Abstract
Background:
The surgical pleth index (SPI) is proposed for titration of analgesic drugs during general anesthesia. Several reports have investigated the effect of SPI on the consumption of opioids including remifentanil, fentanyl, and sufentanil during anesthesia, but there are no reports about oxycodone. We aimed to investigate intravenous oxycodone consumption between SPI-guided analgesia and conventional analgesia practices during sevoflurane anesthesia in patients undergoing thyroidectomy.
Methods:
Forty-five patients undergoing elective thyroidectomy were randomly assigned to an SPI group (SPI-guided analgesia group, n = 23) or a control group (conventional analgesia group, n = 22). Anesthesia was maintained with sevoflurane to achieve bispectral index values between 40 and 60. In the SPI group, oxycodone 1 mg was administered intravenously at SPI values over 50; in the control group, oxycodone 1 mg was administered intravenously at the occurrence of tachycardia or hypertension event. Intraoperative oxycodone consumption and extubation time were recorded. The number of hemodynamic and somatic movement events was recorded, as were postoperative pain and recovery scores.
Results:
Patients’ characteristics were comparable between the groups. Intraoperative oxycodone consumption in the SPI group was significantly lower than the control group (3.5 ± 2.4 vs 5.1 ± 2.4 mg; P = 0.012). Extubation time was significantly shorter in the SPI group (10.6 ± 3.5 vs 13.4 ± 4.6 min; P = 0.026). Hemodynamic and somatic movement events during anesthesia were comparable between the groups, as were numeric rating scales for pain and modified Aldrete scores at postanesthesia care unit.
Conclusions:
SPI-guided analgesia reduces intravenous oxycodone consumption and extubation time compared with conventional analgesia based on clinical parameters during sevoflurane anesthesia in patients undergoing thyroidectomy.
Keywords: analgesia, general anesthesia, inhalation anesthetics, oxycodone, photoplethysmography, pulse oximetry, sevoflurane
1. Introduction
Maintaining a balance between nociceptive stimuli and antinociceptive drugs is essential for providing optimal individual titration of analgesic administration during general anesthesia; however, monitoring to guide analgesic administration remains a challenge.[1,2]
The surgical pleth index (SPI) is a monitoring tool derived from finger photoplethysmographic signals for titration of analgesic administration during general anesthesia.[2–4] SPI has been presented to correlate with both surgical stimuli and analgesic dosing, and, compared with standard evaluation of clinical parameters during total intravenous anesthesia (TIVA), and consequently, SPI has been demonstrated to be more predictable for measuring autonomic responses to nociceptive stimuli and providing effective analgesia.[5–7] There are several reports about the clinical effectiveness of SPI on titration of remifentanil and sufentanil during anesthesia,[5,6,8] but no such report about oxycodone. In TIVA using propofol and remifentanil, SPI-guided opioid administration could reduce opioid consumption, and thus it could reduce extubation time.[6] Remifentanil has a rapid onset and ultrashort duration of action; in comparison, oxycodone has a relatively slower onset and longer duration times, similar to those of sufentanil or fentanyl.[9] Furthermore, unlike TIVA, the effectiveness of SPI-guided analgesia during volatile-based anesthesia may not be valid.[4] Therefore, we aimed to investigate the utility of SPI for analgesia with oxycodone during sevoflurane anesthesia as a clinical setting of balanced anesthesia, one of the most common anesthetic techniques.
The aim of this prospective randomized controlled study was to compare intravenous oxycodone consumption between SPI-guided analgesia and conventional analgesia practices during sevoflurane anesthesia in patients undergoing thyroidectomy. We hypothesized that SPI-guided analgesia would decrease intraoperative oxycodone consumption and shorten extubation time without an increase in unwanted hemodynamic and somatic response events during sevoflurane anesthesia in patients undergoing thyroidectomy.
2. Materials and methods
The trial was registered in the UMIN clinical trials registry (unique trial number: UMIN000015683; registration number: R000018135; date of registration: November 13, 2014) and it was a single-site study performed at Korea University Guro Hospital, Seoul, Republic of Korea from December 2014 to March 2015. All patients were recruited from the Department of Otolaryngology and Breast Endocrine Surgery, Korea University Guro Hospital. Patients were enrolled in the study upon admission to the hospital the day before surgery. After obtaining approval of the Korea University Guro Hospital Institutional Review Board and written informed consent from all the patients, we enrolled 48 patients (age 20–65 years) undergoing thyroidectomy, with an American Society of Anesthesiologists physical status classification of I or II. Exclusion criteria were a history of cardiovascular disease, with the exception of well-controlled hypertension; renal, neuromuscular, or neurological disease; abuse of alcohol or illicit drugs; medication that may affect autonomic regulation (e.g., β-blocker, clonidine); and pregnancy.
