Relationship between BMI and the risk of gastric high-grade dysplasia stratified by the location of the tumor in women.
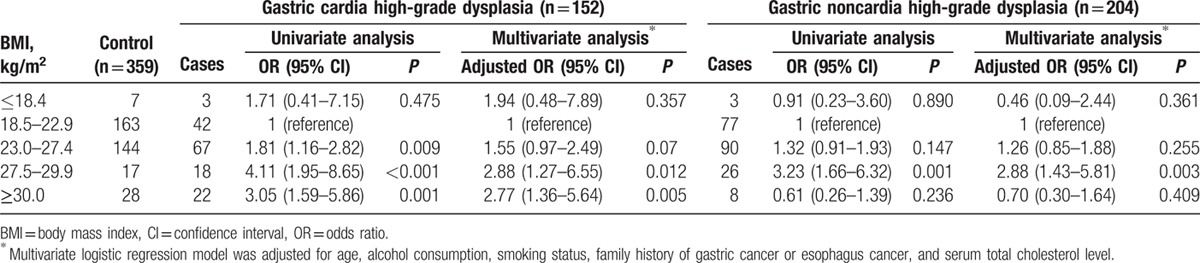
As in men, positive associations between increasing BMI and the risks for gastric cardia and noncardia high-grade dysplasia were observed in women. Compared with the reference group (BMI = 18.5–22.9), the adjusted ORs of the BMIs ranging from 27.5 to 29.9 and those above 30.0 for women with gastric cardia high-grade dysplasia were 2.88 (95% CI = 1.27–6.55) and 2.77 (95% CI = 1.36–5.64). A BMI ranging from 23.0 to 27.4 was not significantly related to an increased risk of gastric cardia high-grade dysplasia in women. Among the women who had gastric noncardia high-grade dysplasia, the adjusted OR of a BMI ranging from 27.5 to 29.9 was 2.88 (95% CI = 1.43–5.81), whereas BMIs ranging from 23.0 to 27.4 or those above 30.0 were not independent risk factors.
3.4. The relationship between serum total cholesterol level and the risk of gastric high-grade dysplasia
In men, serum total cholesterol did not significantly vary between gastric high-grade dysplasia patients and controls (4.58 ± 0.93 vs 4.48 ± 0.96, P = 0.083). Consequently, there were no significant associations between serum total cholesterol level and the increased risk of gastric high-grade dysplasia, including both cardia and noncardia gastric high-grade dysplasia (Tables 7 and 8).
Table 7.
Relationship between serum total cholesterol level and the risk of gastric high-grade dysplasia in men.
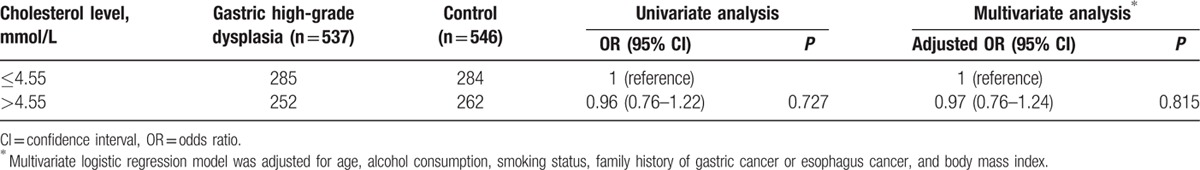
Table 8.
Relationship between serum total cholesterol level and the risk of gastric high-grade dysplasia stratified by the location of the tumor in men.
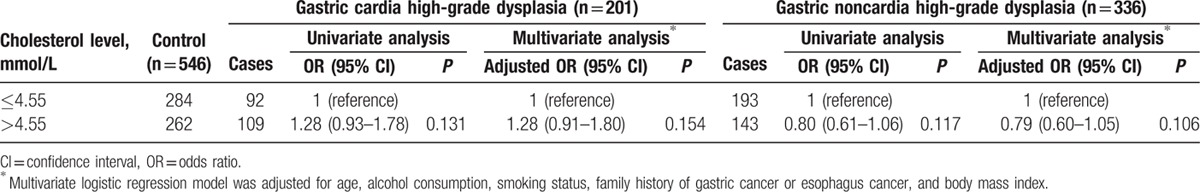
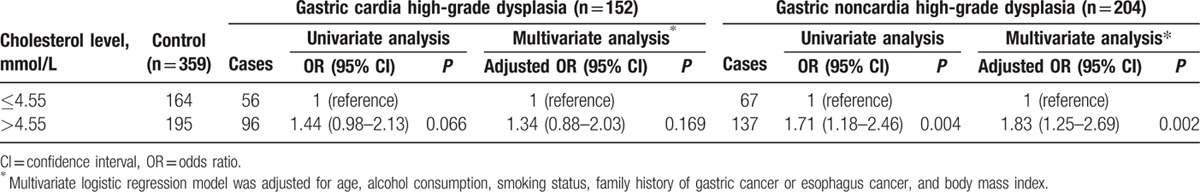
In contrast to men, serum total cholesterol was significantly higher in female patients than in their respective controls (4.91 ± 0.97 vs 4.74 ± 0.89 mmol/L, P = 0.013), specifically in those with gastric noncardia high-grade dysplasia (4.92 ± 0.98 vs 4.74 ± 0.89 mmol/L, P = 0.025) but not those with gastric cardia high-grade dysplasia (4.90 ± 0.96 vs 4.74 ± 0.89 mmol/L, P = 0.066). In addition, high serum total cholesterol (>4.55 mmol/L) was associated with the increased risk of gastric high-grade dysplasia in women after adjusting for age, alcohol consumption, smoking status, family history of GC or esophageal cancer, and BMI. The adjusted ORs were 1.63 (95% CI = 1.19–2.23) for total gastric high-grade dysplasia (Table 9) and 1.83 (95% CI = 1.25–2.69) for gastric noncardia high-grade dysplasia (Table 10). However, higher serum total cholesterol was not a substantial risk factor for gastric cardia high-grade dysplasia in women (adjusted OR = 1.34, 95% CI = 0.88–2.03; Table 10).
Table 9.
Relationship between serum total cholesterol level and the risk of gastric high-grade dysplasia in women.
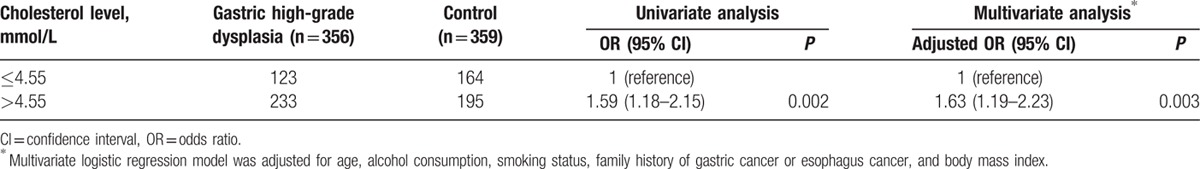
Table 10.
Relationship between serum total cholesterol level and the risk of gastric high-grade dysplasia stratified by the location of the tumor in women.
4. Discussion
This case–control study consistently demonstrated that a BMI ranging from 27.5 to 29.9 was an independent risk factor for gastric high-grade dysplasia in both men and women. Concurrently, the 2 highest BMI categories (27.5–29.9 and 30.0 or more) were positively associated with an increased risk of gastric cardia high-grade dysplasia in both men and women; only a BMI ranging from 27.5 to 29.9 increased the risk of gastric noncardia high-grade dysplasia in both men and women. In addition, we observed that a higher serum total cholesterol level was related to an increased risk of gastric noncardia high-grade dysplasia in women. In contrast, our study did not find an association between serum total cholesterol and gastric cardia high-grade dysplasia in either men or women. The role of serum total cholesterol in gastric cardia high-grade dysplasia requires additional investigation. Old age, alcohol use, cigarette smoking, and family history of GC were proved to be indisputable risk factors for GC. To avoid attenuating the relationships among BMI category, serum total cholesterol level, and the risk of gastric high-grade dysplasia, multivariate logistic regression models were performed to adjust for these potential confounders.