All patients received intramuscular midazolam 2 mg for premedication 30 minutes before induction of anesthesia. For randomization, a computer-generated list that is in a website for randomization (www.randomization.com) was used, and the random numbers were kept in opaque sealed envelopes. Generation of the random allocation sequence, enrollment of participants and assignment of participants to interventions were performed by a single investigator not involved in the anesthetic procedure and assessment of the participants during the study period. In the operating room, intraoperative monitoring devices (e.g., devices for noninvasive blood pressure measurement, electrocardiogram leads, pulse oximeters [CARESCAPE monitor B650, GE Healthcare Finland Oy, Finland] and bispectral index [BIS] monitors [BIS-Vista, Aspect Medical Systems, Newton, MA]) were attached to all patients, and the patients were randomized either to the SPI group (SPI-guided analgesia group) or the control group (conventional analgesia group). The SPI sensor was attached to all patients on an index finger. The baseline values for mean arterial blood pressure (MAP), heart rate (HR), SPI, and BIS were recorded before anesthesia induction.
All anesthetic procedures were carried out by 2 anesthesiologists. One independent anesthesiologist not involved in the study and not blinded to the group allocation performed anesthesia induction and maintenance and analgesic administration. The other blinded independent anesthesiologist assessed extubation time and postoperative pain in patients during the emergence and recovery phases.
Patients were preoxygenated for 5 minutes with 100% oxygen. Then, intravenous oxycodone 1 mg and thiopental sodium 5 mg/kg were administered for anesthesia induction. After loss of consciousness, each patient received rocuronium 0.6 mg/kg and the trachea was intubated. Ventilation was adjusted with 50% oxygen in air to maintain a partial pressure of end-tidal carbon dioxide between 30 and 35 mm Hg. In all patients, sevoflurane was started initially with 2 vol% and adjusted to maintain a BIS value between 40 and 60.
The SPI was calculated using methodology described previously.[3] SPI values range from 0 to 100, with higher scores indicating higher stress level. A value of 50 indicates a mean stress level during anesthesia, and an SPI range between 20 and 50 has been used to guide analgesia.[5] The guideline of analgesic administration in each group was as follows: in the SPI group, an oxycodone 1 mg bolus was given at a 5-minute interval, at each occurrence of an SPI value > 50 for more than 15 seconds. In the control group, an oxycodone 1 mg bolus was administered at a 5-minute interval—based on the change in hemodynamic parameters—at the occurrence of tachycardia or hypertension event. In both groups, a rescue medication (additional thiopental sodium 1 mg/kg) was applied when somatic movement such as coughing, grimacing, and effort of breathing occurred, despite BIS and SPI values within the predefined range during the surgery.[5]
Hemodynamic instability events were defined identically for both groups. Tachycardia was defined as HR of >90 beats per minute, if baseline HR was below 75 beats per minute, or as an increase of over 20% of the baseline HR, if it was >75 beats per min. Hypertension was defined as MAP over 100 mm Hg, if baseline MAP was < 83 mm Hg, or as an increase of >20% of the baseline MAP, if it was >83 mm Hg.
Bradycardia was defined as HR <45 beats per minute. Hypotension was defined as MAP <60 mm Hg. For the SPI group patients, labetalol 5 mg was administered at a 5-minute interval at the occurrence of tachycardia or hypertension event, despite an SPI value <50. For the control-group patients, labetalol 5 mg was administered if hypertension or tachycardia was persistent despite 3sequential oxycodone bolus doses (total 3 mg) within 15 minutes.
Rocuronium 5 mg was administered every 30 minutes until the removal of the thyroid gland was completed and neuromuscular monitoring was performed by using train-of-four (TOF) stimulation. After the end of skin suture, pyridostigmine 10 mg and glycopyrrolate 0.4 mg were administered for reversal of neuromuscular blockade after confirming TOF count of 4. In addition, sevoflurane was discontinued at the end of skin suture, and fresh gas flow was increased to 8 L/min. The extubation was performed when the respiration and consciousness of the patient were recovered, and extubation time was measured from the discontinuation of anesthetics to extubation by the investigator blinded to the patient's assignment.