Obesity has been reported to be associated with an increased risk of many types of cancer. Several prospective and retrospective studies have provided support for the hypothesis that higher BMIs in patients are positively associated with a higher incidence of cardiac GC.[17,36,39–44] In contrast, no significant link was found between obesity and an increased risk for noncardiac GC. The mechanisms that underlie obesity-induced GC are under investigation. Gastroesophageal reflux has been commonly accepted as a potential mechanism underlying the relationship between obesity and cardiac GC.[45] In addition, obese patients often have a high level of insulin-like growth factor 1, which has been associated with the development of many types of cancers including GC.[46] In addition, the accumulation of adipose tissue induces imbalances of adiponectin and leptin, which are related to an increased risk for GC.[47–49] Concurrently, obesity is associated with low-grade systemic inflammation, and recent studies have suggested that common inflammatory cytokines such as IL-6, IL-17, TNFα, and MCP-1 can either induce the growth or suppress the apoptosis of human GC cell lines.[50–53]
Previous studies performed in Western countries categorized BMI using the following guidelines proposed by the WHO: <18.5 kg/m2 (underweight), 18.5 to 24.9 kg/m2 (normal weight), 25 to 29.9 kg/m2 (overweight), 30 to 34.9 kg/m2 (obese class I), 35 to 39.9 kg/m2 (obese class II), and 40 kg/m2 and above (obese class III).[37] In certain Asian populations, specific BMIs reflect higher percentages of body fat than they do in White European populations. The proportion of Asians with risk factors for cardiovascular disease and type 2 diabetes was substantial below the current WHO BMI cut-off point of 25 kg/m2. Consequently, the present WHO BMI cut-off does not provide a sufficient basis for taking action on the risks related to overweight and obesity in many Asian populations.[37] In the present analysis, the BMI categories for overweight and obesity were changed to 23.0 to 27.4 kg/m2 (overweight), 27.5 to 29.9 kg/m2 (obesity), and 30.0 kg/m2, and above (severe obesity). The BMI cut-off point of ≥27.5 kg/m2 was used as a cut-off point for obesity.
Relationships between serum total cholesterol and several types of cancer have been observed in modern research. These results suggest a positive association between high serum total cholesterol and an increased risk for rectal cancer.[54] Among smokers, high serum total cholesterol was related to an increased risk of advanced prostate cancer, but high serum high-density lipoprotein (HDL) cholesterol reduced the risk of prostate cancer overall.[55] In contrast, inverse relationships have been found between serum total cholesterol and cancers of the pancreas, liver, intrahepatic bile duct, hematopoietic tissue, and skin (nonmelanoma) among men as well as between serum total cholesterol and cancers of the breast, gallbladder, hematopoietic tissue, and skin (melanoma) among women.[56,57] With regard to gastric malignancy, Tornberg et al[58] found that low serum cholesterol was a risk factor for developing GC. Asano et al[35] observed a similar result, especially with regard to intestinal-type GC. On the contrary, Wulaningsih et al[54] observed a positive association between higher apolipoprotein B/apolipoprotein A–I ratio and the risk of GC. However, no significant association between serum cholesterol levels and GC was found in a Finnish cohort study.[59] The large European cohort, Me-Can, also reported no association between triglycerides and GC risk.[60] Several mechanisms have been hypothesized to explain the relationship between total cholesterol and cancer risk. Hypercholesteremia has been related to increased inflammatory activity, which might be conducive to cancer development.[61] Furthermore, several pathways that play important roles in tumorigenesis, such as the Akt and sonic hedgehog signaling pathways, are cholesterol sensitive. Thus, higher cholesterol levels might induce their pro-carcinogenic activities.
The clinical significance of gastric dysplasia, especially that of gastric high-grade dysplasia, as a direct precursor lesion of GC has been stressed recently. Gastric high-grade dysplasia has progressed to GC in a high percentage of cases (74%),[62] and some lesions with gastric high-grade dysplasia have been associated with submucosal invasion.[63] Consequently, gastric high-grade dysplasia might be considered as a histological marker for gastric carcinoma. However, data were scarce concerning the relationship between obesity and the risk of gastric high-grade dysplasia or the relationship between serum total cholesterol level and the risk of gastric high-grade dysplasia. Kim et al[32] indicated that obesity was related to an increased risk of gastric dysplasia in women. In contrast, 2 other Korean case–control studies indicated no significant association between obesity and gastric dysplasia,[64,65] which was inconsistent with our findings. However, these studies enrolled fewer patients than did the present study, and the previous studies did not focus exclusively on gastric high-grade dysplasia.
In the present study, female patients with higher BMIs showed higher serum total cholesterol levels (Tables S1 and S2). The increase in serum total cholesterol level might be an effect of high BMIs. However, elevated serum total cholesterol was identified as an independent risk factor for gastric noncardia high-grade dysplasia regardless of BMI classification using a multivariate logistic regression model. This observational study cannot establish cause and effect. Several studies have suggested that dyslipidemia might enhance oxidative stress and chronic inflammation, which are highly related to mechanisms linked to carcinogenesis including gastrointestinal malignancy.[66,67] Higher serum total cholesterol was proved to increase the risk of gastric noncardia high-grade dysplasia only in women not man in the present study, which might be attributed to the more smokers and drinkers in male controls compared with female controls. Cigarette smoking and alcohol consumption were associated with high serum non-HDL cholesterol level,[68] which might attenuate the difference of serum total cholesterol between male gastric noncardia high-grade dysplasia patients and controls. The present analysis extended the findings of previous analyses of the association between serum total cholesterol and the risk of gastric dysplasia. Kim identified higher total cholesterol as an independent risk factor for gastric dysplasia with an OR of 6.299 (95% CI = 1.277–31.076),[64] which was consistent with the present study. However, total cholesterol was not associated with an increased risk of gastric dysplasia in the study conducted by Jung; however, gastric dysplasia risk significantly increased among individuals with higher low-density lipoprotein levels.[65] Differences in diet related to GC risk might explain these conflicting findings.
To the best of our knowledge, this study was the first to assess the relationships among BMI, serum total cholesterol level, and the risk of gastric high-grade dysplasia. However, several limitations were present regarding this analysis. First, observational studies cannot establish causal relationships because other associated factors might serve as confounds. Nevertheless, if the main risk for gastric high-grade dysplasia was a higher BMI (which successively increased the risk of GC), then we might expect gastric high-grade dysplasia to lead to weight reduction. Under this scenario, the association between obesity and gastric high-grade dysplasia would become weaker. Helicobacter pylori infection was a well-established risk factor for GC.[69] However, H pylori status as well as environmental, occupational, and dietary exposures were not available for all of the patients or controls. In particular, H pylori status was available for few controls. Consequently, the present study did not exclude confounding because of unmeasured factors (e.g., H pylori infection status, physical activity, and differences in diet and ethnicities). A similar limitation existed for the analyses of serum total cholesterol and gastric high-grade dysplasia risk. Second, we used self-reported body weight and height to calculate the BMIs of the participants who lost weight over the last 3 years. Although self-reported body weight and height are highly consistent with measured body weight and height,[70] the small error that exists is generally systematic, with an overestimation of height and an underestimation of weight, especially at higher weights.[71] Consequently, our measure of BMI most likely underestimated the true BMIs of the participants who lost weight over the last 3 years. In addition, we had no direct measure of lean body mass or central adiposity (e.g., abdominal circumference or waist-to-hip ratio). Third, the present study found that a BMI of 30.0 or more was not an independent risk factor for gastric noncardia high-grade dysplasia in either men or women; the small number of patients with BMIs of 30.0 or more might account for this result. Fourth, serum total cholesterol was determined at a single time point, which might lead to the misclassification of a participant's usual cholesterol level because of intraindividual variation in serum total cholesterol over the long term. Such a misclassification had a tendency to attenuate the observed association.[35] In addition, BMIs were also measured at a single point, although BMI does not typically decrease with age. We collected the usual data for body weight and height before weight loss for participants who lost weight within 3 years, but we were unable to evaluate long-term patterns of BMI.