All patients were transferred to the postanesthesia care unit (PACU) after extubation. In the PACU, the same investigator who was blinded to the patient's assignment assessed the recovery score and pain score. A modified Aldrete score and numeric rating scale (NRS) for pain (0–10) were recorded in each 10 minutes from arrival of PACU until 60 minutes. If NRS was >5, ketorolac 15 mg was administered and repeated at a 10-min interval. Postoperative nausea (none, slight, moderate, severe) and vomiting (yes, no) and other adverse events, including respiratory depression, also were monitored. Metoclopramide 10 mg was administrated when patients complained of nausea or when vomiting occurred. The total cumulative amounts of rescue ketorolac and incidence of rescue metoclopramide administration were recorded.
The primary endpoint of this study was to evaluate intraoperative oxycodone consumption; secondary endpoints were extubation time, the incidences of intraoperative hemodynamic events including hypertension or tachycardia, and somatic movement, trends of intraoperative SPI, BIS, a partial pressure of end-tidal carbon dioxide, changes of the recovery score (a modified Aldrete score) and NRS for pain, total rescue analgesic consumption and incidences of rescue antiemetic administration and adverse events in the PACU.
2.1. Statistical analysis
The primary endpoint in this study was the oxycodone consumption administered during surgery. The sample size calculation was based on the results of a pilot study with 6 cases in each group. In the pilot study, intraoperative oxycodone doses (mean ± standard deviation) were 5.0 ± 1.4 mg in the SPI group and 6.5 ± 1.8 mg in the control group, respectively. Therefore, the effect size of 2-groups was 0.93. On the assumption that the allocation ratio of 2-groups was 1, a sample size for each group was 20, calculated by Student's t test, 2-sided test, a level of significance of 0.05, and a power of 0.8. Considering a 20% dropout rate, the sample size for final enrollment was 24 in each group (total 48 patients).
Statistical analysis was performed with SPSS 18 software (SPSS Inc., IBM, Chicago, IL). Continuous data such as intraoperative total oxycodone consumption, extubation time, intraoperative labetalol dose, and total ketorolac consumption in the PACU were analyzed using the 2-tailed Student's t test (normally distributed data) or the Mann–Whitney U test (abnormally distributed data). Incidences of intraoperative hemodynamic and somatic movement events between the groups were compared using a chi-squared test or Fisher's exact test. Postoperative nausea and vomiting and incidence of rescue metoclopramide administration also were compared by the χ2 test. Trends of SPI, a partial pressure of end-tidal carbon dioxide and BIS values during surgery, and changes of a modified Aldrete score and NRS for pain in the PACU were compared between the groups by repeated measures analysis of variance (ANOVA) factoring for time and group assignment. The data are expressed as the mean ± standard deviation, median [25; 75 quartiles], or number of patients (%). A P value < 0.05 was considered statistically significant.
3. Results
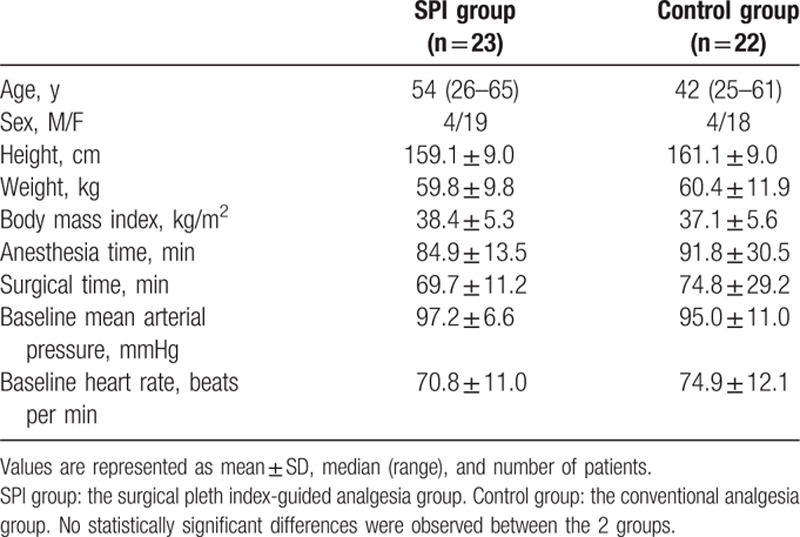
A total of 48 patients were enrolled in this study, and 3 patients were excluded for noncompliance with the study protocol. Finally, 45 patients were evaluated (23 patients in the SPI group and 22 patients in the control group) (Fig. 1). There was no significant difference between the 2 groups in patients’ characteristic data, surgical time, anesthesia time, and baseline MAP, or baseline HR (Table 1).
Figure 1.
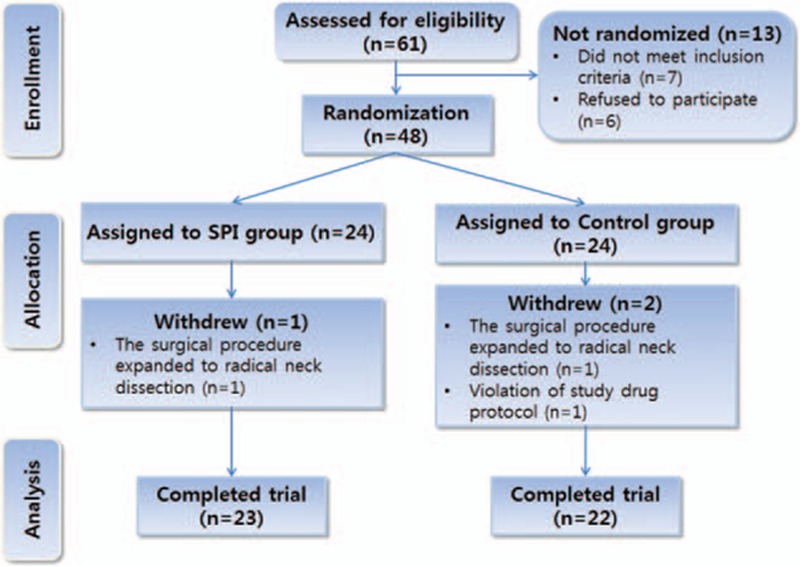
A flowchart describing patient recruitment, randomization, and withdrawal. Initially, 48 patients were randomly assigned to 1 of 2 groups as follows: the surgical pleth index-guided analgesia group (SPI group) or the conventional analgesia group (control group). Finally, 45 patients (23 in the SPI group and 22 in the control group) completed this study. SPI = surgical pleth index.
Table 1.
Demographic and clinical data.
Intraoperative oxycodone dose was significantly lower in the SPI group than in the control group (3.5 ± 2.4 vs 5.1 ± 2.4 mg, respectively; mean difference: –1.61 (95% confidence interval [CI]: –2.86 to –0.37, P = 0.012; Table 2).
Table 2.
Oxycodone consumption, extubation time, hemodynamic events, somatic movement, and labetalol consumption during surgery.
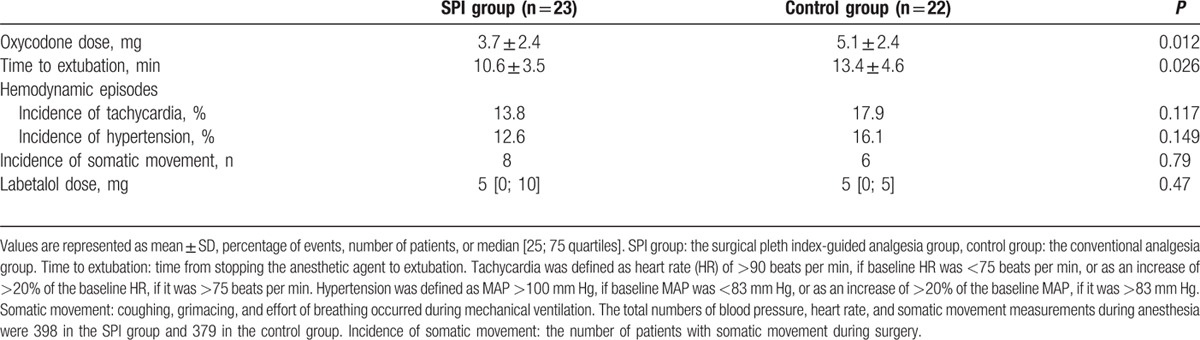
The time to extubation was significantly shorter in the SPI group than in the control group (10.6 ± 3.5 vs 13.4 ± 4.6 min, respectively; mean difference: –2.80 [95% CI: –5.25 to –0.35], P = 0.026). The total numbers of blood pressure and heart rate measurements during anesthesia were 398 in the SPI group and 379 in the control group. The number of hypertension events in the SPI group was 55 (13.8%), whereas that in the control group was 68 (17.9%) (P = 0.117). Similarly, the number of tachycardia events in the SPI group was 49 (12.6 %), and that in the control group was 61 (16.1 %) (P = 0.149). The number of patients with somatic movement during surgery was 8 in the SPI group and 6 in the control group (P = 0.79). Incidences of intraoperative hemodynamic events such as hypertension or tachycardia and somatic movement events were comparable between the 2 groups. Total labetalol dose during the surgery was not significantly different between the 2 groups (5 [0; 10] vs 5 [0; 5], respectively, P = 0.47; Table 2).