In summary, the present study suggests that increased BMI is a risk factor for the development of gastric high-grade dysplasia, especially gastric cardia high-grade dysplasia, in both men and women. Concurrently, high serum total cholesterol was related to an increased risk of gastric noncardia high-grade dysplasia in women. Given the limitations of the present study, large-scale and well-designed cohort studies should be conducted to investigate the relationships among obesity, serum total cholesterol level, and the risk of gastric high-grade dysplasia in different populations.
Supplementary Material
Acknowledgments
The authors thank Dr Tao Xu (Department of Epidemiology and Statistics, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and Peking Union Medical College) for the integrity and accuracy of the data analysis.
Footnotes
Abbreviations: BMI = body mass index, GC = gastric cancer, HDL = high-density lipoprotein, OR = odds ratio, WHO = World Health Organization.
The Beijing Municipal Natural Science Foundation of China financially supported the present study (No. 7132209; http://www.bjnsf.org/nsf_xmsq/nsf_zzxm/). The funders played no role in the study design, data collection or analysis, the decision to publish, or the preparation of the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Huang YK, Yu JC, Kang WM, et al. Significance of serum pepsinogens as a biomarker for gastric cancer and atrophic gastritis screening: a systematic review and meta-analysis. PLoS ONE 2015; 10:e0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang YK, Yu JC. Circulating microRNAs and long non-coding RNAs in gastric cancer diagnosis: an update and review. World J Gastroenterol 2015; 21:9863–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 2002; 11:235–256. [DOI] [PubMed] [Google Scholar]
- 4.Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prevent 2014; 23:700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007; 168:1–64. [DOI] [PubMed] [Google Scholar]
- 6.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC Monogr Eval Carcinog Risks Hum 2010; 94:v–vii. [PMC free article] [PubMed] [Google Scholar]
- 7.Bernini M, Barbi S, Roviello F, et al. Family history of gastric cancer: a correlation between epidemiologic findings and clinical data. Gastric Cancer 2006; 9:9–13. [DOI] [PubMed] [Google Scholar]
- 8.La Vecchia C, Negri E, Franceschi S, et al. Family history and the risk of stomach and colorectal cancer. Cancer 1992; 70:50–55. [DOI] [PubMed] [Google Scholar]
- 9.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345:784–789. [DOI] [PubMed] [Google Scholar]
- 10.Khatami F, Karbakhsh M. Socioeconomic position and incidence of gastric cancer: a systematic review and meta-analysis. J Epidemiol Community Health 2015; 69:818–819. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Park S, Nam BH. Gastric cancer and salt preference: a population-based cohort study in Korea. Am J Clin Nutr 2010; 91:1289–1293. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008; 67:253–256. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Zhuang W, Hu W, et al. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011; 141:80–89. [DOI] [PubMed] [Google Scholar]
- 14.Ye W, Chow WH, Lagergren J, et al. Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology 2001; 121:1286–1293. [DOI] [PubMed] [Google Scholar]
- 15.Derakhshan MH, Malekzadeh R, Watabe H, et al. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut 2008; 57:298–305. [DOI] [PubMed] [Google Scholar]
- 16.Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol 2012; 41:1706–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Liu L, Wang X, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prevent 2013; 22:1395–1408. [DOI] [PubMed] [Google Scholar]
- 18.Niknam R, Manafi A, Fattahi MR, et al. The association between gastric endoscopic findings and histologic premalignant lesions in the Iranian rural population. Medicine 2015; 94:e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauwers GY, Riddell RH. Gastric epithelial dysplasia. Gut 1999; 45:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lansdown M, Quirke P, Dixon MF, et al. High grade dysplasia of the gastric mucosa: a marker for gastric carcinoma. Gut 1990; 31:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter 2007; 12:1–15. [DOI] [PubMed] [Google Scholar]