Trends of SPI, BIS, and a partial pressure of end-tidal carbon dioxide during anesthesia are presented in Fig. 2 and, for all variables, there were no significant differences between the groups (P = 0.756, 0.78, 0.103, respectively).
Figure 2.
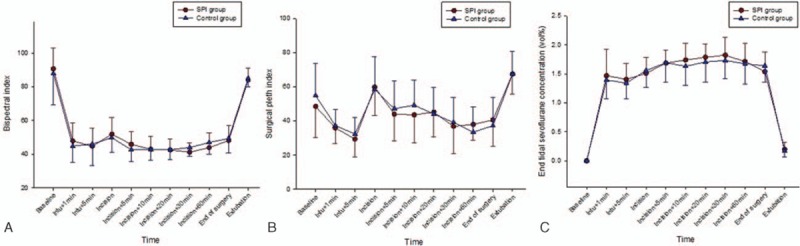
Change of bispectral index (BIS) (A), surgical pleth index (SPI) values (B), and end-tidal sevoflurane concentrations (C) at major time points during general anesthesia in each group. The graphs show mean values ± standard deviation of each variable for every time points during general anesthesia. All data were collected at baseline, 1 minute after intubation (Intu+1 min), 5 minute after intubation (Intu+5 min), incision, 5 minute after incision (incision+5 min), 10 minute after incision (incision+10 min), 20 minute after incision (incision+20 min), 30 minute after incision (incision+30 min), 60 minute after incision (incision+60 min), end of surgery, and extubation. BIS values were well maintained between 40 and 60 during the surgery in both groups. For the SPI value and end-tidal sevoflurane concentration, there were no significant differences between the groups. SPI group: the surgical pleth index-guided analgesia group; control group: the conventional analgesia group. BIS = bispectral index, SPI = surgical pleth index.
In the PACU, trends of the recovery score (a modified Aldrete score) and NRS for pain were comparable between the groups (P = 0.154, 0.941, respectively; Fig. 3).
Figure 3.
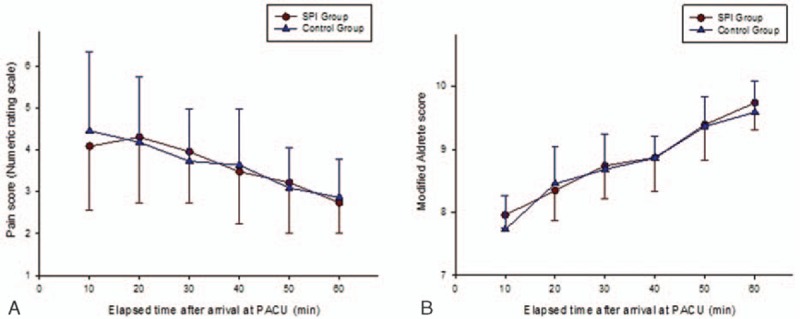
Change of numeric rating scale (NRS) scores (A) and modified Aldrete scores (B) at postanesthesia care unit (PACU) in each group. The graphs show mean values ± standard deviation of each variable for every time points. For each score, there were no significant differences between the groups. SPI group: the surgical pleth index-guided analgesia group, control group: the conventional analgesia group. NRS = numeric rating scale, PACU = postanesthesia care unit, SPI = surgical pleth index.
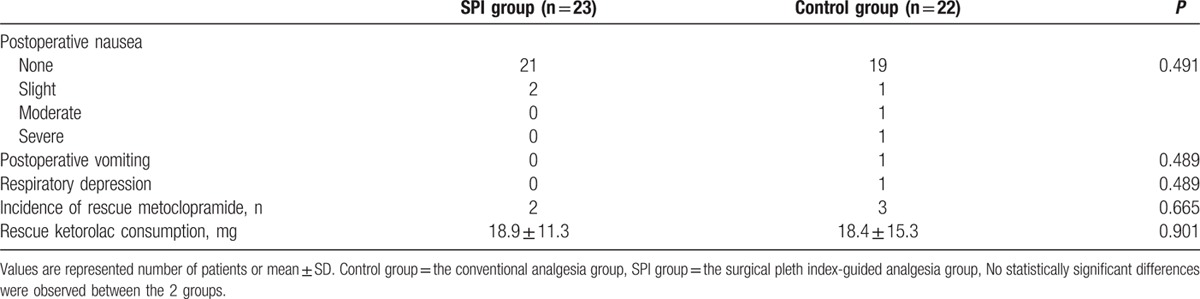
Regarding adverse events, in the SPI group, only 2 cases of slight nausea without vomiting occurred in the PACU. In the control group, 1 case of transient respiratory depression and 1 case each of severe, moderate, and slight nausea were reported, as was 1 case of vomiting. There were no significant differences between the groups for all adverse events, which generally were infrequent in both groups. Rescue ketorolac consumption was not significantly different between the SPI and control groups (18.9 ± 11.3 vs 18.4 ± 15.3 mg, respectively; mean difference: 0.50 (95% CI: –7.55 to 8.56, P = 0.901). Moreover, the number of patients given rescue metoclopramide was not significantly different between the SPI and control groups (2 vs 3, respectively; P = 0.665) (Table 3).
Table 3.
Postoperative nausea and vomiting, total antiemetic, and analgesic consumption.
4. Discussion
In this study, we found that SPI-guided oxycodone administration during general anesthesia for thyroidectomy reduced cumulative intravenous oxycodone consumption during surgery and shortened the time of extubation, without compromising postoperative pain and recovery in PACU. These results suggest several important points about SPI-guided analgesia.
Firstly, in SPI-guided analgesia, intravenous oxycodone administration with an intermittent bolus dose as well as remifentanil infusion can be effective for titration of analgesia. Previously, analgesic drug administration during general anesthesia had been guided clinically by somatic or autonomic responses such as movement, sweating, lacrimation, increase of HR, or arterial blood pressure (conventional analgesic practices). However, the superiority of SPI to conventional analgesic practices has been demonstrated for managing the nociception-antinociception balance or detecting the intensity of both surgical stimuli and antinociceptor drug effect.[7,10,11] Until now, the studies on the clinical utility of SPI have been performed mainly in the settings of TIVA with propofol and remifentanil in adult patients undergoing general anesthesia.[5,6] Those studies have shown that SPI-guided titration of remifentanil infusion during general anesthesia can reduce intraoperative remifentanil consumption without difference in postoperative pain intensity.25,[6] In contrast, there are few reports of studies of other opioids administered via the intermittent bolus method for titration of analgesia by SPI guidance.[8] Gruenewald et al[8] reported that SPI-guided intermittent bolus administration of sufentanil was clinically feasible but was not able to significantly reduce intraoperative sufentanil consumption. However, the present study demonstrated that SPI-guided administration of intravenous oxycodone with an intermittent bolus dose can reduce cumulative oxycodone consumption during anesthesia without an increase in postoperative pain, compared with conventional analgesic practices, in patients undergoing thyroidectomy.
Secondly, SPI-guided analgesia can be effective in general anesthesia using volatile anesthetics such as sevoflurane as well as TIVA. The study by Gruenewald et al[8] that used sufentanil as an analgesic and sevoflurane as a general anesthetic suggested that different anesthesia regimens have different impact on beneficial effects of SPI guidance, thereby raising questions over the efficacy of SPI guidance in anesthesia using volatile anesthetics in contrast to TIVA. In contrast, the present study indicated that SPI-guided oxycodone administration can provide effective analgesia during sevoflurane anesthesia.