- 23.Ogden CL, Yanovski SZ, Carroll MD, et al. The epidemiology of obesity. Gastroenterology 2007; 132:2087–2102. [DOI] [PubMed] [Google Scholar]
- 24.Di Pietro P, Bavaresco TPF, dos Santos R, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines of women during breast cancer treatment. Faseb J 2015; 29 (1 Supplement):918.917. [Google Scholar]
- 25.Nagle CM, Marquart L, Bain CJ, et al. Impact of weight change and weight cycling on risk of different subtypes of endometrial cancer. Eur J Cancer 2013; 49:2717–2726. [DOI] [PubMed] [Google Scholar]
- 26.Lahmann PH, Cust AE, Friedenreich CM, et al. Anthropometric measures and epithelial ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2010; 126:2404–2415. [DOI] [PubMed] [Google Scholar]
- 27.Robsahm TE, Aagnes B, Hjartaker A, et al. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev 2013; 22:492–505. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11:1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Wang B, Shen F, et al. Body mass index and risk of primary liver cancer: a meta-analysis of prospective studies. Oncologist 2012; 17:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genkinger JM, Spiegelman D, Anderson KE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer 2011; 129:1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose–response meta-analysis of published cohort studies. Int J Cancer 2014; 135:1673–1686. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Kim N, Kim HY, et al. Relationship between body mass index and the risk of early gastric cancer and dysplasia regardless of Helicobacter pylori infection. Gastric Cancer 2015; 18:762–773. [DOI] [PubMed] [Google Scholar]
- 33.Law MR, Wald NJ, Wu T, et al. Systematic underestimation of association between serum cholesterol concentration and ischaemic heart disease in observational studies: data from the BUPA study. Bmj 1994; 308:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherwin RW, Wentworth DN, Cutler JA, et al. Serum cholesterol levels and cancer mortality in 361,662 men screened for the Multiple Risk Factor Intervention Trial. Jama 1987; 257:943–948. [PubMed] [Google Scholar]
- 35.Asano K, Kubo M, Yonemoto K, et al. Impact of serum total cholesterol on the incidence of gastric cancer in a population-based prospective study: the Hisayama study. Int J Cancer 2008; 122:909–914. [DOI] [PubMed] [Google Scholar]
- 36.Kuriyama S, Tsubono Y, Hozawa A, et al. Obesity and risk of cancer in Japan. Int J Cancer 2005; 113:148–157. [DOI] [PubMed] [Google Scholar]
- 37.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. [DOI] [PubMed] [Google Scholar]
- 38.Janitzky AA, Akyol C, Kesapli M, et al. Anterior shoulder dislocations in busy emergency departments: the external rotation without sedation and analgesia (ERWOSA) method may be the first choice for reduction. Medicine 2015; 94:e1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case–control study. Cancer Causes Control 2005; 16:285–294. [DOI] [PubMed] [Google Scholar]
- 40.Merry AH, Schouten LJ, Goldbohm RA, et al. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut 2007; 56:1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prevent 2008; 17:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Su XQ, Wu XJ, et al. Effect of body mass index on adenocarcinoma of gastric cardia. World J Gastroenterol 2003; 9:2658–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang P, Zhou Y, Chen B, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer 2009; 45:2867–2873. [DOI] [PubMed] [Google Scholar]
- 44.Lin XJ, Wang CP, Liu XD, et al. Body mass index and risk of gastric cancer: a meta-analysis. Jpn J Clin Oncol 2014; 44:783–791. [DOI] [PubMed] [Google Scholar]
- 45.Terry P, Lagergren J, Wolk A, et al. Reflux-inducing dietary factors and risk of adenocarcinoma of the esophagus and gastric cardia. Nutr Cancer 2000; 38:186–191. [DOI] [PubMed] [Google Scholar]
- 46.Farahani RK, Azimzadeh P, Rostami E, et al. Evaluation of insulin like growth facror-1 genetic polymorphism with gastric cancer susceptibility and clinicopathological features. Asian Pac J Cancer Prev 2015; 16:4215–4218. [DOI] [PubMed] [Google Scholar]
- 47.Capelle LG, de Vries AC, Haringsma J, et al. Serum levels of leptin as marker for patients at high risk of gastric cancer. Helicobacter 2009; 14:596–604. [DOI] [PubMed] [Google Scholar]