Thirdly, a target range of SPI between 20 and 50 is adequate for titration of intravenous oxycodone. Previous studies on the utility of SPI all used the SPI target range of 20 to 50.[5,7,11,12] Some of these studies reported that the target range of SPI between 20 and 50 had been suboptimal for the analgesic titration.[13,14]
Fourthly, SPI-guided administration of intravenous oxycodone can reduce extubation time compared with conventional analgesic practices. Although we should consider many clinical aspects including the study patient group, types of surgery, and anesthetics’ effects on recovery times, several previous studies on the utility of SPI reported that SPI-guided analgesia could not reduce recovery times, as measured by extubation time or emergence time.[5,8,15]
Lastly, SPI-guided administration of intravenous oxycodone can be safe with regard to postoperative adverse events associated with opioids. Intraoperative opioid bolus administration can cause overdose-related problems such as respiratory depression, sedation, bradycardia, and nausea or vomiting at an immediate postoperative period in a dose-dependent correlation.[16,17] The risk can be greater with the use of opioids with a quite long duration such as oxycodone (the onset time of 2–3 minute after injection, a half-life: 4 hours 52 minutes[9]), whereas the surgical stress not attenuated with opioids also results in other complications in the long-term postoperative recovery period.[17,18] Our study suggests that SPI-guided bolus administration of oxycodone can titrate adequately the quantity of individualized oxycodone consumption, leading to favorable PACU outcomes.
A limitation in this study was administration of labetalol that may affect the SPI value. Labetalol has a long elimination half-life (> 5 h) as compared to shorter acting agents such as esmolol. It is conceivable that in some patients in whom labetalol was used, might not have demonstrated subsequent elevations of HR and blood pressure despite nociceptive perceptions, thus leading to fewer oxycodone dosing in the control group, where such dosing depended entirely on HR and blood pressure changes. Thus, we might have been better served using esmolol instead of longer acting agents such as labetalol, which might have been easier to titrate. Nevertheless, labetalol can attenuate MAP and HR via combined α- and β-receptor blockade (mainly via β-blockade at low dose) without analgesic effect.[19] Ahonen et al[20] reported that beta blockade does not affect SPI values because effects of beta blockade target only the beta receptors of the cardiovascular system, rather than exerting an effect of blunting nociceptive reaction. Furthermore, in this study, labetalol cumulative dose during surgery was small in both groups (median 5 mg, respectively) and it was not significantly different between the groups. Also, the administration of labetalol was essential for maintaining the patients’ hemodynamics stable. Considering these points, we suggest that labetalol might have little effect on SPI values, thus indicating that SPI values in the SPI-guided analgesia group could truly reflect the nociception during anesthesia.
Another limitation in the study was use of thiopental sodium bolus as a rescue medication of somatic movement occurred during the surgery. Although the incidence of intraoperative somatic movement events was not high in both groups and it was comparable between the 2 groups, barbiturates such as thiopental sodium sometimes appear to lower the pain threshold. Thus, it may be argued that this pharmacological effect of thiopental sodium might potentially have affected the use of incremental doses of oxycodone in patients who received thiopental sodium. In addition, barbiturates do not produce muscle relaxation, but some, such as methohexital, might produce involuntary muscle contractions.
In conclusion, intravenous oxycodone bolus administration by SPI guidance reduces cumulative oxycodone consumption during surgery and shortens extubation time without negative impact on pain scores and adverse events in the PACU, compared with conventional analgesia practices by clinical parameters, during sevoflurane anesthesia in patients undergoing thyroidectomy. We suggest that SPI guidance can be useful for analgesic titration with intravenous oxycodone during sevoflurane anesthesia as a clinical setting of balanced anesthesia, one of the most common anesthetic techniques.
Footnotes
Abbreviations: ANOVA = analysis of variance, BIS = bispectral index, CI = confidence interval, HR = heart rate, MAP = mean arterial blood pressure, NRS = numeric rating scale, PACU = postanesthesia care unit, SPI = surgical pleth index, TIVA = total intravenous anesthesia, TOF = train-of-four.
Authorship: YJW—patient recruitment, data collection, data analysis, and writing up of the first draft of the paper; BGL—study design, data collection, data analysis, and revision of the first draft of the paper; SHL, SP, and HK—study design, data analysis, and revision of the first draft of the paper; IOL—patient recruitment, data collection, and data analysis; MHK—patient recruitment and data collection. All authors approved the final manuscript.
The trial was registered in the UMIN clinical trials registry (unique trial number: UMIN000015683; registration number: R000018135; date of registration: November 13, 2014).
The authors have no funding and conflicts of interest to disclose.
References
- 1.Gruenewald M, Ilies C. Monitoring the nociception-anti-nociception balance. Best Pract Res Clin Anaesthesiol 2013; 27:235–247. [DOI] [PubMed] [Google Scholar]
- 2.Hamunen K, Kontinen V, Hakala E, et al. Effect of pain on autonomic nervous system indices derived from photoplethysmography in healthy volunteers. Br J Anaesth 2012; 108:838–844. [DOI] [PubMed] [Google Scholar]
- 3.Huiku M, Uutela K, van Gils M, et al. Assessment of surgical stress during general anaesthesia. Br J Anaesth 2007; 98:447–455. [DOI] [PubMed] [Google Scholar]
- 4.Thee C, Ilies C, Gruenewald M, et al. Reliability of the surgical pleth index for assessment of postoperative pain: a pilot study. Eur J Anaesthesiol 2015; 32:44–48. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Thee C, Gruenewald M, et al. Comparison of surgical stress index-guided analgesia with standard clinical practice during routine general anesthesia: a pilot study. Anesthesiology 2010; 112:1175–1183. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann I, Gohner A, Crozier TA, et al. Surgical pleth index-guided remifentanil administration reduces remifentanil and propofol consumption and shortens recovery times in outpatient anaesthesia. Br J Anaesth 2013; 110:622–628. [DOI] [PubMed] [Google Scholar]
- 7.Struys MM, Vanpeteghem C, Huiku M, et al. Changes in a surgical stress index in response to standardized pain stimuli during propofol-remifentanil infusion. Br J Anaesth 2007; 99:359–367. [DOI] [PubMed] [Google Scholar]
- 8.Gruenewald M, Willms S, Broch O, et al. Sufentanil administration guided by surgical pleth index vs standard practice during sevoflurane anaesthesia: a randomized controlled pilot study. Br J Anaesth 2014; 112:898–905. [DOI] [PubMed] [Google Scholar]
- 9.Koch S, Ahlburg P, Spangsberg N, et al. Oxycodone vs. fentanyl in the treatment of early post-operative pain after laparoscopic cholecystectomy: a randomised double-blind study. Acta Anaesthesiol Scand 2008; 52:845–850. [DOI] [PubMed] [Google Scholar]
- 10.Wennervirta J, Hynynen M, Koivusalo AM, et al. Surgical stress index as a measure of nociception/antinociception balance during general anesthesia. Acta Anaesthesiol Scand 2008; 52:1038–1045. [DOI] [PubMed] [Google Scholar]
- 11.Gruenewald M, Meybohm P, Ilies C, et al. Influence of different remifentanil concentrations on the performance of the surgical stress index to detect a standardized painful stimulus during sevoflurane anaesthesia. Br J Anaesth 2009; 103:586–593. [DOI] [PubMed] [Google Scholar]
- 12.Colombo R, Raimondi F, Corona A, et al. Comparison of the surgical pleth index with autonomic nervous system modulation on cardiac activity during general anaesthesia: a randomised cross-over study. Eur J Anaesthesiol 2014; 31:76–84. [DOI] [PubMed] [Google Scholar]
- 13.Ledowski T, Ang B, Schmarbeck T, et al. Monitoring of sympathetic tone to assess postoperative pain: skin conductance vs surgical stress index. Anaesthesia 2009; 64:727–731. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Lim BG, Kim H, et al. Comparison of surgical pleth index-guided analgesia with conventional analgesia practices in children: a randomized controlled trial. Anesthesiology 2015; 122:1280–1287. [DOI] [PubMed] [Google Scholar]
- 15.Colombo R, Raimondi F, Rech R, et al. Surgical pleth index guided analgesia blunts the intraoperative sympathetic response to laparoscopic cholecystectomy. Minerva Anestesiol 2015; 81:837–845. [PubMed] [Google Scholar]
- 16.Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth 2005; 94:505–513. [DOI] [PubMed] [Google Scholar]
- 17.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997; 78:606–617. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Thee C, Gruenewald M, et al. Correlation of surgical pleth index with stress hormones during propofol-remifentanil anaesthesia. Sci World J 2012; 2012:879158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magee LA, Namouz-Haddad S, Cao V, et al. Labetalol for hypertension in pregnancy. Expert Opin Drug Saf 2015; 14:453–461. [DOI] [PubMed] [Google Scholar]
- 20.Ahonen J, Jokela R, Uutela K, et al. Surgical stress index reflects surgical stress in gynaecological laparoscopic day-case surgery. Br J Anaesth 2007; 98:456–461. [DOI] [PubMed] [Google Scholar]