- 48.Kitayama J, Tabuchi M, Tsurita G, et al. Adiposity and gastrointestinal malignancy. Digestion 2009; 79 suppl 1:26–32. [DOI] [PubMed] [Google Scholar]
- 49.Dong Z, Fu S, Xu X, et al. Leptin-mediated regulation of ICAM-1 is Rho/ROCK dependent and enhances gastric cancer cell migration. Br J Cancer 2014; 110:1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kai H, Kitadai Y, Kodama M, et al. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res 2005; 25:709–713. [PubMed] [Google Scholar]
- 51.Kuroda T, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res 2005; 11:7629–7636. [DOI] [PubMed] [Google Scholar]
- 52.Chen J. The IL-23/IL-17 axis may be important in obesity-associated cancer by way of the activation of multiple signal pathways. Int J Obes 2010; 34:1227–1228. [DOI] [PubMed] [Google Scholar]
- 53.Huang YK, Liu H, Wang XZ, et al. Annexin A2 and CD105 expression in pancreatic ductal adenocarcinoma is associated with tumor recurrence and prognosis. Asian Pac J Cancer Prev 2014; 15:9921–9926. [DOI] [PubMed] [Google Scholar]
- 54.Wulaningsih W, Garmo H, Holmberg L, et al. Serum lipids and the risk of gastrointestinal malignancies in the Swedish AMORIS study. J Cancer Epidemiol 2012; 2012:792034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mondul AM, Weinstein SJ, Virtamo J, et al. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control 2011; 22:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strohmaier S, Edlinger M, Manjer J, et al. Total serum cholesterol and cancer incidence in the metabolic syndrome and cancer project (Me-Can). PLoS ONE 2013; 8:e54242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iribarren C, Reed DM, Chen R, et al. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation 1995; 92:2396–2403. [DOI] [PubMed] [Google Scholar]
- 58.Tornberg SA, Carstensen JM, Holm LE. Risk of stomach cancer in association with serum cholesterol and beta-lipoprotein. Acta Oncol 1988; 27:39–42. [DOI] [PubMed] [Google Scholar]
- 59.Knekt P, Reunanen A, Aromaa A, et al. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. J Clin Epidemiol 1988; 41:519–530. [DOI] [PubMed] [Google Scholar]
- 60.Borena W, Stocks T, Jonsson H, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control 2011; 22:291–299. [DOI] [PubMed] [Google Scholar]
- 61.Rivera JJ, Nasir K, Campbell C, et al. Relation of plasma lipoprotein levels with low-grade inflammation in white men without clinical evidence of myocardial ischemia. Am J Cardiol 2007; 100:450–454. [DOI] [PubMed] [Google Scholar]
- 62.Testino G. Gastric precancerous changes: carcinogenesis, clinical behaviour immunophenotype study and surveillance. Panminerva Med 2006; 48:109–118. [PubMed] [Google Scholar]
- 63.Sakurai U, Lauwers GY, Vieth M, et al. Gastric high-grade dysplasia can be associated with submucosal invasion: evaluation of its prevalence in a series of 121 endoscopically resected specimens. Am J Surg Pathol 2014; 38:1545–1550. [DOI] [PubMed] [Google Scholar]
- 64.Kim HY. Metabolic syndrome is associated with gastric dysplasia. Eur J Gastroenterol Hepatol 2011; 23:871–875. [DOI] [PubMed] [Google Scholar]
- 65.Jung MK, Jeon SW, Cho CM, et al. Hyperglycaemia, hypercholesterolaemia and the risk for developing gastric dysplasia. Dig Liver Dis 2008; 40:361–365. [DOI] [PubMed] [Google Scholar]
- 66.Ji Y, Sakata Y, Tso P. Nutrient-induced inflammation in the intestine. Curr Opin Clin Nutr Metab Care 2011; 14:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erdelyi I, Levenkova N, Lin EY, et al. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr 2009; 139:2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wakabayashi I, Groschner K. Age-dependent associations of smoking and drinking with non-high-density lipoprotein cholesterol. Metabolism 2010; 59:1074–1081. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization, International Agency for Research on Cancer. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 70.Stevens J, Keil JE, Waid LR, et al. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol 1990; 132:1156–1163. [DOI] [PubMed] [Google Scholar]
- 71.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348:1625–1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


